Abstract
This paper presents the results of a phytosociological study on the Afroalpine vegetation of the Ruwenzori Mountains, one of the most prominent mountain ranges in Africa. This study marks the pioneering comprehensive investigation into the plant communities of this region, which holds significant phytogeographic importance. Through statistical analyses, eight distinct plant communities, three new alliances, two new orders, and one new class were identified within the altitudinal range of 3500 to 4600 m above sea level. These communities are well-defined from both floristic and ecological perspectives. Hierarchical classification was conducted using the quantitative Sørensen (Bray-Curtis) distance measure and the beta flexible linkage method. Furthermore, indicator species for each group were determined by calculating fidelity and constancy (occurrence frequency) within the classified dataset. To assess the validity of the classification results, non-metric multidimensional scaling (NMDS) was carried out. These analyses provide the first phytosociological arrangement of the Afroalpine vegetation of the Ruwenzori Mountains, providing a solid framework and valuable insights into its floristic and ecological characteristics.
1. Introduction
The Ruwenzori Mountains, located along the border between Uganda and the Democratic Republic of Congo, are one of the main high-altitude areas of Africa. In fact, this mountain range reaches an altitude of 5109 m above sea level (ASL) at the top of Mount Stanley (Cima Margherita), making it the third-highest peak on the African continent, after Kilimanjaro (5895 m) and Mount Kenya (5199 m). The vegetation of this area was first documented by European explorers [1], who noted the presence of distinct altitudinal belts with very characteristic plant communities. The upper limit of the montane rainforest generally occurs between 2400 and 2700 m [2,3], followed by a bamboo-dominated belt and ericaceous forests and scrublands extending up to 3000–3100 m. At higher elevations, a woodland dominated by Rapanea rhododendroides (Gilg) Mez and Hagenia abyssinica (Bruce) J.F.Gmel becomes prevalent, while, above 3600–3800 m, the Afroalpine belt begins, extending nearly to the highest peaks, which are characterized by the presence of glaciers. From a phytogeographic point of view, the upper areas of the Ruwenzori Mountains hold significant importance due to their distinctive Afroalpine flora, with a high presence of local endemics [4]. In fact, the Afroalpine region consists of isolated areas surrounded by a vast expanse of tropical and subtropical climate zones [5]. This flora likely originated in the cold-temperate areas of Eurasia and spread southwards over the past 5 million years through several colonization events [6,7]. The flora of the Afroalpine areas shows numerous examples of evolutionary convergence, particularly within genera such as Dendrosenecio (Hauman ex Hedberg) B. Nord. and Lobelia L., which are notable for unusual phenomena of gigantism, contributing to the unique physiognomy of Afroalpine vegetation [4,8]. The first studies specifically addressing the alpine vegetation of the Ruwenzori were carried out by Woosnam [9], followed by Engler [10] and Ross [2,3], who provided initial data on the main life zones and altitudinal belts. More detailed and in-depth surveys of plant communities were later carried out by Hedberg [4] in his extensive review of the Afroalpine flora and vegetation. Additional local data have been reported by Loveridge [11] for the Nyamagasani Valley, Schmidt & Beck [12] for the Butawu Valley, and Fishlock & Hancock for the Bujuku Valley [13]. Raimànkovà & Rejmànek [14] also provide limited information on the vegetation with Carex runssoroensis K. Schum. However, these studies are largely ecological and physiognomic, typically presenting lists of species and rarely vegetation relevés. Similar data on Afroalpine vegetation have been reported by various authors for other areas, such as Kilimanjaro [15,16] and Mount Kenya [17,18]. To date, no phytosociological studies have been conducted in the Ruwenzori Mountains or other mountain regions with Afroalpine vegetation. The phytosociological method has been widely applied to the study of alpine vegetation in temperate regions of Europe and Asia [19,20,21,22], and it has also proven efficient in studying mountain vegetation in tropical regions, particularly in the Andes [23,24]. Therefore, the aim of this study is to propose a phytosociological classification of the plant communities occurring in the Ruwenzori Mountains between 3500 and 4600 m. The proposed phytosociological framework is based on multivariate analysis, utilizing a hierarchical classification of vegetation. The identified communities were characterized from a floristic, ecological, structural, and distributive point of view. This study, therefore, aims to identify and analyze, using a well-known and tested method, the main types of vegetation present in the area. It will also allow the identification of an altitudinal gradient of the different communities.
This paper may represent a starting point for further studies in other mountain areas of Africa with similar vegetation, which may provide a more extensive phytosociological classification of Afroalpine plant communities.
2. Materials and Methods
2.1. Study Area
The research was carried out on the south-eastern side of the Ruwenzori mountains, within the territory of Uganda (Western Region), near its border with the Democratic Republic of Congo. On the Ugandan side, the mountains lie within the Ruwenzori Mountains National Park (UNESCO World Heritage site). The surveyed area covers approximately 3000 km2, at an altitudinal range of 3500 to 4600 m above sea level. The study area is situated at 0°19′4.58″ N and 29°52′54.14″ E (Figure 1). Due to the lack of long-term climate data, statistically significant trends could not be determined. However, data from the Fresh Field Pass meteorological station (4200 m) recorded over a four-year period (2009 to 2012) indicate an average temperature of −1.3 °C and an average annual precipitation of 535. 8 mm. Additionally, data from the Bujuku Hut station (3900 m) collected over a two-year period (2011–2012) show an average temperature of 3.9 °C and average annual precipitation of 526.1 mm [25]. Precipitation is primarily concentrated in two rainy seasons (March–May and September–December), during which fog, rain, and snow at the highest elevations persist for long periods. Frosts are frequent throughout most of the year at altitudes above 4000 m [26]. However, it must be highlighted that the lack of long-term climate data limits our ability to understand the ecology of the studied communities, and it is hoped that this gap can be filled in the future.
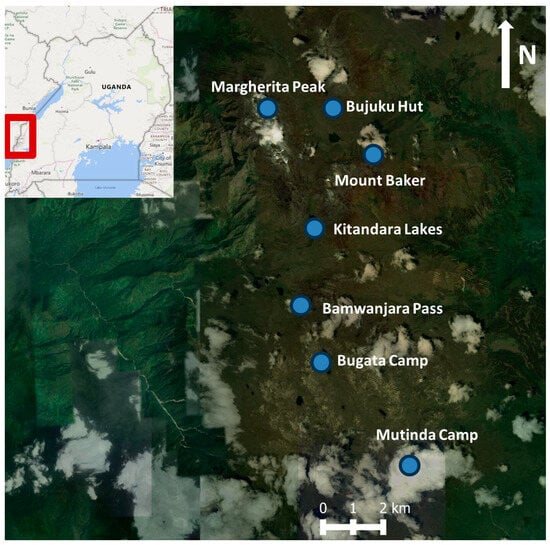
Figure 1.
Ruwenzori Mountains, with indications of the locations where the surveys were carried out (blue dots), Uganda, maps from ESRI base map imagery, and OpenStreetMap (modified).
Geologically, the Ruwenzori Mountains form a narrow mountain range, exceeding 5000 m in elevation, within the western branch of the East African Rift System. Unlike the volcanic origins of Kilimanjaro and Mount Kenya, the Ruwenzori Mountains consist of Precambrian metamorphic rocks [27].
2.2. Data Collection
The plant species were identified and photographed directly in the field. Species identification, as well as other data on taxonomy and distribution, were obtained from the literature. The nomenclature of the taxa follows Flora of Tropical East Africa [28] and Flore du Rwanda [29]. Additional information on the taxonomy and species distribution was sourced from various references [30,31]. Fieldwork was conducted over a two-year period (2016–2017) during the months of December and January.
According to the Braun-Blanquet methodology [32], a total of 27 relevés were conducted in 12 sites within an altitudinal range of 3500–4600 m ASL. Plot size varied from 50 to 100 m2. Vegetation relevés were sampled in different biotopes, which were randomly selected and positioned according to the principle of homogeneity. For each relevé, species presence was recorded. The coverage was obtained by a rough estimate of the percentage of surface covered by vegetation.
Due to insufficient expertise, the species of bryophytes and lichens were not identified.
2.3. Data Analysis and Vegetation Classification
All statistical analyses were performed using R 4.2.3 software [33]. According to van der Maarel [34], we converted the combined cover-abundance data into an ordinal scale (1–9). In particular, the hierarchical classification was carried out using the “vegan” package [35]. The phytosociological relevés were analyzed using the Bray-Curtis distance and beta flexible method (beta = −0.25). The Bray-Curtis distance was chosen as it is widely recognized as one of the most effective metrics for analyzing ecological communities. It minimizes the impact of outliers and maintains sensitivity even when dealing with heterogeneous datasets [36]. The beta flexible method was employed as the linkage criterion due to its compatibility with Bray-Curtis distance and its property of conserving spatial relationships. This method preserves the integrity of the original dissimilarity matrix throughout the clustering process. In contrast, space-distorting methods may introduce issues like excessive chaining, where individual items are incrementally added to existing clusters [36,37]. The optimal number of clusters was determined using the silhouette index, calculated with the “cluster” package [38]. The indicator species analysis was carried out utilizing the “multipatt” function of the “indicspecies” package [39]. We calculated Pearson’s phi coefficient [40] in order to identify the vegetation type-specific species for the different cluster groups [37,41]. Non-metric multidimensional scaling (NMDS), using Bray-Curtis dissimilarity, was applied to depict the main trends in the floristic differences. This iterative approach is particularly suitable for ecological data as it performs well with non-normal distributions, supports non-Euclidean distance measures, and does not assume linear or unimodal species responses to environmental gradients [36]. The nomenclature of the new syntaxa proposed (listed in Appendix A) follows the 4th edition of the International Code of Phytosociological Nomenclature [42]. The nomenclature and chorotype of the taxa recorded (listed in Appendix B) are in accordance with POWO [43].
3. Results and Discussion
Figure 2 presents a dendrogram derived from the classification of phytosociological relevés, which were divided into two primary groups (Cluster A and B). According to the silhouette width, 8 different plant communities were identified (Figure 3A). At each fusion level, the average silhouette width can be used as a measure of the quality of the partition (Rousseeuw quality index), and the best partition turns out to be exactly 8 clusters (Figure 3B).
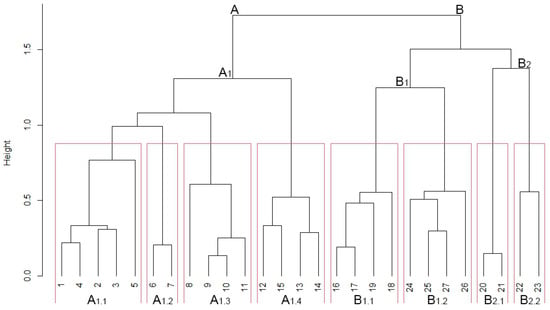
Figure 2.
Hierarchical clustering of phytosociological relevés from the Ruwenzori Mountains (Uganda). The cophenetic correlation coefficient was 0.734. A. Dendrosenecionetalia erici-rosenii; A1. Dendrosenecionion erici-rosenii; A1.1. Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii; A1.2. Alchemilletum argyphyllae; A1.3. Helichrysetum stuhlmannii; A1.4. Erico trimerae-Hypericetum bequaertii; B. Dendrosenecionetalia adnivalis; B1. Dendrosenecionion adnivalis; B1.1. Lobelio wollastonii-Dendrosenecionetum adnivalis; B1.2. Caricetum runssoroensis; B2. Alchemillion stuhlmannii; B2.1. Alchemilletum subnivalis; B2.2. Senecio mattirolii-Sedetum ruwenzoriensis.

Figure 3.
Average silhouette widths. (A). Silhouette plot of the clustering. (B). Bar plot showing the average silhouette widths for k = 2 to 29 groups. The best partition by this criterion is highlighted in red.
The non-metric multidimensional scaling (NMDS) analysis of the entire vegetation using species abundance confirmed the separation of the clusters of the dendrogram for each syntaxa (Figure 4). From a phytosociological viewpoint, a total of 8 associations, 3 alliances, 2 orders, and 1 class are presented. These represent new proposed syntaxa for the Ruwenzori Mountains, considering the lack of previous phytosociological studies for the area. Based on our phytosociological reléves, a reduction in species richness and vegetation cover with increasing elevation was noted. In fact, the communities occurring in the lower belt of the Afroalpine area (below 4000 m) show a floristic richness of up to 9–11 species for a 50 m2 plot, while on the surfaces of equal size above 4000 m, no more than 7 species were detected, with a minimum of 5 species at around 4300 m. Furthermore, the vegetation of the Ruwenzori Mountains is significantly affected by the steep slopes, limited availability of substrate, and the harshest weather conditions being at higher altitudes. These data are consistent with the findings of Ssali et al. [44].
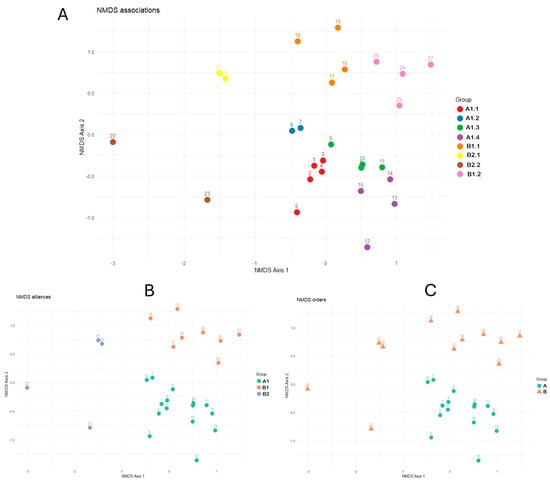
Figure 4.
Non−metric multidimensional scaling (NMDS) of the phytosociological relevés (stress value = 0.14). (A). Associations; (B). alliances; (C). orders. For the legend of the syntaxa, see Figure 2.
Below is provided an overview of these vegetation units, with information about the floristic set, structure, composition, ecology, and distribution. After comparison with the literature [2,3,10,11,12,13], all these vegetation units could be described as new syntaxa.
3.1. Description of Vegetation Units
- Helichrysetea stuhlmannii class nov. hoc loco
- Holotypus: Dendrosenecionetalia erici-rosenii ord. nov.
Physiognomy and ecology: This class includes all Afroalpine vegetation communities found in the Ruwenzori Mountains. Similar vicariant syntaxa are likely present in the other central-eastern Africa mountains ranges, such as Kilimanjaro, Mount Elgon, Mount Kenya, etc. Floristically, this vegetation is characterized by the consistent presence and often dominance of Helichrysum stuhlmannii.
Indicator species: Hypericum bequartii, Helichrysum stuhlmannii, Senecio transmarinus, Poa ruwenzoriensis.
Distribution: This vegetation is widely distributed across the Afroalpine region of the Ruwenzori Mountains, occurring between 3000 (more typically 3500) and 4600 m above sea level.
- A.
- Dendrosenecionetalia erici-rosenii ord. nov. hoc loco
- Holotypus: Dendrosenecionion erici-rosenii all. nov. hoc loco
Physiognomy and ecology: This order includes plant communities linked to the lowest altitudinal zone of the Afroalpine belt. This vegetation is characterized by a dense structure and dominated by tall woody species, such as Dendrosenecio erici-rosenii, Erica trimera subsp. Trimera, and Hypericum bequartii.
Indicator species: Alchemilla argyrophylla subsp. argyrophylloides, Dendrosenecio erici-rosenii, Erica trimera subsp. trimera, Hypericum bequartii.
Distribution: This vegetation is widespread in the Afroalpine region of the Ruwenzori mountains between (3000) 3500 and 4000 m ASL.
- A1.
- Dendrosenecion erici-rosenii all. nov. hoc loco
- Holotypus: Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii ass. nov. hoc loco
Physiognomy and ecology: See order.
Indicator species: Hypericum bequartii, Dendrosenecio erici-rosenii, Alchemilla argyrophylla subsp. argyrophylla, Erica trimera subsp. trimera.
Distribution: See order.
- A1.1.
- Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii ass. nov. hoc loco (Table 1, Figure 2—Cluster A1.1, Figure 5A)
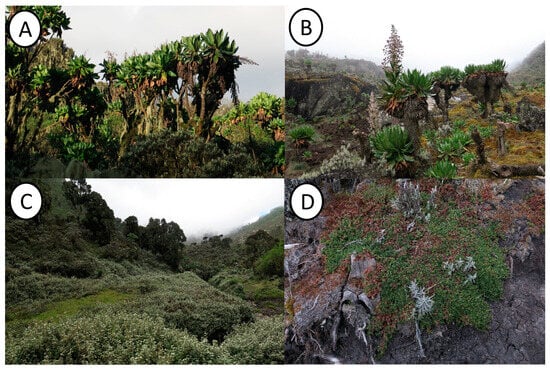 Figure 5. Afroalpine communities of Ruwenzori: (A) Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii; (B) Lobelio wollastonii-Dendrosenecionetum adnivalis; (C) Alchemilletum argyphyllae; (D) Alchemilletum subnivalis. Photos by Salvatore Cambria.
Figure 5. Afroalpine communities of Ruwenzori: (A) Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii; (B) Lobelio wollastonii-Dendrosenecionetum adnivalis; (C) Alchemilletum argyphyllae; (D) Alchemilletum subnivalis. Photos by Salvatore Cambria.
- Holotypus: Rel. 5, Table 1
Physiognomy and ecology: This vegetation type consists of tall woody species, reaching up to 8 m in height, and is associated with slightly sloping and moderately humid surfaces between 3600 and 4000 m. This plant community partly corresponds to the “Dendrosenecio woodland” quoted by Hedberg [4] and represents one of the most distinctive vegetation types of the Afroalpine areas. It is present in all the major mountains of central-eastern Africa, featuring various vicariant species of the genus Dendrosenecio. In the Ruwenzori Mountains, the dominant species is the local endemic D. erici-rosenii, which constitutes dense arboreal-shrub formations with high degrees of coverage, contrasting with similar vegetation on Mount Kenya and Kilimanjaro. Other species commonly found in this vegetation type are Galium ruwenzoriense, Afrosciadium kerstenii, Helichrysum stuhlmannii, Lobelia stuhlmannii, and Alchemilla argyrophylla. At higher altitudes (above 4000 m), D. erici rosenii becomes less frequent, being replaced by D. adnivalis, which prefers more humid and deeper soils.
Indicator species: Dendrosenecio erici-rosenii, Galium ruwenzoriense, Lobelia stuhlmannii.
Distribution: This vegetation was widely detected in various locations of the lower Afroalpine belt below 4000 m.

Table 1.
Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii ass. nov. hoc loco.
Table 1.
Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii ass. nov. hoc loco.
| Relevés | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Altitude (m) | 3600 | 3600 | 3700 | 3700 | 4000 |
| Surface (m2) | 100 | 100 | 50 | 50 | 100 |
| Coverage (%) | 90 | 80 | 90 | 90 | 90 |
| Inclination (°) | 60 | 60 | 70 | 70 | 70 |
| Exposure | SO | SO | NO | NO | NE |
| Char. Ass. | |||||
| Dendrosenecio erici-rosenii | 4 | 4 | 4 | 3 | 3 |
| Lobelia stuhlmannii | 2 | + | + | 1 | . |
| Galium ruwenzoriense | . | + | . | + | 1 |
| Char. Ord. and All. | |||||
| Alchemilla argyrophylla subsp. argyrophylloides | + | 1 | 1 | 1 | . |
| Afrosciadium kerstenii | 1 | . | 1 | + | . |
| Hypericum bequaertii | . | . | 1 | + | 1 |
| Erica trimera subsp. trimera | + | + | + | . | . |
| Char. Class | |||||
| Helichrysum stuhlmannii | 2 | 1 | 3 | 2 | . |
| Senecio transmarinus | + | . | . | + | . |
| Other species | |||||
| Cardamine obliqua | . | + | + | . | + |
| Senecio sp. | + | + | . | + | . |
| Alchemilla johnstonii | . | + | . | . | . |
| Luzula johnstonii | + | . | . | . | . |
| Poa ruwenzoriensis | . | . | + | . | . |
| Lobelia wollastonii | . | . | . | . | 1 |
| Arabis alpina | . | . | . | . | + |
| Poa annua | . | . | . | . | + |
- A1.2.
- Holotypus: Rel. 6, Table 2
Physiognomy and ecology: This vegetation represents a typical community referred to as the “Alchemilla scrublands”, which was previously investigated by Hauman [32] and Hedberg [4] and represents one of the most widespread vegetation types in all Afroalpine areas. From a phytogeographical point of view, it is notable for the presence of some genera commonly found in temperate areas of Europe and Asia, such as Alchemilla, Cardamine, Poa, Deschampsia, Senecio, Hypericum, etc. This community colonizes flat and humid surfaces, often on slightly raised areas above marshes, between 3600 and 4000 m. It is dominated by A. argyrophylla subsp. argyrophylloides, which, especially at lower altitudes, grows with A. johnstonii. The herbaceous layer frequently includes Cardamine obliqua, Poa ruwenzoriensis, and Senecio transmarinus.
Indicator species: Afrosciadium kerstenii, Alchemilla argyrophylla subsp. argyrophylloides, A. johnstonii, Poa schimperana.
Distribution: This vegetation is very frequent on the eastern slopes of the Ruwenzori Mountains.

Table 2.
Alchemilletum argyphyllae ass. nov. hoc loco.
Table 2.
Alchemilletum argyphyllae ass. nov. hoc loco.
| Relevés | 6 | 7 |
|---|---|---|
| Altitude (m) | 3700 | 3800 |
| Surface (m2) | 50 | 50 |
| Coverage (%) | 90 | 90 |
| Inclination (°) | 50 | 60 |
| Exposure | NE | NE |
| Char. Ass. | ||
| Alchemilla argyrophylla subsp. argyrophylloides | 4 | 3 |
| Alchemilla johnstonii | 1 | 1 |
| Afrosciadium kerstenii | 1 | + |
| Poa schimperana | . | + |
| Char. Ord. and All. | ||
| Dendrosenecio erici-rosenii | + | . |
| Hypericum bequaertii | . | + |
| Char. Class | ||
| Helichrysum stuhlmannii | + | 1 |
| Senecio trasmarinus | + | + |
| Poa ruwenzoriensis | + | + |
| Other species | ||
| Cardamine obliqua | 1 | 1 |
| Alchemilla triphylla | . | + |
- A1.3.
 Figure 6. Afroalpine communities of Ruwenzori: (A) Helichrysetum stuhlmannii; (B) Erico trimerae-Hypericetum bequaertii; (C) Caricetum runssoroensis; (D) Senecio mattirolii-Sedetum ruwenzoriensis. Photos by Salvatore Cambria.
Figure 6. Afroalpine communities of Ruwenzori: (A) Helichrysetum stuhlmannii; (B) Erico trimerae-Hypericetum bequaertii; (C) Caricetum runssoroensis; (D) Senecio mattirolii-Sedetum ruwenzoriensis. Photos by Salvatore Cambria.
- Holotypus: Rel. 10, Table 3
Physiognomy and ecology: This scrub community generally represents secondary vegetation, originating from the degradation of Dendrosenecio woodlands. In particular, it primarily colonizes recently burned areas up to 4400 m and constitutes one of the initial stages of plant recolonization after a fire [45].
The plant community is dense but floristically poor, dominated by Helichrysum stuhlmannii, a large shrub reaching up to 2 m in height, often associated with Erica trimera subsp. trimera, Helichrysum guilelmi, Lobelia bequaertii, etc. This vegetation likely plays a primary role in gently sloping, rocky areas with thin soil layers.. At altitudes above 4000 m, this vegetation tends to become shorter and sparser.
Indicator species: Helichrysum stuhlmannii, Helichrysum guilelmi, Lobelia bequaertii.
Distribution: This community represents one of the most typical vegetations of the Ruwenzori Mountains, particularly in the most disturbed areas of the Afroalpine zone.

Table 3.
Helichrysetum stuhlmannii ass. nov. hoc loco.
Table 3.
Helichrysetum stuhlmannii ass. nov. hoc loco.
| Relevés | 8 | 9 | 10 | 11 |
|---|---|---|---|---|
| Altitude (m) | 3900 | 4000 | 4000 | 3900 |
| Surface (m2) | 50 | 100 | 100 | 100 |
| Coverage (%) | 70 | 80 | 80 | 90 |
| Inclination (°) | 60 | 70 | 70 | 60 |
| Exposure | NO | SE | SE | NO |
| Char. Ass. | ||||
| Helichrysum stuhlmannii | 3 | 3 | 4 | 4 |
| Lobelia bequaertii | . | + | + | 1 |
| Helichrysum guilelmi | + | . | + | + |
| Char. Ord. and All. | ||||
| Alchemilla argyrophylla subsp. argyrophylloides | 1 | 1 | 1 | . |
| Erica trimera subsp. trimera | . | 1 | + | + |
| Hypericum bequaertii | . | + | 1 | + |
| Dendrosenecio erici-rosenii | + | . | . | . |
| Char. Class | ||||
| Senecio trasmarinus | 1 | . | . | . |
| Other species | ||||
| Alchemilla johnstonii | + | . | . | . |
| Senecio sp. | + | . | . | . |
| Hedbergia longiflora subsp. macrophylla | . | . | . | + |
- A1.4.
- Holotypus: Rel. 14, Table 4
Physiognomy and ecology: This association is characterized by a very peculiar woodland dominated by Hypericum bequaertii, an endemic species of Ruwenzori and Elgon Mt. It was first identified in the Ruwenzori Mountains by Schmitt & Beck [12]. This community occurs on steep, wet slopes in the lower belt of the Afroalpine zone, between 3300 and 4000 m. Usually, it forms a dense and tall vegetation reaching up to 12 m in height. The shrubby layer is mainly composed of Erica trimera subsp. trimera. From the floristic point of view, the occurrence of Galium ruwenzoriense is particularly noteworthy.
Indicator species: Hypericum bequartii.
Distribution: This community is quite rare in the western and eastern slopes of the Ruwenzori Mountains.

Table 4.
Erico trimerae-Hypericetum bequaertii ass. nov. hoc loco.
Table 4.
Erico trimerae-Hypericetum bequaertii ass. nov. hoc loco.
| Relevés | 12 | 13 | 14 | 15 |
|---|---|---|---|---|
| Altitude (m) | 3600 | 4000 | 4000 | 4000 |
| Surface (m2) | 50 | 100 | 100 | 50 |
| Coverage (%) | 90 | 80 | 80 | 70 |
| Inclination (°) | 80 | 70 | 70 | 70 |
| Exposure | NO | SE | SE | NE |
| Char. Ass. | ||||
| Hypericum bequaertii | 4 | 3 | 4 | 3 |
| Crassocephalum ducis-apruti | . | + | + | . |
| Char. Ord. and All. | ||||
| Erica trimera subsp. trimera | 2 | + | + | . |
| Dendrosenecio erici-rosenii | + | + | . | + |
| Lobelia bequaertii | . | + | + | . |
| Galium ruwenzoriense | . | + | . | . |
| Char. Class | ||||
| Helichrysum stuhlmannii | . | . | + | 1 |
| Senecio trasmarinus | . | . | + | . |
| Other species | ||||
| Lobelia wollastonii | . | 1 | 1 | + |
| Senecio sp. | + | . | . | + |
| Helichrysum forskahlii | . | . | + | . |
| Luzula johanstonii | . | . | + | . |
- B.
- Dendrosenecionetalia adnivalis ord. nov. hoc loco
- Holotypus: Dendrosenecion adnivalis all. nov.
Physiognomy and ecology: This syntaxon includes the communities found in the upper altitudinal zone of the Afroalpine belt. This vegetation generally shows a loose structure and is floristically dominated by woody shrubs such as Dendrosenecio adnivalis and Helichrysum stuhlmannii. Additionally, various herbaceous plants are prominent, including Carex runssoroensis, Deschampsia flexuosa, Festuca abyssinica, and Senecio transmarinus.
Indicator species: Dendrosenecio adnivalis, Carex runssoroensis, Alchemilla subnivalis.
Distribution: This vegetation is widespread in the Afroalpine region of the Ruwenzori mountains between 4000 and 4600 m ASL.
- B1.
- Dendrosenecionion adnivalis all. nov. hoc loco
- Holotypus: Lobelio wollastonii-Dendrosenecionetum adnivalis ass. nov. hoc
Physiognomy and ecology: This alliance includes woody or herbaceous plant communities found on moderately moist, deep soils, typically on flat or slightly sloping surfaces. This vegetation occurs at elevations between 4000 and 4500 m ASL.
Diagnostic species: Dendrosenecio adnivalis, Carex runssoroensis, Lobelia wollastonii.
Distribution: See order.
- B1.1.
- Holotypus: Rel. 16, Table 5
Physiognomy and ecology: This community replaces the previous one in wet and deep soils, within the altitudinal range of 4000–4500 m. It consists of relatively tall vegetation, reaching up to 6–8 m in height, dominated by Dendrosenecio adnivalis, which constitutes a dense tree layer. Floristically, the physiognomy of this vegetation is characterized by the regular presence of Lobelia wollastonii, typically intermixed with D. adnivalis as individual plants or small groups. The herbaceous layer is sparse and composed of a few species, such as Poa ruwezoriensis, Festuca abyssinica, Alchemilla sp. pl., etc. A continuous moss carpet covers both the soil and the stems of Dendrosenecio.
Indicator species: Dendrosenecio adnivalis, Alchemilla adnivalis, Lobelia wollastonii.
Distribution: This vegetation is quite spread in the upper Afroalpine belt, particularly near the Bamwanjara Pass.

Table 5.
Lobelio wollastonii-Dendrosenecionetum adnivalis ass. nov. hoc loco.
Table 5.
Lobelio wollastonii-Dendrosenecionetum adnivalis ass. nov. hoc loco.
| Relevés | 16 | 17 | 18 | 19 |
|---|---|---|---|---|
| Altitude (m) | 4300 | 4300 | 4500 | 4200 |
| Surface (m2) | 100 | 100 | 50 | 50 |
| Coverage (%) | 60 | 50 | 40 | 50 |
| Inclination (°) | 10 | 10 | 30 | 20 |
| Exposure | NE | NE | NE | NE |
| Char. Ass. | ||||
| Dendrosenecio adnivalis | 4 | 3 | 2 | 3 |
| Char, All. and Ord. | ||||
| Lobelia wollastonii | 2 | 1 | . | + |
| Carex runssoroensis | + | . | . | + |
| Char. Class | ||||
| Helichrysum stuhlmannii | 2 | 2 | + | . |
| Alchemilla subnivalis | + | + | 1 | . |
| Afrosciadium kerstenii | + | + | . | . |
| Poa ruwenzoriensis | . | + | 1 | . |
| Festuca abyssinica | . | . | + | . |
| Other species | ||||
| Alchemilla triphylla | + | . | . | . |
| Lobelia stuhlmannii | . | . | . | + |
- B1.2.
- Holotypus: Rel. 24, Table 6
Physiognomy and ecology: The flat or gently sloping surfaces with poor drainage and waterlogging are colonized by herbaceous vegetation dominated by Carex runssoroensis, a species spread in all Afroalpine areas. This bog vegetation occurs between 3300 and 4200 m and is characterized by a taller layer of sedges and grasses, mainly including C. runssoroensis, Deschampsia cespitosa, and Agrostis gracilifolia, along with a lower moss carpet composed of Sphagnum sp.pl. and other herbaceous plants such as Subularia monticola, Cerastium afromontanum, Ranunculus oreophytus, R. volkensii, Huperzia saururus, etc. The tussocks of C. runssoroensis constitute a thick peat layer (up to 1 m), determining low values of pH [4]. This vegetation is often found in the outer belt of alpine lakes, transitioning inward to highly hygrophilous herbaceous communities dominated by Subularia monticola. Outwardly, this vegetation forms catenal contact with Alchemilletum argyphyllae, occurring on the dryer raised surfaces.
Indicator species: Carex runssoroensis, Huperzia saururus, Ranunculus oreophytus, Subularia monticola.
Distribution: This vegetation is very frequent in the Ruwenzori Mountains, particularly along the eastern slopes. Similar communities probably occur in the other Afroalpine areas.

Table 6.
Caricetum runssoroensis ass. nov. hoc loco.
Table 6.
Caricetum runssoroensis ass. nov. hoc loco.
| Relevés | 24 | 25 | 26 | 27 |
|---|---|---|---|---|
| Altitude (m) | 3800 | 4100 | 4000 | 3900 |
| Surface (m2) | 50 | 50 | 100 | 50 |
| Coverage (%) | 90 | 80 | 90 | 90 |
| Inclination (°) | 0 | 10 | 0 | 0 |
| Exposure | SO | SO | NE | NE |
| Char. Ass. | ||||
| Carex runssoroensis | 4 | 4 | 1 | 4 |
| Huperzia saururus | . | + | + | + |
| Subularia monticola | . | + | 1 | + |
| Ranunculus oreophytus | + | . | + | + |
| Cerastium afromontanum | + | + | ||
| Deschampsia cespitosa | + | . | 3 | . |
| Char. All. | ||||
| Lobelia wollastonii | 1 | + | + | . |
| Char. Ord. | ||||
| Dendrosenecio adnivalis | + | . | ||
| Char. Class | ||||
| Helichrysum stuhlmannii | + | + | + | + |
| Senecio trasmarinus | . | + | . | . |
| Other species | ||||
| Alchemilla subnivalis | + | . | + | . |
| Crassocephalum ducis-aprutii. | + | . | . | . |
| Erica trimera subsp. trimera | . | + | . | . |
| Helichrysum guilelmii | + | . | . | . |
| Agrostis gracilifolia | + | . | . | . |
| Alchemilla argyrophylla subsp. argyrophylloides | . | . | 1 | . |
| Ranunculus volkensii | . | . | . | + |
- B2.
- Alchemillion stuhlmannii all. nov. hoc loco
- Holotypus: Alchemilletum subnivalis ass. nov. hoc loco
Physiognomy and ecology: This syntaxon includes low shrubby or herbaceous plant communities. This vegetation occurs on shallow soils, often with rocky outcrops, at elevations ranging from 4000 m to the upper limit of vegetation.
Indicator species: Alchemilla stuhlmannii, A. triphylla, Arabis alpina, Festuca abyssinica.
Distribution: See order.
- B2.1.
- Holotypus: Rel. 20, Table 7
Physiognomy and ecology: This community replaces the Alchemilletum argyphyllae at higher altitudes, occurring between 4000 and 4750 m, often on fresh moraines near the upper vegetation limit below glaciers. Floristically, it is a rather species-poor community with low coverage. In fact, Alchemilla subnivalis is associated with only a few other herbaceous species, including A. triphylla, A. stuhlmannii, Arabis alpina, Poa ruwenzoriensis, and Cardamine obliqua.
Indicator species: Alchemilla subnivalis, Arabis alpina.
Distribution: This vegetation is sparsely distributed across the upper slopes of the Ruwenzori Mountains.

Table 7.
Alchemilletum subnivalis ass. nov. hoc loco.
Table 7.
Alchemilletum subnivalis ass. nov. hoc loco.
| Relevés | 20 | 21 |
|---|---|---|
| Altitude (m) | 4300 | 4300 |
| Surface (m2) | 50 | 50 |
| Coverage (%) | 90 | 90 |
| Inclination (°) | 40 | 30 |
| Exposure | NO | NO |
| Char. Ass. | ||
| Alchemilla subnivalis | 3 | 2 |
| Char. All., Ord. and Class | ||
| Alchemilla stuhlmannii | + | 1 |
| Arabis alpina | + | + |
| Poa ruwenzoriensis | + | . |
| Other species | ||
| Alchemilla triphylla | 1 | 1 |
| Cardamine obliqua | + | + |
- B2.2.
- Holotypus: Rel. 22, Table 8
Physiognomy and ecology: This is a chasmophilous community linked to rocky outcrops or stony slopes with very shallow soils, occurring between 3300 and 4500 m. It is characterized by the dominance of Sedum ruwenzoriensis, often with low coverage values. Floristically, this community shows very low species richness. Among the few other species present are Senecio mattirolii, S. x pirottae, S. trasmarinus, and Festuca abyssinica.
Indicator species: Sedum ruzeworiense, Senecio mattirolii, S. x pirottae.
Distribution: This vegetation is relatively uncommon and covers only small surfaces in the Ruwenzori Mountains.

Table 8.
Senecio mattirolii-Sedetum ruwenzoriensis ass. nov. hoc loco.
Table 8.
Senecio mattirolii-Sedetum ruwenzoriensis ass. nov. hoc loco.
| Relevés | 22 | 23 |
|---|---|---|
| Altitude (m) | 4000 | 4000 |
| Surface (m2) | 50 | 50 |
| Coverage (%) | 40 | 40 |
| Inclination (°) | 80 | 90 |
| Exposure | NE | NE |
| Char. Ass. | ||
| Sedum ruwenzoriense | 2 | 2 |
| Senecio mattirolii | 1 | 1 |
| Senecio x pirottae | + | + |
| Char. Ord. and All. | ||
| Arabis alpina | + | + |
| Char. Class. | ||
| Festuca abyssinica | 1 | + |
| Senecio trasmarinus | . | + |
| Other species | ||
| Avenella flexuosa subsp. flexuosa | + | . |
3.2. Floristic Remarks
A total of 39 vascular species (Appendix C, Figure 7) belonging to 21 genera and 15 families were recorded. The flora of the total relevé dataset is dominated by Asteraceae (10 taxa), Poaceae (7 taxa), Rosaceae (6 taxa), and Campanulaceae and Brassicaceae (3 taxa). These results concerning plant diversity are also in line with those observed, for example, in the study conducted by Ssali et al. [44]. Such a low number of recorded species is in agreement with the hypothesis proposed by [7]: that the Afroalpine flora is isolated, young, and unsaturated because the habitat disturbance caused by the Pleistocene climate oscillations likely induced cycles of colonization, speciation, extinction, and recolonization, probably not yet finished. A low number of species and a high rate of endemism are also observed in the high-altitude areas of mountains in other territories, which are also subject to Pleistocene climatic oscillations [45,46].

Figure 7.
Endemic species and of phytogeographical interest in the Ruwenzori flora: (A) Dendrosenecio adnivalis: A1. habitus; A2. flower detail (B) Dendrosenecio erici-rosenii; (C) Lobelia wollastonii; (D) Lobelia bequaertii; (E) Hypericum bequaertii; (F) Sedum ruwenzoriense. Photos by Salvatore Cambria.
In terms of life forms (Figure 8), hemicryptophytes are the most represented (59%), followed by phanerophytes (16%), chamaephytes (15%), geophytes, and therophytes (2%). From a chorological perspective (Figure 9), the narrow endemics of the Ruwenzori mountains are represented by 11 taxa (28%), while 17 species (44%) are shared with other mountain areas of Central and Eastern Africa. A significant contingent consists of species mainly distributed in the temperate regions of Europe and Asia (5%). Furthermore, the vegetation and flora of the Ruwenzori Mountains, including many endemic species identified during our phytosociological surveys, may be at high risk of extinction due to ongoing climate change [47]. This phenomenon is becoming increasingly apparent in East Africa’s high mountain regions, as evidenced by the rapidly receding glaciers [48]. The difference in plant species between the lowest and highest summits of the Ruwenzori Mountains highlights the restricted elevation ranges of many species. Several studies have demonstrated that plant species growing in tropical alpine zones are predominantly dispersed by birds and wind, which enables them to overcome topographical barriers [49,50,51].
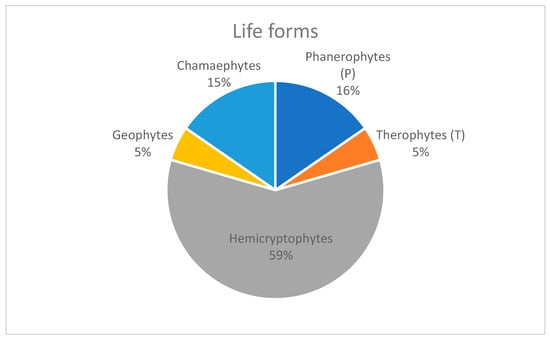
Figure 8.
Life forms of the species recorded in the study area.
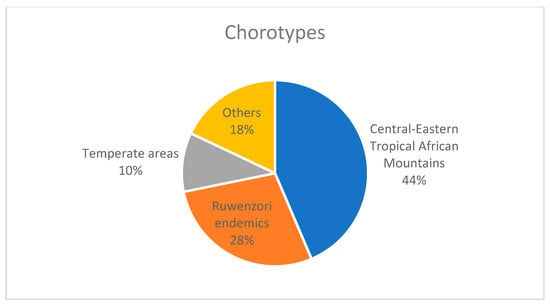
Figure 9.
Chorotypes of the species investigated from the Ruwenzori mountains (Uganda).
4. Conclusions
The results of this study represent the first comprehensive classification of the Afroalpine vegetation of the Ruwenzori Mountains. The dominance of hemicryptophytes (59%) in the flora, followed by phanerophytes (16%) and chamaephytes (15%), reflects the significant adaptation of these life forms to the harsh climatic conditions at elevations above 3500 m. These adaptations, including frost tolerance and wind resistance, are critical for survival in extreme environments. The vegetation communities display distinct vertical zoning in correspondence with altitudinal gradients (Figure 10). Identifying new plant groups, including new associations and alliances, contributes significantly to understanding Afroalpine vegetation.
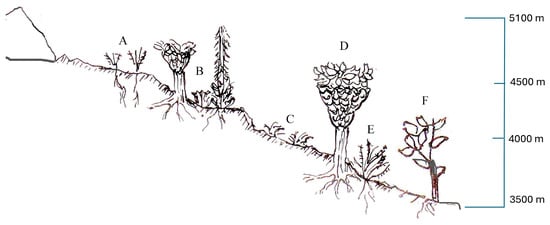
Figure 10.
Altitudinal zonation of Afroalpine plant communities in the Ruwenzori Mountains. The summit area devoid of vegetation represents the zones devoid of vascular flora and coincides largely with the surface occupied by glaciers. It is followed towards lower altitudes by various communities: (A). Alchemilletum subnivalis: a low and floristically poor vegetation linked to the highest altitudes of the Afroalpine belt, developing up to 4750 m on fairly steep stands; (B). Lobelio wollastonii-Dendrosenecionetum adnivalis: a sparse scrub growing above 4000 m on deep, moist soils; (C). Caricetum runssoroensis: a grassland linked to humid stations on flat surfaces; (D). Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii: a dense woodland occurring below 4000 m; (E). Alchemilletum argyphyllae: a low, shrubby vegetation linked to dry ridges; (F). Erico trimerae-Hypericetum bequaertii: a tall woodland occurring in the lower belt of the Afroalpine area. Drawing by Rosaria Di Cicca.
The presence of unique and vicariant species in the Ruwenzori Mountains, such as Dendrosenecio erici-rosenii and Helichrysum stuhlmannii, supports previous findings on the evolutionary significance of these isolated mountain ecosystems. Based on our results, the number of unique species highlights the biogeographical importance of the Ruwenzori Mountains as a center for endemism and a potential refuge during historical climatic shifts. The flora likely represents a mix of ancient lineages that survived past climatic fluctuations. The ecological significance of the identified plant communities is reflected in their roles in ecosystem function, particularly in water regulation and soil stabilization. The bog vegetation dominated by Carex runssoroensis, which occurs in poorly drained areas between 3300 and 4200 m, is crucial for peat formation and water retention. Despite the limitations of this study, including the sample size, constraints of the investigation period, and potential long-term effects of environmental changes, our findings provide a baseline for future research on Afroalpine vegetation.
Further investigations are needed to monitor the dynamics of these plant communities in response to environmental changes, particularly those driven by climate change. In conclusion, this study represents a significant step forward in the classification of the Afroalpine vegetation of the Ruwenzori Mountains. The identification of new plant communities and their ecological roles underscores the importance of these ecosystems for biodiversity conservation. Immediate conservation actions, combined with continued research, will be essential to preserve the unique flora of this remarkable mountain range.
Supplementary Materials
The following supporting information can be downloaded at: www. Mdpi.com/xxx/s1.
Author Contributions
Conceptualization, S.C. and G.T.; methodology, S.C., P.M. and G.T.; software, G.T.; validation, G.T. and S.C.; formal analysis, G.T.; investigation, S.C.; resources, P.M.; data curation, S.C.; writing—original draft preparation, S.C., G.T. and P.M.; writing—review and editing, S.C. and G.T.; visualization, G.T.; supervision, P.M.; project administration, P.M.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the research program of University of Catania (PIA.CE.RI. 2020–2022) Line 2 cod. 22722132189.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request. The geographical coordinates of all the relevés are available in the Supplementary Materials.
Acknowledgments
We thank Rosaria Di Cicca for the illustration and Rwenzori Trekking Services for the support in the field activities.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
| Conspectus of the Syntaxa |
| HELICHRYSETEA STUHLMANNII Cambria, Minissale & Tavilla |
| DENDROSENECIONETALIA ERICI-ROSENII Cambria, Minissale & Tavilla |
| DENDROSENECIONION ERICI-ROSENII Cambria, Minissale & Tavilla |
| Galio ruwenzoriensis-Dendrosenecionetum erici-rosenii Cambria, Minissale & Tavilla |
| Alchemilletum argyphyllae Cambria, Minissale & Tavilla |
| Helichrysetum bequaertii Cambria, Minissale & Tavilla |
| Erico trimerae-Hypericetum bequaertii Cambria, Minissale & Tavilla |
| DENDROSENECIONETALIA ADNIVALIS Cambria, Minissale & Tavilla |
| DENDROSENECIONION ADNIVALIS Cambria, Minissale & Tavilla |
| Lobelio wollastonii-Dendrosenecionetum adnivalis Cambria, Minissale & Tavilla |
| Caricetum runssoroensis Cambria, Minissale & Tavilla |
| ALCHEMILLION STUHLMANNII Cambria, Minissale & Tavilla |
| Alchemilletum subnivalis Cambria, Minissale & Tavilla |
| Senecio mattirolii-Sedetum ruwenzoriensis Cambria, Minissale & Tavilla |
Appendix B
Localities and dates of relevés. Table 1—Rels 1–2: 23 December 2016, Mutinda Camp (Uganda); Rels 3–4: 23 December 2016, sampling between Mutinda Camp and Bugata (Uganda); Rel 5: 26 December 2016, above Kachope Lakes (Uganda), Table 2—Rels 6–7: 23 December 2016, between Mutinda Camp and Bugata (Uganda). Table 3—Rel 8: 23 December 2016, between Mutinda Camp and Bugata (Uganda); Rels 9–10: 27 December 2016, near Butawu Camp (Uganda); Rel 11: 27 December 2016, near Kachope Lakes (Uganda). Table 4—Rel 12: 23 December 2016, above Mutinda Camp (Uganda); Rels 13–14: 27 December 2016, near Kachope Lakes (Uganda); Rel 15: 27 December 2016, near Kitandara Lake (Uganda). Table 5—Rels 16–17: 26 December 2016, near Bamwanjara Pass (Uganda); Rel 18: 27 December 2016, near Bamwanjara Pass (Uganda); Rel 19: 27 December 2016, between Bugata and Bamwanjara (Uganda). Table 6—Rels 24–25: 24 December 2016, near Bugata Camp (Uganda); Rel 26: 24 December 2016, valley above Kachope Lakes (Uganda); Rel 27: 27 December 2016, near Butawu Camp (Uganda). Table 7—Rels 20–21: 26 December 2016, near Bamwanjara Pass (Uganda). Table 8—Rels 22–23: 23 December 2016, near Bugata Camp (Uganda).
Appendix C

| Species | Family | Life Forms | Chorotypes |
|---|---|---|---|
| Afrosciadium kerstenii (Engl.) P.J.D.Winter | Apiaceae | G | Central-Eastern Tropical African Mountains |
| Agrostis gracilifolia C.E.Hubb. | Poaceae | H | Central-Eastern Tropical African Mountains |
| Alchemilla argyrophylla Oliv. subsp. argyrophylloides (Baker f.) Rothm. | Rosaceae | Ch | Endemic Ruwenzori |
| Alchemilla johnstonii Oliv. | Rosaceae | H | Central-Eastern Tropical African Mountains |
| Alchemilla stuhlmannii Engl. | Rosaceae | Ch | Endemic Ruwenzori |
| Alchemilla subnivalis Baker f. | Rosaceae | Ch | Endemic Ruwenzori |
| Alchemilla triphylla Rothm. | Rosaceae | Ch | Endemic Ruwenzori |
| Arabis alpina L. | Brassicaceae | H | Temperate areas of Old World |
| Avenella flexuosa (L.) Drejer subsp. Flexuosa | Poaceae | H | Temperate areas |
| Cardamine obliqua Hochst. ex A.Rich. | Brassicaceae | H | Tropical mountains of America and Africa |
| Carex runssoroensis K.Schum. | Cyperaceae | H | Central-Eastern Tropical African Mountains |
| Crassocephalum ducis-apruti S. Moore | Asteraceae | H | Central-Eastern Tropical African Mountains |
| Dendrosenecio adnivalis (Stapf) E.B.Knox | Asteraceae | P | Endemic Ruwenzori |
| Dendrosenecio erici-rosenii (R.E.Fr. & T.C.E.Fr.) E.B.Knox | Asteraceae | P | Endemic Ruwenzori |
| Deschampsia cespitosa (L.) P. Beauv. | Poaceae | H | Temperate areas |
| Erica trimera (Engl.) Beentje subsp. trimera | Ericaceae | P | Endemic Ruwenzori |
| Festuca abyssinica A. Rich | Poaceae | H | Africa |
| Galium ruwenzoriense (Cortesi) Ehrend. | Rubiaceae | H | Central-Eastern Tropical African Mountains |
| Hedbergia longiflora (Hochst. ex Benth.) A.Fleischm. & Heubl subsp. macrophylla (Hedberg) A.Fleischm. & Heubl | Orobanchaceae | G | Central-Eastern Tropical African Mountains |
| Helichrysum forskahlii (J.F.Gmel.) Hilliard & B.L.Burtt | Asteraceae | Ch | Africa and SW Asia |
| Helichrysum guilelmi Engl. | Asteraceae | P | Central-Eastern Tropical African Mountains |
| Helichrysum stuhlmannii O.Hoffm. | Asteraceae | P | Endemic Ruwenzori |
| Huperzia saururus (Lam.) Trevis. | Lycopodiaceae | H | Tropical areas of Africa and America |
| Hypericum bequaertii De Wild. | Hypericaceae | P | Central-Eastern Tropical African Mountains |
| Lobelia bequaertii De Wild. | Campanulaceae | H | Endemic Ruwenzori |
| Lobelia stuhlmannii Schweinf. ex Stuhlmann | Campanulaceae | H | Central-Eastern Tropical African Mountains |
| Lobelia wollastonii Baker f. | Campanulaceae | H | Central-Eastern Tropical African Mountains |
| Luzula johnstonii Buchenau | Juncaceae | H | Eastern and Central Africa |
| Poa annua L. | Poaceae | T | Temperate areas |
| Poa ruwenzoriensis Robyns & Tournay | Poaceae | H | Central-Eastern Tropical African Mountains |
| Poa schimperiana Hochst. ex A.Rich. | Poaceae | H | Tropical Africa and SW Asia |
| Ranunculus oreophytus Delile | Ranunculaceae | H | Central-Eastern Tropical African Mountains |
| Ranunculus volkensii Engl. | Ranunculaceae | H | Central-Eastern Tropical African Mountains |
| Sedum ruwenzoriense Baker f. | Crassulaceae | Ch | Central-Eastern Tropical African Mountains |
| Senecio mattirolii Chiov. | Asteraceae | H | Endemic Ruwenzori |
| Senecio sp. | Asteraceae | H | - |
| Senecio transmarinus S. Moore | Asteraceae | H | Central-Eastern Tropical African Mountains |
| Senecio x pirottae Chiov. | Asteraceae | H | Endemic Ruwenzori |
| Subularia monticola A.Braun ex Schweinf. | Brassicaceae | T | Central-Eastern Tropical African Mountains |
List of taxa surveyed in the phytosociological study. The following abbreviations are used for life forms: G = Geophyte; H = Hemicryptophyte; Ch = Chamaephyte; P = Phanerophyte; T = Phanerophyte.
References
- Bere, R.M. The exploration of Ruwenzori. Alp. J. 1952, 285, 483–495. [Google Scholar]
- Ross, R. Some aspects of the vegetation of the sub-alpine zone on Ruwenzori. Proc. Linn. Soc. Lond. 1953, 165, 136–140. [Google Scholar] [CrossRef]
- Ross, R. Some aspects of the vegetation of the Ruwenzori. Webbia 1955, 11, 451–457. [Google Scholar] [CrossRef]
- Hedberg, O. Features of Afroalpine Plant Ecology; Swedish Science Press: Stockholm, Sweden, 1964; pp. 1–144. [Google Scholar]
- Hedberg, O. Evolution and speciation in tropical high mountain flora. Biol. J. Linn. Soc. 1969, 1, 135–149. [Google Scholar] [CrossRef]
- Brochmann, C.; Gizaw, A.; Chala, D.; Kandziora, M.; Eilu, G.; Popp, M.; Pirie, M.B.; Gehrke, B. History and evolution of the afroalpine flora: In the footsteps of Olov Hedberg. Alp. Bot. 2022, 132, 65–87. [Google Scholar] [CrossRef]
- Kandziora, M.; Gehrke, B.; Popp, M.; Gizaw, A.; Brochmann, C.; Pirie, M.D. The enigmatic tropical alpine flora on the African sky islands is young, disturbed, and unsaturated. Proc. Natl. Acad. Sci. USA 2022, 119, e2112737119. [Google Scholar] [CrossRef]
- Hedberg, O. The phytogeographical position of the afroalpine flora. Rec. Adv. Bot. 1961, 1, 914–919. [Google Scholar]
- Woosnam, R.B. Ruwenzori and its life Zones. Geogr. J. 1907, 30, 616–629. [Google Scholar] [CrossRef]
- Engler, A. Die Pflanzenwelt Afrikas, Part 5; W. Engelmann: Leipzig, Germany, 1925; pp. 1–341. [Google Scholar]
- Loveridge, J.P. Plant ecological investigations in the Nyamagasani Valley, Ruwenzori Mountains, Uganda. Kirkia 1968, 6, 153–168. [Google Scholar]
- Schmitt, K.; Beck, E. On the Afroalpine vegetation of the Ruwenzori Mountain, Uganda. Phytocoenologia 1992, 21, 313–332. [Google Scholar] [CrossRef]
- Fishlock, C.W.L.; Hancock, G.L.R. Notes on the flora and fauna of Ruwenzori with special reference to the Bujuku valley. J. East Afr. Nat. Hist. Soc. 1932, 44, 205–229. [Google Scholar]
- Rejmànkovà, E.; Rejmànek, M.A. Comparison of Carex runssoroensis Fens on Ruwenzori Mountains and Mount Elgon, Uganda. Biotropica 1995, 27, 37–46. [Google Scholar] [CrossRef]
- Beck, E. Plant Life on Top of Mt. Kilimanjaro (Tanzania). Flora 1988, 181, 379–381. [Google Scholar] [CrossRef]
- Hemp, A. Vegetation of Kilimanjaro: Hidden Endemics and Missing Bamboo. Afr. J. Ecol. 2006, 44, 305–328. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Vegetation analysis of the upper Teleki valley (Mount Kenya) and adjacent areas. J. East Afr. Nat. Hist. 1981, 171, 1–8. [Google Scholar]
- Young, T.P.; Peacock, M.M. Giant Senecios and Alpine Vegetation of Mount Kenya. J. Ecol. 1992, 80, 141–148. [Google Scholar] [CrossRef]
- Kaul, V.; Sarin, Y.K. The phytosociology of some alpine meadows in N.W. Himalayas. Vegetatio 1971, 23, 361–368. [Google Scholar] [CrossRef]
- Parolly, G. The High Mountain Vegetation of Turkey—A State of the Art Report, Including a First Annotated Conspectus of the Major Syntaxa. Turk. J. Bot. 2004, 28, 39–63. [Google Scholar]
- Noroozi, J.; Hülber, K.; Willner, W. Phytosociological and ecological description of the high alpine vegetation of NW Iran. Phytocoenologia 2004, 47, 233–259. [Google Scholar] [CrossRef]
- Malfasi, F.; Cannone, N. Phytosociology of the vegetation communities of the Stelvio Pass area. J. Maps 2021, 17, 367–375. [Google Scholar] [CrossRef]
- Zarate, N. The database on high Andean páramo vegetation in Colombia. Biodivers. Ecol. 2012, 4, 275–286. [Google Scholar]
- Montesinos-Tubée, D.B.; Cleef, A.M.; Sýkora, K.V. The Subnival Vegetation of Moquegua, South Peru: Chasmophytes, Grasslands and Cushion Communities. Ecologies 2021, 2, 71–111. [Google Scholar] [CrossRef]
- Eilu, G.; Galabuzi, C.; Waiswa, D.; Oriekot, J.; Kakuri, W.; Mwavu, E.N.; Orikiriza, L.B.; Turyahabwe, N.; Shofuna, A.; Kasangaki, A.; et al. Impact of Climate Change on the Species of Restricted Range in RMNP. Final Report November 2013; Makerere University: Kampala, Uganda, 2013; pp. 1–61. [Google Scholar]
- Eggermont, H.; van Damme, K.; Russell, J.M. Rwenzori Mountains (Mountains of the Moon): Headwaters of the White Nile. In The Nile: Origin, Environments, Limology and Human Use; Dumont, H.J., Ed.; Springer Science + Business Media B.V.: Berlin, Germany, 2009; pp. 243–261. [Google Scholar]
- Bauer, F.U.; Karl, M.; Glasmacher, U.A.; Nagudi, B.; Schumann, A.; Mroszewski, L. The Rwenzori Mountains of western Uganda—Aspects on the evolution of their remarkable morphology within the Albertine Rift. J. Afr. Earth Sci. 2012, 73–74, 44–56. [Google Scholar] [CrossRef]
- Milne-Redhead, E.; Bertram Turrill, W.; Hubbard, C.E.; Polhill, R.M.; Beentje, H.; Ghazanfar, S.A. (Eds.) Flora of Tropical East Africa; 263 parts; Crown Agents for Overseas Governments and Administration & Rotterdam: London, UK, 1952–2012. [Google Scholar]
- Troupin, G. (Ed.) Flore du Rwanda. Spermatophytes; Koninklijk Mseum voor Midden-Afrika: Tervuren, Belgium, 1978–1987. [Google Scholar]
- König, P. Field Guide to the Upland Plants of Uganda; Meise Botanic Garden: Greifswald, Germany, 2023; pp. 4–511. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie; Grundzüge der Vegetationskunde: Berlin, Germany, 1928; pp. 1–888. [Google Scholar]
- Hauman, L. Esquisse de la vegetation des hautes altitudes sur le Ruwenzori. Bull. Acad. Belg. Cl. Sci. V 1933, 19, 301–368. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 26 July 2024).
- van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. 2015. Available online: https://john-quensen.com/wp-content/uploads/2018/10/Oksanen-Jari-vegantutor.pdf (accessed on 23 September 2024).
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P.; Gonzalez, J.; Kozlowski, K.; Schubert, E.; et al. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.1. Available online: https://CRAN.R-project.org/package=cluster (accessed on 23 September 2024).
- De Cáceres, M. indicspecies: Relationship Between Species and Groups of Sites. Available online: https://cran.r-project.org/web/packages/indicspecies/index.html (accessed on 23 September 2024).
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Theurillat, J.P.; Willner, W.; Fernández-González, F.; Bültmann, H.; Čarni, A.; Gigante, D.; Mucina, L.; Weber, H. International code of phytosociological nomenclature. Appl. Veg. Sci. 2021, 24, e12491. [Google Scholar] [CrossRef]
- POWO (Plants of the World Online). Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 27 September 2024).
- Ssali, F.; Mugerwa, B.; van Heist, M.; Nkurunziza, V.; Mutabazi, M.; Arinaitwe, H.; Bitariho, R.; Uwimana, A.; Oryem-Origa, H.; Gilbert, G. Plant diversity and composition vary with elevation on two equatorial high mountains in Uganda: Baselines for assessing the influence of climate change. Alp. Bot. 2023, 133, 149–161. [Google Scholar] [CrossRef]
- Sciandrello, S.; Minissale, P.; Giusso del Galdo, G. Vascular plant species diversity of Mt. Etna (Sicily): Endemicity, insularity and spatial patterns along the altitudinal gradient of the highest active volcano in Europe. PeerJ 2020, 8, e9875. [Google Scholar] [CrossRef]
- Raduła, M.; Świerszcz, S.; Nobis, M.; Nowak, S.; Nobis, A.; Nowak, A. Palaeoclimate has a major effect on the diversity of endemic species in the hotspot of mountain biodiversity in Tajikistan. Sci. Rep. 2021, 11, 18684. [Google Scholar] [CrossRef] [PubMed]
- Eltringham, S.K. The frequency and extent of uncontrolled grass fires in the Rwenzori National Park, Uganda. Afr. J. Ecol. 1976, 14, 215–222. [Google Scholar] [CrossRef]
- Chala, D.; Brochmann, C.; Psomas, A.; Ehrich, D.; Gizaw, A.; Masao, C.A.; Bakkestuen, V.; Zimmermann, N.E. Good-bye to tropical alpine plant giants under warmer climates? Loss of range and genetic diversity in Lobelia rhynchopetalum. Ecol. Evol. 2016, 6, 8931–8941. [Google Scholar] [CrossRef] [PubMed]
- Tovar, C.; Melcher, I.; Kusumoto, B.; Cuesta, F.; Cleef, A.M.; Meneses, R.I.; Halloy, S.; Llambí, L.D.; Beck, S.; Muriel, P.; et al. Plant dispersal strategies of high tropical alpine communities across the Andes. J. Ecol. 2020, 108, 1910–1922. [Google Scholar] [CrossRef]
- Tovar, C.; Hudson, L.; Cuesta, F.; Meneses, R.I.; Muriel, P.; Hidalgo, O.; Palazzesi, L.; Suárez Ballesteros, C.; Hammond Hunt, E.; Diazgranados, M.; et al. Strategies of diaspore dispersal investment in Compositae: The case of the Andean highlands. Ann. Bot. 2023, 132, 255–267. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Zhang, M.; Donohue, K.; Hou, M.; Li, J.; Ge, W.; Zhou, H.; Ma, L.; Yang, L.; et al. Climate and plant traits alter the relationship between seed dispersal and seed dormancy in alpine environment. Environ. Exp. Bot. 2024, 219, 105660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).