Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile
Abstract
1. Introduction
1.1. Agroecology, Functional Ecology, Biocultural Diversity, and Sociocological Approach
1.2. Agroecology, Biodiversity, and Agroecosystems Design
1.3. Situated Knowledge and Local Resources Valorization
1.4. Multifunctional Plants: Unknown Uses and Ignored Contexts
2. Methodology
3. Results: Experiences of Local Agroecologies
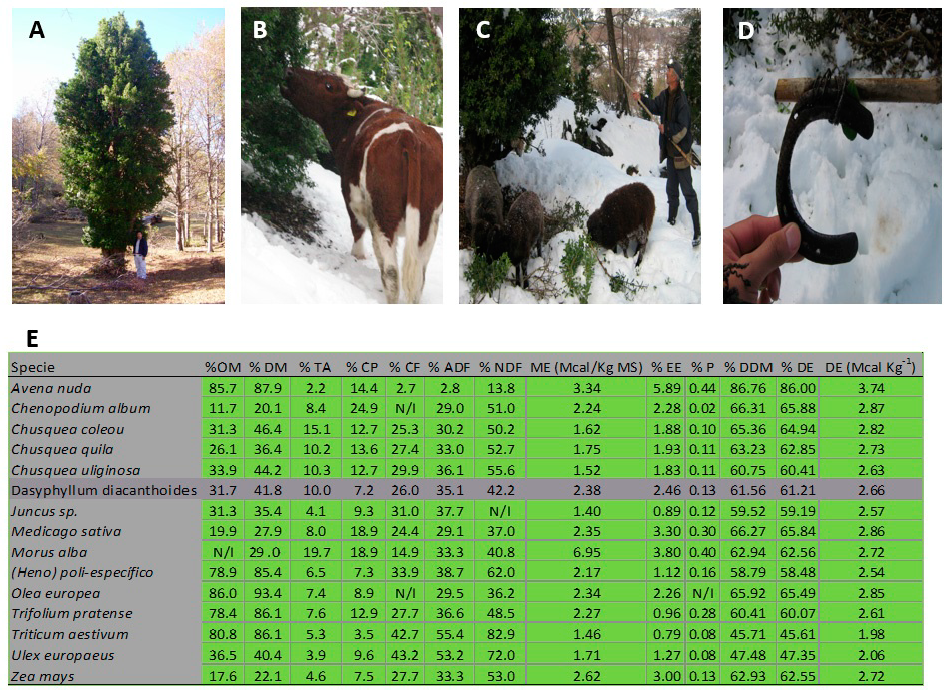
3.1. Dasyphylum Diacanthoides: The Lesser-Known Fodder of the “White Earthquake”
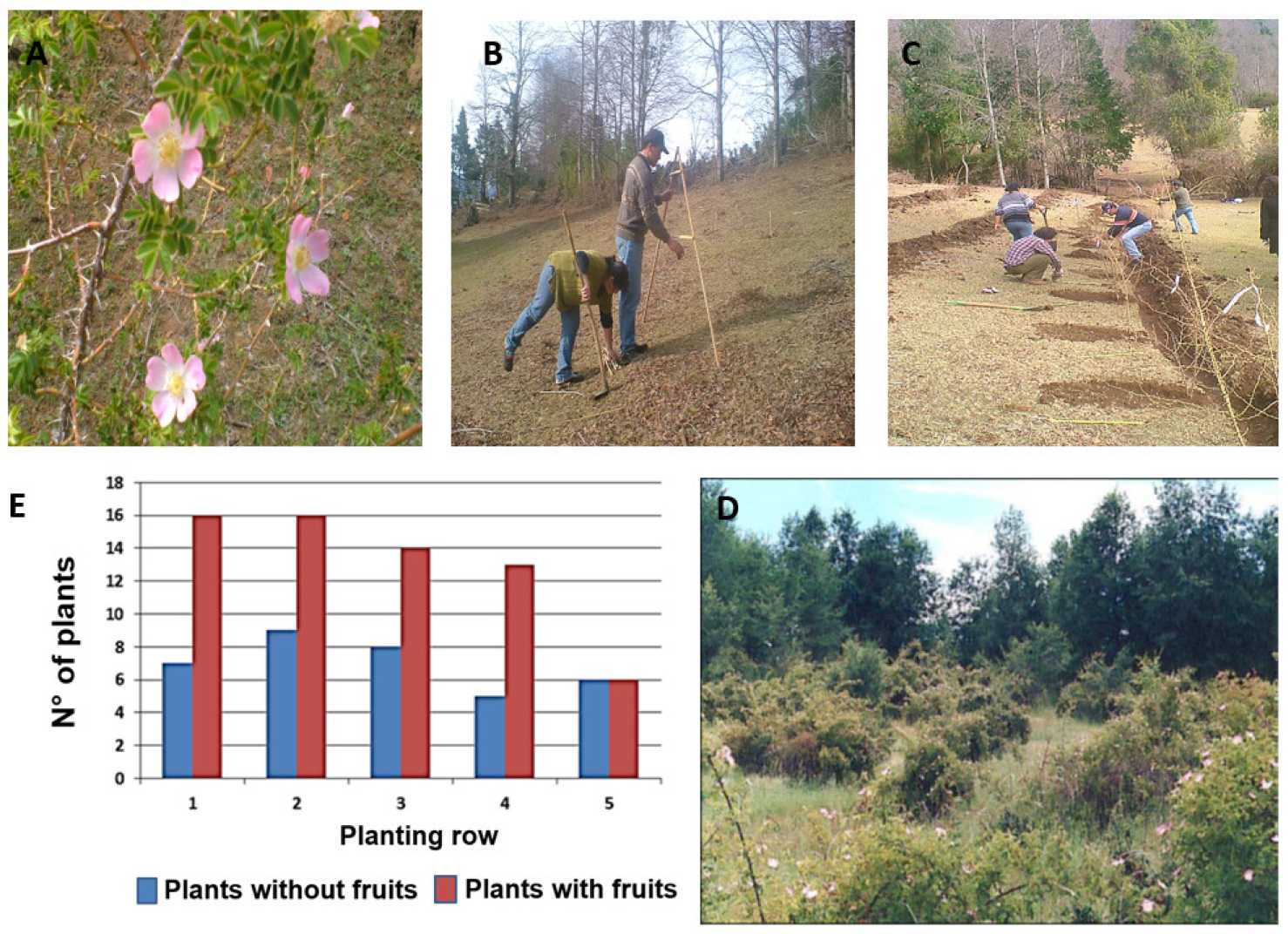
3.2. Rosa spp.: Agroecological Cultivation of an Invasive Plant
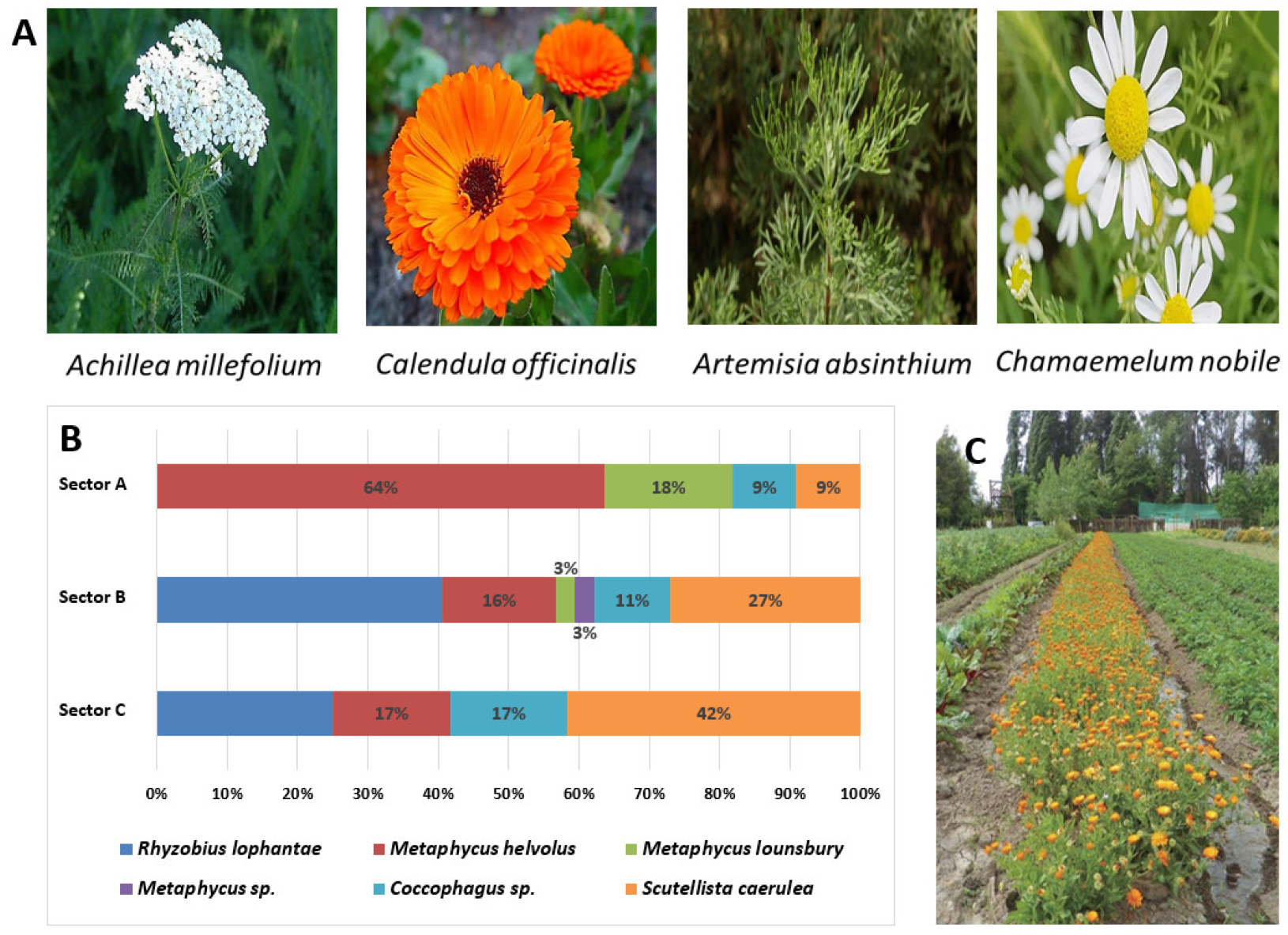
3.3. Aromatic and Medicinal Plants as Hosts of Natural Friends
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrera, C.; Peredo, S. Plantas Multifuncionales: Conocimiento situado y valorización de los recursos locales para el manejo sustentable de los sistemas agroecológicos en Chile. In Sembramos, Comemos y Vivimos. Saberes Agroecológicos desde los Sures; Ortega Santos, A., Ed.; COMARES: Granada, Spain, 2022; pp. 47–67. [Google Scholar]
- Cruz-García, G.; Lore, V. El manejo de plantas silvestres alimenticias en escenarios de deforestación, ilustrado por una comunidad mestiza de la Amazonía Peruana Kehl, Paulo Brack and Débora B. da Silva. Plantas Alimentícias Não Convencionais (Pancs). Hortaliças Espontâneas e Nativas. In Domesticación en el Continente Americano. Investigación para el Manejo Sustentable de Recursos Genéticos en el Nuevo Mundo; Casas, A., Torres-Guevara, J., Parra, F., Eds.; UNAM: México City, Mexico, 2017; pp. 327–344. [Google Scholar]
- Reis, G. Guía Práctica Sobre PANC: Plantas Alimenticias não Tradicionais; Kairós: São Paulo, Brazil, 2017. [Google Scholar]
- Jiménez, A.; Vela, M. Plantas Multifuncionales. Guía de usos, Cultivo y Recetas; Ecoherentes: Málaga, Spain, 2016. [Google Scholar]
- Kelen, M.E.B.; Nouhuys, I.A.S.; Kehl, L.C.; Brack, P.; da Silva, D.B. Plantas Alimentícias Não Convencionais (Pancs). Hortaliças Espontâneas e Nativas; UFRGS: Porto Alegre, Brazil, 2015. [Google Scholar]
- Cilia, V.; Celia Aradillas, C.; Díaz-Barriga, F. Las plantas comestibles de una comunidad indígena de la Huasteca Potosina, San Luis Potosí. Entreciencias 2015, 3, 143–152. [Google Scholar]
- Kinupp, V.F.; Lorenzi, H. Plantas Alimentícias não Convencionais PANC No Brasil; Plantarum: São Paulo, Brazil, 2014. [Google Scholar]
- Pereira, S.R.M.; Bohrer, S.; Uriartt, A.E. Alimentos da Biodiversidade: Receitas com Plantas Alimentícias não Convencionais; UFRGS: Porto Alegre, Brazil, 2011. [Google Scholar]
- Chávez, E. Plantas Comestibles no Convencionales en Chiapas; UNICACH: Chiapas, Mexico, 2010. [Google Scholar]
- Méndez, V.; Bacon, C.; Cohen, R. Agroecology as a transdisciplinary, participatory, and action-oriented approach. Agroecol. Sustain. Food Syst. 2013, 37, 3–18. [Google Scholar] [CrossRef]
- Rezende de Paula, G.A. Perspectiva histórica e estudo de conceitos em ecologia funcional. Oecol. Aust. 2013, 17, 331–346. [Google Scholar] [CrossRef][Green Version]
- González, M.; Salgado, B.; Baptiste, M.P.; Cortés, A.; Ruíz, C.; Ruíz, C.; Urbina, N.; García, H. Ecología funcional: Una herramienta para la generación de conocimiento científico frente a la gestión integral de la biodiversidad y sus servicios ecosistémicos. In La Ecología Funcional como Aproximación al Estudio, Manejo y Conservación de la Biodiversidad: Protocolos y Aplicaciones; Salgado-Negret, B., Ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2015; pp. 212, 234. [Google Scholar]
- Rodríguez, S.; Campanello, P.; Carrasco, O.; Goldstein, L.; Bucci, G. La ecología funcional, una herramienta de manejo forestal. In Ciencia y Tecnología Forestal en la Argentina; Area, M., Lupi, A., Escobar, P., Eds.; CONICET: Buenos Aires, Argentina, 2021; pp. 209–213. [Google Scholar]
- Cardinale, B.J.; Dufy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Turner, R.K.; Morling, P. Defyning and classifying ecosystem services for decision making. Ecol. Econ. 2009, 68, 643–653. [Google Scholar] [CrossRef]
- Fu, B.-J.; Wang, S.; Su, C.; Forsius, M. Linking ecosystem processes and ecosystem services. Curr. Opin. Environ. Sustain. 2013, 5, 4–10. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.; Bardgett, R.D.; Berg, M.; Cipriotti, P.; Feld, C.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Toledo, V.M.; Barrera, N.; Boege, E. ¿Qué es el Diversidad Biocultural? Universidad Nacional Autónoma de México: Morelia, Mexico, 2019; 64p. [Google Scholar]
- Swiderska, K. Banishing the Biopirates: A New Approach to Protecting Traditional Knowledge; International Institute for Environment and Development, Sustainable Agriculture and Rural Livelihoods Programme: London, UK, 2006; 129p. [Google Scholar]
- Davidson-Hunt, I.J.; Turner, K.L.; Te Pareake, M.A.; Cabrera López, J.; Bolton, R.; Idrobo, C.J. Biocultural design: A new conceptual framework for sustainable development in rural indigenous and local communities. Sapiens 2012, 5, 33–45. [Google Scholar]
- Raymond, C.M.; Singh, G.G.; Benessaiah, K.; Bernhardt, J.R.; Levine, J.; Nelson, H.; Turner, N.J.; Norton, B.; Tam, J.; Chan, K.M. Ecosystem services and beyond: Using multiple metaphors to understand human–environment relationships. BioScience 2013, 63, 536–546. [Google Scholar] [CrossRef]
- Caillon, S.; Cullman, G.; Verschuuren, B.; Sterling, E. Moving beyond the human–nature dichotomy through biocultural approaches: Including ecological well-being in resilience indicators. Ecol. Soc. 2017, 22, 27. [Google Scholar] [CrossRef]
- Sterling, E.J.; Filardi, C.; Toomey, A.; Sigouin, A.; Betley, E.; Gazit, N.; Newell, J.; Albert, S.; Alvira, D.; Bergamini, N.; et al. Biocultural approaches to well-being and sustainability indicators across scales. Nat. Ecol. Evol. 2017, 1, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Idrobo, C.J.; Turner, K.L.; Lara, D.M. Navegando el desarrollo económico local desde la diversidad biocultural. In Repensando el Desarrollo Económico Local Desde Colombia; Montero, S., Ed.; Universidad de los Andes: Bogotá, Colombia, 2021; pp. 85–112. [Google Scholar]
- Maffi, L.; Woodley, E. Biocultural Diversity Conservation: A Global Sourcebook; Routledge Taylor & Francis Group: London, UK, 2010; 312p. [Google Scholar]
- Nemogá, G.B. Diversidad biocultural: Innovando en investigación para la conservación. Acta Biol. Colomb. 2016, 21, 311–319. [Google Scholar] [CrossRef]
- Skewes, J.C. A medio camino en la reconciliación con el bosque nativo: Los aportes de Elinor Ostrom y la socioecología. In Hacia una Socioecología del Bosque Nativo en Chile; Reyes, R., Razeto, J., Barreau, A., Müller-Using, S., Eds.; Social-Ediciones; Instituto Forestal: Santiago, Chile, 2020; pp. 17–32. [Google Scholar]
- Gómez, A.; Cadenas, H. Sistemas socio-ecológicos: Elementos teóricos y conceptuales para la discusión en torno a vulnerabilidad hídrica. Ordin. Des. Amériques 2015, 218, 1–18. [Google Scholar]
- Folke, C.; Biggs, R.; Norström, A.V.; Reyers, B.; Rockström, J. Social-ecological resilience and biosphere-based sustainability science. Ecol. Soc. 2016, 21, 41. [Google Scholar] [CrossRef]
- Escalera, J. ¿Servicios de los ecosistemas o en los socioecosistemas?: Una mirada crítica al marco de los servicios ecosistémicos desde la Antropología. In Antropología Ambiental, Conocimientos y Prácticas Locales a las Puertas del Antropoceno; Santamarina, B., Coca, A., Beltrán, O., Eds.; Icaria: Barcelona, Spain, 2018; pp. 71–82. [Google Scholar]
- Nicholls, C.; Altieri, M.; Vázquez, L. Agroecología: Principios para la conversión y el rediseño de sistemas agrícolas. Agroecología 2015, 10, 61–72. [Google Scholar]
- Folke, C. Resilience: The emergence of a perspective for social ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Nicholls, C.; Altieri, M. Bases agroecológicas para la adaptación de la agricultura al cambio climático. Cuad. Inv. Uned 2019, 11, 55–61. [Google Scholar] [CrossRef]
- Moonen, A.C.; Barberi, P. Functional biodiversity: An agroecosystem approach. Agric. Ecosyst. Environ. 2008, 127, 7–21. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Agroecology: Scaling up for food sovereignty and resiliency. Sustain. Agric. Rev. 2012, 11, 1–29. [Google Scholar]
- Nicholls, C.; Altieri, M. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Power, A.G.; Flecker, A.S. The role of biodiversity in tropical managed ecosystems. In Biodiversity and Ecosystem Processes in Tropical Forests. Ecological Studies (Analysis and Synthesis); Gordon, H., Dirzo, R., Cushman, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Vázquez, L.; Matienzo Brito, Y.; Simonetti, J.A.; Veitia Rubio, M.; Paredes, E.; Fernandez, E. Contribución al diseño agroecologico de sistemas de producción urbanos y suburbanos para favorecer procesos ecológicos. Agric. Orgán. 2012, 18, 14–19. [Google Scholar]
- Paleologos, M.F.; Iermanó, M.J.; Blandi, M.L.; Sarandón, S. Las relaciones ecológicas: Un aspecto central en el rediseño de agroecosistemas sustentables, a partir de la Agroecología. Redes 2016, 22, 92–115. [Google Scholar]
- Malezieux, E. Designing cropping systems from nature. Agron. Sustain. Dev. 2012, 32, 15–29. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Agroecology: Challenges and opportunities for farming in the Anthropocene Review. IJANR 2020, 47, 204–215. [Google Scholar] [CrossRef]
- Gliessmann, S.; Mendez, E.; Izzo, V.; Engles, V.W. Agroecology: Leading the Transformation to a Just and Sustainable Food System, 4th ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2022; p. 480. [Google Scholar] [CrossRef]
- Noguera-Talavera, A.; Salmerón, F.; Reyes-Sánchez, N. Bases teórico-metodológicas para el diseño de sistemas agroecológicos. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2019, 51, 273–293. [Google Scholar]
- Salembier, C.; Elverdín, J.H.; Meynard, J.M. Tracking on-farm innovations to un earth alternatives to the dominant soybean-based system in the Argentinean Pampa”. Agron. Sustain. Dev. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Peredo, S.; Barrera, C. Usos etnobotánicos, estrategias de acción y transmisión cultural de los recursos florísticos en la localidad de Armerillo, Región del Maule (Chile). Bol. Latinoam. Caribe Plantas Med. Aromát. 2017, 16, 398–409. [Google Scholar]
- Barrera, C.; Acuña, B.; Baeza, C.; Peredo, S. Sabiduría ancestral: Rescate de conocimientos agroecológicos de mujeres del Valle del Loa, desierto de Atacama, Chile. In Proceedings of the VII Congreso Internacional de Agroecología, Vigo, Spain, 1–3 July 2020. [Google Scholar]
- Peredo, S.; Barrera, C. Agroecology, Local Knowledge and Participatory Research: Articulation of Knowledge for Sustainable Use of Plant Resources in Agroecosystems. In Ethnobotany: Local Knowledge and Traditions; Martínez, J.L., Muñoz Acevedo, A., Rai, M., Eds.; CRC Press-Taylor & Francis: Boca Ratón, FL, USA, 2019; pp. 19–33. [Google Scholar]
- Arrioja, L. Sentido de la agroecología: Una aproximación reflexiva de la producción ecosocial desde los sujetos académicos. Rev. Venez. Investig. 2019, 19, 45–52. [Google Scholar]
- Kansanga, M.M.; Luginaah, I.; Bezner Kerr, R.; Lupafya, E.; Dakishoni, L. Beyond ecological synergies: Examining the impact of participatory agroecology on social capital in smallholder farming communities. Int. J. Sustain. Dev. World Ecol. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Peredo, S.; Barrera, C. Desarrollo Rural Endógeno: Condiciones para una transición agroecológica desde una experiencia de producción orgánica. CUHSO 2002, 6, 71–90. [Google Scholar] [CrossRef]
- Flick, U. Introducción a la Investigación Cualitativa; Morata: Madrid, Spain, 2004; 45p. [Google Scholar]
- Domené-Painenao, O.; Herrera, F.F. Situated agroecology: Massification and reclaiming university programs in Venezuela. Agroecol. Sustain. Food Syst. 2019, 43, 936–953. [Google Scholar] [CrossRef]
- Spivak, G.C. ¿Puede hablar el sujeto subalterno? Orb. Tert. 1998, 3, 175–235. [Google Scholar]
- Toledo, V.M. El Juego de la Supervivencia, Un Manual para la Inivestigación Etnoecológica en Latinoamérica; CLADES: Berkelet, CA, USA, 1991; 75p. [Google Scholar]
- Peredo, S.; Barrera, C.; Burbi, S.; Rocha, D. Agroforestry in the Andean Araucanía: An Experience of Agroecological Transition withWomen from Cherquén in Southern Chile. Sustainability 2020, 12, 10401. [Google Scholar] [CrossRef]
- Mosbach, E.W. Botánica Indígena de Chile; Andrés Bello: Santiago, Chile, 1992. [Google Scholar]
- García, N.; Ormazabal, C. Árboles Nativos de Chile; Enersis S.A: Santiago, Chile, 2008. [Google Scholar]
- Abarzúa, A.; Donoso, P.; Donoso, C. Dasyphylum diacanthoides (Asteraceae). Trevo, tayo, tevo, palo santo, palo blanco, Familia Asteraceae (Compositae). In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina. Autoecología; Donoso, C., Ed.; Marisa Cueno Ediciones: Valdivia, Chile, 2007; pp. 212–215. [Google Scholar]
- Peredo, S.; Alvarez, R.; Barrera, C.; Parada, E. Nutritional value of Dasyphyllum diacanthoides (Less.) Carb.: An endemic tree used as suplementary forage in agroforestry systems. Bioagro 2020, 30, 139–144. [Google Scholar]
- Gut, B. Árboles Nativos e Introducidos en Patagonia; Vázquez Mazzini: Buenos Aires, Argentina, 2017; p. 416. [Google Scholar]
- Muñoz, M.; Barrera, E. El uso Medicinal y Alimenticio de Plantas Nativas y Naturalizadas en Chile; MNHN: Santiago, Chile, 1981. [Google Scholar]
- Zampini, I.C.; Cudmani, N.; Isla, M.I. Actividad antimicrobiana de plantas medicinales argentinas sobre bacterias antibiótico-resistentes. Acta Bioquim Clin. Latinoam 2007, 41, 385–393. [Google Scholar]
- Valencia Galindo, E. Validación y Actualización del uso de Plantas Medicinales Presentes en la Selva Valdiviana. Bachelor’s Thesis (Chemistry and Pharmacy), Universidad Austral de Chile, Valdivia, Chile, 2013. [Google Scholar]
- Hoffmann, A. Plantas Medicinales de Uso Común en Chile, 3rd ed.; Fundación Claudio Gay: Santiago, Chile, 2003. [Google Scholar]
- Estomba, D.; Ladio, A.; Lozada, M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J. Ethnopharmacol. 2006, 103, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Corporación Nacional Forestal (CONAF). El Estado de los Recuros Genéticos en el Mundo. Informe Regional Chile; FAO: Santiago, Chile, 2011. [Google Scholar]
- Benoit, I. El Libro Rojo de la Flora Terrestre de Chile; INFOR: Santiago, Chile, 1989. [Google Scholar]
- Vergara, R. La Variabilidad Poblacional. In Mejora Genética Forestal Operativa; Ipinza, R., Gutierrez, B., Emhart, V., Eds.; UACh: Valdivia, Chile, 1998; pp. 39–48. [Google Scholar]
- Salazar, E.; León, P.; Rosas, M.; Muño, C. Estado de la Conservación ex situ de los Recursos Fitogenéticos Cultivados y Silvestres en Chile; INIA: Santiago, Chile, 2006. [Google Scholar]
- Hechenleitner, P.; Gardner, M.E.; Thomas, P.I.; Echeverría, C.; Escobar, B.; Brownless, P.; Martínez, C. Plantas Amenazadas del Centro-Sur de Chile. Distribución, Conservación y Propagación; UACH: Valdivia, Chile, 2005. [Google Scholar]
- Peredo, S.; Parada, E.; Alvarez, R.; Barrera, C. Propagación vegetativa por estacas de Dasyphylum diacanthoides mediante recursos endógenos. Una aproximación agroecológica. Bol. Latinoam Caribe Plant Med. Aromat. 2015, 14, 301–307. [Google Scholar]
- Santelices, R.; García, C. Efecto del ácido indolbutírico y la ubicación de la estaca en el rebrote de tocón sobre la rizogénesis de Nothofagus alessandri Espinoza. Bosque 2013, 24, 53–61. [Google Scholar] [CrossRef]
- Latsague, M.; Saéz, P.; Hauenstein, E. Inducción de enraizamiento en estacas de Berberidopsis corallina con ácido indolbutírico. Bosque 2008, 29, 227–230. [Google Scholar] [CrossRef]
- Latsague, M.; Saéz, P.; Yáñez, J. Efecto del ácido indolbutírico en la capacidad rizogénica de estacas de Eucryphia glutinosa. Bosque 2009, 30, 102–105. [Google Scholar] [CrossRef]
- Jordan, M.; Prehn, D.; Gebahuer, M.; Neumann, J.; Parada, G.M.; Veloso, J.; San Martín, R. Iniciación adventicia de raíces en estacas adultas y juveniles de Guindilia trinervis, una planta endémica de Chile, apta para producción de biodiesel. Bosque 2010, 31, 195–201. [Google Scholar] [CrossRef]
- Cabrera, A. Revisión del Género Dasyphyllum (Compositae). Botánica 1959, 9, 21–108. [Google Scholar]
- Villagran, C.; Hinojosa, L.F. Historia de los bosques del sur de Sudamérica, II: Análisis fitogeográfico. Rev. Chil. Hist. Nat. 1997, 70, 241–267. [Google Scholar]
- Brasovan, A.; Mandroc, V.; Campean, R.; Petean, I.; Codrea, V.; Arghir, G. Calcium and magnesium content in brier (Rosa canina L.) “Fruits at the “campul lui neag” sterile coal dump (Hunedoara county, Romania). Fasc. Biol. 2011, 18, 5–9. [Google Scholar]
- Galaz, A. Relación entre Momento de Cosecha y Algunos Parámetros de Calidad en dos Especies de Rosa Mosqueta; UdeC: Chillán, Chile, 1999. [Google Scholar]
- Cavallero, L.; Raffaele, E. Fire enhances the ‘competition-free’ space of an invader shrub: Rosa rubiginosa in northwestern Patagonia. Biol. Invasions 2010, 12, 3395–3404. [Google Scholar] [CrossRef]
- Joublan, J.P.; Ríos, D. Rose culture and industry in Chile. Acta Hortic 2005, 690, 65–69. [Google Scholar] [CrossRef]
- Espinosa, N. Malezas Presentes en Chile; INIA: Temuco, Chile, 1996. [Google Scholar]
- Matthei, O. Manual de las Malezas que Crecen en Chile; Alfabeto: Concepción, Chile, 1995. [Google Scholar]
- Espinoza, T.; Valencia, R.; Quevedo, L.; Díaz, O. Importancia y propiedades físico química de la rosa mosqueta (R. canina, R. rubiginosa): Una revisión. Sci. Agropecu. 2016, 7, 67–78. [Google Scholar] [CrossRef][Green Version]
- Benaiges, A. Aceite de rosa mosqueta: Composición y aplicaciones dermocosméticas. Offarm 2008, 27, 94–97. [Google Scholar]
- Pirones, B.N.; Ochoa, M.R.; Kesseler, A.G.; De Michelis, A. Evolución de la concentración de ácidos ascórbico durante el proceso de deshidratación de frutos de la rosa mosqueta. RIA 2002, 31, 85–98. [Google Scholar]
- da Silva Carlos, E.; Vandenabeele, P.; Edwards, G.M.; Cappa de Oliveira, L.F. NIR-FT-Raman spectroscopic analytical characterization of the fruits, seeds, and phytotherapeutic oils from rosehips. Anal. Bioanal. Chem. 2008, 392, 1489–1496. [Google Scholar] [CrossRef]
- Dourado, F.; Vasco, P.; Gama, F.M.; Coimbra, M.; Mota, M. Characterisation of Rosa Mosqueta seeds: Cell wall polysaccharide composition and light microscopy observations. J. Sci. Food Agric. 2000, 80, 1859–1865. [Google Scholar] [CrossRef]
- Franco, D.; Pinelo, M.; Sineiro, J.; Núnez, M. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 2007, 98, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Romero, N.; Ortiz, J.; Masson, L.; Barrera-Arellano, D. Effect of rosa mosqueta (Rosa rubiginosa) extract on the performance of Chilean hazelnut oil (Gevuina avellana mol.) at high temperature. JAOCS 2006, 83, 691–695. [Google Scholar] [CrossRef]
- Črnivec, G.O.; Muri, P.; Djinovic, P.; Pintar, A. Biogas production from spent rose hips (Rosa canina L.): Fraction separation, organic loading and co-digestion with N-rich microbial biomass. Bioresour. Technol. 2014, 171, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.; Coburn, T.; Shofoluwe, A.; Martin, P. Rosehip (Rosa canina) Extracts Prevent Cell Proliferation and Migration in Triple Negative Breast Cancer Cells. FASEB J. 2015, 29, 629.14. [Google Scholar] [CrossRef]
- Avello, M.; Cisternas, I. Fitoterapia, sus orígenes, características y situación en Chile. Rev. Med. Chile 2010, 138, 1288–1293. [Google Scholar] [CrossRef]
- Chrubasik, C.; Wiesner, L.; Black, A.; Müller-Ladner, U.; Chrubasik, S. A one-year Survey on the Use of a Powder from Rosa canina lito in Acute Exacerbations of Chronic Pain. Phytother. Res. 2008, 22, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Warholm, O.; Skaar, S.; Hedman, E.; Mølmen, H.M.; Erik, L. The effects of a standardized herbal remedy made from a subtype of Rosa canina in patients with osteoarthritis: A double-blind, randomized, placebo-controlled clinical trial. Curr. Ther. 2003, 64, 21–32. [Google Scholar] [CrossRef]
- Azón López, E.; Hernández Pérez, J.; Mir Ramos, E. Evidencia científica sobre el uso del aceite de rosa mosqueta en el embarazo: Una revisión de la bibliografía. Med. Nat. 2013, 7, 94–98. [Google Scholar]
- Valenzuela, A.; Valenzuela, R. Ácidos grasos omega-3 en la nutrición ¿cómo aportarlos? Rev. Chil. Nutr. 2014, 41, 205–211. [Google Scholar] [CrossRef]
- Parejas, B.; Horst, K. Contribucion a la identificacion de los principios activos en el aceite de rosa off Rubiginosa L. Real Acad. Farm 1990, 56, 283–294. [Google Scholar]
- Soare, R.; Bonea, D.; Iancu, P.; Niculescu, M. Biochemical and technological properties of Rosa canina l. Fruits from spontaneous flora of Oltenia, Romania. Bull. UASVM Hortic. 2015, 72, 182–186. [Google Scholar] [CrossRef][Green Version]
- Crețescu, I.; Ropciuc, S.; Leahu, A. Evaluation of rosehip fruit productivity and total acidity in response to climatic factors. Rom. Biotechnol. Lett. 2013, 18, 8403–8412. [Google Scholar]
- Moure, A.; Franco, D.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Lema, J.M. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res. Int. 2001, 34, 103–109. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Vieira, A.B.D.; KamadaI, I. La Rosa Mosqueta en el tratamiento de heridas abiertas: Una revisión. Rev. Bras. Enferm. 2009, 62, 457–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duvaldo, E.; Silva, L.A.; Daleck, C.R.; Freitas, M.; Alves, L.B. Efecto del extracto de óleo de rosa mosqueta (Rosa aff. rubiginosa) en la cicatrización de heridas cutáneas. Redvet 2011, 1695, 7504. [Google Scholar]
- Cañellas, M.; Espada, N.; Ogalla, J.M. Estudio del aceite de rosa mosqueta en cicatrices postquirúrgicas. El Peu 2008, 28, 9–13. [Google Scholar]
- Tacón, A. Cuadernos de Campo de Buenas Prácticas de Recolección Sustentable para Productos Forestales No Madereros Prioritarios. Rosa mosqueta (Rosa spp.); FIA: Santiago, Chile, 2017. [Google Scholar]
- Muñoz, J.; Mardones, R. Autogestión e Intercambio Territorial Mapuche en la Comuna de Melipeuco (2003–2008): Una mirada desde el Desarrollo Local. In Proceedings of the XXIX Congreso de la Asociación Latinoamericana de Sociología, Santiago, Chile, 29 September–4 October 2013. [Google Scholar]
- Barros Biscardi, R. Los saberes colectivos locales como factores del anclaje territorial. El SIAL de la rosa mosqueta rubiginosa de la Patagonia Argentina. In Proceedings of the 116th EAAE Seminar Spatial Dynamics in Agri-food Systems: Implications for Sustainability and Consumer Welfare, Parma, Italy, 27–30 October 2010. [Google Scholar]
- Fundación para la Innovación Agraria. Plantas Medicinales y Aromáticas Evaluadas en Chile; FIA: Santiago, Chile, 2003. [Google Scholar]
- Cruzat, R.; Bellolio, C. Producción y Comercialización de Hierbas Medicinales bajo Manejo Orgánico; Ograma Ltd.: Santiago, Chile, 2009. [Google Scholar]
- Délano, G.; Zamorano, M.E.; Ormeño, J.; Sepúlveda, P.; Hewstone, N.; Estay, P.; Hinrichsen, P. Cultivo de Plantas Medicinales como Alternativa para el secano de la Sexta Región; Boletín INIA N° 31; INIA: Santiago, Chile, 2000. [Google Scholar]
- Aguilera, M.; Navarro, R. Inocuidad en Hierbas Aromáticas, Medicinales y Culinarias. Implementación de Protocolos de Inocuidad en la Producción y Procesamiento de Hierbas Aromáticas, Medicinales y Culinarias; Ograma Ltd.: Santiago, Chile, 2016. [Google Scholar]
- Herrera Campos, H. Diseño de un modelo de alianza estratégica productiva para la agricultura familiar campesina: Caso productores de plantas medicinales de Casablanca. Bachelor’s Thesis (Engineering), Universidad de Viña del Mar, Viña del Mar, Chile, 2011. [Google Scholar]
- Peredo, S.; Barrera, C.; Martínez, J.L.; Romo, J. Plantas medicinales y aromáticas como hospederas de enemigos naturales de Saissetia oleae en arreglos espacio-temporales para el cultivo agroecológico de Olea europea. Bol. Latinoam Caribe Plant Med. Aromat. 2020, 19, 482–491. [Google Scholar] [CrossRef]
- Peredo, S.; Barrera, C. Democratizando el consumo ecológico: Elementos para la acción y aprendizaje colectivo en procesos de investigación acción participativa. Agroecologia 2018, 13, 57–69. [Google Scholar]
- Trujillo, R.; García, L. Conocimiento indígena del efecto de plantas medicinales locales sobre las plagas agrícolas de Los Altos de Chiapas, México. Agrociencia 2001, 35, 685–692. [Google Scholar]
- Prado, E. Artrópodos y sus Enemigos Naturales Asociadas a Plantas Cultivadas en Chile; INIA: Santiago, Chile, 1991. [Google Scholar]
- Cazanga, R.; Leiva, C. Antecedentes Técnicos y Económicos para la Producción de Olivo en la Región del Maule; CIREN: Santiago, Chile, 2013. [Google Scholar]
- Quiroz, C.; Erica, G. Manual de Manejo de Huerto de Olivo; INIA: Santiago de Chile, Chile, 2017. [Google Scholar]
- Zúñiga, E. Ochenta años de control biológico en Chile. Revisión histórica y evaluación de los proyectos realizados (1903–1983). Agric. Téc. 1985, 3, 175–183. [Google Scholar]
- Klein, C. Aspectos generales del control biológico e integrado de plagas en Chile. Bol. Serv. Plagas 1997, 3, 121–132. [Google Scholar]
- Skewes, J.C.; Guerra, D.E. Sobre árboles y personas: La presencia del roble (Nothofagus obliqua) en la vida cordillerana mapuche de la cuenca del río Valdivia. Atenea 2015, 512, 189–210. [Google Scholar] [CrossRef]
- Jaksic, F.M.; Castro, S. Invasiones Biológicas en Chile; Ediciones UC: Santiago, Chile, 2014; 526p. [Google Scholar]
- Lago, P.F. A Consciencia Ecológica. A luta pelo Futuro; Universidade Federal Santa Caterina: Florianópolis, Brazil, 1986; 232p. [Google Scholar]
- Guzmán, D. Diversidad biocultural y género: Trayectorias productivas de mujeres campesinas de Chiloé. Rev. Austral Cienc. Soc. 2016, 31, 25–42. [Google Scholar] [CrossRef]
- Marchant, C.; Fuentes, N.; Kaulen, S.; Ibarra, J. Local knowledge in montane homegardens in the southern Andes: A refuge of Mapuche Pewenche biocultural memory. Pirin. Rev. Ecol. Mont. 2020, 175, e060. [Google Scholar]
- Riquelme, W. Árboles y geografías sagradas de la espiritualidad mapuche contemporánea. In Geografías de lo Sagrado en la Contemporaneidad; Carballo, C., Flores, C., Eds.; Universidad Nacional de Quilmes: Bernal, Argentina, 2019; pp. 573–596. [Google Scholar]
- Olivares, F.; Marchant, C.; Ibarra, J. The climate itself must have hidden some medicines: Traditional veterinary medicine of indigenous and non-indigenous campesinos of the southern Andes. J. Ethnobiol. Ethnomed. 2022, 18, 36. [Google Scholar] [CrossRef]
- Marchant, C.; Rodríguez, P.; Morales, L.; Paz, L.; Ortega, L. Practices and strategies for adaptation to climate variability in family farming. An analysis of cases of rural communities in the Andes mountains of Colombia and Chile. Agriculture 2021, 11, 1096. [Google Scholar] [CrossRef]
- Barreau, A.; Ibarra, J.T.; Wyndham, F.S.; Rojas, A.; Kozak, R.A. How can we teach our children if we cannot access the forest? Generational change in Mapuche knowledge of wild edible plants in Andean temperate ecosystems of Chile. J. Ethnobiol. 2016, 36, 412–432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo Parada, S.; Barrera Salas, C. Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile. Land 2024, 13, 39. https://doi.org/10.3390/land13010039
Peredo Parada S, Barrera Salas C. Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile. Land. 2024; 13(1):39. https://doi.org/10.3390/land13010039
Chicago/Turabian StylePeredo Parada, Santiago, and Claudia Barrera Salas. 2024. "Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile" Land 13, no. 1: 39. https://doi.org/10.3390/land13010039
APA StylePeredo Parada, S., & Barrera Salas, C. (2024). Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile. Land, 13(1), 39. https://doi.org/10.3390/land13010039




