Drought Stress Responses of Some Prairie Landscape C4 Grass Species for Xeric Urban Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Site Description
2.2. Plant Material and Planting Conditions
2.3. Drought Treatments and Measured Parameters
2.3.1. Physiological Parameters
2.3.2. Qualitative Parameters
2.3.3. Plant Growth and Morphological Parameters
2.4. Statistical Analyses
3. Results
3.1. Physiological Parameters
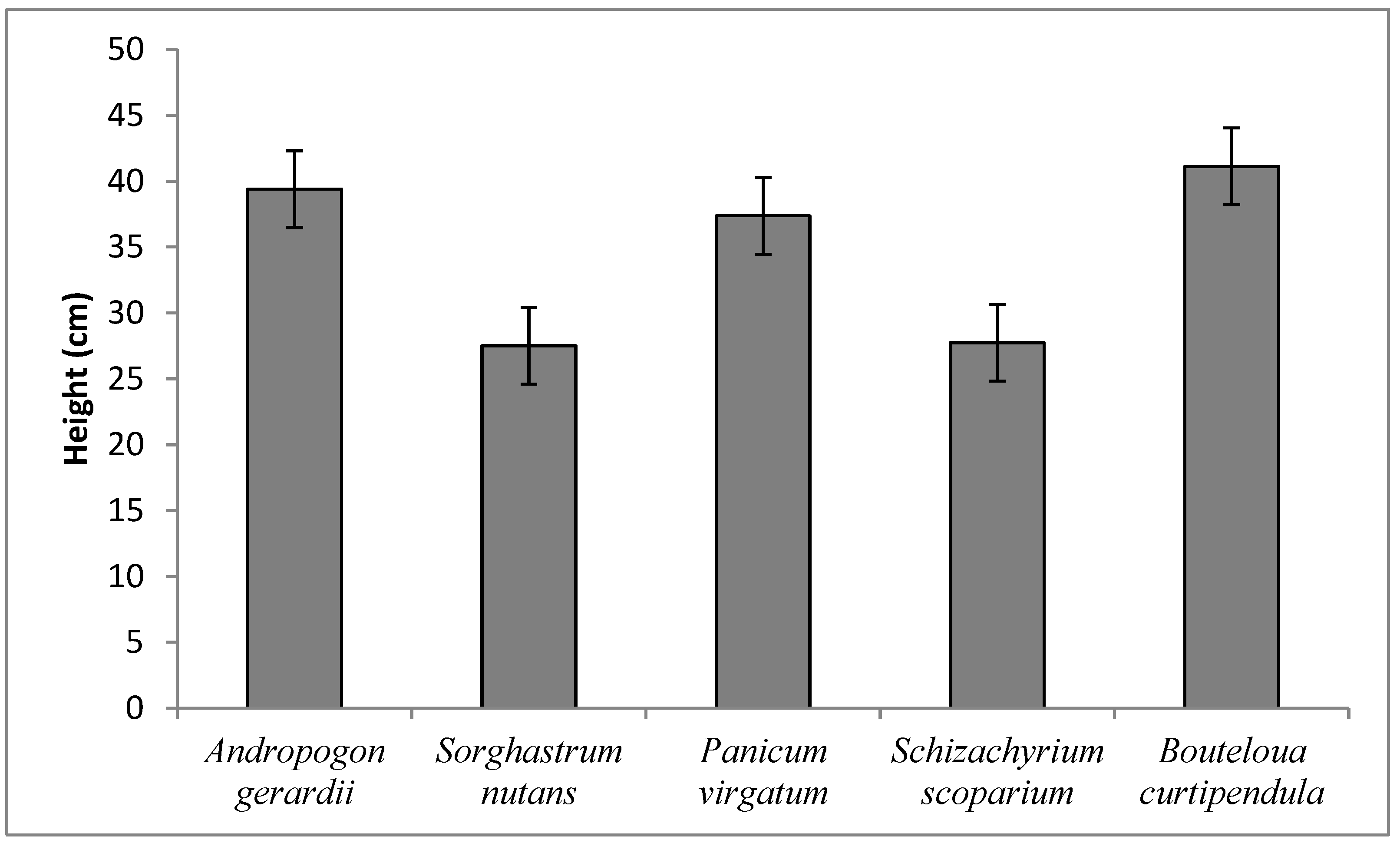
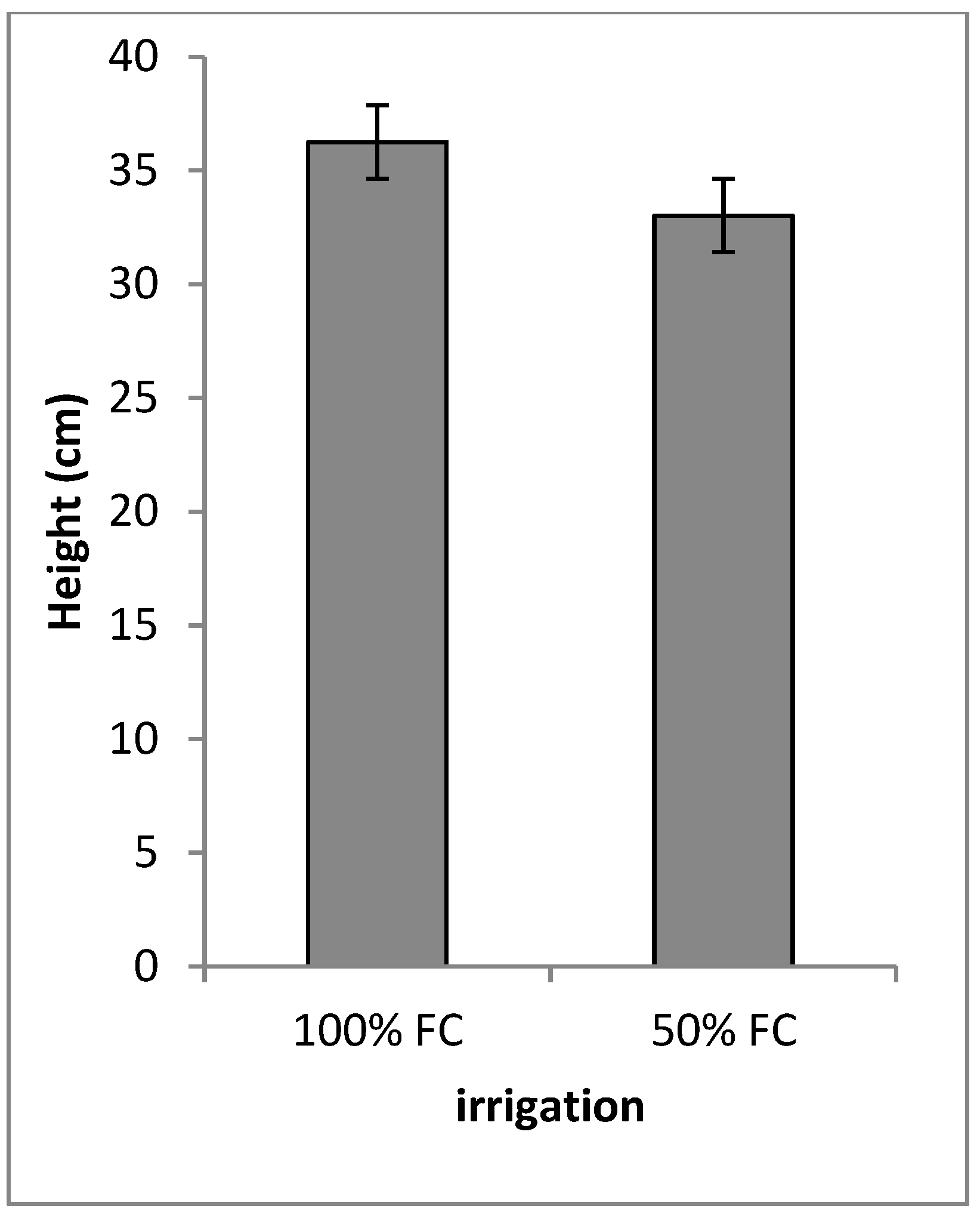
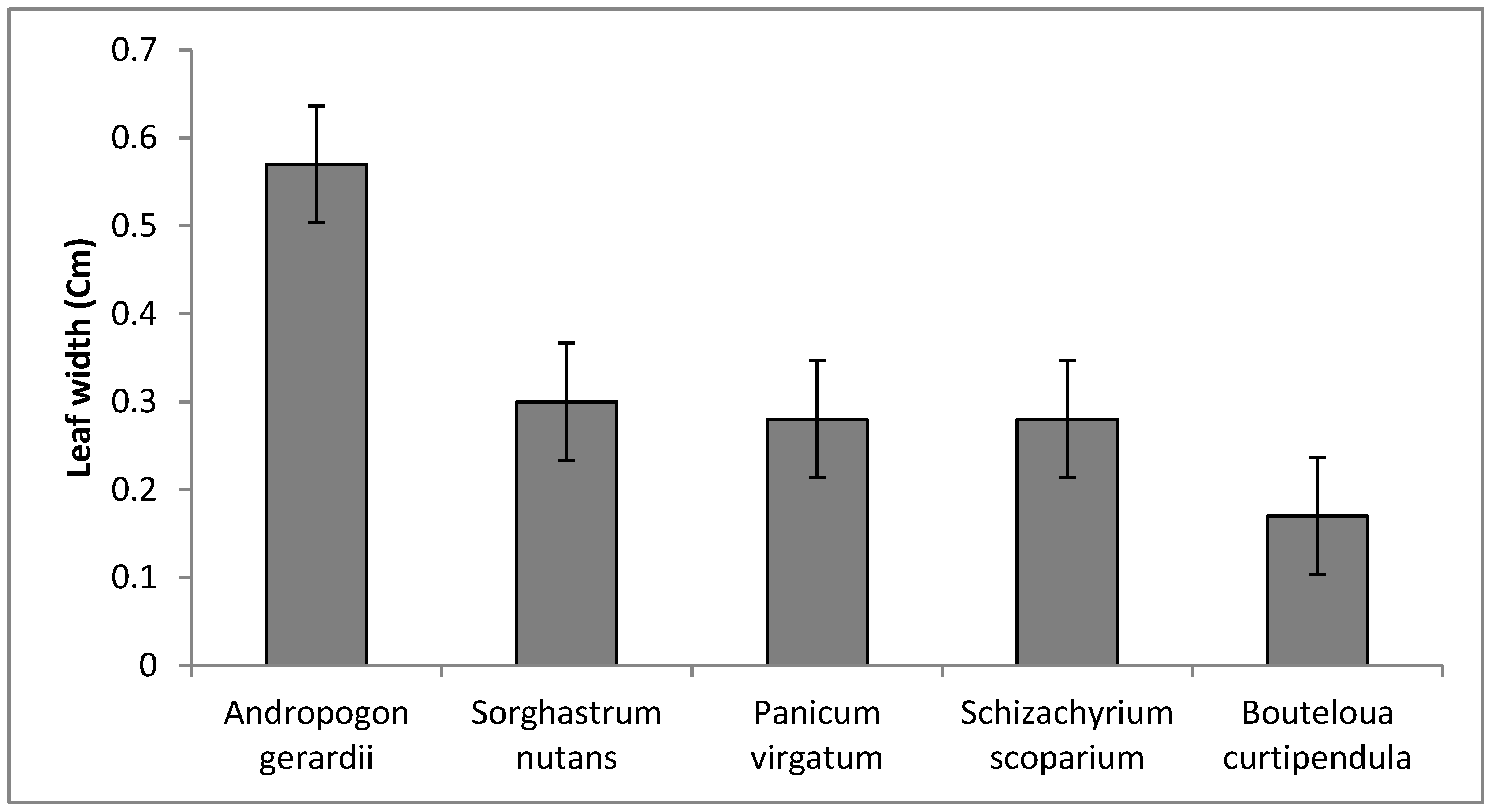
3.2. Qualitative, Morphological and Plant Growth Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hitchmough, J.D.; De la Fleur, M. Establishing North American prairie vegetation in urban parks in northern England: Effect of management and soil type on long-term community development. Landsc. Urban Plan. 2006, 78, 386–397. [Google Scholar] [CrossRef]
- Hitchmough, J.; Paraskevopoulou, A.; Dunnett, N. Influence of grass suppression and sowing rate on the establishment and persistence of forb dominated urban meadow. Urban Ecosyst. 2008, 11, 33–44. [Google Scholar] [CrossRef]
- Ignatieva, M.; Haase, D.; Dushkova, D.; Haase, A. Lawns in cities: From a globalised urban green space phenomenon to sustainable nature-based solutions. Land 2020, 9, 73. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Knowles-Yanez, K.; Hope, D. Urban ecology as an interdisciplinary field; differences in the use of “urban” between the social and natural sciences. Urban Ecosyst. 2000, 4, 5–24. [Google Scholar] [CrossRef]
- Hoffman, M.A.; Smith, D. Nonlinear drought plasticity reveals intraspecific diversity in a dominant grass species. Funct. Ecol. 2021, 35, 463–474. [Google Scholar] [CrossRef]
- De Carvalho, C.A.; Raposo, M.; Pinto-Gomes, C.; Matos, R. Native or exotic: A bibliographical review of the debate on ecological science methodologies: Valuable lessons for urban green space design. Land 2022, 11, 1201. [Google Scholar] [CrossRef]
- Shi, Y.; Guofu, Y.; Du, Y.; Ren, Y.; Lu, Y.; Fan, L.; Chang, J.; Ge, Y.; Bao, Z. Estimating irrigation water demand for green spaces in humid areas: Seeking a sustainable water management strategy. Urban Water J. 2017, 15, 16–22. [Google Scholar] [CrossRef]
- Kazemi, F.; Mohorko, R. Review on the roles and effects of growing media on plant performance in green roofs in world climates. Urban For. Urban Green. 2017, 23, 13–26. [Google Scholar] [CrossRef]
- Baganz, G.F.M.; Baganz, D. Compensating for loss of nature and landscape in a growing city—Berlin case study. Land 2023, 12, 567. [Google Scholar] [CrossRef]
- Ross, C.E.; McIntyre, S.; Barton, P.S.; Evans, M.J.; Cunningham, S.A.; Manning, A.D. A reintroduced ecosystem engineer provides a germination niche for native plant species. Biodivers. Conserv. 2020, 29, 817–837. [Google Scholar] [CrossRef]
- Hitchmough, J. Exotic plants and plantings in the sustainable, designed urban landscape. Landsc. Urban Plan. 2011, 100, 380–382. [Google Scholar] [CrossRef]
- Bahrani, M.J.; Bahrami, H.; Kamgar Haghighi, A.A. Effect of water stress on ten forage grasses native or introduced to Iran. Jpn. Soc. Grassl. Sci. 2010, 56, 1–5. [Google Scholar] [CrossRef]
- Nazemi Rafi, Z.; Kazemi, F.; Tehranifar, A. Effects of various irrigation regimes on water use efficiency and visual quality of some ornamental herbaceous plants in the field. Agric. Water Manag. 2019, 212, 78–87. [Google Scholar] [CrossRef]
- Kazemi, F.; Hill, K. Effect of permeable pavement basecourse aggregates on stormwater quality for irrigation reuse. Ecol. Eng. 2015, 77, 189–195. [Google Scholar] [CrossRef]
- Kazemi, F.; Beecham, S.; Gibbs, J. Streetscape biodiversity and the role of bioretention swales in an Australian urban environment. Landsc. Urban Plan. 2011, 101, 139–148. [Google Scholar] [CrossRef]
- Kazemi, F.; Beecham, S.; Gibbs, J.; Clay, R. Factors affecting terrestrial invertebrate diversity in bioretention basins in an Australian urban environment. Landsc. Urban Plan. 2009, 92, 304–313. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Rabbani, S.M.; Kazemi, F. Investigating strategies for optimum water usage in green spaces covered with lawn. Desert 2015, 20, 217–230. [Google Scholar]
- Khoshkholghsima, N.A.; Rohollahi, I. Evaluating biochemical response of some selected perennial grasses under drought stress in Iran. Hortic. Environ. Biotechnol. 2015, 56, 383–390. [Google Scholar] [CrossRef]
- Hedblom, M.; Lindberg, F.; Vogel, E.; Wissman, j.; Ahrne, K. Estimating urban lawn cover in space and time: Case studies in three Swedish cities. Urban Ecosyst. 2017, 20, 1109–1119. [Google Scholar] [CrossRef]
- Dunnett, N.; Hitchmough, J. (Eds.) The Dynamic Landscape: Design, Ecology and Management of Naturalistic Urban Planting; Spon Press: London, UK; Spon Press: New York, NY, USA, 2004. [Google Scholar]
- Hitchmough, J.; De la Fleur, M.; Findlay, C. Establishing North American prairie vegetation in urban parks in Northern England, Part 1. Effect of sowing season, sowing rate and soil type. Landsc. Urban Plan. 2004, 66, 75–90. [Google Scholar] [CrossRef]
- Amiri Fedradi, R. Evaluation of the Establishment and Adoption of Several Species of Ornamental Grasses for Use in Green Space. Master’s Thesis, Mashhad University, Mashhad, Iran, 2015. [Google Scholar]
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Nazemi Rafi, Z.; Kazemi, F.; Tehranifar, A. Public preferences toward water-wise landscape design in a summer season. Urban For. Urban Green. 2020, 48, 126563. [Google Scholar] [CrossRef]
- Barney, J.N.; Mann, J.J.; Kyser, G.B.; Blumwald, E.; Van Deynze, A.; DiTomaso, J.M. Tolerance of switchgrass to extreme soil moisture stress: Ecological implications. Plant Sci. 2009, 177, 724–732. [Google Scholar] [CrossRef]
- Cathey, S.E.; Kruse, J.K.; Sinclair, T.R.; Dukes, M.D. Transpiration and visual appearance of warm season turfgrasses during soil drying. Environ. Exp. Bot. 2013, 89, 36–43. [Google Scholar] [CrossRef]
- Kazemi, F.; Jozay, M.; Salahshoor, F.; Farhadi, H. Application of soil mulches on establishment and growth of native and commercial tall fescue (Festuca arundinacea Schreb.) in an arid environment. Desert 2020, 26, 55–69. [Google Scholar]
- Ma, Y.; An, Y.; Shui, J.; Sun, Z. Adaptability evaluation of switchgrass (Panicum virgatum L.) cultivars on the Loess Plateau of China. Plant Sci. 2011, 181, 638–643. [Google Scholar] [CrossRef]
- Maricle, B.R.; Adler, P.B. Effects of precipitation on photosynthesis and water potential in Andropogon gerardii and Schizachyrium scoparium in a southern mixed grass prairie. Environ. Exp. Bot 2011, 72, 223–231. [Google Scholar] [CrossRef]
- Hatamzadeh, A.; Molaahmad Nalousi, A.; Ghasemnezhad, M.; Biglouei, M.H. The potential of nitric oxide for reducing oxidative damage induced by drought stress in two turfgrass species, creeping bentgrass and tall fescue. Grass Forage Sci. 2015, 70, 538–548. [Google Scholar] [CrossRef]
- Pirnajmedin, F.; Majidi, M.M.; Gheysari, M. Root and physiological characteristics associated with drought tolerance in Iranian tall fescue. Euphytica 2015, 202, 141–155. [Google Scholar] [CrossRef]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Parrish, D.J.; Fike, J.H.; Bransby, D.I.; Samson, R. Establishing and managing switchgrass as an energy crop. Forage Grazinglands 2008. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Santos, S. Sustainable approach to weed management: The role of precision weed management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Scherer, J.; Brede, A. Cost-Effectiveness of Increased Mowing Frequency on Turfgrass Maintenance. Hort. Technol. 2011, 21, 619–626. [Google Scholar]
- Invasive Species Specialist Group ISSG. Global Invasive Species Database. 2023. Available online: https://www.gbif.org/dataset/b351a324-77c4-41c9-a909-f30f77268bc4 (accessed on 19 May 2023).
- Salter, P.J.; Haworth, F. The available water capacity of a sandy loam soil: I. A critical comparison of methods of determining the moisture content of soil at field capacity and at the permanent wilting percentage. J. Soil Sci. 1961, 12, 326–334. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee-Stadelmann, O.Y. Water relations and cell wall elasticity quantities in Phaseolus vulgaris leaves. J. Exp. Bot. 1984, 35, 841–858. [Google Scholar] [CrossRef]
- Mansourifar, S.; Shaban, M.; Ghobadi, M.; Sabaghpoor, S.H. Physiological characteristics of chickpea (Cicer arietinum L.) cultivars under drought stress and nitrogen fertilizer as starter. Iran. J. Pul. Res. 2012, 3, 53–66. [Google Scholar]
- Dedio, W. Water relations in wheat leaves as screening tests for drought resistance. Can. J. Plant Sci 1975, 55, 369–378. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Physiological responses to heat stress alone or in combination with drought: A comparison between tall fescue and perennial ryegrass. HortScience 2001, 36, 682–686. [Google Scholar] [CrossRef]
- Dere, S.; Günes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Shearman, R.C.; Morris, K.N. NTEP Turfgrass Evaluation Guidelines. 2000. Available online: https://www.ntep.org/pdf/ratings.pdf (accessed on 16 April 2023).
- National Turfgrass Evaluation Program (NTEP). National Bermudagrass Test. Final Report NTEP No. 18-14. 2013. Available online: http://www.ntep.org/reports/bg13/bg13_18-14f/bg13_18-14f.htm (accessed on 16 April 2023).
- Yu, S.; Fang, T.; Dong, H.; Yan, L.; Martin, D.L.; Moss, J.Q.; Fontanie, C.H.; Wu, Y. Genetic and QTL mapping in African bermudagrass. Plant Genom. 2021, 14, e20073. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, S.; Shabanpour, M.; Isfahani, M. The effect of soil density and soil texture on root growth and shoot of wheat. J. Water Soil 2012, 26, 727–735. [Google Scholar]
- Israel, W.K.; Watson-Lazowski, A.; Chen, Z.; Ghannoum, O. High intrinsic water use efficiency is underpinned by high stomatal aperture and guard cell potassium flux in C3 and C4 grasses grown at glacial CO2 and low light. J. Exp. Bot. 2022, 73, 1546–1565. [Google Scholar] [CrossRef]
- Kazemi, F.; Salahshoor, F.; Farhadi, H. Effect of humic acid and mulches on some characteristics of tall fescue (Festuca arundinacea Schreb.). Desert 2019, 24, 51–59. [Google Scholar]
- Heckathorn, S.A.; DeLucia, E.H. Ammonia volatilization during drought in perennial C4 grasses of tallgrass prairie. Oecologia 1995, 101, 361–365. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Meizhen, S. High nitrogen enhances drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plant 2020, 9, 178. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; DeLucia, E.H.; Zielinski, R.E. The contribution of drought-related decreases in foliar nitrogen concentration to decreases in photosynthetic capacity during and after drought in prairie grasses. Physiol. Plant 1997, 101, 173–182. [Google Scholar] [CrossRef]
- Knapp, A.K. Effect of fire and drought on the ecophysiology of Andropogon gerardii and Panicum virgatum in a tall grass prairie. Ecology 1985, 66, 1309–1320. [Google Scholar] [CrossRef]
- Gupta, S.K.; Yadav, R.K.; Kumar, A.; Yadav, N.; Singh, S.P.; Singh, G. Mechanisms of drought tolerance in C4 warm-season grasses: A review. Plant Cell Rep. 2022, 41, 1–16. [Google Scholar]
- Saha, S.; Chakraborty, P.; Bera, R.; Sengupta, D. Drought tolerance mechanisms in C4 warm season grasses: Insights from Pennisetum species. Plant Physiol. Biochem. 2022, 169, 516–527. [Google Scholar] [CrossRef]
- Alkadi, H. A review on free radicals and antioxidants. Drug Targets 2020, 20, 16–26. [Google Scholar]
- Keles, Y.; Öncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Faros, M.; Al-juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol 2009, 11, 100–105. [Google Scholar]
- Gitz III, D.C.; Baker, J.T. C4 grasses and cereals: Growth, development, and stress response. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms; Springer: Cham, Switzerland, 2018; pp. 431–448. [Google Scholar]
- Polley, H.W.; Norman, J.A.; Arkebauer, T.J.; Walter-Shea, E.A.; Greego, J.R.; Bramer, B. Leaf gas exchange of Andropogon gerardii Vitman, Panicum virgatum L. and Sorghastrum nutans L. Nash in a tall grass prairie. J. Geophys. Res 1992, 97, 18837–18844. [Google Scholar] [CrossRef]
- Silletti, A.M.; Knapp, A.K. Responses of the codominant grassland species Andropogon gerardii and Sorghastrum nutans to long-term manipulations of nitrogen and water. Am. Midl. Nat. 2001, 145, 159–167. [Google Scholar] [CrossRef]
- Derner, J.D.; Polley, H.W.; Johnson, H.B.; Tischler, C.R. Root system response of C4 grass seedlings to CO2 and soil water. J. Plant Soil 2001, 231, 97–104. [Google Scholar] [CrossRef]
- Aimar, D.; Calafat, M.; Andrade, A.M.; Carassay, L.; Bouteau, F.; Abdala, G.; Molas, M.L. Drought effects on the early development stages of Panicum virgatum L.: Cultivar differences. Biomass Bioenergy 2014, 66, 49–59. [Google Scholar] [CrossRef]
- Knapp, A.K. Water relations and growth of three grasses during wet and drought years in a tallgrass prairie. Oecologia 1984, 65, 35–43. [Google Scholar] [CrossRef]
- Bustami, R.A.; Beecham, S.; Hopeward, J. The influence of plant type, substrate and irrigation regime on living wall performance in a semi-arid climate. Environments 2023, 10, 26. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Liu, Q.; Zhang, L. Construction and optimization of ecological network based on landscape ecological risk assessment: A case study in Jinan. Land 2023, 12, 743. [Google Scholar] [CrossRef]
- Wilson, J.R.; Caplat, P.; Dickie, I.A.; Hui, C.; Maxwell, B.D.; Nunez, M.A.; Pauchard, A.; Rejmánek, M.; Richardson, D.M.; Robertson, M.P.; et al. A standardized set of metrics to assess and monitor tree invasions. Biol. Invasions 2014, 16, 535–551. [Google Scholar] [CrossRef]
| Factors | df | RWC (%) | RSD (%) | RWL (%) | EL (%) | Chla (mgg−1FW) | Chlb (mgg−1FW) | ChlT (mgg−1FW) | Carotenoid (mgg−1FW) |
|---|---|---|---|---|---|---|---|---|---|
| Block | 3 | 6.08 ns | 0.15 ns | 2.82 ns | 0.46 ns | 1.01 * | 30.43 ns | 22.68 ns | 36.62 ns |
| Species | 4 | 1014.54 ** | 1.88 ** | 25.99 ** | 5.64 ** | 33.28 ** | 1004.59 ** | 341.39 ** | 325.40 ** |
| Irrigation | 1 | 3768.45 ** | 2.65 ** | 415.76 ** | 33.00 ** | 214.23 ** | 7019.32 ** | 5351.55 ** | 3612.28 ** |
| Species × irrigation | 4 | 376. 62 ** | 1.84 ** | 39.67 ** | 11.62 ** | 8.98 ** | 2870.35 ** | 284.10 ** | 266.02 ** |
| Error | 27 | 15.71 | 0.09 | 1.61 | 0.32 | 0.59 | 20.90 | 15.83 | 10.89 |
| Grass Species | Irrigation Level (FC) (%) | RWC (%) | RSD (%) | RWL (%) | EL (%) | Chla (mgg−1FW) | Chlb (mgg−1FW) | Total chl. (mgg−1FW) | Carotenoid (mgg−1FW) |
|---|---|---|---|---|---|---|---|---|---|
| A. gerardii | 100 | 51.62 b | 16.26 d | 68.43 a | 38.11 f | 11.35 a | 2.04 c | 13.4 b | 1.04 d |
| A. gerardii | 50 | 25.05 e | 26.95 c | 53 cd | 82.56 b | 8.86 cd | 1.47 cd | 10.34 d | 1.11 cd |
| S. nutans | 100 | 33.92 d | 15.97 d | 51.25 cd | 24.47 g | 10.77 ab | 1.56 cd | 12.33 bc | 1.1 cd |
| S. nutans | 50 | 17.09 f | 48.12 a | 19.82 f | 63.77 c | 8.55 d | 1.56 c | 11.53 cd | 1.42 bcd |
| P. virgatum | 100 | 60.17 a | 25.4 c | 62.13 b | 94.61 a | 8.06 d | 3.23 b | 11.29 cd | 1.49 bc |
| P. virgatum | 50 | 20.91 ef | 43.18 b | 31.87 e | 55.41 d | 2.5 f | 1.45 cd | 3.96 f | 1.64 b |
| Sch. scoparium | 100 | 59.02 a | 17.73 d | 55.62 c | 47.78 e | 10.77 ab | 5.33 a | 16.1 a | 2.77 a |
| Sch. scoparium | 50 | 53.06 ab | 23.06 c | 49.76 d | 78.92 b | 4.65 e | 11.93 cd | 6.58 e | 1.39 bcd |
| B. curtipendula | 100 | 51.12 b | 16.37 d | 61.87 b | 27.93 g | 9.94 bc | 5.69 a | 15.9 a | 3.04 a |
| B. curtipendula | 50 | 42.68 bc | 45.41 ab | 29.19 e | 84.71 b | 3.19 f | 1.19 d | 4.37 f | 1.31 bcd |
| Factors | df | Color (1–9 Scores) | Texture (1–9 Scores) | Height (cm) | Leaf Width (cm) | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Block | 3 | 0.02 ns | 0.03 ns | 8.07 ns | 0.00 ns | 0.77 * | 0.26 ns | 0.02 ns | 0.21 ns | 0.00 ns |
| Species | 4 | 26.27 ** | 9.90 ** | 341.39 ** | 0.18 ** | 79.57 ** | 17.36 ** | 12.02 ** | 2.81 ** | 0.45 ** |
| Irrigation | 1 | 3.02 ** | 8.10 ** | 104.65 ** | 0.00 ns | 548.41 ** | 53.10 ** | 13.87 ** | 9.86 ** | 0.54 ** |
| Species × irrigation | 4 | 0.27 * | 0.10 * | 13.49 ns | 0.00 ns | 53.68 ** | 13.67 ** | 4.69 ** | 2.54 ** | 0.05 ** |
| Error | 27 | 0.09 | 0.03 | 7.44 | 0 | 2.19 | 0.39 | 0.14 | 0.13 | 0 |
| Grass Species | Irrigation Levels (FC) (%) | Leaf Color (1–9 Scores) | Leaf Texture (1–9 Scores) | Highest Length of Root (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|
| A. gerardii | 100 | 5 c | 4 e | 28.3 b | 12.99 ef | 7.01 a | 2.72 d | 1.01 a |
| A. gerardii | 50 | 4.5 cd | 3 f | 12.01g | 11.66 f | 5.3 b | 2.36 d | 0.7 cd |
| S. nutans | 100 | 4 de | 6 b | 22.28 cd | 18.72 a | 6.33 b | 4.96 a | 0.62 de |
| S. nutans | 50 | 3.9 de | 5 c | 17.76 f | 12.88 e | 2.87 e | 2.48 d | 0.25 f |
| P.virgatum | 100 | 6 b | 5 c | 30.75 a | 17.55 b | 4.52 d | 4.74 a | 0.88 b |
| P. virgatum | 50 | 5 c | 4.5 d | 24.09 c | 13.37 e | 5.07 cd | 2.8 d | 0.91 ab |
| Sch. scoparium | 100 | 4 de | 7 a | 27.56 b | 15.45 cd | 3.28 e | 3.6 c | 0.6 e |
| Sch. scoparium | 50 | 3.25 e | 6 b | 21.09 de | 14.93 d | 2.24 f | 3.52 c | 0.23 f |
| B.curtipendula | 100 | 7 a | 6 b | 23.14 cd | 15.65 cd | 5.15 c | 4.16 b | 0.9 b |
| B. curtipendula | 50 | 6.5 ab | 5 c | 20.04 e | 16 c | 4.93 cd | 4.05 bc | 0.76 c |
| Days to Germination | Chla (mgg−1FW) | Chlb (mgg−1FW) | Total chl. (mgg−1FW) | Carot. (mgg−1FW) | Leaf Width (cm) | No. of Tillers | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | RWC (%) | RSD (%) | RWL (%) | El. (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days to germination | ||||||||||||||||
| Chla (mgg−1FW) | 0.25 ns | |||||||||||||||
| Chlb (mgg−1FW) | −0.36 * | 0.51 ** | ||||||||||||||
| Total chl. (mgg−1FW) | 0.047 ns | 0.94 ** | 0.77 ** | |||||||||||||
| Carot. (mgg−1FW) | −0.47 ** | 0.25 ns | 0.92 ** | 0.54 ** | ||||||||||||
| Leaf width (cm) | 0.88 ** | 0.33 * | −0.37 * | 0.1 ns | −0.47 ** | |||||||||||
| No. of tillers | −0.13 ns | 0.08 ns | 0.26 ns | 0.16 ns | 0.29 * | 0.03 ns | ||||||||||
| Root length (cm) | −0.05 ns | 0.17 ns | 0.32 * | 0.24 ns | 0.23 ns | −0.25 ns | −0.08 ns | |||||||||
| Shoot fresh weight (g) | −0.55 ** | 0.81 ns | 0.15 ns | 0.12 ns | 0.12 ns | −0.5 ** | −0.11 ns | 0.45 ** | ||||||||
| Root fresh weight (g) | 0.43 ** | 0.3 ns | −0.24 ns | 0.13 ns | −0.25 ns | 0.39 * | −0.49 ** | 0.13 ns | 0.10 ns | |||||||
| Shoot dry weight (g) | −0.52 ** | 0.07 ns | 0.17 ns | 0.12 ns | 0.14 ns | −0.48 ** | −0.11 ns | 0.43 ** | 0.97 ** | 0.15 ns | ||||||
| Root dry weight (g) | 0.32 * | 0.1 ns | 0.1 ns | 0.1 ns | 0.1 ns | 0.15 ns | −0.43 ** | 0.42 ** | 0.07 ns | 0.77 ** | 0.18 ns | |||||
| RWC (%) | −0.15 ns | 0.24 ns | 0.3 ** | 0.35 * | 0.33 * | −0.20 ns | 0.43 ** | 0.66 ** | 0.44 ** | −0.07 ns | 0.46 ** | 0.2 ns | ||||
| RSD (%) | −0.18 ns | −0.66 ** | −0.35 * | −0.63 ** | −0.23 ns | −0.21 ns | −0.33 * | −0.35 * | −0.29 * | −0.27 ns | −0.31 * | −0.21 ns | −0.6 ** | |||
| RWL (%) | 0.37 * | 0.58 ** | 0.31 * | 0.6 ** | 0.17 ns | 0.30 ns | 0.21 ns | 0.45 ** | 0.19 ns | 0.37 * | 0.26 ns | 0.46 ** | 0.68 ** | −0.88 ** | ||
| El. (%) | 0.12 ns | −0.52 ** | −0.34 * | −0.52 ** | −0.34 * | 0.04 ns | 0.02 ns | −0.21ns | −0.14 ns | −0.41 ** | −0.1 ns | −0.2 ns | 0.005 ns | 0.46 ** | −0.26 ns |
| Grass Species | Color | Texture | Root Length | Shoot Fresh Weight | Root Fresh Weight | Shoot Dry Weight | Root Dry Weight | RWC | RSD | RWL | EL | Chla | Chlb | Total Chl. | Carot. | No. Sig. Responses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. gerardii | 9 | |||||||||||||||
| S. nutans | 12 | |||||||||||||||
| P. virgatum | 12 | |||||||||||||||
| Sch. scoparium | 9 | |||||||||||||||
| B. curtipendula | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemi, F.; Jozay, M.; Salahshoor, F.; van Etten, E.; Rezaie, S. Drought Stress Responses of Some Prairie Landscape C4 Grass Species for Xeric Urban Applications. Land 2023, 12, 1195. https://doi.org/10.3390/land12061195
Kazemi F, Jozay M, Salahshoor F, van Etten E, Rezaie S. Drought Stress Responses of Some Prairie Landscape C4 Grass Species for Xeric Urban Applications. Land. 2023; 12(6):1195. https://doi.org/10.3390/land12061195
Chicago/Turabian StyleKazemi, Fatemeh, Mansoure Jozay, Farzaneh Salahshoor, Eddie van Etten, and Sahar Rezaie. 2023. "Drought Stress Responses of Some Prairie Landscape C4 Grass Species for Xeric Urban Applications" Land 12, no. 6: 1195. https://doi.org/10.3390/land12061195
APA StyleKazemi, F., Jozay, M., Salahshoor, F., van Etten, E., & Rezaie, S. (2023). Drought Stress Responses of Some Prairie Landscape C4 Grass Species for Xeric Urban Applications. Land, 12(6), 1195. https://doi.org/10.3390/land12061195







