Storage and Stability of Soil Organic Carbon in Two Temperate Forests in Northeastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Soil Sampling
2.2. Soil fractionations
2.3. Analyses of Soil Physicochemical Properties
2.4. Data and Analyses
3. Results
3.1. Bulk Soil C Concentration and Stock
3.2. Soil Aggregates: Mass Distribution, C Concentration, and C Stock
3.3. POM and MAOM: Mass Distribution, and Fraction-Based C Concentration and Stock
3.4. C/N Ratios and δ13C Values of Soil Aggregates, POM and MAOM
4. Discussion
4.1. Soil Organic C Storage and Distribution in the Mixed Forest and Larch Forest
4.2. Soil C Stability along Soil Depth in the Mixed Forest and Larch Forest
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Schnecker, J.; Borken, W.; Schindlbacher, A.; Wanek, W. Little effects on soil organic matter chemistry of density fractions after seven years of forest soil warming. Soil Biol. Biochem. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- García-Palacios, P.; Crowther, T.W.; Dacal, M.; Hartley, I.P.; Reinsch, S.; Rinnan, R.; Rousk, J.; Hoogen, J.V.D.; Ye, J.-S.; Bradford, M.A. Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nat. Rev. Earth Environ. 2021, 2, 507–517. [Google Scholar] [CrossRef]
- Liu, X.J.A.; Pold, G.; Domeignoz-Horta, L.A.; Geyer, K.M.; Caris, H.; Nicolson, H.; Kemner, K.M.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Soil aggregate-mediated microbial responses to long-term warming. Soil Biol. Biochem. 2020, 152, 108055. [Google Scholar] [CrossRef]
- Chen, Y.; Han, M.; Yuan, X.; Zhou, H.; Zhao, X.; Schimel, J.P.; Zhu, B. Long-term warming reduces surface soil organic carbon by reducing mineral-associated carbon rather than “free” particulate carbon. Soil Biol. Biochem. 2023, 177, 108905. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.-D. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, M.; Huang, J.; Yang, S.; Guo, L.; Yang, L.; Ahirwal, J.; Peng, Z.; Liu, W.; Liu, L. Global patterns in mycorrhizal mediation of soil carbon storage, stability, and nitrogen demand: A meta-analysis. Soil Biol. Biochem. 2022, 166, 108578. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Pathways of litter C by formation of aggregates and SOM density fractions: Implications from 13C natural abundance. Soil Biol. Biochem. 2014, 71, 95–104. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Soong, J.L.; Castanha, C.; Pries, C.E.H.; Ofiti, N.; Porras, R.C.; Riley, W.J.; Schmidt, M.W.; Torn, M.S. Five years of whole-soil warming led to loss of subsoil carbon stocks and increased CO2 efflux. Sci. Adv. 2021, 7, abd1343. [Google Scholar] [CrossRef]
- Lyu, M.; Xie, J.; Ukonmaanaho, L.; Jiang, M.; Li, Y.; Chen, Y.; Yang, Z.; Zhou, Y.; Lin, W.; Yang, Y. Land use change exerts a strong impact on deep soil C stabilization in subtropical forests. J. Soils Sediments 2016, 17, 2305–2317. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Relating the biological stability of soil organic matter to energy availability in deep tropical soil profiles. Soil Biol. Biochem. 2015, 89, 162–171. [Google Scholar] [CrossRef]
- Qin, S.; Chen, L.; Fang, K.; Zhang, Q.; Wang, J.; Liu, F.; Yu, J.; Yang, Y. Temperature sensitivity of SOM decomposition governed by aggregate protection and microbial communities. Sci. Adv. 2019, 5, eaau1218. [Google Scholar] [CrossRef] [PubMed]
- Mobley, M.L.; Lajtha, K.; Kramer, M.G.; Bacon, A.R.; Heine, P.R.; Richter, D.D. Surficial gains and subsoil losses of soil carbon and nitrogen during secondary forest development. Glob. Chang. Biol. 2015, 21, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Luo, Y.; Xu, X.; Sherry, R.; Zhang, Q. Soil organic matter dynamics in a North America tallgrass prairie after 9 yr of experimental warming. Biogeosciences 2011, 8, 1487–1498. [Google Scholar] [CrossRef]
- Breulmann, M.; Boettger, T.; Buscot, F.; Gruendling, R.; Schulz, E. Carbon storage potential in size–density fractions from semi-natural grassland ecosystems with different productivities over varying soil depths. Sci. Total. Environ. 2016, 545–546, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Schwendenmann, L.; Pendall, E. Effects of forest conversion into grassland on soil aggregate structure and carbon storage in Panama: Evidence from soil carbon fractionation and stable isotopes. Plant Soil 2006, 288, 217–232. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Houghton, R.A.; Hall, F.; Goetz, S.J. Importance of biomass in the global carbon cycle. J. Geophys. Res. Biogeosciences 2009, 114, G00E03. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Palmer, P.I.; Liu, Y.; Fang, S.; Bösch, H.; O’dell, C.W.; Tang, X.; Yang, D.; Liu, L.; et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 2020, 586, 720–723. [Google Scholar] [CrossRef]
- Cabon, A.; Kannenberg, S.A.; Arain, A.; Babst, F.; Baldocchi, D.; Belmecheri, S.; Delpierre, N.; Guerrieri, R.; Maxwell, J.T.; McKenzie, S.; et al. Cross-biome synthesis of source versus sink limits to tree growth. Science 2022, 376, 758–761. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.L.; Zhu, J.J. Silviculture of Planted Forests Managed for Multi-functional Objectives: Lessons from Chinese and British Experiences. In Challenges and Opportunities for the World’s Forests in the 21st Century; Fenning, T., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 37–54. [Google Scholar]
- National Forestry and Grassland Administration. China Forest Resources Report; (In chinese). China Forestry Publisher: Beijing, China, 2019; pp. 326–397.
- Yang, K.; Shi, W.; Zhu, J.-J. The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 2013, 368, 535–546. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Hu, Y.-L.; Bol, R.; Ma, X.-Q.; Jin, S.-F.; Mgelwa, A.S. Effects of land use change from natural forest to plantation on C, N and natural abundance of (13)C and (15)N along a climate gradient in eastern China. Sci. Rep. 2019, 9, 16516. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Yuangang, Z.U. Temporal changes in SOM, N, P, K, and their stoichiometric ratios during reforestation in China and interactions with soil depths: Importance of deep-layer soil and management implications. For. Ecol. Manag. 2014, 325, 8–17. [Google Scholar] [CrossRef]
- Huang, S.; Elliott, E.M.; Felix, J.D.; Pan, Y.; Liu, D.; Li, S.; Li, Z.; Zhu, F.; Zhang, N.; Fu, P.; et al. Seasonal pattern of ammonium 15N natural abundance in precipitation at a rural forested site and implications for NH3 source partitioning. Environ. Pollut. 2019, 247, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gurmesa, G.A.; Zhu, W.; Gundersen, P.; Zhang, S.; Xi, D.; Huang, S.; Wang, A.; Zhu, F.; Jiang, Y.; et al. Fate of atmospherically deposited NH4+ and NO3− in two temperate forests in China: Temporal pattern and redistribution. Ecol. Appl. 2019, 29, e01920. [Google Scholar]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.; Paustian, K.; Doran, J.W. Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, W.; Chen, H. Changes of soil C stocks and stability after 70-year afforestation in the Northeast USA. Plant Soil 2015, 401, 319–329. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.; Combrink, C. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Xu, S.; Zheng, X. Conversion from temperate secondary forests into plantations (Larix spp.): Impact on belowground carbon and nutrient pools in northeastern China. Land Degrad. Dev. 2018, 29, 4129–4139. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Kurmi, B.; Nath, A.J.; Lal, R.; Das, A.K. Water stable aggregates and the associated active and recalcitrant carbon in soil under rubber plantation. Sci. Total. Environ. 2020, 703, 135498. [Google Scholar] [CrossRef]
- Garten, C.T. Comparison of forest soil carbon dynamics at five sites along a latitudinal gradient. Geoderma 2011, 167–168, 30–40. [Google Scholar] [CrossRef]
- Schweizer, S.A.; Bucka, F.B.; Graf-Rosenfellner, M.; Kögel-Knabner, I. Soil microaggregate size composition and organic matter distribution as affected by clay content. Geoderma 2019, 355, 113901. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Chen, X.; He, K.; Zhu, B. Changes in soil organic matter stability with depth in two alpine ecosystems on the Tibetan Plateau. Geoderma 2019, 351, 153–162. [Google Scholar] [CrossRef]
- Wynn, J.G.; Harden, J.W.; Fries, T.L. Stable carbon isotope depth profiles and soil organic carbon dynamics in the lower Mississippi Basin. Geoderma 2006, 131, 89–109. [Google Scholar] [CrossRef]
- Wang, C.; Wei, H.; Liu, D.; Luo, W.; Hou, J.; Cheng, W.; Han, X.; Bai, E. Depth profiles of soil carbon isotopes along a semi-arid grassland transect in northern China. Plant Soil 2017, 417, 43–52. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Yang, K.; Yu, L.; Zhang, J. Nutrient removal under different harvesting scenarios for larch plantations in northeast China: Implications for nutrient conservation and management. For. Ecol. Manag. 2017, 400, 150–158. [Google Scholar] [CrossRef]
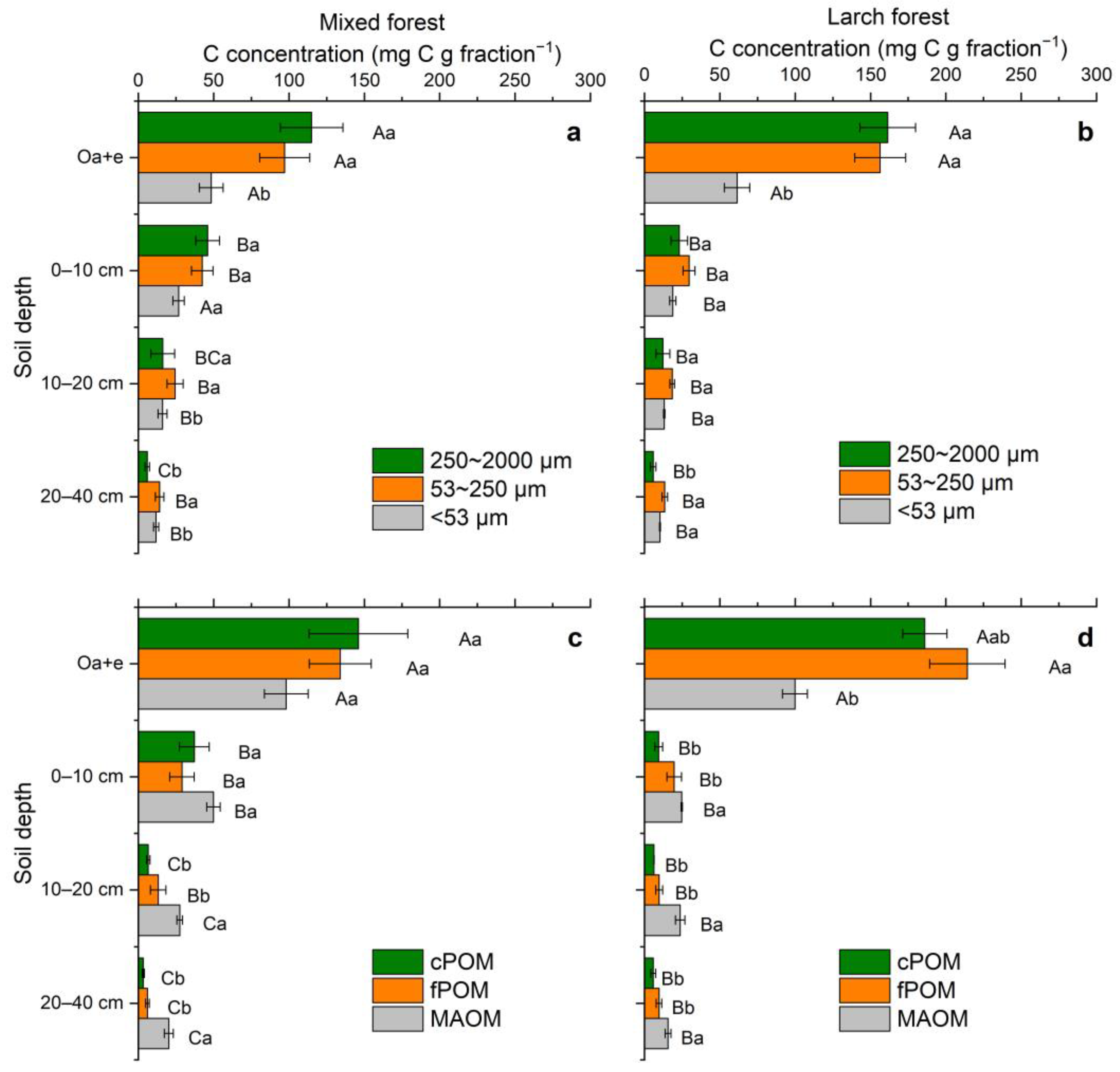

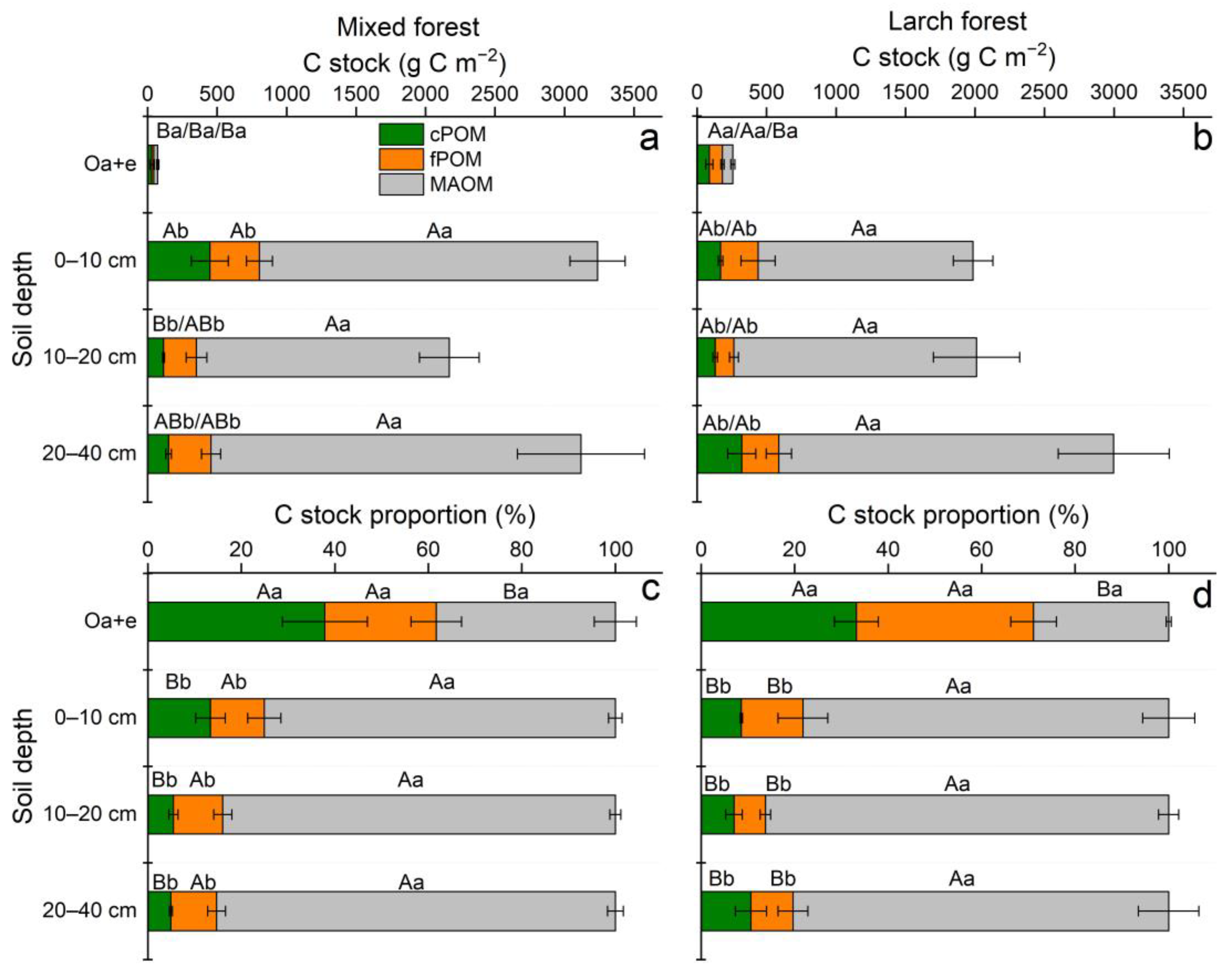

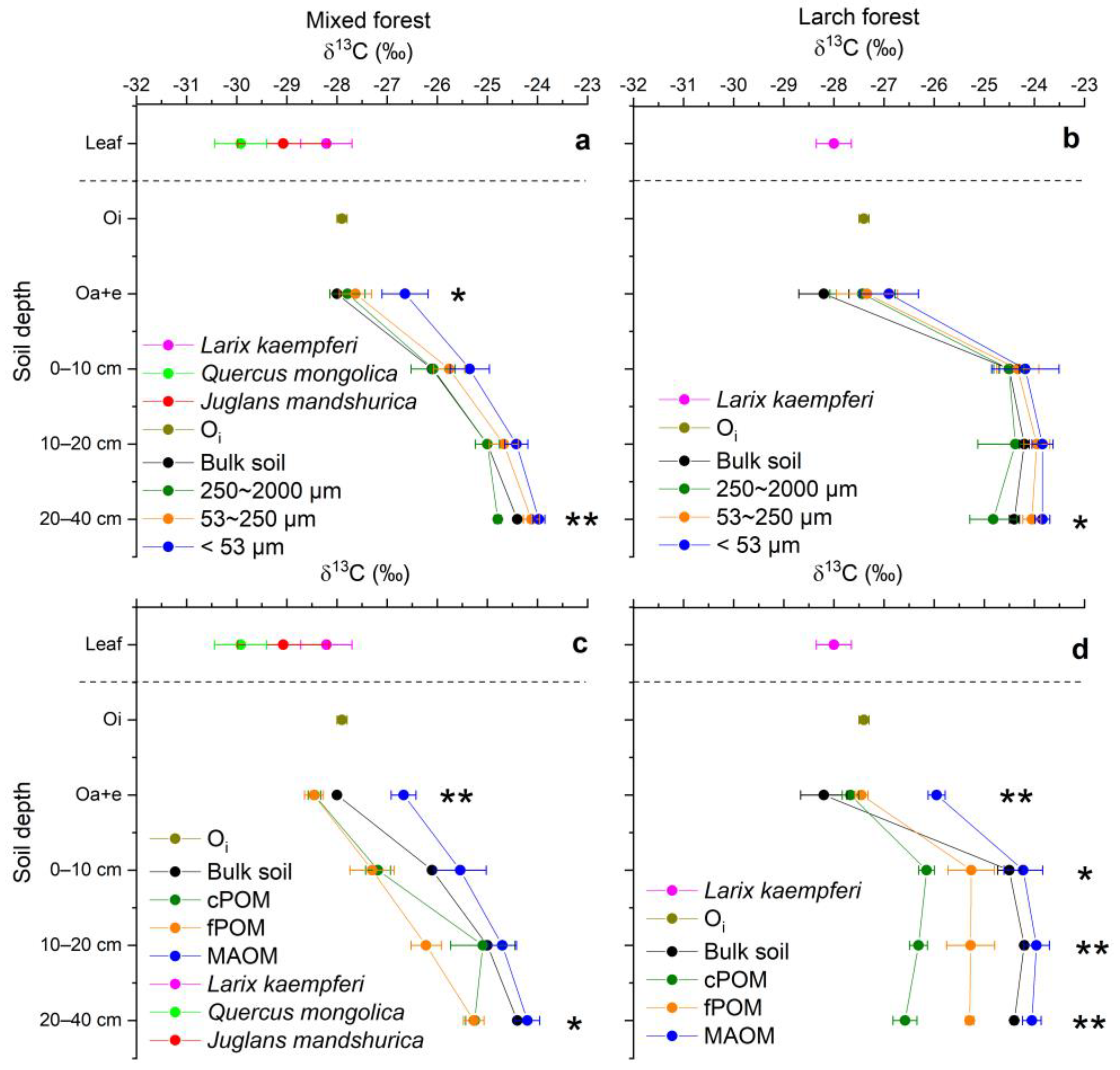
| Forest/ Depth | Sand (%) | Silt (%) | Clay (%) | Bulk Density (g cm−3) | C (mg g−1 soil) | C/N | C Stock (g C m−2) | δ13C (‰) |
|---|---|---|---|---|---|---|---|---|
| MF | ||||||||
| Oi | 390.8 ± 5.7 A | 24.8 ± 2.1 A | 447 ± 58 B | −27.9 ± 0.1 C | ||||
| Oa+e | 115.6 ± 6.9 B | 13.0 ± 0.3 B | 68 ± 14 B | −28.0 ± 0.3 C | ||||
| O layer | 515 ± 46 | |||||||
| 0–10 cm | 17.2 ± 0.4 cB | 52.8 ± 0.5 aA | 30.0 ± 0.2 bA | 0.7 ± 0.03 A | 45.7 ± 8.3 C | 10.9 ± 0.1 BC | 3343 ± 608 A | −26.1 ± 0.3 B |
| 10–20 cm | 16.8 ± 0.6 cB | 54.4 ± 0.7 aA | 28.8 ± 0.5 bB | 1.0 ± 0.05 B | 24.0 ± 4.1 D | 9.7 ± 0.1 C | 2481 ± 426 A | −25.0 ± 0.2 A |
| 20–40 cm | 21.0 ± 1.5 cA | 53.0 ± 1.2 aA | 26.0 ± 0.6 bB | 1.1 ± 0.08 B | 15.9 ± 4.7 D | 9.4 ± 0.2 C | 3630 ± 1081 A | −24.4 ± 0.2 A |
| 0–40 cm | 9454 ± 2114 | |||||||
| Total | 9969 ± 2084 | |||||||
| LF | ||||||||
| Oi | 424.9 ± 3.8 A | 27.5 ± 1.0 A | 591 ± 16 B | −27.4 ± 0.1 B | ||||
| Oa+e | 164.1 ± 9.0 B | 14.3 ± 0.1 B | 273 ± 46 B | −28.2 ± 0.5 C | ||||
| O layer | 864 ± 60 | |||||||
| 0–10 cm | 27.6 ± 1.1 bA | 46.4 ± 1.0 aA | 26.1 ± 0.1 bA | 1.0 ± 0.07 A | 23.2 ± 1.9 C | 9.7 ± 0.3 C | 2239 ± 181 A | −24.5 ± 0.2 A |
| 10–20 cm | 27.4 ± 1.4 bA | 47.7 ± 1.5 aA | 24.9 ± 1.2 bA | 1.1 ± 0.04 AB | 22.4 ± 5.8 C | 9.7 ± 0.2 C | 2409 ± 628 A | −24.2 ± 0.1 A |
| 20–40 cm | 32.7 ± 4.4 bA | 44.1 ± 3.1 aA | 23.2 ± 1.3 bA | 1.2 ± 0.03 B | 13.1 ± 2.8 C | 9.1 ± 0.2 C | 3097 ± 650 A | −24.4 ± 0.1 A |
| 0–40 cm | 7745 ± 1123 | |||||||
| Total | 8609 ± 1180 |
| Mixed Forest | Larch Forest | |||
|---|---|---|---|---|
| Species /Mycorrhizal Type | Total Density (Stems ha−1) | Biomass (t ha−1) | Total Density (Stems ha−1) | Biomass (t ha−1) |
| Larix kaempferi (ECM) | ||||
| 442 ± 76 | 68.9 ± 16.5 | 567 ± 36 | 147.9 ± 3.8 | |
| Juglans mandshurica (AM) | ||||
| 109 ± 21 | 49.5 ± 7.8 | 33 ± 27 | 9.1 ± 5.3 | |
| Quercus mongolica (ECM) | ||||
| 59 ± 13 | 8.4 ± 1.7 | - | - | |
| Other species | 201 ± 51 | 8.9 ± 1.2 | 327 ± 51 | 6.9 ± 0.5 |
| AM | 84 ±20 | 6.7 ± 2.1 | 250 ± 51 | 4.7 ± 0.6 |
| AM and ECM | 117 ± 24 | 2.2 ± 0.9 | 42 ± 10 | 0.6 ± 0.1 |
| ECM | - | - | 34 ± 10 | 1.6 ± 0.5 |
| Total | 811 ± 53 | 135.7 ± 13.5 | 927 ± 72 | 163.9 ± 3.6 |
| Forest/ Depth | Proportion of Total Mass of Soil Aggregates and Non-Aggregate (%) | Proportion of Total Mass of POM and MAOM (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Macroaggre. (250~2000 μm) | Microaggre. (53~250 μm) | Silt and Clay (<53 μm) | Recovery (%) | cPOM (250~2000 μm) | fPOM (53~250 μm) | MAOM (<53 μm) | Recovery (%) | |
| MF | ||||||||
| Oa+e | 71.2 ± 3.3 Aa | 21.3 ± 3.2 Cb | 3.5 ± 0.5 Bc | 96.0 ± 2.3 | 27.4 ± 4.3 Ab | 19.1 ± 4.3 Ab | 41.1 ± 2.4 Ba | 95.4 ± 4.3 |
| 0–10 cm | 50.9 ± 4.1 Ba | 34.9 ± 4.0 Bb | 7.3 ± 0.7 ABc | 93.2 ± 0.7 | 14.7 ± 1.3 Bb | 15.7 ± 0.9 Ab | 61.2 ± 1.2 Aa | 90.9 ± 0.9 |
| 10–20 cm | 28.9 ± 3.1 Cb | 53.7 ± 2.5 Aa | 10.1 ± 1.8 Ac | 92.7 ± 1.4 | 16.9 ± 3.6 ABb | 17.4 ± 1.4 Ab | 59.8 ± 4.5 Aa | 90.4 ± 2.4 |
| 20–40 cm | 24.8 ± 2.0 Cb | 59.5 ± 2.3 Aa | 10.6 ± 1.6 Ac | 94.9 ± 1.4 | 19.9 ± 2.3 ABb | 20.7 ± 0.6 Ab | 54.0 ± 1.4 Aa | 96.7 ± 3.3 |
| LF | ||||||||
| Oa+e | 69.2 ± 3.1 Aa | 26.3 ± 3.9 Bb | 2.5 ± 0.3 Bc | 98.0 ± 1.3 | 26.4 ± 4.3 Aa | 26.1 ± 4.3 Aa | 42.9 ± 2.4 Ba | 87.6 ± 6.4 |
| 0–10 cm | 53.0 ± 4.5 Ba | 30.4 ± 4.4 Bb | 10.8 ± 0.1 Ac | 94.1 ± 0.1 | 18.5 ± 2.9 Ab | 13.4 ± 5.4 Bb | 59.0 ± 6.2 Aa | 91.6 ± 1.2 |
| 10–20 cm | 30.6 ± 5.5 Cb | 51.0 ± 3.5 Aa | 13.9 ± 4.4 Ac | 95.5 ± 0.6 | 17.7 ± 2.8 Ab | 11.7 ± 1.2 Bb | 61.0 ± 1.2 Aa | 94.1 ± 0.6 |
| 20–40 cm | 28.4 ± 2.9 Cb | 58.8 ± 2.5 Aa | 8.2 ± 1.3 ABc | 95.4 ± 0.4 | 23.1 ± 4.8 Ab | 10.8 ± 2.0 Bb | 62.8 ± 4.3 Aa | 94.6 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, S.; Zhu, W.; Wang, Y.; Zhang, S.; Fang, Y. Storage and Stability of Soil Organic Carbon in Two Temperate Forests in Northeastern China. Land 2023, 12, 1019. https://doi.org/10.3390/land12051019
Liu D, Li S, Zhu W, Wang Y, Zhang S, Fang Y. Storage and Stability of Soil Organic Carbon in Two Temperate Forests in Northeastern China. Land. 2023; 12(5):1019. https://doi.org/10.3390/land12051019
Chicago/Turabian StyleLiu, Dongwei, Shanlong Li, Weixing Zhu, Yongyang Wang, Shasha Zhang, and Yunting Fang. 2023. "Storage and Stability of Soil Organic Carbon in Two Temperate Forests in Northeastern China" Land 12, no. 5: 1019. https://doi.org/10.3390/land12051019
APA StyleLiu, D., Li, S., Zhu, W., Wang, Y., Zhang, S., & Fang, Y. (2023). Storage and Stability of Soil Organic Carbon in Two Temperate Forests in Northeastern China. Land, 12(5), 1019. https://doi.org/10.3390/land12051019







