Renaturalization of Ex-Arable Arenosols: Phytocenosis Development and the Dynamics of Sandy Soil Properties
Abstract
1. Introduction
2. Materials and Methods
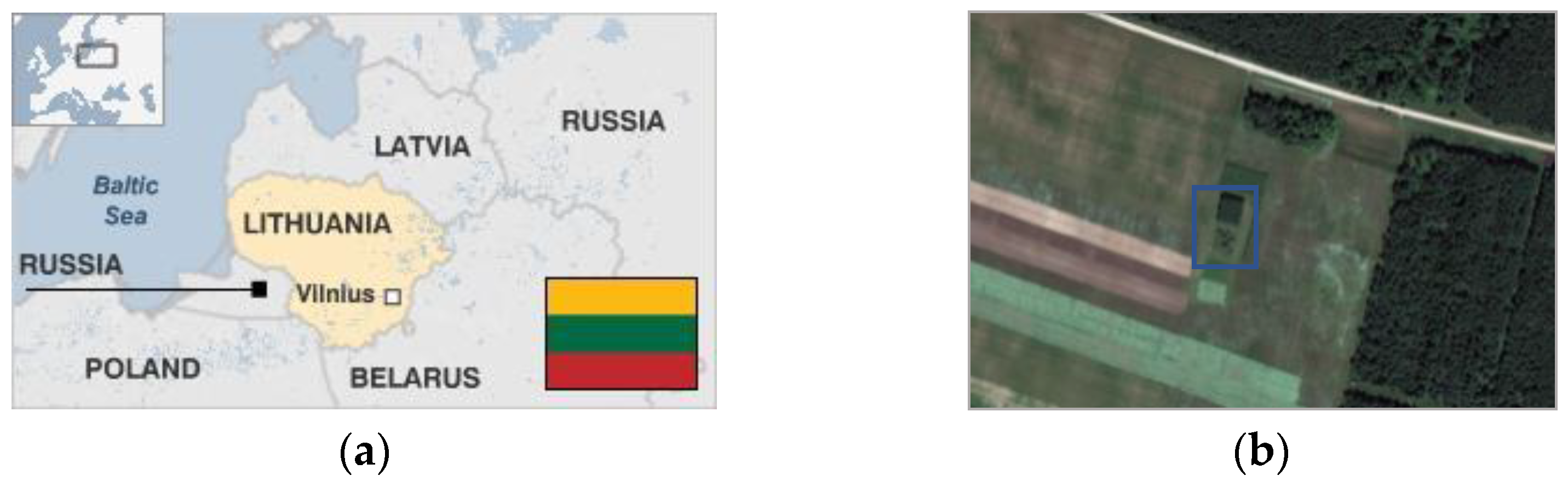
2.1. Experimental Site Description
2.2. Treatment and Experimental Design
2.3. Soil Sampling and Analytical Methods
2.4. Determination of the Species Composition and Biomass of Plants
2.5. Data Analyses and Statistics
3. Results
3.1. Diversity of Plant Species
3.2. Plant Biomass and Soil Properties as Related Factors Together with Plant Species
4. Discussion
4.1. Plant Richness
4.2. Plant Biomass and Richness-Driving Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayet, C.M.J.; Reilly, K.H.; Ham, C.V.; Verburg, P.H. What is the future of abandoned agricultural lands? A systematic review of alternative trajectories in Europe. Land Use Policy 2022, 112, 105833. [Google Scholar] [CrossRef]
- Prishchepov, A.V. Agricultural Land Abandonment. Oxford Bibliographies; In Environmental Science; Oxford University Press: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Bell, S.M.; Barriocanal, C.; Terrer, C.; Rosell-Melé, A. Management opportunities for soil carbon sequestration following agricultural land abandonment. Environ. Sci. Policy 2020, 108, 104–111. [Google Scholar] [CrossRef]
- Ryzhova, I.M.; Erokhova, A.A.; Podvezennaya, M.A. Dynamics and structure of carbon storage in the postagrogenic ecosystems of the southern taiga. Eurasian Soil Sci. 2014, 47, 1207–1215. [Google Scholar] [CrossRef]
- Kalinina, O.; Barmin, A.; Chertov, O.; Dolgikh, A.; Goryachkin, S.; Lyuri, D.; Giani, L. Self-restoration of post-agrogenic soils of Calcisol–Solonetz complex: Soil development, carbon stock dynamics of carbon pools. Geoderma 2015, 237, 117–128. [Google Scholar] [CrossRef]
- Unchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Svirskis, A. Conversion into natural grassland of infertile and abandoned land in Lithuania. In Proceedings of the 20th General Meeting of the European Grassland Federation “Land Use Systems in Grassland Dominated Regions”, Luzern, Switzerland, 21–24 June 2004; pp. 219–221. [Google Scholar]
- Davis, A.S.; Renner, K.A.; Gross, K.L. Weed seedbank and community shifts in a long-term cropping systems experiment. Weed Sci. 2005, 53, 296–306. [Google Scholar] [CrossRef]
- Liebman, M.; Mohler, C.L.; Staver, C.P. Ecological Management of Agricultural Weeds; Liebman, M., Mohler, C.L., Staver, C.P., Eds.; Cambridge University Press: Cambridge, UK, 2001; 532p. [Google Scholar]
- Pérez-Hernández, J.; Gavilán, R.G. Impacts of Land-Use Changes on Vegetation and Ecosystem Functioning: Old-Field Secondary Succession. Plants 2021, 10, 990. [Google Scholar] [CrossRef]
- Kudryashova, S.Y.; Chumbaev, A.S.; Tanasienko, A.A.; Solovyev, S.V.; Miller, G.F.; Bezborodova, A.N.; Filimonova, D.A. Post-agrogenic dynamics of soil properties of eroded agrochernozems in the forest-steppe zone of Western Siberia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 862, p. 012101. [Google Scholar] [CrossRef]
- Mironycheva-Tokareva, N.P.; Kudryashova, S.Y.; Chumbaev, A.S.; Ermakov, N.B.; Kurbatskaya, S.S.; Samdan, A.M.; Kurbatskaya, S.G. Structure of vegetation and phytomass and mortmass stocks in Mongun-Taiga mountain tundra-steppe ecosystems. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 232, p. 012006. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 212. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 1 November 2022).
- Guidelines for Soil Description FAO, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; p. 97.
- Alissow, B.P. Die Klimate der Erde. Deuts cher Verlage der Wissenschaften; Reinhard, H., Dt Verl, d., Eds.; Wissenschaften: Berlin, Germany, 1954; 277p. [Google Scholar]
- Lithuania’s 7th UNFCCC Report, Lithuania’s 7th National Communication under the United Nations Framework Convention on Climate Change. Vilnius 2017. Available online: https://unfccc.int/files/national_reports/national_communications_and_biennial_reports/application/pdf/142035_lithuania-nc7-1-7th_national_communication.pdf (accessed on 1 November 2022).
- Martens, D.A.; Reedy, T.E.; Lewis, D.T. Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements. Glob. Change Biol. 2003, 10, 65–78. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- McKenzie, N.J.; Jacquier, D.J.; Isbell, R.F.; Brown, K.L. Australian Soils and Landscapes: An Illustrated Compendium; CSIRO Publishing: Clayton, Australia, 2004. [Google Scholar]
- Poeplau, C.; Vos, C.; Don, A. Soil organic carbon stocks are systematically overestimated by misuse of the parameters bulk density and rock fragment content. Soil 2017, 3, 61–66. [Google Scholar] [CrossRef]
- Dierschke, H. Pflanzensoziologie–Grundlagen und Methoden; Editor Verlag Eugen Ulmer: Stuttgart, Germany, 1994; 683p. [Google Scholar]
- Vilkonis, K.K. Lietuvos Žaliasis Rūbas [in Lithuanian]. Lithuanian Green Clothes. Atlas [in English]; “Latute” Publishing House: Paris, France, 2008; 406p. [Google Scholar]
- Chazdon, R.L.; Lindenmayer, D.; Guariguata, M.R.; Crouzeilles, R.; Rey Benayas, J.M.; Lazos Chavero, E. Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ. Res. Lett. 2020, 15, 043002. [Google Scholar] [CrossRef]
- Hossain, M.M.; Begum, M. Soil weed seed bank: Importance and management for sustainable crop production—A Review. J. Bangladesh Agril. Univ. 2015, 13, 221–228. [Google Scholar] [CrossRef]
- Valachovič, M. Succession model with Corynephorus canescens in abandoned sandy fields (W Slovakia). Hacquetia 2012, 11, 5–15. [Google Scholar] [CrossRef]
- Sanchez-Moreiras, A.M.; Weiss, O.A.; Reigosa-Roger, M.J. Allelopathic Evidence in the Poaceae; Source: Botanical Review; Springer on behalf of New York Botanical Garden Press: New York, NY, USA, 2003; Volume 69, pp. 300–319. [Google Scholar]
- Murphy, S.D.; Aarssen, L.W. Allelopathic Pollen Extract from Phleum pratense L. (Poaceae) Reduces Seed Set in Sympatric Species. Int. J. Plant Sci. 1995, 156, 435–444. [Google Scholar]
- Rusina, S.; Bambe, B.; Daugaviete, M. Changes in ground vegetation of arable land under afforestation in Latvia. Balt. For. 2011, 17, 243–255. [Google Scholar]
- Nawrocki, T. Mouse-Ear Hawkweed Hieracium pilosella L. Alaska Natural Heritage Program; University of Alaska Anchorage: Anchorage, AK, USA, 2011; 4p, Available online: https://accs.uaa.alaska.edu/wp-content/uploads/Hieracium_pilosella_BIO_HIPIP.pdf (accessed on 1 December 2022).
- French, K. Invasion by hawkweeds. Biol. Invasions 2021, 23, 3641–3652. [Google Scholar] [CrossRef]
- French, K.; Watts, E. Differences in vegetative growth of two invasive hawkweeds at temperatures simulating invaded habitats at two altitudes. Sci. Rep. 2020, 10, 2180. [Google Scholar] [CrossRef]
- Chapman, H.M.; Parh, D.; Oraguzie, N. Genetic structure and colonizing success of a clonal, weedy species, Pilosella Officinarum (Asteraceae). Heredity 2000, 84, 401–409. [Google Scholar] [CrossRef]
- Murphy, S.D. The Role of Pollen Allelopathy in Weed Ecology. Weed Technol. 2001, 15, 867–872. [Google Scholar] [CrossRef]
- Gibson, D.J.; Newman, J.A. Festuca arundinacea Schreber (F. elatior L. ssp. arundinacea (Schreber) Hackel). J. Ecol. 2001, 89, 304–324. [Google Scholar] [CrossRef]
- Zobel, M.; Suurkask, M.; Rosén, E.; Pärtel, M. The dynamics of species richness in an experimentally restored calcareous grassland. J. Veg. Sci. 1996, 7, 203–210. [Google Scholar] [CrossRef]
- Jankowska, J.; Ciepiela, G.A.; Jankowski, K.; Kolczarek, R.; Sosnowski, J.; Wisniewska-Kadzajan, B. The allelopathic influence of Taraxacum officinale on the initial growth and developments of Festuca rubra L. J. Ecol. Eng. 2014, 15, 38–44. [Google Scholar] [CrossRef]
- Myster, R.W.; Pickett, S.T.A. Dynamics of associations between plants in ten old fields during 31 years of succession. J. Ecol. 1992, 80, 291–302. [Google Scholar] [CrossRef]
- Gu, M.; Liu, S.; Duan, H.; Wang, T.; Gu, Z. Dynamics of Community Biomass and Soil Nutrients in the Process of Vegetation Succession of Abandoned Farmland in the Loess Plateau. Front. Environ. Sci. 2021, 9, 580775. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, S.; Zhang, R.; Qi, W.; Zhang, R.; Knops, J.M.H.; Lu, J. Magnitude of species diversity effect on aboveground plant biomass increases through successional time of abandoned farmlands on the eastern Tibetan plateau of China. Land Degrad. Dev. 2016, 28, 370–378. [Google Scholar] [CrossRef]
- Ryspekov, T.; Jandák, J.; Balkozha, M.; Winkler, J. Vegetation of Abandoned Fields on Soil Types of Kastanozems in Northern Kazakhstan (Kostanay Region). J. Ecol. Eng. 2021, 22, 176–184. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; An, S. Environmental driving factors affecting plant biomass in natural grassland in the Loess Plateau, China. Ecol. Indic. 2017, 82, 250–259. [Google Scholar] [CrossRef]
- Bukantis, A.; Kažys, J. Klimatas Vilniuje per 330 Metu˛: Istorija Ir Perspektyvos [330 Years of Vilnius’ Climate: History and Future]; Vilniaus Universiteto Leidykla: Vilnius, Lithuania, 2020; Volume 10. [Google Scholar]
- Li, J.; Li, M.; Dong, L.; Wang, K.; Liu, Y.; Hai, X.; Pan, Y.; Lv, W.; Wang, X.; Shangguan, Z.; et al. Plant productivity and microbial composition drive soil carbon and nitrogen sequestrations following cropland abandonment. Sci. Total Environ. 2020, 744, 140802. [Google Scholar] [CrossRef]
- Ovsepyan, L.; Kurganova, L.; Lopes de Gerenyu, V.; Kuzyakov, Y. Recovery of organic matter and microbial biomass after abandonment of degraded agricultural soils: The influence of climate. Land Degrad. Dev. 2019, 30, 1861–1874. [Google Scholar] [CrossRef]
- Thibault, M.; Thiffault, E.; Bergeron, Y.; Ouimet, R.; Tremblay, S. Afforestation of abandoned agricultural lands for carbon sequestration: How does it compare with natural succession? Plant Soil 2022, 475, 605–621. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; Sala, G.; Galati, A.; Crescimanno, M.; Cerdà, A.; Badalamenti, E.; La Mantia, T. Agricultural land abandonment in Mediterranean environment provides ecosystem services via soil carbon sequestration. Sci. Total Environ. 2017, 576, 420–429. [Google Scholar] [CrossRef]
- Kämpf, I.; Hölzel, N.; Störrle, M.; Broll, G.; Kiehl, K. Potential of temperate agricultural soils for carbon sequestration: A meta-analysis of land-use effects. Sci. Total Environ. 2016, 566–567, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Tripolskaja, L.; Mašauskas, V.; Adomaitis, A.; Karčiauskienė, D.; Vaišvila, Z. (Eds.) Management of Agroecosystem Components. Results of Long-Term Agrochemical Research. Agroekosistemų Komponentų Valdymas. Ilgalaikių Agrocheminių Tyrimų Rezultatai [in Lithuanian]; Akademija: Kėdainiai, Lithuania, 2010; 567p. [Google Scholar]
- Abakumov, E.; Morgun, E.; Pechkin, A.; Polyakov, V. Abandoned agricultural soils from the central part of the Yamal region of Russia: Morphology, diversity, and chemical properties. Open Agric. 2020, 5, 94–106. [Google Scholar] [CrossRef]
- Du, F.; Liang, Z.; Xu, X.; Shan, L.; Zhang, X. Community biomass of abandoned farmland and its effects on soil nutrition in the Loess hilly region of Northern Shaanxi, China. Acta Ecol. Sinica 2007, 27, 1673–1683. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.-M.; An, S.-S. Changes in soil properties across a chronosequence of vegetation restoration on the Loess Plateau of China. Catena 2011, 86, 110–116. [Google Scholar] [CrossRef]
- Li, Y.Y.; Shao, M.A. Change of soil physical properties under long-term natural vegetation restoration in the Loess Plateau of China. J. Arid. Environ. 2006, 64, 77–96. [Google Scholar] [CrossRef]
- Nunes, A.; Figueiredo, A.; Almeida, A. The effects of farmland abandonment and plant succession on soil properties and erosion processes: A study case in central of Portugal. Geogr. Spat. Plan. J. 2012, 2, 165–190. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.B.; Xue, S.; Zhu, B. Changes in soil physico-chemical and microbiological properties during natural succession on abandoned farmland in the Loess Plateau. Environ. Earth Sci. 2011, 62, 915–925. [Google Scholar] [CrossRef]

| Horizon | Depth | Texture | ||
|---|---|---|---|---|
| Sand | Silt | Clay | ||
| (cm) | % | |||
| Ah | 0–28 | 88.58 | 7.67 | 3.75 |
| AB | 28–43 | 86.89 | 8.66 | 4.45 |
| B1 | 43–70 | 84.55 | 7.95 | 7.50 |
| B2 | 70–96 | 86.80 | 4.55 | 8.65 |
| C1 | 96–107 | 91.43 | 3.53 | 5.04 |
| C2 | 107–121 | 98.42 | 1.16 | 0.42 |
| Family Name | Latin Name of Species | Life Type | Abandonment Time | ||||
|---|---|---|---|---|---|---|---|
| 1 Year | 6 Years | 10 Years | 20 Years | 27 Years | |||
| Amaranthaceae | Amaranthus retroflexus L. | Y | r | ||||
| Apiaceae | Pimpinella saxifraga L. | P | r | + | r | ||
| Asteraceae | Achillea millefolium L. | P | + | r | 1 | 1 | 1 |
| Artemisia vulgaris L. | P | + | + | 1 | r | ||
| Carduus acanthoides L. | B | r | r | 1 | |||
| Carlina vulgaris L. | P/B | + | |||||
| Centaurea cyanus L. | Y | + | |||||
| Cirsium vulgare L. | P | + | |||||
| Conyza canadensis L. | Y | + | 1 | ||||
| Erigeron acris L. | B | r | + | ||||
| Filago arvensis L. | Y | r | |||||
| Gnaphalium silvaticum L. | P | r | + | ||||
| Helichrysum arenarium L. | P | + | |||||
| Hieracium vulgatum L. | P | 1 | |||||
| Pilosella officinarum F. W. Schultz et Sch. Bip. | P | 1 | 2 | 3 | |||
| Phalacroloma annuum L. | P/B | r | |||||
| Senecio jacobaea L. | P/B | + | |||||
| Solidago virgaurea L. | P | r | r | ||||
| Taraxacum officinale L. | P | r | + | r | |||
| Thlaspi arvense L. | Y | r | |||||
| Tripleurospermum inodorum L. | Y/B | 1 | |||||
| Boraginaceae | Echium vulgare L. | B | + | ||||
| Myosotis micrantha Pall. ex Lehm. | P | r | |||||
| Brassicaceae | Berteroa incana L. | B | r | ||||
| Camelina microcarpa L. | Y | r | |||||
| Capsella bursa pastoralis (L.) Medik. | Y | r | |||||
| Erophyla verna L. | Y | + | |||||
| Erucastrum gallicum L. | Y/B | + | |||||
| Raphanus raphanistrurm L. | Y | r | |||||
| Campanulaceae | Jasione montana L. | B | + | ||||
| Campanula patula L. | P | r | + | ||||
| Campanula glomerata L. | P | r | |||||
| Caryophyllaceae | Arenaria serpyllifolia L. | Y | + | + | |||
| Cerastium arvense L. | P | + | r | ||||
| Cerastium holosteoides L. | P | r | |||||
| Psammophiliella muralis L. | Y | ||||||
| Scleranthus annuus L. | Y | 1 | |||||
| Silene pratensis L. | P | + | + | + | |||
| Silene vulgaris L. | P | + | + | + | |||
| Spergula arvensis L. | Y | + | |||||
| Spergularia rubra (L.) J. Pressl et C. Presl. | P/Y/B | ||||||
| Stellaria holostea L. | P | 1 | |||||
| Chenopodiaceae | Chenopodium album L. | Y | + | ||||
| Convolvulaceae | Convolvulus arvensis L. | P | + | + | + | ||
| Equisetaceae | Equisetum arvense L. | P | + | + | + | 2 | |
| Fabaceae | Lotus corniculatus L. | P | 1 | 2 | + | ||
| Medicago falcata L. | P | 1 | 1 | ||||
| Medicago lupulina L. | P/Y | 1 | |||||
| Melilotus albus Medik. | P/Y | r | |||||
| Trifolium arvense L. | Y | r | r | + | 1 | ||
| Trifolium repens L. | P | r | + | ||||
| Vicia angustifolia L. | Y | r | |||||
| Vicia cracca L. | P | r | r | + | 1 | ||
| Vicia lathyroides L. | Y | + | |||||
| Fumariaceae | Fumaria officinalis L. | Y | 1 | ||||
| Geraniaceae | Erodium cicutarium L. | Y | r | ||||
| Globulariaceae | Globularia punctat Lapeyr. | P | r | ||||
| Hypericaceae | Hypericum perforatum L. | P | r | + | 1 | r | |
| Illecebraceae | Herniaria glabra L. | P | r | ||||
| Poaceae | Agrostis gigantea L. | P | r | ||||
| Bromus inermis L. | P | r | |||||
| Dactylis glomerata L. | P | 1 | + | ||||
| Elytrigia repens L. | P | 3 | 3 | 3 | r | ||
| Festuca arundinacea Schreb. | P | 4 | 3 | ||||
| Festuca ovina L. | P | 2 | |||||
| Festuca rubra L. | P | + | r | ||||
| Phleum pratense L. | P | r | + | ||||
| Secale cereale L. | Y | + | |||||
| Setaria viridis L. | Y | + | r | r | |||
| Polygonaceae | Polygonum aviculare L. | Y | + | ||||
| Rumex acetosella L. | P | + | |||||
| Rumex obtusifolius L. | P | + | |||||
| Plantaginaceae | Plantago lanceolata L. | P | r | r | + | ||
| Plantago major L. | P | r | |||||
| Ranunculaceae | Consolida regalis Gray. | Y | + | + | |||
| Rosaceae | Fragaria vesca L. | P | r | ||||
| Geum urbanum L. | P | + | |||||
| Potentilla argentea L. | P | 1 | r | + | |||
| Scrophulariaceae | Linaria vulgaris L. | P | r | r | + | ||
| Veronica arvensis L. | Y | r | |||||
| Violaceale | Viola arvensis Murray. | Y | 1 | r | |||
| Ah Depth cm | Soil Bulk Density | pHKCl | Available P mg kg−1 | Available K mg kg−1 | SOC g kg−1 | SOC Mg ha−1 | |
|---|---|---|---|---|---|---|---|
| 1995 | 28.0 | 1.33 g ha K | 6.0 ± 0.08 | 157 ± 4.9 | 170 ± 4.7 | 9.9 ± 0.08 | 36.8 ± 0.86 |
| 2022 | 31.0 | 1.37 8 ha K | 6.1 ± 0.11 | 131 ± 7.3 | 123 ± 8.7 | 14.5 ± 1.17 | 61.4 ± 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazlauskaite-Jadzevice, A.; Tripolskaja, L.; Baksiene, E. Renaturalization of Ex-Arable Arenosols: Phytocenosis Development and the Dynamics of Sandy Soil Properties. Land 2023, 12, 271. https://doi.org/10.3390/land12020271
Kazlauskaite-Jadzevice A, Tripolskaja L, Baksiene E. Renaturalization of Ex-Arable Arenosols: Phytocenosis Development and the Dynamics of Sandy Soil Properties. Land. 2023; 12(2):271. https://doi.org/10.3390/land12020271
Chicago/Turabian StyleKazlauskaite-Jadzevice, Asta, Liudmila Tripolskaja, and Eugenija Baksiene. 2023. "Renaturalization of Ex-Arable Arenosols: Phytocenosis Development and the Dynamics of Sandy Soil Properties" Land 12, no. 2: 271. https://doi.org/10.3390/land12020271
APA StyleKazlauskaite-Jadzevice, A., Tripolskaja, L., & Baksiene, E. (2023). Renaturalization of Ex-Arable Arenosols: Phytocenosis Development and the Dynamics of Sandy Soil Properties. Land, 12(2), 271. https://doi.org/10.3390/land12020271







