Nutrients and Contaminants in Soils of Current and Former Oil Palm Production Systems from Indonesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.2. Analytical Methods
2.2.1. Sample Collection and Preparation
2.2.2. Pseudo-Total Element Concentrations
2.2.3. Ca(NO3)2-Extractable Element Concentrations
2.2.4. pH
2.2.5. Cation Exchange Capacity and Base Saturation
2.2.6. Total Carbon and Nitrogen
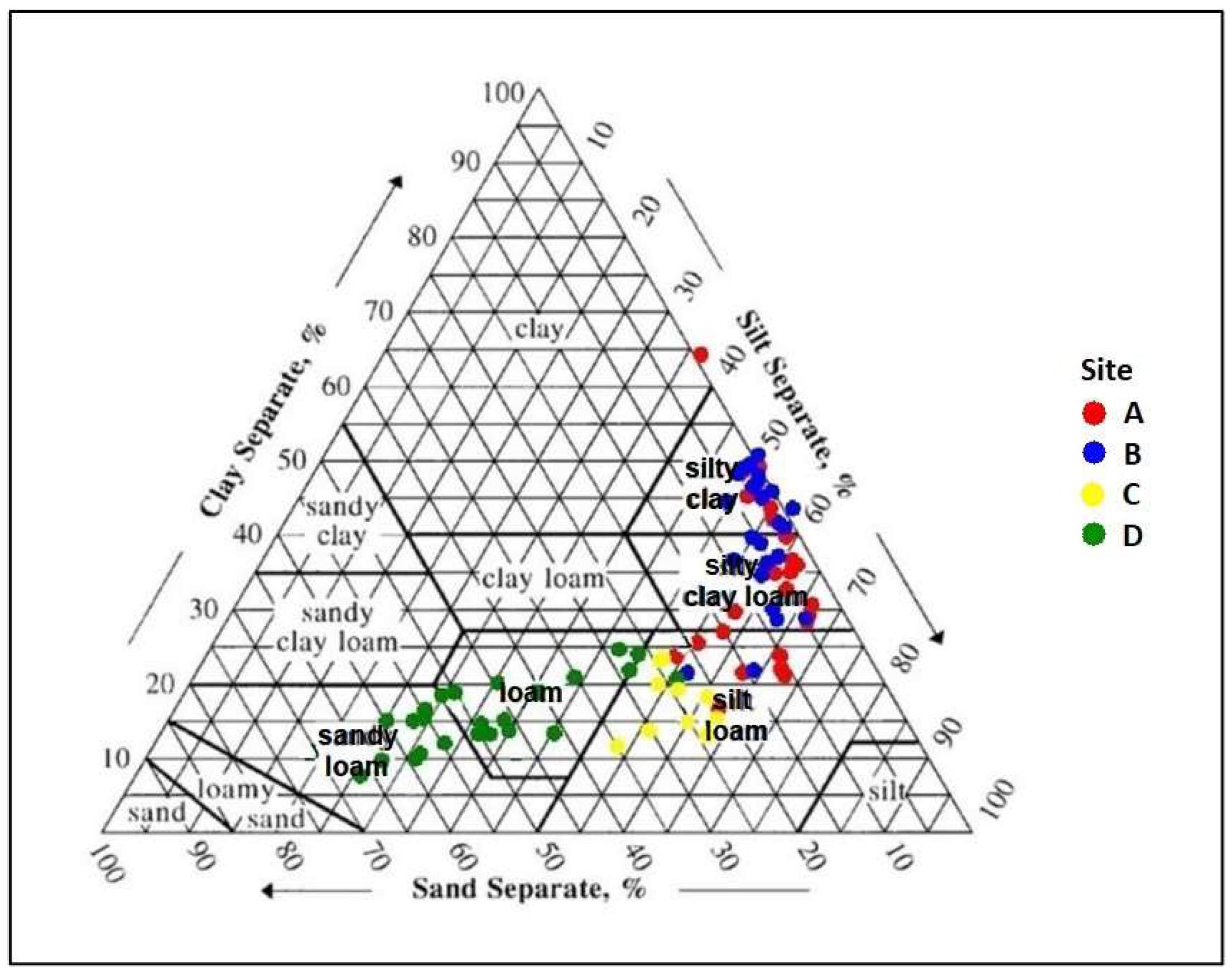
2.2.7. Particle Size Analysis
2.2.8. Elemental Determination
2.3. Statistical Analysis
3. Results and Discussion
3.1. General Soil Properties and Soil Carbon
3.2. Plant Macronutrients
3.3. Total Trace Element Concentrations
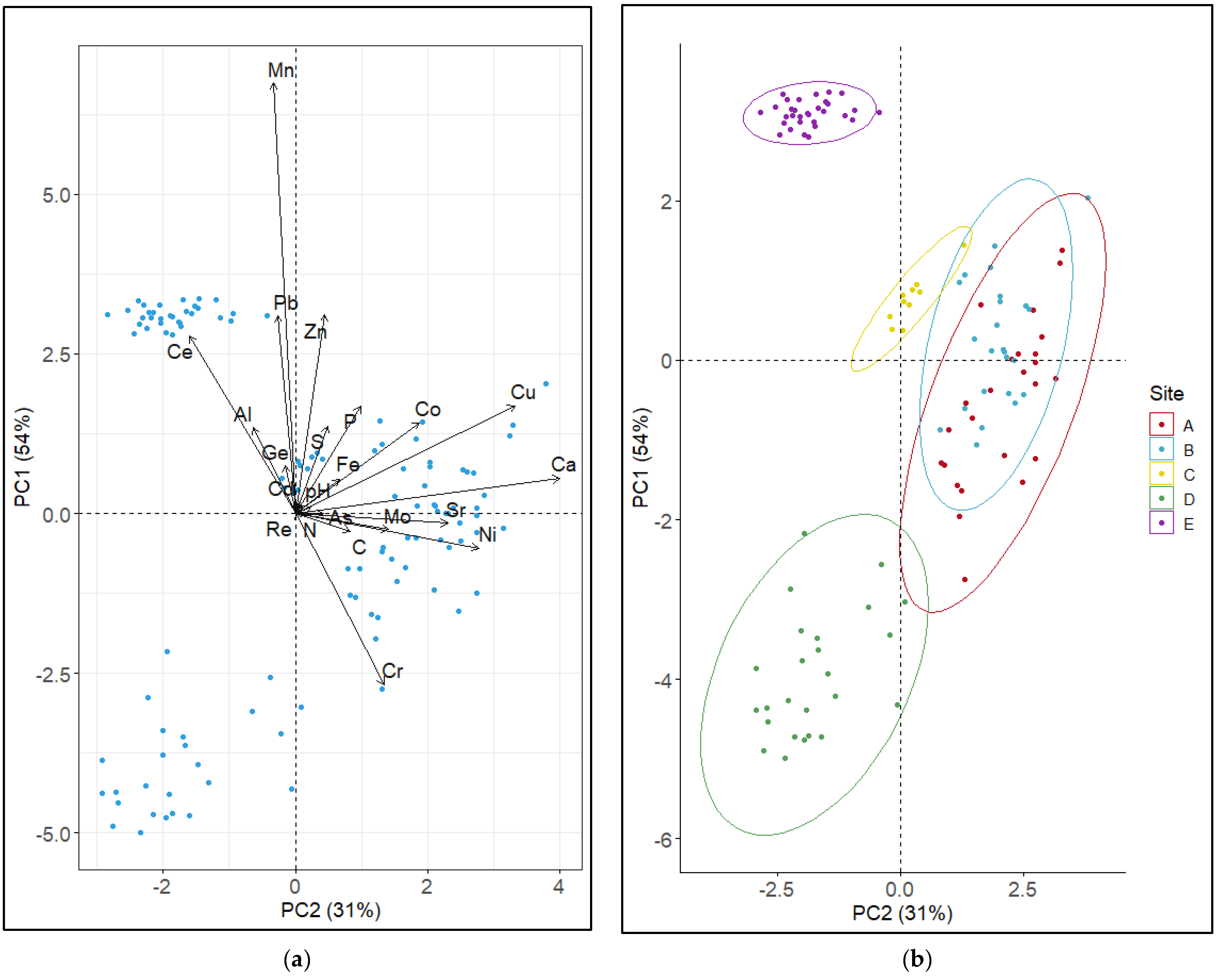
3.4. Factors Controlling the Variation between Sites
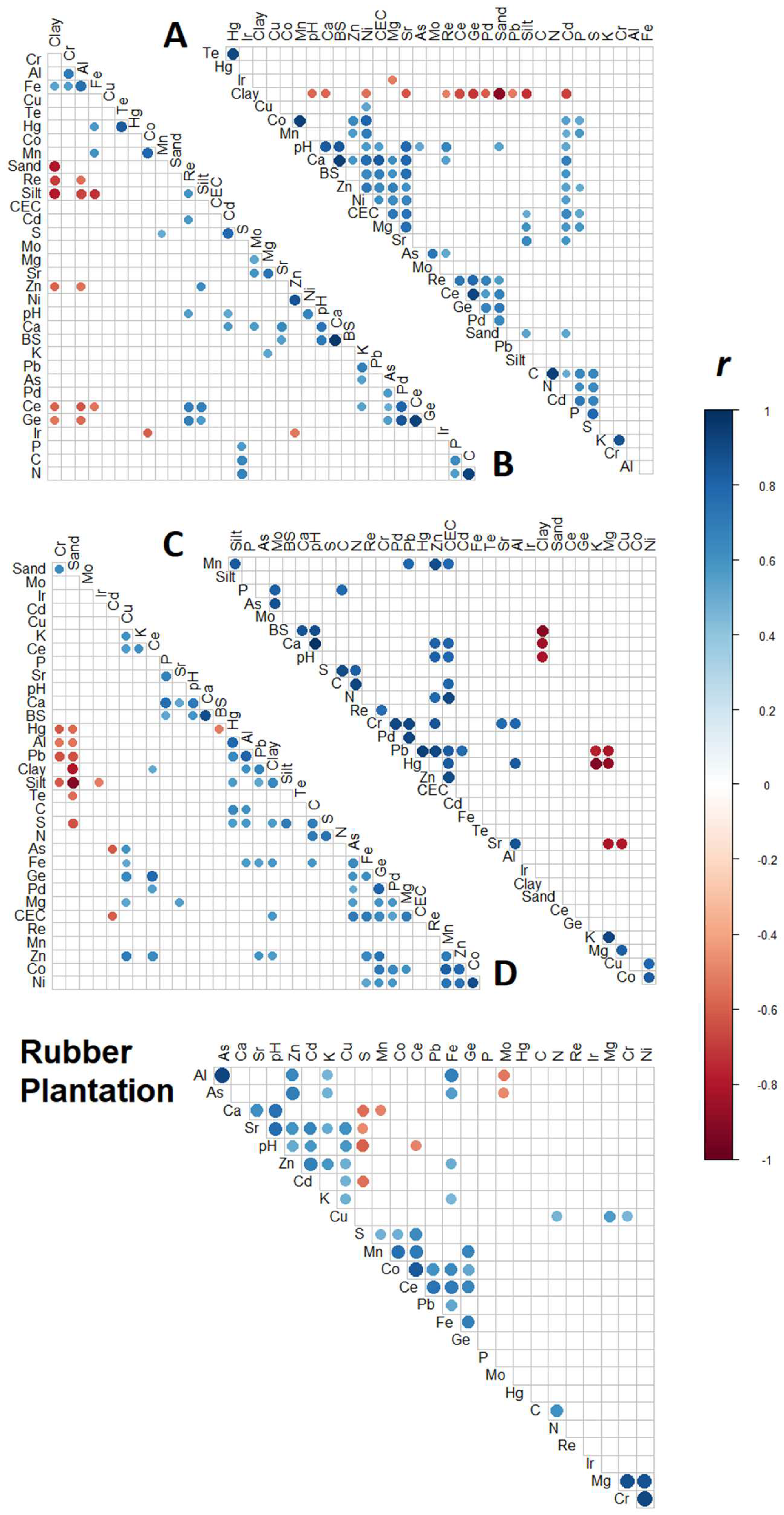
3.5. Intra-Site Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | Mineral N (NH4 and NO3) could not be measured due to the inability to import fresh soil into our laboratory. |
| 2 | This SGV applies to soil with pH 5.5, CEC 15 cmol (+) kg−1 and 15% clay content. |
| 3 | SGVs specific to Indonesia were not identified. |
References
- Gibbs, H.K.; Ruesch, A.S.; Achard, F.; Clayton, M.K.; Holmgren, P.; Ramankutty, N.; Foley, J.A. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef]
- Vancutsem, C.; Achard, F.; Pekel, J.-F.; Vieilledent, G.; Carboni, S.; Simonetti, D.; Gallego, J.; Aragão LE, O.C.; Nasi, R. Long-term (1990–2019) monitoring of forest cover changes in the humid tropics. Sci. Adv. 2021, 7, eabe1603. [Google Scholar] [CrossRef] [PubMed]
- Corley, R.H.V.; Tinker, P.B.H. The Oil Palm, 5th ed.; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Kissinger, G. Fiscal Incentives for Indonesian Palm oil Production: Pathways for Alignment with Green Growth; United Nations Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- Bateman, I.J.; Fisher, B.; Fitzherbert, E.; Glew, D.; Naidoo, R. Tigers, markets and palm oil: Market potential for conservation. Oryx 2010, 44, 230–234. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef]
- Morgans, C.L.; Meijaard, E.; Santika, T.; Law, E.; Budiharta, S.; Ancrenaz, M.; Wilson, K.A. Evaluating the effectiveness of palm oil certification in delivering multiple sustainability objectives. Environ. Res. Lett. 2018, 13, 064032. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Koh, L.P. Addressing the threats to biodiversity from oil-palm agriculture. Biodivers. Conserv. 2010, 19, 999–1007. [Google Scholar] [CrossRef]
- Padfield, R.; Hansen, S.; Davies, Z.G.; Ehrensperger, A.; Slade, E.M.; Evers, S.; Papargyropoulou, E.; Bessou, C.; Abdullah, N.; Page, S.; et al. Co-producing a research agenda for sustainable palm oil [Original Research]. Front. For. Glob. Chang. 2019, 2, 1–17. [Google Scholar] [CrossRef]
- Robinson, B.; Bolan, N.; Mahimairaja, S.; Clothier, B. Solubility, mobility, and bioaccumulation of trace elements: Abiotic processes in the rhizosphere. In Trace Elements in the Environment: Biogeochemistry, Biotechnology, and Bioremediation; CRC Press: Boca Raton, FL, USA, 2005; pp. 97–110. [Google Scholar]
- Chapman, P.M. Determining When Contamination Is Pollution—Weight of Evidence Determinations for Sediments and Effluents. Environ. Int. 2007, 33, 492–501. [Google Scholar] [CrossRef]
- Taylor, M.; Kim, N.; Smidt, G.; McNally, S.; Robinson, B.; Kratz, S.; Schnug, E. Trace element contaminants and radioactivity from phosphate fertilizer. In Phosphorus in Agriculture: 100% Zero; Schnug, E., De Kok, L.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 231–266. [Google Scholar] [CrossRef]
- Bivi MS, H.R.; Paiko, A.S.; Khairulmazmi, A.; Akhtar, M.S.; Idris, A.S. Control of basal stem rot disease in oil palm by supplementation of calcium, copper, and salicylic acid. Plant Pathol. J. 2016, 32, 396–406. [Google Scholar] [CrossRef]
- Taghavi, M.; Darvishiyan, M.; Momeni, M.; Eslami, H.; Fallahzadeh, R.A.; Zarei, A. Ecological risk assessment of trace elements (TEs) pollution and human health risk exposure in agricultural soils used for saffron cultivation. Sci. Rep. 2023, 13, 4556. [Google Scholar] [CrossRef]
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int. J. Environ. Res. Public Health 2019, 16, 4604. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Cavanagh, J.E. The state of knowledge of cadmium in New Zealand agricultural systems: 2021. N. Z. J. Agric. Res. 2022, 66, 285–335. [Google Scholar] [CrossRef]
- Besnard, E.; Chenu, C.; Robert, M. Distribution of copper in champagne vineyards soils, as influenced by organic amendments. In Proceedings of the 5th International Conference on the Biogeochemistry of Trace Elements, Vienna, Austria, 11–15 July 1999; p. 416. [Google Scholar]
- Foy, C.D. Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In Agronomy Monograph; Adams, F., Ed.; Wiley: New York, NY, USA, 1984. [Google Scholar] [CrossRef]
- Hartemink, A.E. Soil science in tropical and temperate regions—Some differences and similarities. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 269–292. Available online: https://www.sciencedirect.com/science/article/pii/S0065211302770168 (accessed on 25 May 2021).
- Cooper, H.V.; Evers, S.; Aplin, P.; Crout, N.; Dahalan MP, B.; Sjogersten, S. Greenhouse gas emissions resulting from conversion of peat swamp forest to oil palm plantation. Nat. Commun. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Woomer, P.L.; Martin, A.; Albrecht, A.; Resck DV, S.; Scharpens, H.W. The importance and management of soil organic matter in the tropics. In The Biological Management of Tropical Soil Fertility; Woomer, P.L., Swift, M.J., Eds.; Wiley-Sayce: New York, NY, USA, 1994. [Google Scholar]
- Rahman, N.; Neergaard A d Magid, J.; Ven, G.W.; Giller, K.E.; Bruun, T.B. Changes in soil organic carbon stocks after conversion from forest to oil palm plantations in Malaysian Borneo. Environ. Res. Lett. 2018, 13, 105001. [Google Scholar] [CrossRef]
- Woittiez, L.S.; Slingerland, M.; Giller, K.E. Yield gaps in Indonesian smallholder plantations: Causes and solutions. In Proceedings of the International Palm Oil Congress and Exhibition, Kuala Lumpur, Malaysia, 6–8 October 2015. [Google Scholar]
- Ng, S.K. Nutrition and nutrient management of oil palm—New thrust for the future perspective. In Potassium for Sustainable Crop production: International Symposium on the Role of Potassium; Pasricha, N.S., Bansal, S.K., Eds.; Potash Research Institute of India and International Potash Institute: Haryana, India, 2002; pp. 415–429. [Google Scholar]
- MPOB. Chemical Fertilizer Ganocare™ as Preventive Treatment in Controlling Ganoderma Disease of oil Palm; Malaysian Palm Oil Board TT No. 564. 2015. Available online: http://palmoilis.mpob.gov.my/publications/TOT/TT564-Idris.pdf (accessed on 25 May 2021).
- Woittiez, L.S.; Slingerland, M.; Rafik, R.; Giller, K.E. Nutritional imbalance in smallholder oil palm plantations in Indonesia. Nutr. Cycl. Agroecosyst. 2018, 111, 73–86. [Google Scholar] [CrossRef]
- Thompson-Morrison, H.; Gaw, S.; Robinson, B. An assessment of trace element accumulation in palm oil production. Sustainability 2022, 14, 4553. [Google Scholar] [CrossRef]
- Wahid, M.B.; Abdullah, S.N.A.; Henson, I.E. Oil palm—Achievements and potential. Plant Prod. Sci. 2005, 8, 288–297. [Google Scholar] [CrossRef]
- Woittiez, L.S.; Van Wijk, M.; Slingerland, M.; Van Noordwijk, M.; Giller, K. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Afriyanti, D.; Hein, L.; Kroeze, C.; Zuhdi, M.; Saad, A. Scenarios for withdrawal of oil palm plantations from peatlands in Jambi Province, Sumatra, Indonesia. Reg. Environ. Chang 2019, 19, 1201–1215. [Google Scholar] [CrossRef]
- Pradipta, T. Abandoned Oil Palm Plantations to be Converted into Forests. The Palm Scribe. 2018. Available online: https://thepalmscribe.id/abandoned-oil-palm-plantations-to-be-converted-into-forests/ (accessed on 17 March 2022).
- Sari DA, P.; Falatehan, A.F.; Ramadhonah, R.Y. The social and economic impacts of peat land palm oil plantation in Indonesia. J. Phys. Conf. Ser. 2019, 1364, 012017. [Google Scholar] [CrossRef]
- Rochayati, S.; Verloo, M.; Laing, G.D. Availability of cadmium and zinc as affected by the use of reactive phosphate rock, lime, and chicken manure on an indonesian acidic upland soil under field conditions. Commun. Soil Sci. Plant Anal. 2010, 41, 1986–2003. [Google Scholar] [CrossRef]
- FAO; UNESCO. Soil Map of the World; FAO: Québec City, QC, Canada, 1976. [Google Scholar]
- Encyclopedia Britannica. Acrisol. 2016. Available online: https://www.britannica.com/science/Acrisol (accessed on 25 May 2021).
- Du, Z.; Yu, L.; Yang, J.; Xu, Y.; Chen, B.; Peng, S.; Zhang, T.; Fu, H.; Harris, N.; Gong, P. A global map of planting years of plantations. Sci. Data 2022, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Asnur, P.; Thoha, A.S.; Elfiati, D. Mapping of Degraded Soil Properties in Gunung Leuser National Park. J. Sylva Indones. 2019, 2, 70–79. [Google Scholar] [CrossRef]
- Wasis, B. Soil properties in natural forest destruction and conversion to agricultural land, in Gunung Leuser National Park, North Sumatera province. J. Trop. For. Manag. 2012, 18, 206–212. [Google Scholar] [CrossRef]
- Schwarze, S.; Euler, M.; Gatto, M.; Hein, J.; Hettig, E.; Holtkamp, A.M.; Izhar, L.; Kunz, Y.; Lay, J.; Merten, J.; et al. Rubber vs. Oil Palm: An Analysis of Factors Influencing Smallholders’ Crop Choice in Jambi, Indonesia; EFForTS Discussion Paper Series, Issue. Georg-August-Universität. 2015. Available online: https://www.econstor.eu/bitstream/10419/117323/1/EFForTS_dp-11.pdf (accessed on 25 May 2021).
- Ultisol. 2012. Available online: https://www.britannica.com/science/Ultisol (accessed on 25 May 2021).
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for chemical analysis of soils. N. Z. Soil Bur. Sci. Rep. 1987, 80. [Google Scholar]
- US EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1; US EPA: Washington, DC, USA, 2007.
- Milestone. Digestion Method for Environmental Samples; Milestone: Williston, ND, USA, 2011. [Google Scholar]
- NIST. Certificate of Analysis: Standard Reference Material 2710 Montana Soil; National Institute of Standards & Technology: Gaithersburg, MA, USA, 2003.
- NIST. Certificate of Analysis: Standard Reference Material 2706 New Jersey Soil; National Institute of Standards & Technology: Gaithersburg, MA, USA, 2018.
- Gray, C.W.; McLaren, R.G.; Roberts AH, C.; Condron, L.M. The effect of long-term phosphatic fertilizer applications on the amounts and forms of cadmium in soils under pasture in New Zealand. Nutr. Cycl. Agroecosyst. 1999, 54, 267–277. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determing cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- LECO. Instrument: CN828 Determination of Carbon and Nitrogen in Soil. LECO Corportaion. 2021. Available online: https://eu.leco.com/images/Analytical-Application-Library/CN828_SOIL_203-821-627.pdf (accessed on 3 March 2022).
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- USDA. USDA Textural Soil Classification; USD: Washington, DC, USA, 1987. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1044818.pdf (accessed on 6 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 6 June 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 6 June 2021).
- Harrell, F.E. Hmisc: Harrell Miscellaneous. R Package Version 4.6-0. 2021. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 6 June 2021).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 20 September 2023).
- Rao, I.M.; Zeigler, R.S.; Vera, R.; Sarkarung, S. Selection and breeding for acid-soil tolerance in crops. Bioscience 1993, 43, 454–465. [Google Scholar] [CrossRef]
- Shofiyati, R.; Las, I.; Agus, F. Indonesian soil data base and predicted stock of soil carbon. In Proceedings of the International Workshop on Evaluation and Sustainable Management of Soil Carbon Sequestration in Asian Countries, Bogor, Indonesia, 28–29 September 2010. [Google Scholar]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- van Noordwijk, M.; Cerri, C.; Woomer, P.L.; Nugroho, K.; Bernoux, M. Soil carbon dynamics in the humid tropical forest zone. Geoderma 1997, 79, 187–225. [Google Scholar] [CrossRef]
- Goh, K.; Po, S.B. Fertilizer recommendation systems for oil palm: Estimating the fertilizer rates. In Proceedings of the MOSTA Best Practices Workshops-Agronomy and Crop Management, Kuala Lumpur, Malaysia, 27 March–14 August 2004. [Google Scholar]
- Obeng, A.S.; Sadick, A.; Hanyabui, E.; Musah, M.; Marius, M.; Acheampong, M.K. Evaluation of soil fertility status in oil palm plantations in the Western Region of Ghana. Agric. Food 2020, 5, 938–949. [Google Scholar] [CrossRef]
- Kahle, M.; Kleber, M.; Jahn, R. Predicting carbon content in illitic clay fractions from surface area, cation exchange capacity and dithionite-extractable iron. Eur. J. Soil Sci. 2002, 53, 639–644. [Google Scholar] [CrossRef]
- Matus, F.; Rumpel, C.; Neculman, R.; Panichini, M.; Mora, M. Soil carbon storage and stabilisation in andic soils: A review. Catena 2014, 120, 102–110. [Google Scholar] [CrossRef]
- Feng, W.; Plante, A.F.; Six, J. Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 2013, 112, 81–93. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Aweto, A.O.; Enaruvbe, G.O. Catenary properties of soil properties under oil palm plantations in southwetsern Nigeria. Ethiop. J. Environ. Stud. Manag. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Mason, B.H.; Moore, C.B. Principles of Geochemistry; Wiley: New York, NY, USA, 1982; Available online: https://books.google.co.nz/books?id=K75gIAAACAAJ (accessed on 20 September 2023).
- McLaren, R.G.; Cameron, K.C. Soil Ccience: Sustainable Production and Environmental Protection; Oxford University Press: Oxford, UK, 1996; Available online: https://books.google.co.nz/books?id=AYVFAQAAIAAJ (accessed on 20 September 2023).
- Fageria, N.K.; Nascente, A.S. Chapter Six—Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 128, pp. 221–275. Available online: https://www.sciencedirect.com/science/article/pii/B9780128021392000068 (accessed on 6 June 2021).
- Foster, H.L.; Tayeb DM, T. The effect of different methods of placement and frequency of application of fertilizer to oil palm on an inland soil in Peninsula Malaysia. Palm Oil Res. Inst. Malays. Bull. 1986, 12, 1–11. [Google Scholar]
- Goh, K.J.; Härdter, R.; Fairhurst, T. Fertilizing for maximum return. In The Oil Palm: Management for Large and Sustainable Yields; Fairhurst, T., Härdter, R., Eds.; Phosphate Institute of Canada, Potash & Phosphate Institute, International Potash Institute: Zug, Switzerland, 2003; pp. 279–306. [Google Scholar]
- Maene, L.M.; Thong, K.C.; Ong, T.S.; Mokhtaruddin, A.M.; Pushparajah, E. Surface wash under mature oil palm. In Proceedings of the 1979 Symposium on Water in Malaysian Agriculture, Kuala Lumpur, Malaysia, 16–17 March 1979. [Google Scholar]
- Climate: North Sumatra. Available online: https://en.climate-data.org/asia/indonesia/north-sumatra-1204/ (accessed on 30 March 2022).
- Aflizar Aprisal Alarima, C.I.; Masunaga, T. Effect of soil erosion and topography on distribution of cadmium (Cd) in Sumani watershed, west Sumatra, Indonesia. MATEC Web Conf. 2018, 229, 03001. [Google Scholar] [CrossRef][Green Version]
- Kvakić, M.; Pellerin, S.; Ciais, P.; Achat, D.L.; Augusto, L.; Denoroy, P.; Gerber, J.S.; Goll, D.; Mollier, A.; Mueller, N.D.; et al. Quantifying the Limitation to World Cereal Production Due To Soil Phosphorus Status. Glob. Biogeochem. Cycles 2018, 32, 143–157. [Google Scholar] [CrossRef]
- Van De Wiel CC, M.; Van Der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef]
- Manning DA, C. How will minerals feed the world in 2050? Proc. Geol. Assoc. 2015, 126, 14–17. [Google Scholar] [CrossRef]
- Nokes, C. A Guide to the Ministry of Health Drinking-Water Standards for New Zealand; Environmental Science & Research Ltd.: Kenepuru, Porirua, 2008. [Google Scholar]
- Aini Azura, A.; Fauziah, C.I.; Samsuri, A.W. Cadmium and zinc concentrations in soils and oil palm tissues as affected by long-term application of phosphate rock fertilizers. Soil Sediment Contam. Int. J. 2012, 21, 586–603. [Google Scholar] [CrossRef]
- Olafisoye, B.O.; Oguntibeju, O.O.; Osibote, O.A. An assessment of the bioavailability of metals in soils on oil palm plantations in Nigeria. Pol. J. Environ. Stud. 2016, 25, 1125–1140. [Google Scholar] [CrossRef]
- Hooda, P. Trace Elements in Soils; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Cavanagh, J.; Munir, K. Development of Soil Guideline Values for the Protection of Ecological Receptors (Eco-SGVs): Technical Document; Landcare Research: Lincoln, New Zealand, 2016. [Google Scholar]
- Kiwifruit Vine Health. KVH Information Sheet: Copper Spray Information. 2019. Available online: https://www.kvh.org.nz/vdb/document/91314 (accessed on 25 May 2021).
- Golow, A.A.; Agyemang, O.; Ackah, M. Soil fertility analysis in two oil plam plantation towns in Assin, north district of the central region of Ghana. Aust. J. Basic Appl. Sci. 2010, 4, 1650–1655. [Google Scholar]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar] [CrossRef]
- Kaiser, S.; Wagner, S.; Moschner, C.; Funke, C.; Wiche, O. Accumulation of germanium (Ge) in plant tissues of grasses is not solely driven by its incorporation in phytoliths. Biogeochemistry 2020, 148, 49–68. [Google Scholar] [CrossRef]
- Sheffer, F.; Schachtschabel, P.; Blume, H.-P. Scheffer/Schachtschabel Soil Science; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Cole, B.; Craven, J. Impacts of Oil Plam Activities in the Kokonda and Popondetta Catchments: An initial Environmental Examination. 2006. Available online: https://aarhusclearinghouse.unece.org/resources/impacts-oil-palm-activities-kokoda-and-popondetta-catchments-initial-environmental (accessed on 25 May 2021).
- Orobator, P.; Ashiriba, H.; Aighewi, I. Assessment of Heavy Metals Concentration in Soils under Selected Oil Palm (Elaeis guineensis) Plantations in Edo State, Nigeria. Academix. 2018. Available online: http://www.academix.ng/documents/papers/1524666819_6831.pdf (accessed on 6 June 2021).
- Perelomov, L.V. Interaction of rare-earth elements with biotic and abiotic soil components. Agrokhimiya 2007, 11, 85–96. [Google Scholar]
- Suryatmojo, H.; Fujimoto, M.; Kosugi, K.; Mizuyama, T. Runoff and soil erosion characteristics in different periods of an intensive forest management system in a tropical indonesian rainforest. Int. J. Sustain. Dev. Plan. 2014, 9, 830–846. [Google Scholar] [CrossRef]
- Maynard, J.B. 7.11—Manganiferous Sediments, Rocks, and Ores. In Treatise on Geochemistr; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 289–308. Available online: https://www.sciencedirect.com/science/article/pii/B0080437516070997 (accessed on 25 May 2021).
- Chaney, R.L. Zinc phytotoxicity. In Zinc in Soils and Plants; Robson, A.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 55. [Google Scholar] [CrossRef]
- Fernando TH, P.S.; Jayasinghe, C.K.; Wijesundera RL, C.; Siriwardana, D. Screening of fungicides against Corynespora leaf fall disease of rubber under nursery conditions [Screening von Fungizidwirkungen gegenüber der Corynespora-Blattfallkrankheit in Baumschulen]. J. Plant Dis. Prot. 2010, 117, 117–121. [Google Scholar] [CrossRef]
- Hurt, S.; Ollinger, J.; Arce, G.; Bui, Q.; Tobia, A.J.; Ravenswaay, B. Chapter 78—Dialkyldithiocarbamates (EBDCs). In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 1689–1710. [Google Scholar]
- Mancino, C.R.; Holdren, D.D.; Tredway, L.; Soika, M.D.; Soika, M.D. Influence of mancozeb fungicides, chlorothalonil, manganese and zinc on bentgrass color and disease incidence. J. Turfgrass Manag. 1999, 3, 13–22. [Google Scholar]
- Shi, F.; Li, X.; Bai, Y.; Li, L.; Pu, M.; Liu, L.; Lei, M. Mechanism of the Zinc Dithiocarbamate-Activated Rubber Vulcanization Process: A Density Functional Theory Study. ACS Appl. Polym. Mater. 2021, 3, 5188–5196. [Google Scholar] [CrossRef]
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb: Past, present, and future. Plant Dis. 2010, 94, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Savichev, A.T.; Vodyanitskii, Y.N. Determination of barium, lanthanum, and cerium contents in soils by the X-ray radiometric method. Eurasian Soil Sci. 2009, 42, 1461–1469. [Google Scholar] [CrossRef]
- Moreira, C.G.; de Carvalho, T.S.; de Carvalho, T.S.; de Oliveira, C.; de Abreu, L.B.; de Castro, A.C.S.; Ribeiro, P.G.; Bispo, F.H.A.; Boutin, C.; Guilherme, L.R.G. Ecological risk assessment of cerium for tropical agroecosystems. Chemosphere 2019, 221, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef]
- Setyorini, D.; Prihatini, T.; Kurnia, U.; No, J.I.J. Pollution of Soil by Agricultural and Industrial Waste; Food and Fertilizer Technology Center: Taipei, Taiwan, 2002. [Google Scholar]
- Sheppard, S.; Long, J.; Sanipelli, B.; Sohlenius, G. Solid/Liquid Partition Coefficients (Kd) for Selected Soils and Sediments at Forsmark and Laxemar-Simpevarp. Geological Survey of Sweden. 2009. Available online: http://www.skb.com/publication/1951648/R-09-27.pdf (accessed on 25 May 2021).
- Reiser, R.; Simmler, M.; Portmann, D.; Clucas, L.; Schulin, R.; Robinson, B. Cadmium concentrations in New Zealand pastures: Relationships to soil and climate variables. J. Environ. Qual. 2014, 43, 917–925. [Google Scholar] [CrossRef]
- Oorts, K. Copper. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 367–394. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Sienkiewicz, S.; Zalewska, M.; Żarczyński, P.; Krzebietke, S. Phosphorus fractions in soil fertilised with organic waste. Environ. Monit. Assess. 2020, 192, 315. [Google Scholar] [CrossRef]
- Németh, T.; Kiss, Z.; Kismányoky, T.; Lehoczky, É. Effect of Long-Term Fertilization on the Strontium Content of Soil. Commun. Soil Sci. Plant Anal. 2006, 37, 2751–2758. [Google Scholar] [CrossRef]
- Born, I.; Jansson, G.; Johnsson, L. A field study on the influence of soil pH on trace element levels in spring wheat (Triticum aestivum), potatoes (Solanum tuberosum) and carrots (Daucus carota). Water Air Soil Pollut. 1995, 85, 835–840. [Google Scholar] [CrossRef]
- Liu, W.; Yang, X.; Duan, L.; Naidu, R.; Yan, K.; Liu, Y.; Wang, X.; Gao, Y.; Chen, Y. Variability in plant trace element uptake across different crops, soil contamination levels and soil properties in the Xinjiang Uygur Autonomous Region of northwest China. Sci. Rep. 2021, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; Kim, J.-G.; Park, J.-S.; Kim, M.-S.; Owens, G.; Youn, G.-H.; Lee, J.-S. Immobilizer-assisted management of metal-contaminated agricultural soils for safer food production. J. Environ. Manag. 2012, 102, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Min, H.-G.; Lee, S.-H.; Kim, J.-G. The effects of various amendments on trace element stabilization in acidic, neutral, and alkali soil with similar pollution index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef] [PubMed]

| Site Abbreviation | Site A | Site B | Site C | Site D |
|---|---|---|---|---|
| Site status | Abandoned, trees remaining | Abandoned, trees remaining | Active, smallholder | Ex-plantation, no trees remaining |
| Soil type (FAO classification) | Acrisol | Acrisol | Acrisol | Acrisol |
| Soil type (USDA classification) | Oxisol | Oxisol | Oxisol | Oxisol |
| Slope | ≤12° | ≤12° | <4° | ≤9° |
| Years in production | ca. 30 | ca. 30 | ca. 20 | ca. 12 |
| Recent management history | No amendments applied and unharvested for past 3 years | No amendments applied and unharvested for past 3 years | Amendments applied and weeds cleared with pesticides within last year | Oil palm trees Removed, area under regeneration with no amendments applied for past 3 years |
| Number of samples collected | 25 | 25 | 10 | 25 |
| Site | A (n = 25) | B (n = 25) | C (n = 10) | D (n = 25) | Rubber Plantation (n = 32) | |
|---|---|---|---|---|---|---|
| Particle size distribution | Sand (%) | 8.3(1.4) a | 5.6(1.0) a | 27(1.3) b | 50(2.5) b | — |
| Silt (%) | 58(1.4) b | 55(0.99) b | 57(1.3) b | 35(1.8) a | — | |
| Clay (%) | 33(2.2) b | 40(1.7) b | 17(1.3) a | 15(0.92) a | — | |
| pH | 5.4(0.11) c | 4.8(0.061) ab | 5.1(0.097) bc | 4.6(0.047) a | 5.2(0.076) c | |
| CEC (cmol (+) kg−1) | 25(1.5) c | 30(0.80) d | 4.3(0.63) a | 10(0.67) b | — | |
| BS (%) | 70(5.5) c | 33(4.7) b | 43(8.5) bc | 21(3.4) a | — | |
| Total Al (mg kg−1) | 29,207(868) b | 31,022(1144) b | 32,307(1241) b | 25,174(1855) a | 67,371(2379) c | |
| Ca(NO3)2 extractable Al (mg kg−1) | 110(24) ab | 308(30) c | 36(7.1) a | 194(16) b | — | |
| Total Fe (mg kg−1) | 28,530(696) c | 31,149(1017) c | 23,084(420) b | 16,847(1007) a | 23,921(334) b | |
| Ca(NO3)2 extractable Fe (mg kg−1) | 0.42(0.095) a | 0.64(0.18) a | 0.045(0.015) a | 4.2(0.95) b | — | |
| Site | A (n = 25) | B (n = 25) | C (n = 10) | D (n = 25) | Rubber Plantation (n = 32) |
|---|---|---|---|---|---|
| Total C (%) | 3.2(0.23) c | 3.3(0.23) c | 2.1(0.11) b | 2.0(0.14) b | 1.3(0.027) a |
| Total N (%) | 0.23(0.023) c | 0.23(0.020) c | 0.035(0.0090) a | 0.021(0.0057) a | 0.12(0.0032) b |
| C:N ratio | 14 | 14 | 60 | 95 | 11 |
| Total P (mg kg−1) | 296(21) b | 305(17) b | 299(13) b | 118(30) a | 323(14) b |
| Ca(NO3)2 extractable P (mg kg−1) | 0.030(0.0040) b | 0.021(0.0029) ab | 0.037(0.0031) b | 0.013(0.0033) a | — |
| Total K (mg kg−1) | 4252(303) d | 2316(106)c | 1883(176) bc | 778(67) a | 1612(25) b |
| Total S (mg kg−1) | 266(16) c | 358(139) bc | 170(9.5) b | 116(7.6) a | 306(6.5) c |
| Ca(NO3)2 extractable S (mg kg−1) | 2.6(1.3) a | 1.8(0.85) a | 0.45(0.39) a | 1.1(0.42) a | — |
| Total Ca (mg kg−1) | 9422(1400) d | 5468(996) cd | 2439(564) bc | 1286(416) a | 1202(117) ab |
| Ca(NO3)2 extractable Ca (mg kg−1) | 65(5.6) c | 6.6(2.4) b | 0.0001(<0.001) ab | 1.9(1.9) a | — |
| Total Mg (mg kg−1) | 3005(156) c | 2637(101) c | 2747(405) c | 1023(102) a | 1185(49) b |
| Site | A (n = 25) | B (n = 25) | C (n = 10) | D (n = 25) | Rubber Plantation (n = 32) |
|---|---|---|---|---|---|
| Cr | 34(1.1) c | 25(0.90) b | 27(1.4) bc | 50(3.7) d | 5.03(0.20) a |
| Mn | 281(56) b | 519(87) c | 876(82) d | 28(3.7) a | 2995(52) e |
| Co | 19(2.3) c | 19(1.5) c | 16(0.36) c | 3.4(0.42) a | 8.9(0.12) b |
| Ni | 29(2.0) d | 30(1.5) d | 13(0.61) c | 7.9(0.58) b | 4.5(0.40) a |
| Cu | 19(1.4) c | 38(0.86) d | 5.6(0.69) b | 1.1(0.22) a | 6.6(0.49) b |
| Zn | 91(3.7) b | 91(3.8) b | 75(5.2) b | 19(1.4) a | 213(5.7) c |
| Ge | 0.86(0.051) b | 1.0(0.79) b | 1.05(0.36) b | 0.54(0.028) a | 1.7(0.034) c |
| As | 3.8(0.14) bc | 4.3(0.16) c | 9.0(0.59) d | 3.7(0.28) ab | 3.1(0.12) a |
| Sr | 24(2.2) c | 14(1.4) b | 48(13) d | 6.1(0.67) a | 4.3(0.16) a |
| Mo | 1.5(0.17) b | 2.0(0.15) c | 0.13(0.0068) a | 0.30(0.080) a | 0.095(0.0038) a |
| Cd | 0.14(0.026) bc | 0.087(0.024) ac | 0.085(0.029) ab | 0.026(0.0073) a | 0.16(0.012) b |
| Ce | 22(1.9) a | 23(2.5) a | 55(1.6) b | 17(0.83) a | 139(1.9) c |
| Pb | 16(0.70) b | 17(1.2) b | 28(1.8) c | 5.8(1.2) a | 51(0.53) d |
| Site | A | B | C | D |
|---|---|---|---|---|
| Cr | 3179 | 2651 | 2900 | 5849 |
| Co | 279 | 195 | 2344 | 156 |
| Ni | 46 | 26 | 1389 | 25 |
| Cu | 904 | 358 | 5643 | 933 |
| Zn | 72 | 42 | 172 | 52 |
| Ge | 649 | 128 | 481 | 148 |
| As | 127 | 46 | 150 | 61 |
| Sr | 23 | 26 | 83 | 35 |
| Mo | 420 | 1615 | 43 | 28 |
| Ce | 658 | 102 | 615 | 106 |
| Pb | 175 | 76 | 257 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson-Morrison, H.; Ariantiningsih, F.; Arief, S.M.; Gaw, S.; Robinson, B. Nutrients and Contaminants in Soils of Current and Former Oil Palm Production Systems from Indonesia. Land 2023, 12, 2144. https://doi.org/10.3390/land12122144
Thompson-Morrison H, Ariantiningsih F, Arief SM, Gaw S, Robinson B. Nutrients and Contaminants in Soils of Current and Former Oil Palm Production Systems from Indonesia. Land. 2023; 12(12):2144. https://doi.org/10.3390/land12122144
Chicago/Turabian StyleThompson-Morrison, Hadee, Fransisca Ariantiningsih, Sugesti Muhammad Arief, Sally Gaw, and Brett Robinson. 2023. "Nutrients and Contaminants in Soils of Current and Former Oil Palm Production Systems from Indonesia" Land 12, no. 12: 2144. https://doi.org/10.3390/land12122144
APA StyleThompson-Morrison, H., Ariantiningsih, F., Arief, S. M., Gaw, S., & Robinson, B. (2023). Nutrients and Contaminants in Soils of Current and Former Oil Palm Production Systems from Indonesia. Land, 12(12), 2144. https://doi.org/10.3390/land12122144





