Abstract
Coastal ecosystems, encompassing land and marine environments and hosting substantial biodiversity, are among the most threatened worldwide. The European Habitats Directive prioritises coastal habitats and species, requiring legislative, direct protection, monitoring, and informational measures. Accurate habitat and species monitoring is crucial to conservation efforts, yet biodiversity research in complex, ever-changing environments like coastal areas is difficult. Citizen Science may bridge biodiversity assessment and eco-friendly monitoring by incorporating non-scientists into the data collection for scientists and stakeholders. A Citizen Science approach supported by a dedicated iNaturalist project (called Wild Coast CASCADE) was implemented to obtain a complete monitoring framework that includes observations of many taxa in terrestrial, aquatic, and transitional dynamic coastal environments in the Central Italian Adriatic coast. We explored data gathered focusing on the IUCN Red List species, the species and habitats of European conservation concern, and the non-native species. Between 2020 and 2023, we collected 3784 records covering 742 species, with 81% meeting the “research grade criteria”, and these were retained for subsequent research. Citizen Science volunteers have collected 291 georeferenced animal records from the global IUCN Red List, 51 plant species from 14 species that are indicators of the presence of habitats of European Conservation Concern, and 44 non-native plants and animals. Our results provide evidence that citizen research projects can effectively assist in monitoring coastal–marine habitats and species. They also underline the potential of Citizen Science for biodiversity conservation and emphasize the importance of public engagement in conservation efforts.
1. Introduction
Coastal ecosystems are characterised by dynamic transitional zones between terrestrial and marine environments, exhibiting significant levels of specialised biodiversity [1,2]. Not only do they possess significant quantities of unique flora and fauna, but they also offer a multitude of benefits to society and ecosystem services [3,4]. Simultaneously, coastal areas are ranked as one of the most at risk in the world [5,6], with the Mediterranean region being particularly vulnerable [7,8]. To mitigate the ongoing deterioration of these imperilled habitats, they are subject to conservation restrictions that have been imposed at both the global and regional level. The Convention on Biological Diversity [9] underscores the importance of allocating focused attention and financial resources to the preservation of biodiversity in coastal areas. The Member States of the European Union have collectively embraced the Council Directive 92/43/EEC, more often known as the Habitats Directive (HD). This legislation represents an important step forward in the development of a European approach to nature conservation. The HD designates numerous coastal ecosystems as habitats that require conservation efforts. These habitats are specifically mentioned in Annex I of the directive. Additionally, the HD identifies certain coastal species of flora and fauna that are of conservation importance. These species are included in Annexes II, IV, and V of the Habitats Directive, deserving either direct (the species) or indirect (their habitat) protection. Art. 17 of the directive impose all member states to monitor and report every six years on the distribution and conservation status of all species and habitats listed in Annexes I, II, IV and V. Furthermore, it establishes a comprehensive network of biologically significant areas referred to as the Natura 2000 network, aiming to preserve these ecosystems and species in a state of “good conservation status”. Each Natura 2000 site is obligated to develop and implement the essential measures and actions required to guarantee the enduring preservation of the designated species and habitats as stipulated by HD. These measures must encompass regulatory, direct protection, monitoring, and information-based strategies. The importance of precise monitoring of habitats and species is widely acknowledged as a crucial element of successful conservation strategies [10,11]. However, conducting periodic biodiversity assessments in complex and ever-changing environments such as coastal transitions can be problematic. This difficulty stems from a variety of factors, including the high costs involved [12,13], poor accessibility to specific sampling locations [14], and the intricate nature of organising field campaigns that require frequent and regular intervals [15].
Citizen Science (CS) is a potential solution for bridging the divide between biodiversity assessment and the implementation of efficient and economical monitoring practises [16]. Citizen Science encompasses the participation of non-professional citizens in many scientific activities, including data collection and analysis, technology development, species occurrence assessment, and the dissemination of these endeavours [16]. The continuous expansion of online platforms and the introduction of novel digital technologies are facilitating a significant increase in the engagement of CS activities [16,17]. Volunteer participation in environmental data collection fosters their connection to research subjects, while also raising public awareness about pressing issues such as biodiversity loss, climate change, environmental contamination, and the spread of invasive species [18]. Furthermore, CS efforts have shown favourable improvements in citizen behaviour, encouraging a more environmentally concerned and scientifically engaged citizenry [19]. At the same time, there is empirical support for the impact of data acquired by CS endeavours in the surveillance of distinct ecological systems, such as forests or savannas [20,21,22]. CS may also contribute to increasing public awareness in long-term monitoring areas [23,24]. Certain studies have specifically focused on investigating the application of CS in the monitoring of fauna, e.g., [25,26], or flora [27]. Additionally, there are studies that have explored the use of CS for monitoring specific domains, such as water or land [28,29]. CS initiatives are frequently aimed at specific tasks, such as implementing environmental education for youth [30,31,32], studying the presence of rare or less attractive species [33,34], or even monitoring biological invasions [35,36]. Yet, the studies that simultaneously analyse organisms belonging to distinct kingdoms are still scarce [37,38,39]. Moreover, the use of CS to support the implementation of a comprehensive observation framework, which includes observations of many taxa in various terrestrial, aquatic, and transitional dynamic systems in coastal regions, has not been attempted thus far, and it is necessary to enhance our comprehension of how CS can function in such a context.
iNaturalist [40] is a social networking platform that was founded in 2008 to facilitate the collaboration of individuals from various scientific backgrounds, including professionals and laypeople, to collectively document and disseminate discoveries related to biodiversity [40]. This platform is well recognised as an efficient networking service for the purpose of observing biodiversity and preserving associated data within the realm of CS activities. iNaturalist relies on a substantial user base of over six million registered participants, serving as a hub for scientists, naturalists, biologists, and citizens alike who are interested in advancing online CS endeavours and facilitating the gathering of biodiversity data [40]. The collection consists of more than 160 million verifiable observations, with a continuous growth rate, and each observation is accompanied by details, including the geographic location and date of observation. The dataset excludes observations of organisms that are held in captivity or those that have been cultivated. Notably, around 100 million of these observations have achieved the designation of “research grade”, indicating their credibility and suitability for scientific applications (as of 10 October 2023). The iNaturalist programme has been recognised as a key tool for collecting biodiversity data in multiple nations globally [41]. Moreover, it has gained significant popularity as a social platform for consolidating nature-related data [12]. According to [42], the platform has the capacity to significantly contribute to the implementation of conservation strategies by providing useful data to many stakeholders, including research initiatives, land managers, environmental organisations, and the wider public.
Within this framework, the current study aims to examine the capacity of CS initiatives facilitated by the iNaturalist platform in monitoring biodiversity in coastal–marine regions, with a specific focus on the Central Adriatic Italian coast as a designated experimental site. For this purpose, we established a specialised iNaturalist project known as the “Wild Coast CASCADE” (WCC) and subsequently investigated critical questions pertaining to the preservation of biodiversity within these regions by means of CS. Subsequently, we focused our attention on those data pertaining to taxonomic groups that are of conservation significance. These groups include (a) species that are listed in the IUCN red book and are also present in the annexes of HD, (b) plant species that serve as indicators for the occurrence of habitats that are of conservation concern in Europe (HD), and (c) non-native species. Focusing on these three species groups can meet different needs in conservation biology. Firstly, the updated distribution of vulnerable species might potentially enhance devoted monitoring and conservation efforts. Secondly, investigating the occurrence of plant species that serve as indicators of European habitats may allow us to propose specific management priorities for the analysed coasts. Finally, the inclusion of current and accurate data regarding the occurrence of alien organisms can enhance the effectiveness of the early detection systems for invasive species.
2. Materials and Methods
2.1. Study Area
Biodiversity data were gathered from the coastal areas of the Abruzzo and Molise regions in Central Italy, along the Italian Central Adriatic coast. The study area, spanning a total distance of roughly 166 km and encompassing a landward extent of 300 m and a seaward extent of 500 m, exhibits a Mediterranean climate. This stretch of coast is distinguished by expanses of sandy or gravelly/pebbly beaches that are occasionally interrupted by rocky promontories and interspersed with the mouths of rivers. Previous studies evidenced the high ecological significance of the analysed coasts [43,44]. The southern sector is characterised by seashores mostly composed of gravel and pebbles (Abruzzo), while the southern tract (Molise) is predominantly characterised by low dunes. As far as bathymetry is concerned, the entire study area is characterised by very shallow depths, with the depths sloping gently from the coast towards the open sea [45].
The study area comprises a total of eleven areas under differ types of protection, including 1 marine protected area, 3 regional reserve sites, and 7 Natura 2000 sites (Figure 1), all requiring biodiversity monitoring activities, as mandated national and international regulations. In addition, the area includes two sites of the Long-Term Ecological Research Network (LTER), specifically referred to as “Foce Saccione-Bonifica Ramitelli” (IT20-003-T) and “Foce Trigno-Marina di Petacciato” (IT20-002-T; [46]; https://deims.org/6d7ffd99-40e1-4f0d-ad26-6904581dbe9b (accessed on 1 August 2023)).
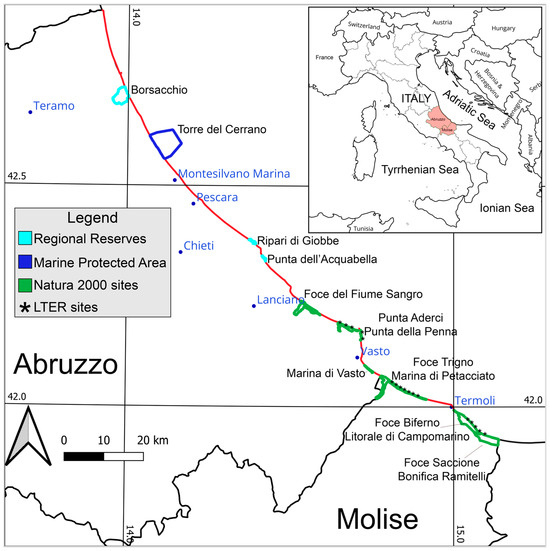
Figure 1.
Central Adriatic coast of the Abruzzo and Molise Region (Italy). Protected areas are reported in different colours, with the Natura 2000 sites in green (IT7140107-Lecceta litoranea di Torino di Sangro e foce del Fiume Sangro; IT7140108-Punta Aderci—Punta della Penna; IT7140109-Marina di Vasto; IT7228221-Foce Trigno—Marina di Petacciato; IT7222216-Foce Biferno—Litorale di Campomarino; and IT7222217-Foce Saccione—Bonifica Ramitelli), regional reserves in cyan (Riserva naturale del Borsacchio; Ripari di Giobbe-Foce Fiume Moro; Punta dell’Acquabella), and the Marine Protected Area in blue (Torre del Cerrano, annexing on emerged dunes a Natura 2000 site IT7120215). Asterisks indicate the LTER sites (IT20-002-T in the north and IT20-003-T in the south). The name of the main cities (with more than 30,000 residents) are reported in blue text as the analysed seashore (Abruzzo and Molise Regions) is indicated with a red line.
2.2. Data Collection
Data were collected through CS initiatives, which included active engagement in public outreach and educational activities that began in August 2020 (for details, see Figure 2). We sent invitations to a diverse group of people, requesting their cooperation and participation in the process of collecting data on the presence of wild species in both natural and semi-natural sites. From 2020 to 2022, a total of 12 CS initiatives along the Central Adriatic coast were coordinated and implemented in collaboration with local NGO’s, such as “Ambiente Basso Molise” (http://ambientebassomolise.blogspot.com/ (accessed on 1 August 2023)) and the Abruzzo Institution for Protected Areas (IAAP, https://www.iaap.it/ (accessed on 1 August 2023)), along with protected area management bodies such as the Marine Protected Area “Torre del Cerrano” and the Special Area of Conservation Punta Aderci–Punta della Penna (IT7140108). Our initiative was partially supported by the INTERREG Italy–Croatia CASCADE project (https://www.italy-croatia.eu/web/cascade (accessed on 1 August 2023)) and the LIFE17 NAT/IT/000565 CALLIOPE project (https://lifecalliope.eu/ (accessed on 1 August 2023)).
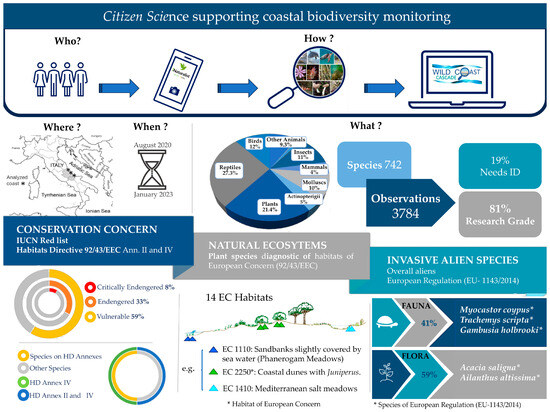
Figure 2.
Flowchart depicting a synthetic overview of the adopted approach.
2.3. Data Storage and Organization
The WCC records were obtained from a diverse range of sources, including organised CS events and the independent leisure activities of individuals. These records were collected from the beginning of August 2020 to the end of January 2023 and involved the participation of hundreds of observers (Figure 2). Data were systematically collected within a dedicated iNaturalist project known as the “Wild Coast CASCADE” (WCC) (https://www.inaturalist.org/projects/wild-coast-cascade (accessed on 1 August 2023)). Each individual observation submitted to the WCC project consisted of one or more field photographs, precise geographic coordinates, and a proposed taxonomic identification provided by the iNaturalist criteria (for the full explanation of how iNaturalist works, please refer to https://www.inaturalist.org/pages/help#general3 (accessed on 1 August 2023)). In addition to the iNaturalist platform, we established a specialised Facebook webpage that offered people with low computer experience the chance to submit observations that the managers of the WCC will then upload to iNaturalist on their behalf.
The comprehensive evaluation of the quality of each observation involved an initial classification of the newly submitted entry as “Needs ID”, indicating the requirement for verification and potential correction by members of the iNaturalist community who possess expertise in the relevant taxonomic categories.
After achieving a consensus among a majority of specialists (i.e., more than two-thirds) who verified the identification of the organism at the species level, the observation was subsequently elevated to the category of “research grade”. This categorization enhances the suitability of the data for scientific utilisation (Figure 2). The data collected consisted of a wide range of living organisms, representing different taxonomic levels. The aforementioned observations were categorised according to the 13 taxonomic groups acknowledged by iNaturalist, namely Actinopterygii, amphibians, arachnids, birds, chromists, fungi and lichens, insects, mammals, molluscs, plants, protozoa, reptiles, and other animals [40].
2.4. Data Extraction and Analysis
We first classified data on iNaturalist taxonomic groups and evaluated the reliability of the observations collected, keeping for further analysis the records that had reached the “research grade”.
The number of threatened species (Figure 2) collected within the WCC project was assessed by relying on the global [47] and Italian [48] IUCN Red Lists. Additionally, the conservation concern level of these species in Europe was determined by referring to the annexes of the HD. The IUCN Red List provides a comprehensive and up-to-date assessment of the conservation status and risk of extinction for various biological species. These species are classified into nine categories, and, among these, we focused on critically endangered (CR), endangered (EN), vulnerable (VU), and near threatened (NT). The annexes of the HD consist of a list of species that member states of the European Union have agreed to safeguard and regulate through a range of measures. These measures include the designation of Areas of Special Conservation Interest (Annex II), the establishment of stringent protection rules (Annex IV), and the development of national management plans (Annex V).
As for the analysis on plant species that represent “diagnostic taxa” (Figure 2) according to the HD, we followed the nomenclature recommended in the updated checklist of “Flora d’Italia” [49]. These species are of outmost importance in influencing the composition and operation of these habitats. Because of their importance to the general functionality and survival of the ecosystem, they are also known as “focal species” [50]. The identification of diagnostic species was based on the “Italian Vegetation Prodrome” [51] and the European Union’s Habitat Interpretation Manual [52].
Subsequently, we shifted our attention towards an analysis of alien and invasive species (IAS; Figure 2) that are included in the European regulation known as “on the prevention and management of the introduction and spread of invasive alien species” (Regulation (EU) No. 1143/2014, hereafter referred to as the IAS Regulation). The IAS Regulation delineates a catalogue of invasive alien species categorised as being of “Union concern”. These species pose a threat to biodiversity and ecosystem services, necessitating coordinated efforts at the European Union level [53]. The determination of the origin of non-native species was also established by Galasso et al. [54] and Loy et al. [55]. In our analysis of plant species, we incorporated the research conducted by Galasso et al. [54] and its subsequent revisions [56,57]. Regarding the fauna, we conducted a thorough examination of the data provided by the Global Biodiversity Information Facility (GBIF) platform (https://www.gbif.org (accessed on 1 August 2023)). Additionally, we conducted a quick analysis of the plant species that are poisonous to humans based on information from the iNaturalist platform and consulting some of the relevant literature (e.g., [58]).
3. Results and Discussion
As of 31 January 2023, the WCC project has documented a total of 3,784 observations by 250 distinct users pertaining to 742 species across diverse ecosystems (terrestrial and aquatic; Table 1). The research has acquired a large number of observations collected systematically through time and across diverse coastal ecosystems (terrestrial and aquatic). These observations provide compelling evidence of the efficacy of the dedicated CS activities, as well as the valuable contributions made by people during their leisure activities. During the summer season, the coastal regions under study attract a significant number of tourists. However, during the winter and fall seasons, these sites are frequented by schools and local people [4]. Scaling with the study area extent, our collection of data showed figures that are comparable to previous studies conducted in various regions, such as California, Scandinavia, South America, and North Asia [59]. Our findings also align with the research conducted by international institutions, including natural history museums [30].

Table 1.
Number of observations that are “research grade” and species per iNaturalist taxa, along with the respective percentages.
As for the number of records per taxonomic group, reptiles account for 27% of total records, making it the most observed group, followed by plants (21%), birds (12%), and insects (11%). The less represented categories (with less than 1%), include amphibians, fungi and lichens, and chromists (Table 1). A substantial percentage of species is also present among the category of other animals, i.e., 13%. On iNaturalist, this macro-category collects a series of taxonomic categories, such as Cnidaria, Arthropoda, and Chordata, without any distinction and in which we have identified species of considerable importance.
The high number of reptile observations is mainly attributed to records of Caretta caretta. In recent years, this species has been recolonising and returning to historical nesting sites along the coasts of Spain, France, and Western Italy [60], with the WCC project providing evidence of this ongoing process also in the Adriatic.
Plants was the most diverse taxonomic category, with 236 species recorded (31%), followed by insects (163 species; 22%). Birds are another taxonomic group that has a significant number of reports and species (82 species, 11%). In this case, it is highly probable that this pattern is associated with the sea–land ecotone, which provides a diverse range of trophic niches [61].
Conversely, the lowest represented taxonomic groups, each comprising less than 1% of the total, were fungi and lichens (two taxa), chromists (two taxa), and amphibians (three taxa) (Table 1). Such a distribution of reports is congruent with earlier iNaturalist studies that have identified plants and insects as the most often observed groups [59]. Plants and insects in the studied area showed a great species diversity, which is indicative of the extensive ecological mosaic influenced by a pronounced environmental sea–land ecotone. This ecotone, found within relatively narrow coastal strips, facilitates substantial turnover rates of plant species [62] and arthropods [63].
The “research grade” status achievement, indicating species that have undergone thorough review, was observed in 81% of the cases (Figure 3). The “research grade” was reached according to iNaturalist criteria of at least 2/3 of users agreeing on an identification, and, in our case, most of the observations (81%) reached “research grade”, with 1 or 2 users in agreement with the submitting user. Less than 1% of the “research grade” observations showed any remaining disagreement among users on the determination of the specimen. This highlights the significant potential of CS activities in involving a high number of well-trained citizens who are able to improve collected data reliability, thus confirming the usefulness of the iNaturalist platform in the context of future research and conservation endeavours, despite some research highlighting limitations and biases in the iNaturalist approach [64,65,66], including errors or undersampling in less known and charismatic taxa, while well-known taxa or charismatic species might be oversampled. Accordingly, it is worth mentioning that both amphibians and reptiles have attained a “research grade” status of 100% for all their recorded observations (Figure 3). The higher quality of these observations can be primarily attributed to the number of records related to a few easily recognizable species. For example, the abundance of reptile observations at the “research grade” is mainly due to the loggerhead turtle (Caretta caretta) and the Italian wall lizard (Podarcis siculus) records, while for amphibians, it includes several observations of the Balearic green toad (Bufotes balearicus) and the marsh frog (Pelophylax ridibundus).
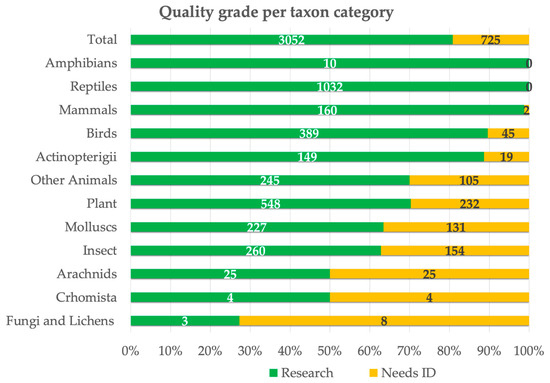
Figure 3.
Number and percentage of observations at research level and on need of ID for each iNaturalist macro-category.
Among the other macro-categories, most taxonomic groups reached the “research grade” for at least the 50% of the records. Only fungi and lichens had less than 30% of their observations at the “research grade” (Figure 3). As observed by Barbato et al. [34] in regard to marine environments, most of the reports that did not reach the “research grade” (19% requiring identification) are not readily identifiable and classifiable by photographic evidence. The 30% of plant records that have not achieved the research status can be ascribed to the phenology and seasonal variability of species features (e.g., flowers and fruits), which enable identification only within a short time frame [26].
3.1. Species of Conservation Concern
The WCC has documented a total of 291 observations (Table 2) of species that fall within the global IUCN Red List [47]. These observations pertain to 17 species that have been classified as critically endangered (CR—5.9%), endangered (EN—17.7%), vulnerable (VU—47.0%), or near threatened (NT—29.4%). Among the most threatened taxa, we documented the occurrence of one critically endangered species (Fan Mussel—Pinna nobilis) and three endangered (EN) species (namely Testudo hermanni, Cladocora caespitosa, and Chelonia mydas).
The critically endangered Fan Mussel (Pinna nobilis Linnaeus, 1758), the largest long-lived endemic bivalve species in the Mediterranean Sea [67], has faced a significant decline since 2016, primarily attributed to infections caused by pathogens. This infection likely heightened the species’ susceptibility to changes in water temperature, resulting in sporadic occurrences around the Adriatic seashore [68]. The reported occurrence of Pinna nobilis within the WCC project can help enhance scientific research and guide future monitoring efforts. The occurrence areas of Pinna nobilis can serve as pilot sites for implementing reinforcement actions aimed at boosting the population of the species [69]. Moreover, they can guide habitat restoration plans and facilitate the implementation of protection actions required by the HD for species listed on Annex IV.
The Hermann’s tortoise (Testudo hermanni Gmelin, 1789) is an endangered terrestrial Testudinidae that is native to the northern coastal region of the Mediterranean and occurs in Italy with a fragmented distribution [70]. The Hermann’s tortoise exhibits a preference for well-preserved coastal dune mosaics throughout the Adriatic coast that consist of many habitats that are of conservation concern [71]. Being highly susceptible to habitat deterioration [72], its occurrence underscores the high conservation status of certain coastal tracts in the Central Adriatic.
Updated data on the presence of Annex II species outside the Natura 2000 network can also help us organise monitoring campaigns targeted at expanding the network. The occurrence data collected within protected areas can aid in the implementation of targeted conservation measures for species listed on Annex IV of the HD.
The WCC also documented the presence of the endangered green sea turtle (Chelonia mydas Linnaeus, 1758), which has a preferential distribution on tropical and subtropical waters and a reduced presence in temperate regions [73]. The green turtle population in the Mediterranean Sea is highly susceptible to the effects of climate change and human activities, which have a detrimental impact on all stages of its life cycle, from eggs to adult individuals [74]. The significance of our observations is heightened by recent research that highlights the strategic importance of the Adriatic basin for the conservation of green turtle populations [60]. Furthermore, the new data on green sea turtle’s occurrence in the Adriatic coastal area provide valuable insights for planning additional monitoring campaigns and developing specific protection measures, as mandated by the European Union for species listed on Annex IV (HD).

Table 2.
Status of threatened species registered on the WCC iNaturalist according to the global and the Italian IUCN Red Lists [48,75] along with their inclusion in HD Annexes II and IV. The number of observations and the iNaturalist taxonomic category are also reported. CR, critically endangered; EN, endangered; VU, vulnerable; NT, near threatened; LC, least concern; NE, not evaluated.
Table 2.
Status of threatened species registered on the WCC iNaturalist according to the global and the Italian IUCN Red Lists [48,75] along with their inclusion in HD Annexes II and IV. The number of observations and the iNaturalist taxonomic category are also reported. CR, critically endangered; EN, endangered; VU, vulnerable; NT, near threatened; LC, least concern; NE, not evaluated.
| Species | GLOBAL IUCN | Italian IUCN | HD Ann. | No. of Obs. | iNaturalist Category |
|---|---|---|---|---|---|
| Pinna nobilis | CR | CR | IV | 3 | Mollusks |
| Cladocora caespitosa | EN | LC | 9 | Other animals | |
| Chelonia mydas | EN | NE | IV | 3 | Reptiles |
| Testudo hermanni | EN | EN | II; IV | 8 | Reptiles |
| Caretta caretta | VU | EN | II; IV | 239 | Reptiles |
| Passer italiae | VU | VU | 8 | Birds | |
| Streptopelia turtur | VU | LC | 5 | Birds | |
| Palinurus elephas | VU | NE | 2 | Other animals | |
| Balaenoptera physalus | VU | EN | IV | 1 | Mammals |
| Epinephelus marginatus | VU | EN | 2 | Fishes | |
| Aythya ferina | VU | VU | 1 | Birds | |
| Cerambyx cerdo | VU | LC | II; IV | 1 | Insects |
| Haematopus ostralegus | NT | VU | 3 | Birds | |
| Calidris ferruginea | NT | NE | 2 | Birds | |
| Aythya nyroca | NT | EN | 1 | Birds | |
| Raja asterias | NT | NE | 2 | Other animals | |
| Sciaena umbra | NT | VU | 1 | Fishes |
The Mediterranean Pillow Coral (Cladocora caespitosa Linnaeus, 1767) is an endangered long-lived reef-building species indigenous to the Mediterranean Sea, where it forms scattered colonies, beds, or vast banks [76,77]. Cladocora caespitosa is declining in the Adriatic Sea due to its great sensitivity to pollution [78], invasive algae [79], eutrophication [80], trawling, dredging [81], and climate change. Cladocora caespitosa was discovered in the setting of infra-littoral algal biocenosis, providing additional evidence of the presence of the Habitat of European Concern 1170 “Reefs” on several coastal tracts where the habitat had not previously been documented. Moreover, the WCC’s new knowledge may enable future scientific campaigns to adequately record and map the Reef EU habitat, updating its distribution range in the Central Adriatic area and extending the execution of necessary conservation actions [77].
The loggerhead sea turtle (Caretta caretta Linnaeus, 1758) has a broad distribution in the Mediterranean Sea [11]. The Italian breeding population of Caretta caretta plays a crucial role in preserving the genetic diversity of the species [82]. The species is classified in the national Red List as endangered [48], as it suffered a serious decline in Italy that were caused by multiple threats, such as urban expansion, human trampling, and pollution [83]. The recorded occurrences and nesting sites for Caretta caretta in the WWC database fill the information gap concerning the Central Adriatic coast, where, until about ten years ago, there was a lack of recorded nesting sites [84]. Furthermore, the current distribution data can be instrumental in guiding scientific research and the development of suitable conservation measures for Italian populations, as advocated for species listed on Annexes II and IV of the Habitats Directive (42/93/EEC).
The investigation of the WCC database, with a focus on endangered species, demonstrates how CS can be used to monitor various types of wildlife in need of conservation. This indicates an essential issue that necessitates additional research [85]. The abundance of georeferenced recordings of species listed on the IUCN Red List and in the HD annexes allows us to generate a comprehensive picture of the priority areas of the Central Adriatic coast that require concerted conservation efforts. Our findings highlight the potential of CS for biodiversity monitoring, a task that is frequently performed for a single taxon. The biodiversity data acquired in these broad areas can play a critical role in the development of integrated coastal management strategies. These strategies should successfully include the conservation needs of each taxon or group of taxa within the geographical framework offered by protected area networks such as the Natura 2000 network and regional/national protected areas.
3.2. EU Habitats
In WCC, we recorded 51 plant species which are diagnostic of 14 habitats of European Conservation Concern (Table 3). Our results reveal the presence of the typical ecosystems of dune zonation from the halo-nitrophilous formations on the drift-line to the fore dune woody formations (e.g., 1210: Annual vegetation of drift lines; 2110: Embryonic shifting dunes; 2120: Shifting dunes with Ammophila arenaria; 2130 *: Fixed coastal dunes with herbaceous vegetation; 2230: Malcolmietalia dune grasslands; 2250 *: Coastal dunes with Juniperus spp.; 2260: Cisto-Lavanduletalia dune sclerophyllous scrubs; and 2270 *: Wooded dunes with Pinus pinea and/or Pinus pinaster), some cliff formations (1240: Vegetated sea cliffs of the Mediterranean coasts with endemic Limonium spp.), a rich and heterogeneous salt marsh mosaic (1410: Mediterranean salt meadows Juncetalia maritimi; 1420: Mediterranean and thermo-Atlantic halophilous scrubs Sarcocornietea fruticosi; 3170*: Mediterranean temporary ponds; and 6420: Mediterranean tall humid herb grasslands of the Molinio-Holoschoenion), and few river (3250: Constantly flowing Mediterranean rivers with Glaucium flavum) and marine habitats (1110: Sandbanks slightly covered by seawater).
The data acquired validates earlier research indicating the great diversity and well-preserved dune mosaic characterising the Molise coast [86], which, in accordance with HD, is in large part included in the Natura 2000 network [4]. It also points to the necessity of restoration efforts along the Abruzzo coastal dunes, where natural ecosystems are fragmented and poorly covered by European protection areas [87]. Conservation actions aimed at reinforcing populations of dune-building perennial grasses (e.g., Ammophila arenaria and Elymus farctus) and reducing trampling pressure, for example, may play a critical role in dune consolidation, ensuring both biodiversity values and ecological functions [88].

Table 3.
List of the recorded diagnostic plant species of habitats of European Conservation Concern, along with the respective habitat code and name [89]. * Priority habitats.
Table 3.
List of the recorded diagnostic plant species of habitats of European Conservation Concern, along with the respective habitat code and name [89]. * Priority habitats.
| Species | Habitat Code and Name |
|---|---|
| Cymodocea nodosa (Ucria) Asch. | 1110: Sandbanks which are slightly covered by seawater all the time |
| Cakile maritima Scop. subsp. Maritima | 1210: Annual vegetation of drift lines |
| Salsola kali L. | |
| Salsola soda L. | |
| Crithmum maritimum L. | 1240: Vegetated Sea cliffs of the Mediterranean coasts with endemic Limonium spp. |
| Calystegia soldanella (L.) R. Br. | 2110: Embryonic shifting dunes |
| Elymus farctus (Viv.) Runemark ex Melderis | |
| Eryngium maritimum L. | |
| Euphorbia paralias L. | |
| Euphorbia peplis L. | |
| Lotus creticus L. | |
| Medicago marina L. | |
| Polygonum maritimum L. | |
| Achillea maritima (L.) Ehrend. et Y.P. Guo | 2120: Shifting dunes along the shoreline with Ammophila arenaria (white dunes) |
| Ammophila arenaria LK. | |
| Anthemis maritima L. | |
| Cyperus capitatus Vand. | |
| Echinophora spinosa L. | |
| Eryngium maritimum L. | |
| Lotus creticus L. | |
| Pancratium maritimum L. | |
| Sixalix atropurpurea (L.) Greuter and Burdet | |
| Glaucium flavum Crantz | 3250: Constantly flowing Mediterranean rivers with Glaucium flavum |
| Euphorbia terracina L. | 2130 *: Fixed coastal dunes with herbaceous vegetation (grey dunes) |
| Verbascum niveum subsp. garganicum (Ten.) Murb. | |
| Artemisia campestris L. | 2230: Malcolmietalia dune grasslands |
| Erodium laciniatum (Cav.) Willd. | |
| Lagurus ovatus L. | |
| Malcolmia ramosissima (Desf.) Thell. | |
| Matthiola incana (L.) R. Br. | |
| Ononis variegata L. | |
| Silene colorata Poir. | |
| Verbascum niveum subsp. garganicum (Ten.) Murb. | |
| Asparagus acutifolius L. | 2250 *: Coastal dunes with Juniperus spp. |
| Juniperus macrocarpa Sm. | |
| Myrtus communis L. | |
| Phillyrea angustifolia L. | |
| Pistacia lentiscus L. | |
| Rhamnus alaternus L. | |
| Smilax aspera L. | |
| Asparagus acutifolius L. | 2260: Cisto-Lavanduletalia dune sclerophyllous scrubs |
| Cistus creticus L. | |
| Cistus salviifolius L. | |
| Phillyrea angustifolia L. | |
| Pistacia lentiscus L. | |
| Rhamnus alaternus L. | |
| Rosmarinus officinalis L. | |
| Smilax aspera L. | |
| Phillyrea angustifolia L. | 2270 *: Wooded dunes with Pinus pinea and/or Pinus pinaster |
| Pinus halepensis Mill. | |
| Pinus pinea L. | |
| Pistacia lentiscus L. | |
| Atriplex prostrata Boucher ex DC. | 1410: Mediterranean salt meadows (Juncetalia maritimi) |
| Blackstonia perfoliata (L.) Huds. | |
| Juncus acutus L. | |
| Limbarda crithmoides (L.) Dumort. | |
| Sarcocornia fruticosa (L.) A.J. Scott | 1420: Mediterranean and thermo-Atlantic halophilous scrubs (Sarcocornietea fruticosi) |
| Schoenus nigricans L. | 6420: Mediterranean tall humid herb grasslands of the Molinio-Holoschoenion |
| Scirpoides holoschoenus (L.) Soják | |
| Tripidium ravennae (L.) H. Scholz |
The coastal dunes with Juniperus spp. form a priority habitat characterised by a distinctive Juniperus scrub which is mainly threatened by urban expansion, tourist pressure [90], and invasive alien plants [91]. These dune juniper thickets, which reach the northern limit of their geographical range in Molise [86], are included in a Natura 2000 site, where they provide suitable habitats for several species of fauna of conservation concern, such as the Hermann tortoise [71].
The recorded salt meadows (Juncetalia maritimi) are characteristic of well-preserved back-dune depressions that are periodically flooded by brackish water. Unfortunately, as with most salt meadows, they are impinged by land reclamation, changes in water regimes, urbanization, invasive species, and coastal erosion [92]. The observed habitat, which represents one of the few remaining coastal wetlands in the Mediterranean [93], is encompassed within a Natura 2000 site. These meadows may play a pivotal role in biodiversity conservation, serving as essential habitats for both resident and migratory avian species, as well as various aquatic organisms, like fish and arthropods [94].
We also have detected the presence of Cymodocea nodosa (Ucria) Ascherson formations on the Central Adriatic coastal seabed. Cymodocea meadows may play a crucial role in supporting fish populations by offering refuge to schools, facilitating the establishment of trophic niches, and serving as nursery sites [95]. Additionally, these meadows have a significant function in safeguarding coastal areas from various environmental impacts [96]. The areas of occurrence, all identified outside the Natura 2000 network, warrant dedicated field campaigns to verify and map the presence of Cymodocea nodosa meadows.
The record of species that are diagnostic of habitats of European concern has enabled their identification on the Central Adriatic coast. Despite the partial degradation of coastal areas, these habitats remain valuable components, serving as important seed banks for the recolonization of degraded areas and providing the natural support for their recovery. Our findings highlight the potential of CS for vegetation monitoring, a task that, in line with the Habitat Directive, is typically scheduled every 5 years. However, this time interval may be inadequate for studying highly dynamic ecosystems like dunes [97]. Citizen Science could also help indirectly to monitor the anthropogenic pressures that most affect habitat and species of conservation concern, providing essential information for their proper management.
3.3. Alien Species
We registered 109 records of 44 non-native species (Table 4 and Table 5). We specifically reported the presence of 25 alien plants (57%) and 19 species of non-native fauna (43%). It is worth noting that six of the alien plants belong to the taxonomic family Asteraceae, which is distinguished by its mostly anemophilous dispersal mechanism, making them particularly effective in the processes of expansion and colonization. The substantial number of Asteraceae species aligns well with the overall composition of alien flora in Italy [54] and Europe [98]. Instead, the number of Poaceae records appears relatively low in comparison to Italy’s alien flora. This is likely due to the difficulty in identifying these species, which poses a challenge for both regular citizens in the field and professionals who rely solely on uploaded photos.
In terms of life forms, we found a significantly higher number of perennial plants, including herbs, shrubs, and trees, than the national average [99]. This is most likely due to their extensive and long-lasting vegetative structures, which allow them to be identified all year. In contrast, we have documented fewer annuals than the national list of non-native plants [54], which can be attributed to the limited season observational window of annuals.
Two of the alien plants, namely Acacia saligna and Ailanthus altissima, have been identified as invasive species of European Union concern [53]. For these two species, the Italian government is required to undertake monitoring, management, and control measures. Acacia saligna is among the most invasive taxa in Italy and Europe, and in the Central Adriatic coast, it mostly colonises back dune habitats, impinging several habitats of conservation concern (e.g., coastal dunes with Juniperus spp., dunes with sclerophyllous vegetation of Cisto-Lavanduletalia, and wooded dunes with Pinus pinea and/or Pinus pinaster). This information is supported by the studies conducted by Marzialetti et al. [100]. Ailanthus altissima, commonly known as the Tree of Heaven, is a highly invasive plant that is present on every continent except Antarctica. Its primary distribution in its invasive range includes urban areas, from where it has expanded into neighbouring natural and semi-natural environments [101]. Ailanthus altissima provides significant negative impacts on biodiversity by altering the vegetation structure, degrading soil properties, and threatening ecosystem stability [102]. The WCC has identified the existence of several invasive species of European Union concern within the Adriatic coast’s network of protected areas. These occurrence data can be used to lead future dedicated field investigations targeted at detecting and mapping alien plants, which are required for the implementation of control or eradication methods outlined in European legislation.

Table 4.
List of non-native species, along with the number of reports, taxonomic family, and life form: “Tree” (phanerophytes); “Shrub” (nano-phanerophytes and woody climbers); “Dwarf” (chamaephytes); “Herb” (hemicryptophytes and geophytes); “Annual” (therophytes) (Sensu [103]) and geographic origin. Bold font highlights invasive alien species of European Union concern [53]. Superscript letters: NI, invasive neophytes; NC, casual neophytes; NN, naturalised neophytes; (Sensu [54]); TX, toxic.
Table 4.
List of non-native species, along with the number of reports, taxonomic family, and life form: “Tree” (phanerophytes); “Shrub” (nano-phanerophytes and woody climbers); “Dwarf” (chamaephytes); “Herb” (hemicryptophytes and geophytes); “Annual” (therophytes) (Sensu [103]) and geographic origin. Bold font highlights invasive alien species of European Union concern [53]. Superscript letters: NI, invasive neophytes; NC, casual neophytes; NN, naturalised neophytes; (Sensu [54]); TX, toxic.
| Species | No. | Family | Life Form | Origin |
|---|---|---|---|---|
| Xanthium orientale subsp. italicum (Moretti) GreuterNI | 11 | Asteraceae | Annual | North America |
| Acacia saligna (Labill.) H.L. Wendl.NI | 9 | Fabaceae | Tree | Western Australia |
| Oxalis pes-caprae L.NI | 7 | Oxalidaceae | Herb | South Africa |
| Carpobrotus acinaciformis (L.) L. BolusNI | 5 | Aizoaceae | Dwarf | South Africa |
| Carpobrotus edulis (L.) N.E. Br.NI | 5 | Aizoaceae | Dwarf | South Africa |
| Erigeron bonariensis L.NI | 3 | Asteraceae | Annual | South America |
| Opuntia stricta (Haw.) Haw.NI | 3 | Cactaceae | Shrub | Central America |
| Robinia pseudoacacia L.NI | 3 | Fabaceae | Tree | North America |
| Symphyotrichum squamatum (Spreng.) G.L. NesomNI | 3 | Asteraceae | Annual | South America |
| Ailanthus altissima (Mill.) SwingleNI | 2 | Simaroubaceae | Tree | China |
| Datura stramonium L.NI-TX | 2 | Solanaceae | Annual | Central/North Am. |
| Euphorbia maculata L.NI | 2 | Euphorbiaceae | Annual | North America |
| Oxalis articulata SavignyNI | 2 | Oxalidaceae | Herb | South America |
| Agave americana L.NI | 1 | Asparagaceae | Shrub | Central/North Am. |
| Amorpha fruticosa L.NI | 1 | Fabaceae | Shrub | North America |
| Erigeron canadensis L.NI | 1 | Asteraceae | Annual | North America |
| Helianthus annuus L.NC | 1 | Asteraceae | Annual | North America |
| Mirabilis jalapa L.NI | 1 | Nyctaginaceae | Herb | South America |
| Nicotiana glauca GrahamNI-TX | 1 | Solanaceae | Shrub | South America |
| Oenothera glazioviana MicheliNI | 1 | Onagraceae | Herb | North America |
| Opuntia ficus-indica (L.) Mill.NI | 1 | Cactaceae | Shrub | Central America |
| Paspalum dilatatum Poir.NI | 1 | Poaceae | Herb | South America |
| Pittosporum tobira (Thunb.) W.T. AitonNN | 1 | Pittosporaceae | Shrub | Eastern Asia |
| Senecio inaequidens DCNI-TX | 1 | Asteraceae | Herb | South Africa |
| Yucca gloriosa L.NI | 1 | Asparagaceae | Shrub | North America |
It is also worth noting the existence of some toxic alien plants (e.g., Datura stramonium, Senecio inaequidens, and Nicotiana glauca) [58] that pose an additional threat to human health. Following the CS approach, special campaigns for their identification might be organised, raising people’s knowledge of the risks connected with their ingestion, usage, or exposure.
The WCC recorded 40 observations of 19 alien species of fauna (Table 5). Myocastor coypus was the most recorded species, followed by Trachemys scripta. It is noteworthy that these two species, in addition to Gambusia holbrooki, are widely recognised as being highly invasive in Europe, thus warranting their inclusion in the European regulation [53].

Table 5.
List of non-native species, along with the relative number of reports (40 for fauna), taxonomic category (taxon), and geographic origin (origin). Bold font highlights invasive alien species of European Union concern [53].
Table 5.
List of non-native species, along with the relative number of reports (40 for fauna), taxonomic category (taxon), and geographic origin (origin). Bold font highlights invasive alien species of European Union concern [53].
| Species | No. | iNaturalist Taxon | Origin |
|---|---|---|---|
| Myocastor coypus (Molina, 1782) | 13 | Mammals | South America |
| Trachemys scripta (Schoepff, 1792) | 4 | Reptiles | North America |
| Hystrix cristata (Linnaeus, 1758) | 2 | Mammals | Africa |
| Magallana gigas (Thunberg, 1793) | 2 | Molluscs | Asia |
| Paysandisia archon (Burmeister, 1880) | 2 | Insects | South America |
| Rapana venosa (Valenciennes, 1846) | 2 | Molluscs | Asia |
| Sceliphron caementarium (Drury, 1773) | 2 | Insects | North America |
| Threskiornis aethiopicus (Latham, 1790) | 2 | Birds | Africa; Asia |
| Anadara transversa (Say, 1822) | 1 | Molluscs | North America |
| Blatta orientalis (Linnaeus, 1758) | 1 | Insects | Asia; Africa |
| Cairina moschata (Linnaeus, 1758) | 1 | Birds | South America |
| Complex Pelophylax ridibundus (Pallas, 1771) | 1 | Amphibians | Europe; Asia |
| Gambusia holbrooki (Girard, 1859) | 1 | Actinopterygii | North America |
| Harmonia axyridis (Pallas, 1773) | 1 | Insects | Asia |
| Isodontia mexicana (de Saussure, 1867) | 1 | Insects | North America |
| Leptoglossus occidentalis (Heidemann, 1910) | 1 | Insects | North America |
| Rhynchophorus ferrugineus (Olivier, 1790) | 1 | Insects | Asia |
| Steatoda nobilis (Thorell, 1875) | 1 | Arachnids | Macaronesia |
| Trichopoda pictipennis (Fabricius, 1781) | 1 | Insects | South America |
The coypu (Myocastor coypus) is an aquatic rodent that is native to South America and was imported to Italy for fur farming [104]. The escaped individuals tend to establish themselves preferentially along riverbanks and in wetlands, leading to detrimental effects on biological diversity, ecosystems, crops, and irrigation systems. Given the severity of this invasion and the high ecological value of the studied coasts, our data underscore the urgent need to implement control and eradication measures. A management and restriction strategy for the coypu should be implemented following a thorough assessment of its current distribution along the Central Adriatic coast [104].
The pond slider (Trachemis scripta) is a tortoise native to North America that was introduced through the pet trade. Mostly sold as small hatchlings, they are often released by owners into natural systems during their adulthood. The pond slider can establish breeding populations and competes with native species for food resources and basking sites, also exerting strong predation pressure on the biodiversity of invaded water bodies [105]. Considering this, a deeper field evaluation aiming at better defining and comprehending the ongoing invasion became critical [106]. Such updated data could be utilised to identify the proper control mechanisms claimed by the European Union and the Italian management plan [106].
4. Conclusions
Citizen Science data from the WCC project showed the promise of this strategy, which directly engages citizens in biodiversity monitoring. Citizen research projects that are supported by the iNaturalist platform have shown that they can monitor habitats and species in complex and ever-changing landscapes, like coastal–marine transitions. Existing and archived data, as well as prospective data from the next phase of the project, may give biodiversity study and management opportunities. The WCC is a pioneering programme that collects multi-taxa and multi-domain data, which may be useful for integrated biodiversity monitoring, especially in long-term observation sites like the LTER network [23] in the examined area.
The WCC submitted new and amended spatial data on various IUCN red book or HD annex species. The simultaneous analysis of the spatial distribution and consistency of several conservation-important species across the coastal gradient can help determine comprehensive habitat restoration strategies and implement specific protective measures claimed by international agreements and policies like the Convention on Biological Diversity [9] and the HD. The high number of species on the WCC, indicating the presence of multiple European habitats of conservation concern (HD), showed a wide range of ecosystems along the Central Adriatic coast, some of which are new to the area. In line with previous research by Drius et al. [4], the WCC’s coastal transition mosaic is still largely intact.
The citizen research strategy using iNaturalist has also been helpful in catching alien species across large areas at a cheap expense. Our findings suggested that CS can help implement targeted monitoring and control actions against invasive alien species, as required by European law [53]. The WCC may help identify foreign plant and animal species, thus enabling early detection and eradication. Additionally, it may help monitor invasive taxa’s regional expansion and population dynamics to ensure effective control. Lozano et al. [107] and Di Febbraro et al. [108] emphasise that the data derived from CS can improve predictions of the spread of invasive alien species, especially when used in nationwide projects.
Author Contributions
Conceptualisation, M.L.C., F.C., M.V. and M.D.F.; methodology, F.C., M.V. and M.D.F.; data collection: F.C., M.V., A.S. and M.C.d.F.; software, M.D.F.; formal analysis, F.C. and M.V.; investigation, F.C., M.V., M.I., M.D.F., A.L., A.S., G.M. and M.L.C.; resources, M.L.C. and A.S.; data curation, F.C. and M.V.; writing—original draft preparation, F.C., M.V., M.I. and M.L.C.; writing—review and editing, F.C., M.V., M.I., M.D.F., A.L., A.S., G.M. and M.L.C.; visualisation, F.C., M.V. and M.I.; supervision, M.L.C., A.S., A.L., M.D.F. and M.I.; project administration, M.L.C. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was partially supported by the INTERREG V-A IT-HR CBC Program (CoAStal and marine Waters Integrated Monitoring Systems for Ecosystems Protection and Management—CASCADE—ID: 10255941) and by LIFE17 NAT/IT CALLIOPE (“Coastal dune hAbitats, subLittoraL sandbanks, marIne reefs: cOnservation, Protection, and thrEats mitigation) grant number 000565.
Data Availability Statement
Data are available on iNaturalist platform (https://www.inaturalist.org/projects/wild-coast-cascade (accessed on 1 August 2023).
Acknowledgments
Thanks to the INPS (National Institute of Social Security) for a PhD scholarship and the LTER network (Long-Term Ecological Research). Thanks to the Italian Ministry of University and Research (MIUR) for supporting a Researcher contract on Green axes. We are thankful to the Basso Molise Association, the Marine Protected Area Torre del Cerrano, the Abruzzo Institute for Protected Areas and Legambiente for their valuable support in involving students, local people, and tourists in Citizen Science activities. Thank you to Denis Carlucci for his collaboration and support. We are also grateful to the reviewers and to the editor for helping us improve this manuscript. Finally, we would like to sincerely thank all the citizens and iNaturalist members who contributed to the success of the project by uploading their observations and/or contributing to the recognition of taxa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walker, S.; Wilson, J.B.; Steel, J.B.; Rapson, G.L.; Smith, B.; King, W.M.; Cottam, Y.H. Properties of ecotones: Evidence from five ecotones objectively determined from a coastal vegetation gradient. J. Veg. Sci. 2003, 14, 579–590. [Google Scholar] [CrossRef]
- Martínez, M.L.; Psuty, N.P.; Lubke, R.A. A Perspective on Coastal Dunes. In Coastal Dunes; Martínez, M.L., Psuty, N.P., Eds.; Ecology and Conservation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–10. [Google Scholar] [CrossRef]
- Carpenter, S.R. Ecosystems and Human Well-Being: Scenarios; Findings of the Scenarios Working Group; Island Press: Washington, DC, USA, 2005; Volume 2. [Google Scholar]
- Drius, M.; Jones, L.; Marzialetti, F.; De Francesco, M.C.; Stanisci, A.; Carranza, M.L. Not just a sandy beach. The multi-service value of Mediterranean coastal dunes. Sci. Total. Environ. 2019, 668, 1139–1155. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Dugan, J.; Schoeman, D.S.; Lastra, M.; Jones, A.; Scapini, F.; McLachlan, A.; Defeo, O. Sandy beaches at the brink. Divers. Distrib. 2007, 13, 556–560. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Hesp, P.A.; Martínez, M.L. Disturbance Processes and Dynamics in Coastal Dunes. In Disturbance Ecology: The Process and the Response; Johnson, E.A., Miyanishi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 215–247. [Google Scholar] [CrossRef]
- Malavasi, M.; Bartak, V.; Carranza, M.L.; Simova, P.; Acosta, A.T.R. Landscape pattern and plant biodiversity in Mediterranean coastal dune ecosystems: Do habitat loss and fragmentation really matter? J. Biogeogr. 2018, 45, 1367–1377. [Google Scholar] [CrossRef]
- CBD 1992. Convention on Biological Diversity. Available online: http://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 1 August 2023).
- Gigante, D.; Acosta, A.T.R.; Agrillo, E.; Armiraglio, S.; Assini, S.; Attorre, F.; Bagella, S.; Buffa, G.; Casella, L.; Giancola, C.; et al. Habitat conservation in Italy: The state of the art in the light of the first European Red List of Terrestrial and Freshwater Habitats. Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 251–265. [Google Scholar] [CrossRef]
- La Mesa, G.; Paglialonga, A.; Tunesi, L. Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE e Direttiva 09/147/CE) in Italia: Ambiente Marino; ISPRA, Serie Manuali e Linee Guida: Rome, Italy, 2019.
- Goldsmith, G.R. The field guide, rebooted Identifying species in the field? There’s an app for that. Science 2015, 349, 594. [Google Scholar] [CrossRef]
- Ellwood, E.R.; Crimmins, T.M.; Miller-Rushing, A.J. Citizen science and conservation: Recommendations for a rapidly moving field. Biol. Conserv. 2017, 208, 1–4. [Google Scholar] [CrossRef]
- Maccherini, S.; Bacaro, G.; Tordoni, E.; Bertacchi, A.; Castagnini, P.; Foggi, B.; Gennai, M.; Mugnai, M.; Sarmati, S.; Angiolini, C. Enough Is Enough? Searching for the Optimal Sample Size to Monitor European Habitats: A Case Study from Coastal Sand Dunes. Diversity 2020, 12, 138. [Google Scholar] [CrossRef]
- Marzialetti, F.; Giulio, S.; Malavasi, M.; Sperandii, M.G.; Acosta, A.T.R.; Carranza, M.L. Capturing Coastal Dune Natural Vegetation Types Using a Phenology-Based Mapping Approach: The Potential of Sentinel-2. Remote Sens. 2019, 11, 1506. [Google Scholar] [CrossRef]
- Silvertown, J. A new dawn for citizen science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, R.; Antoniou, V.; Hummer, P.; Potsiou, C. Citizen Science in the Digital World of Apps. In The Science of Citizen Science; Vohland, K., Land-Zandstra, A., Ceccaroni, L., Lemmens, R., Perelló, J., Ponti, M., Samson, R., Wagenknecht, K., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Peter, M.; Diekötter, T.; Kremer, K. Participant Outcomes of Biodiversity Citizen Science Projects: A Systematic Literature Review. Sustainability 2019, 11, 2780. [Google Scholar] [CrossRef]
- Peter, M.; Diekötter, T.; Höffler, T.; Kremer, K. Biodiversity citizen science: Outcomes for the participating citizens. People Nat. 2021, 3, 294–311. [Google Scholar] [CrossRef]
- Conrad, C.C.; Hilchey, K.G. A review of citizen science and community-based environmental monitoring: Issues and opportunities. Environ. Monit. Assess. 2010, 176, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Steger, C.; Butt, B.; Hooten, M.B. Safari Science: Assessing the reliability of citizen science data for wildlife surveys. J. Appl. Ecol. 2017, 54, 2053–2062. [Google Scholar] [CrossRef]
- de Groot, M.; Pocock, M.J.O.; Bonte, J.; Fernandez-Conradi, P.; Valdés-Correcher, E. Citizen Science and Monitoring Forest Pests: A Beneficial Alliance? Curr. For. Rep. 2022, 9, 15–32. [Google Scholar] [CrossRef]
- Bergami, C.; Campanaro, A.; Davis, C.; L’astorina, A.; Pugnetti, A.; Oggioni, A. Environmental citizen science practices in the ILTER community: Remarks from a case study at global scale. Front. Environ. Sci. 2023, 11, 1130020. [Google Scholar] [CrossRef]
- L’astorina, A.; Davis, C.; Pugnetti, A.; Campanaro, A.; Oggioni, A.; Bergami, C. Scientists’ attitudes about citizen science at Long-Term Ecological Research (LTER) sites. Front. Environ. Sci. 2023, 11, 1130022. [Google Scholar] [CrossRef]
- Mwango’mbe, M.G.; Spilsbury, J.; Trott, S.; Nyunja, J.; Wambiji, N.; Collins, T.; Gomes, I.; Pérez-Jorge, S. Cetacean Research and Citizen Science in Kenya. Front. Mar. Sci. 2021, 8, 642399. [Google Scholar] [CrossRef]
- Rosa, R.M.; Cavallari, D.C.; Salvador, R.B. iNaturalist as a tool in the study of tropical molluscs. PLoS ONE 2022, 17, e0268048. [Google Scholar] [CrossRef]
- Seregin, A.P.; Bochkov, D.A.; Shner, J.V.; Garin, E.V.; Pospelov, I.N.; Prokhorov, V.E.; Golyakov, P.V.; Mayorov, S.R.; Svirin, S.A.; Khimin, A.N.; et al. “Flora of Russia” on iNaturalist: A dataset. Biodivers. Data J. 2020, 8, e59249. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.V.R.; Bessa, E. Dolphin conservation can profit from tourism and Citizen science. Environ. Dev. 2019, 32, 100467. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Somveille, M. Survey completeness of a global citizen-science database of bird occurrence. Ecography 2020, 43, 34–43. [Google Scholar] [CrossRef]
- Aristeidou, M.; Herodotou, C.; Ballard, H.L.; Young, A.N.; Miller, A.E.; Higgins, L.; Johnson, R.F. Exploring the participation of young citizen scientists in scientific research: The case of iNaturalist. PLoS ONE 2021, 16, e0245682. [Google Scholar] [CrossRef]
- Echeverria, A.; Ariz, I.; Moreno, J.; Peralta, J.; Gonzalez, E.M. Learning Plant Biodiversity in Nature: The Use of the Citizen–Science Platform iNaturalist as a Collaborative Tool in Secondary Education. Sustainability 2021, 13, 735. [Google Scholar] [CrossRef]
- Forti, L.R. Students as citizen scientists: Project-based learning through the iNaturalist platform could provide useful biodiversity data. Biodiversity 2023, 24, 76–78. [Google Scholar] [CrossRef]
- Wilson, J.S.; Pan, A.D.; General, D.E.M.; Koch, J.B. More eyes on the prize: An observation of a very rare, threatened species of Philippine Bumble bee, Bombus irisanensis, on iNaturalist and the importance of citizen science in conservation biology. J. Insect Conserv. 2020, 24, 727–729. [Google Scholar] [CrossRef]
- Barbato, D.; Benocci, A.; Guasconi, M.; Manganelli, G. Light and shade of citizen science for less charismatic invertebrate groups: Quality assessment of iNaturalist nonmarine mollusc observations in central Italy. J. Molluscan Stud. 2021, 87, eyab033. [Google Scholar] [CrossRef]
- Hiller, T.; Haelewaters, D. A case of silent invasion: Citizen science confirms the presence of Harmonia axyridis (Coleoptera, Coccinellidae) in Central America. PLoS ONE 2019, 14, e0220082. [Google Scholar] [CrossRef]
- Dimson, M.; Fortini, L.B.; Tingley, M.W.; Gillespie, T.W. Citizen science can complement professional invasive plant surveys and improve estimates of suitable habitat. Divers. Distrib. 2023, 29, 1141–1156. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Ozeroff, I.; Hitchcock, C.; Chandler, M. Capitalizing on opportunistic citizen science data to monitor urban biodiversity: A multi-taxa framework. Biol. Conserv. 2020, 251, 108753. [Google Scholar] [CrossRef]
- Mesaglio, T.; Callaghan, C.T. An overview of the history, current contributions and future outlook of iNaturalist in Australia. Wildl. Res. 2021, 48, 289–303. [Google Scholar] [CrossRef]
- Gorta, S.B.Z.; Callaghan, C.T.; Samonte, F.; Ooi, M.K.J.; Mesaglio, T.; Laffan, S.W.; Cornwell, W.K. Multi-taxon biodiversity responses to the 2019–2020 Australian megafires. Glob. Chang. Biol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- iNaturalist. Available online: http://www.inaturalist.org (accessed on 30 September 2023).
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Bowser, A.; Wiggins, A.; Shanley, L.; Preece, J.; Henderson, S. Sharing data while protecting privacy in citizen science. Interactions 2014, 21, 70–73. [Google Scholar] [CrossRef]
- Miccadei, E.; Mascioli, F.; Piacentini, T.; Ricci, F. Geomorphological Features of Coastal Dunes along the Central Adriatic Coast (Abruzzo, Italy). J. Coast. Res. 2011, 277, 1122–1136. [Google Scholar] [CrossRef]
- Drius, M.; Carranza, M.L.; Stanisci, A.; Jones, L. The role of Italian coastal dunes as carbon sinks and diversity sources. A multi-service perspective. Appl. Geogr. 2016, 75, 127–136. [Google Scholar] [CrossRef]
- Di Paola, G.; Amodio, A.M.; Dilauro, G.; Rodriguez, G.; Rosskopf, C.M. Shoreline Evolution and Erosion Vulnerability Assessment along the Central Adriatic Coast with the Contribution of UAV Beach Monitoring. Geosciences 2022, 12, 353. [Google Scholar] [CrossRef]
- Capotondi, L.; Ravaioli, M.; Acosta, A.; Chiarini, F.; Lami, A.; Stanisci, A.; Tarozzi, L.; Mazzocchi, M.G. (Eds.) La Rete Italiana per la Ricerca Ecologica di Lungo Termine. Lo Studio della Biodiversità e dei Cambiamenti; CNR Edizioni: Roma, Italy, 2011; ISBN 978-88-8080-214-3. [Google Scholar]
- IUCN. Red List of Threatened Species. Available online: http://www.iucnredlist.org (accessed on 30 September 2023).
- Rondinini, C.; Battistoni, A.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani 2022; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Roma, Italy, 2022. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Santoro, R.; Carboni, M.; Carranza, M.L.; Acosta, A.T.R. Focal species diversity patterns can provide diagnostic information on plant invasions. J. Nat. Conserv. 2012, 20, 85–91. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2014, 148, 728–814. [Google Scholar] [CrossRef]
- European Commission DG Enviroment. Interpretation Manual of European Union Habitats. [Eur 28.Nature ENV B.3]. 2013. Available online: https://eunis.eea.europa.eu/references/2435 (accessed on 30 September 2023).
- EUR-Lex Access to European Union Law. Available online: http://data.europa.eu/eli/reg_impl/2022/1203/oj (accessed on 30 September 2023).
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Cozzi, B.; et al. Mammals of Italy: An annotated checklist. Hystrix It. J. Mamm. 2019, 30, 87–106. [Google Scholar] [CrossRef]
- Bartolucci, F.; Galasso, G.; Peruzzi, L.; Conti, F. Report 2020 on plant biodiversity in Italy: Native and alien vascular flora. Nat. Hist. Sci. 2021, 8, 41–54. [Google Scholar] [CrossRef]
- Bartolucci, F.; Galasso, G.; Peruzzi, L.; Conti, F. Report 2021 on plant biodiversity in Italy: Native and alien vascular flora. Nat. Hist. Sci. 2022, 10, 41–50. [Google Scholar] [CrossRef]
- Mazza, G.; Tricarico, E. Invasive Species and Human Health; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Aristeidou, M.; Herodotou, C.; Ballard, H.L.; Higgins, L.; Johnson, R.F.; Miller, A.E.; Young, A.N.; Robinson, L.D. How Do Young Community and Citizen Science Volunteers Support Scientific Research on Biodiversity? The Case of iNaturalist. Diversity 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Casale, P.; Broderick, A.C.; Camiñas, J.A.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.J.; Godley, B.J.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea turtles: Current knowledge and priorities for conservation and research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Gregory, R.D.; Eaton, M.A.; Burfield, I.J.; Grice, P.V.; Howard, C.; Klvaňová, A.; Noble, D.; Šilarová, E.; Staneva, A.; Stephens, P.A.; et al. Drivers of the changing abundance of European birds at two spatial scales. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220198. [Google Scholar] [CrossRef]
- Acosta, A.; Blasi, C.; Carranza, M.L.; Ricotta, C.; Stanisci, A. Quantifying ecological mosaic connectivity and hemeroby with a new topoecological index. Phytocoenologia 2003, 33, 623–631. [Google Scholar] [CrossRef]
- Degli, E.I.; Defeo, O.; Scapini, F. Arthropodofauna richness and abundance across beach–dune systems with contrasting morphodynamics. Reg. Stud. Mar. Sci. 2021, 44, 101722. [Google Scholar] [CrossRef]
- Hochmair, H.H.; Scheffrahn, R.H.; Basille, M.; Boone, M. Evaluating the data quality of iNaturalist termite records. PLoS ONE 2020, 15, e0226534. [Google Scholar] [CrossRef]
- Koo, K.-S.; Oh, J.-M.; Park, S.-J.; Im, J.-Y. Accessing the Accuracy of Citizen Science Data Based on iNaturalist Data. Diversity 2022, 14, 316. [Google Scholar] [CrossRef]
- Campbell, C.J.; Barve, V.; Belitz, M.W.; Doby, R.J.; White, E.; Seltzer, C.; Di Cecco, G.; Hurlbert, A.H.; Guralnick, R. Identifying the identifiers: How iNaturalist facilitates collaborative, research-relevant data generation and why it matters for biodiversity science. BioScience 2023, 73, 533–541. [Google Scholar] [CrossRef]
- Pinna nobilis. The IUCN Red List of Threatened Species 2019. Available online: https://www.iucnredlist.org/species/160075998/160081499 (accessed on 11 August 2023).
- Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Feidantsis, K.; Georgoulis, I.; Karagiannis, D.; Carella, F.; Michaelidis, B. Investigation of the highly endangered Pinna nobilis’ mass mortalities: Seasonal and temperature patterns of health status, antioxidant and heat stress responses. Mar. Environ. Res. 2023, 188, 105977. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Álvarez, E.; Belando, M.D.; Deudero, S.; Catanese, G.; Bernardeau-Esteller, J.; García-Muñoz, R.; Ramos-Segura, A.; Ruiz, J.M.; Vázquez-Luis, M. Living under threat: Will one of the last Pinna nobilis populations be able to survive? Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1–13. [Google Scholar] [CrossRef]
- Corti, C.; Bassu, L.; Biaggini, M.; Bressi, N.; Capula, M.; Di Cerbo, A.R.; Vanni, S. Updated distribution of Testudo hermanni hermanni in Italy. In Proceedings of the International Workshop on the Management and Restoration of Hermann’s Tortoise Habitats and Populations, Soptom, Gonfaron, France, 18–20 September 2013. [Google Scholar]
- Berardo, F.; Carranza, M.L.; Frate, L.; Stanisci, A.; Loy, A. Seasonal habitat preference by the flagship species Testudo hermanni: Implications for the conservation of coastal dunes. Comptes Rendus Biol. 2015, 338, 343–350. [Google Scholar] [CrossRef]
- Cheylan, M.; Corti, C.; Carpaneto, G.M.; Mazzotti, S.; Zuffi, M.A.L. Testudo hermanni Gmelin, 1789. In Fauna d’Italia; Capula, M., Corti, C., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Reptilia, Edizioni Calderini: Bologna, Italy, 2011; Volume XLV, pp. 188–199. [Google Scholar]
- Seminoff, J.A.; Allen, C.D.; Balazs, G.H.; Dutton, P.H.; Eguchi, T.; Haas, H.L.; Hargrove, S.A.; Jensen, M.P.; Klemm, D.L.; Lauritsen, A.M.; et al. Green Turtle (Chelonia mydas) Status Review under the U.S. Endangered Species Act; NOAA Technical Memorandum, NOAA-NMFS-SWFSC: La Jolla, CA, USA, 2015; pp. 539–571.
- Chelonia mydas. The IUCN Red List of Threatened Species 2004. Available online: https://www.iucnredlist.org/species/pdf/11037468/attachmenten (accessed on 11 August 2023).
- IUCN Liste Rosse Italiane. Available online: https://www.iucn.it/liste-rosse-italiane.php (accessed on 30 September 2023).
- Casado de Amezua, P.; Kersting, D.; Linares, C.L.; Bo, M.; Caroselli, E.; Garrabou, J.; Cerrano, C.; Ozalp, B.; Terrón-Sigler, A.; Betti, F. Cladocora caespitosa. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2015. [Google Scholar] [CrossRef]
- Roveta, C.; Coppari, M.; Calcinai, B.; Di Camillo, C.G.; Marrocco, T.; Mantas, T.P.; Puce, S.; Torsani, F.; Valisano, L.; Cerrano, C. What’s the key for success? Translocation, growth and thermal stress mitigation in the Mediterranean coral Cladocora caespitosa (Linnaeus, 1767). Front. Mar. Sci. 2023, 10, 1199048. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Neururer, C.; Pisapia, C.; Spezzaferri, S. Correlation between pollution and decline of Scleractinian Cladocora caespitosa (Linnaeus, 1758) in the Gulf of Gabes. Heliyon 2016, 2, e00195. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.K.; Cebrian, E.; Casado, C.; Teixidó, N.; Garrabou, J.; Linares, C. Experimental evidence of the synergistic effects of warming and invasive algae on a temperate reef-builder coral. Sci. Rep. 2015, 5, 18635. [Google Scholar] [CrossRef]
- Kružić, P.; Požar-Domac, A. Impact of tuna farming on the banks of the coral Cladocora caespitosa in the Adriatic Sea. Coral Reefs 2007, 26, 665. [Google Scholar] [CrossRef]
- Cladocora caespitosa. The IUCN Red List of Threatened Species 2015. Available online: https://www.iucnredlist.org/species/133142/165739749 (accessed on 11 August 2023).
- Garofalo, L.; Mastrogiacomo, A.; Casale, P.; Carlini, R.; Eleni, C.; Freggi, D.; Gelli, D.; Knittweis, L.; Mifsud, C.; Mingozzi, T.; et al. Genetic characterization of central Mediterranean stocks of the loggerhead turtle (Caretta caretta) using mitochondrial and nuclear markers, and conservation implications. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 868–884. [Google Scholar] [CrossRef]
- Giacoma, C.; Balletto, E.; Bentivegna, F.; Guarino, F.M.; Hochscheid, S.; Maio, N.; Mingozzi, A.T.; Piovano & Scaravelli, D. Caretta caretta (Linnaeus, 1758). In Fauna d’Italia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Reptilia: Edizioni Calderini: Bologna, Italy, 2011; Volume XLV. [Google Scholar]
- Di Renzo, L.; Mascilongo, G.; Berti, M.; Bogdanović, T.; Listeš, E.; Brkljača, M.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Olivieri, V.; et al. Potential Impact of Microplastics and Additives on the Health Status of Loggerhead Turtles (Caretta caretta) Stranded along the Central Adriatic Coast. Water Air Soil Pollut. 2021, 232, 98. [Google Scholar] [CrossRef]
- Fontaine, A.; Simard, A.; Brunet, N.; Elliott, K.H. Scientific contributions of citizen science applied to rare or threatened animals. Conserv. Biol. 2022, 36, e13976. [Google Scholar] [CrossRef] [PubMed]
- Stanisci, A.; Acosta, A.T.R.; Carranza, M.L.; de Chiro, M.; Del Vecchio, S.; Di Martino, L.; Frattaroli, A.R.; Fusco, S.; Izzi, C.F.; Pirone, G.; et al. EU habitats monitoring along the coastal dunes of the LTER sites of Abruzzo and Molise (Italy). Plant Sociol. 2014, 51, 51–56. [Google Scholar] [CrossRef]
- de Francesco, M.C.; Cerrano, C.; Pica, D.; D’Onofrio, D.; Stanisci, S. Characterization of Teatina Coast Marine Habitats (Central Adriatic Sea) toward an Integrated Coastal Management. Oceanogr. Fish. Open Access J. 2017, 5, 7–10. [Google Scholar] [CrossRef]
- de Francesco, M.C.; Tozzi, F.P.; Buffa, G.; Fantinato, E.; Innangi, M.; Stanisci, A. Identifying Critical Thresholds in the Impacts of Invasive Alien Plants and Dune Paths on Native Coastal Dune Vegetation. Land 2022, 12, 135. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; et al. Manuale Italiano di Interpretazione degli Habitat della Direttiva 92/43/CEE; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Direzione per la Protezione della Natura: Rome, Italy, 2009; pp. 1–16.
- Carranza, M.L.; Drius, M.; Malavasi, M.; Frate, L.; Stanisci, A.; Acosta, A.T.R. Assessing land take and its effects on dune carbon pools. An insight into the Mediterranean coastline. Ecol. Indic. 2018, 85, 951–955. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Leone, V.; Moya, D.; Lovreglio, R.; Delipetrou, P.; de las Heras, J. Management of threatened, high conservation value, forest hotspots under changing fire regimes. In Post-Fire Management and Restoration of Southern European Forests, Managing Forest Ecosystems; Moreira, F., Arianoutsou, M., Corona, P., de las Heras, J., Eds.; Springer: Amsterdam, The Netherlands, 2012; pp. 257–291. [Google Scholar]
- Adam, P. Salt marsh restoration. In Coastal Wetlands, 2nd ed.; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 817–861. [Google Scholar] [CrossRef]
- Tozzi, F.P.; Varricchione, M.; de Francesco, M.C.; Carranza, M.L.; Stanisci, A. Vegetation Dynamics on a Restored salt Marsh Mosaic: A Re-Visitation Study in a Coastal Wetland in Central Italy. Wetlands 2022, 42, 101. [Google Scholar] [CrossRef]
- Perennou, C.; Gaget, E.; Galewski, T.; Geijzendorffer, I.; Guelmami, A. Evolution of wetlands in Mediterranean region. In Water Resources in the Mediterranean Region; Zribi, M., Brocca, L., Tramblay, Y., Molle, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 297–320. [Google Scholar]
- Casas, E.; Martín-García, L.; Otero-Ferrer, F.; Tuya, F.; Haroun, R.; Arbelo, M. Economic mapping and assessment of Cymodocea nodosa meadows as nursery grounds for commercially important fish species. A case study in the Canary Islands. One Ecosyst. 2021, 6, e70919. [Google Scholar] [CrossRef]
- Da Ros, Z.; Corinaldesi, C.; Dell’Anno, A.; Gambi, C.; Torsani, F.; Danovaro, R. Restoration of Cymodocea nodosa seagrass meadows: Efficiency and ecological implications. Restor. Ecol. 2021, 29, e13313. [Google Scholar] [CrossRef]
- Angelini, P.; Casella, L.; Grignetti, A.; Genovesi, P. Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Habitat; ISPRA Serie Manuali e Linee Guida: Rome, Italy, 2016.
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef] [PubMed]
- Marzialetti, F.; Frate, L.; De Simone, W.; Frattaroli, A.R.; Acosta, A.T.R.; Carranza, M.L. Unmanned Aerial Vehicle (UAV)-Based Mapping of Acacia saligna Invasion in the Mediterranean Coast. Remote Sens. 2021, 13, 3361. [Google Scholar] [CrossRef]
- Espenschied-Reilly, A.L.; Runkle, J.R. Distribution and changes in abundance of Ailanthus altissima (Miller) Swingle in a Southwest Ohio Woodlot. Ohio J. Sci. 2008, 108, 16–22. [Google Scholar]
- Castro-Díez, P.; Fierro-Brunnenmeister, N.; González-Muñoz, N.; Gallardo, A. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 2011, 350, 179–191. [Google Scholar] [CrossRef]
- Guarino, R.; Chytrý, M.; Attorre, F.; Landucci, F.; Marcenò, C. Alien plant invasions in Mediterranean habitats: An assessment for Sicily. Biol. Invasions 2021, 23, 3091–3107. [Google Scholar] [CrossRef]
- Cocchi, R.; Bertolino, S. Piano di Gestione Nazionale della Nutria (Myocastor coypus); Manuali ISPRA: Rome, Italy, 2020.
- Standfuss, B.; Lipovšek, G.; Fritz, U.; Vamberger, M. Threat or fiction: Is the pond slider (Trachemys scripta) really invasive in Central Europe? A case study from Slovenia. Conserv. Genet. 2016, 17, 557–563. [Google Scholar] [CrossRef]
- Macchi, S.; Scali, S.; Bisi, F.; Martinoli, A.; Alonzi, A.; Carnevali, L. Piano Nazionale per la Gestione della Testuggine Palustre Americana (Trachemys scripta); Manuali ISPRA: Rome, Italy, 2020.
- Lozano, V.; Di Febbraro, M.; Brundu, G.; Carranza, M.L.; Alessandrini, A.; Ardenghi, N.M.G.; Barni, E.; Bedini, G.; Celesti-Grapow, L.; Cianfaglione, K.; et al. Plant invasion risk inside and outside protected areas: Propagule pressure, abiotic and biotic factors definitively matter. Sci. Total. Environ. 2023, 877, 162993. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Bosso, L.; Fasola, M.; Santicchia, F.; Aloise, G.; Lioy, S.; Tricarico, E.; Ruggieri, L.; Bovero, S.; Mori, E.; et al. Different facets of the same niche: Integrating citizen science and scientific survey data to predict biological invasion risk under multiple global change drivers. Glob. Chang. Biol. 2023, 29, 5509–5523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).