Conservation Responsibility for Priority Habitats under Future Climate Conditions: A Case Study on Juniperus drupacea Forests in Greece
Abstract
:1. Introduction
2. Materials and Methods
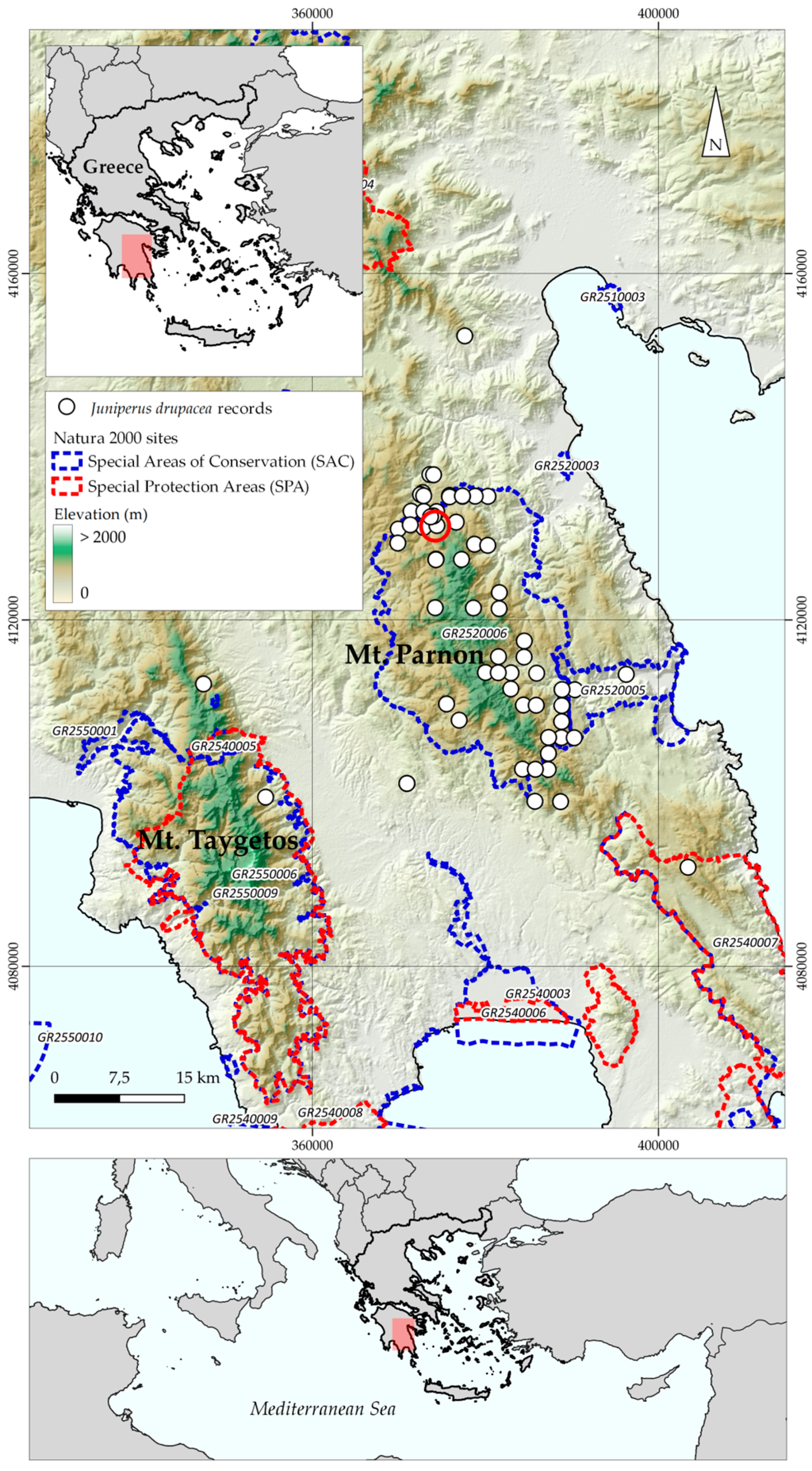
2.1. Species Occurrence Data
2.2. Environmental Data
2.3. Species Distribution Models
2.4. Future IUCN Extinction Risk Assessment
2.5. Sensitivity, Exposure, and Vulnerability to Climate and Land-Use Change
2.6. Fire Danger Impact Assessment
3. Results
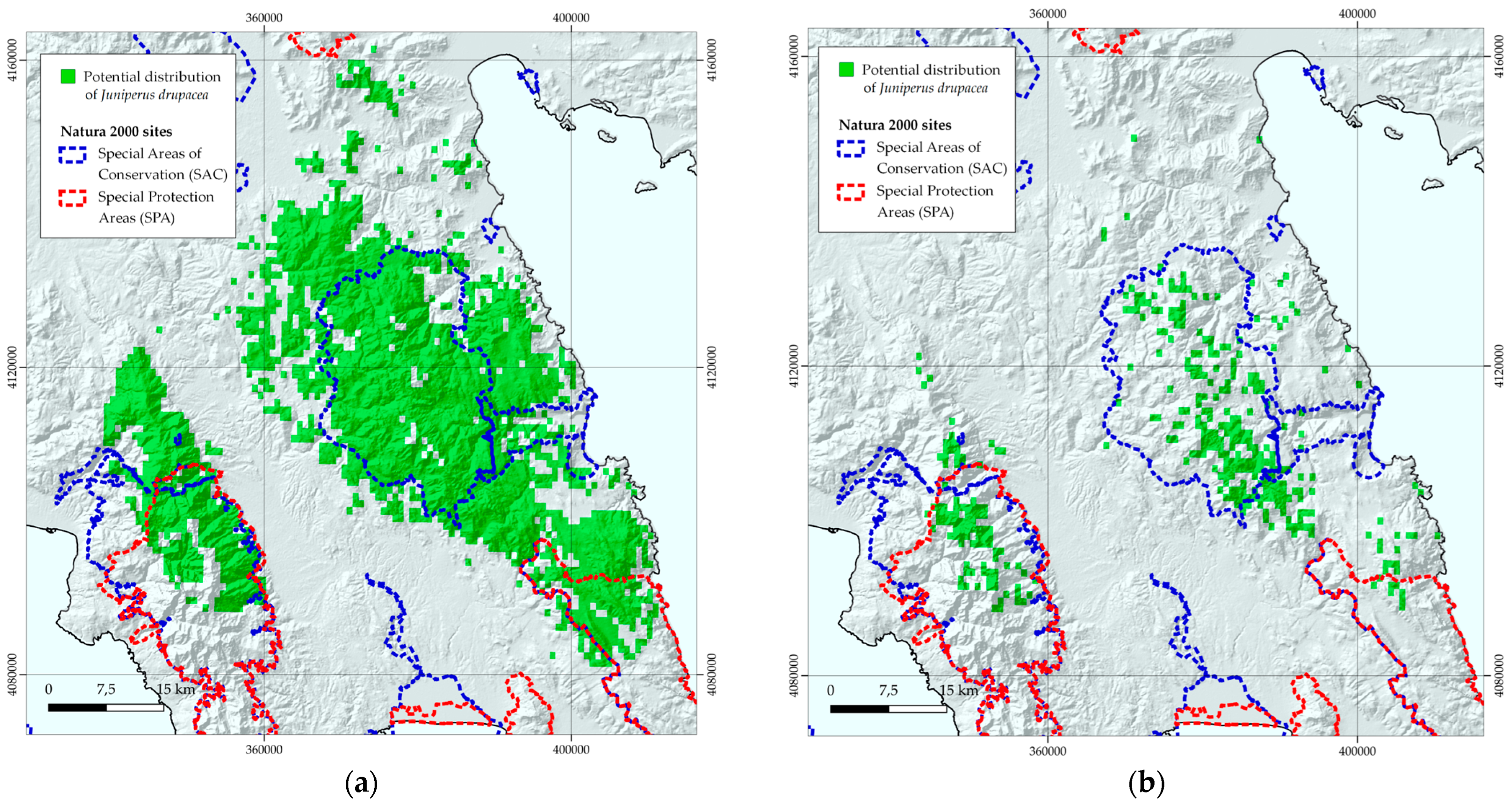
3.1. Species Distribution Models
3.2. Habitat Suitability Range Change
3.3. IUCN Extinction Risk Assessment
- Vulnerable, under the IUCN Criterion A assessment;
- Least Concern or Near Threatened, under the IUCN Criterion B assessment;
- Vulnerable, under the combined IUCN Criteria A and B assessment.
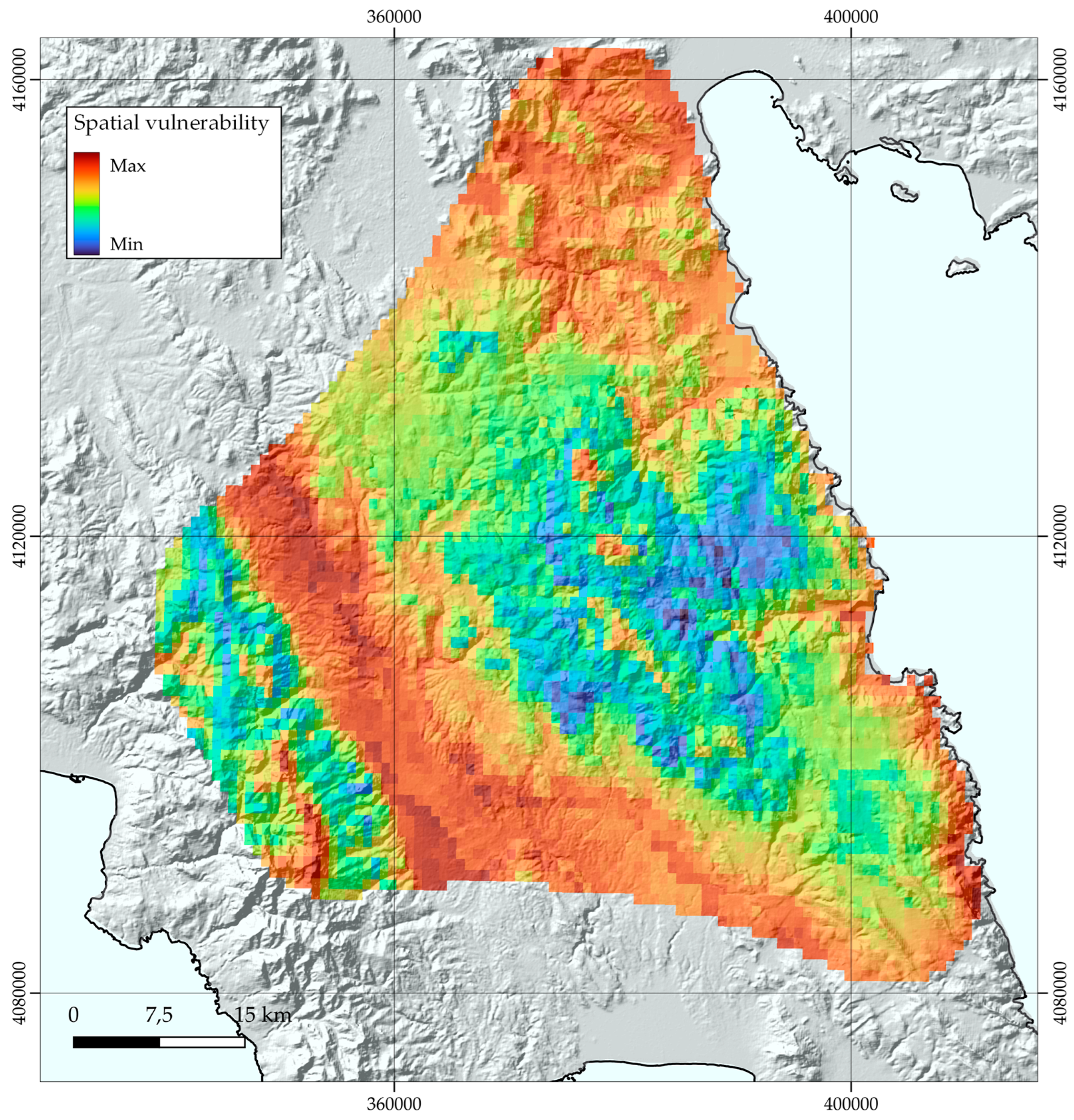
3.4. Sensitivity, Exposure, and Vulnerability to Climate and Land-Use Change
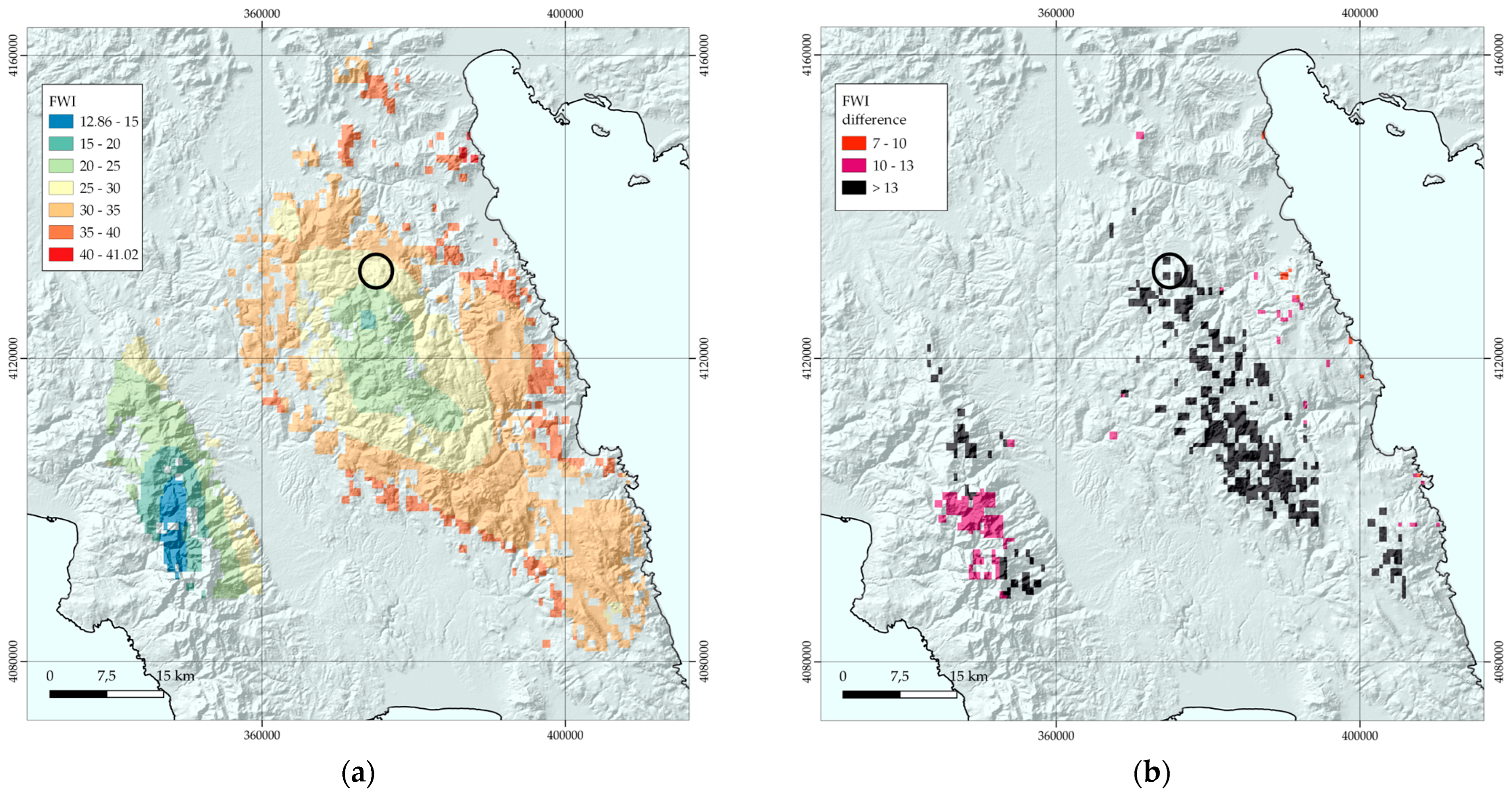
3.5. Wildfire Risk Assessment
- The FWI values range from 12.86 to 41.02, with a mean value of 29.43, for the total area of current potential distribution;
- 57% of the area has a Fire Weather Index (FWI) greater than 30;
- 16% of the area has an FWI exceeding 35;
- Areas with higher FWI values are in Mt Parnon and its wider area (Figure 5);
- In the strictly protected area (Preserved Monument of Nature), FWI values range from 27 to 29.
4. Discussion
4.1. Management Implications
4.2. Protection from Fire
4.3. Awareness Raising
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Commission of the European Union Natura. 2000. Available online: https://ec.europa.eu/environment/nature/natura2000/index_en.htm (accessed on 20 September 2023).
- Charalampopoulos, I.; Droulia, F.; Kokkoris, I.P.; Dimopoulos, P. Future Bioclimatic Change of Agricultural and Natural Areas in Central Europe: An Ultra-High Resolution Analysis of the De Martonne Index. Water 2023, 15, 2563. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial Phylogenetics, Biogeographical Patterns and Conservation Implications of the Endemic Flora of Crete (Aegean, Greece) under Climate Change Scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-change impacts on the southernmost mediterranean arctic-alpine plant populations. Sustainability 2021, 13, 13778. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; Mcguire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Communities (CEC). European Commission Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities 1992, 206, 7–50. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant endemism centres and biodiversity hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef]
- Stévart, T.; Dauby, G.; Lowry, P.P.; Blach-Overgaard, A.; Droissart, V.; Harris, D.J.; Mackinder, B.A.; Schatz, G.E.; Sonké, B.; Sosef, M.S.M.; et al. A third of the tropical African flora is potentially threatened with extinction. Sci. Adv. 2019, 5, eaax9444. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Bergmeier, E.; Fischer, P. Natura 2000 Habitat Types of Greece Evaluated in the Light of Distribution, Threat and Responsibility. Biol. Environ. Proc. R. Ir. Acad. B 2006, 106, 175–187. [Google Scholar] [CrossRef]
- Biodiversity Information System for Europe: Greece. Available online: https://biodiversity.europa.eu/countries/greece (accessed on 25 September 2023).
- Boratynski, A.; Browicz, K.; Zielinski, J. Chorology of Trees and Shrubs in Greece; Institute of Dendrology, Polish Academy of Sciences: Kornik, Poland, 1992; ISBN 83-85599-04-5. [Google Scholar]
- Constantinidis, T.; Kalpoutzakis, E. Plant Guide to Mount Parnon and Moustos Wetland Protected Area: Endemic, Rare and Threatened Species; Management Body Mount Parnon: Moustos Wetland, Greece, 2015; ISBN 960-99424-4-X. [Google Scholar]
- Bergmeier, E. Plant Communities and Habitat Differentiation in the Mediterranean Coniferous Woodlands of Mt. Parnon (Greece). Folia Geobot. 2002, 37, 309–331. [Google Scholar] [CrossRef]
- Presidential Decree οn the declaration of historic and ancient trees or forest areas of exceptional importance as protected natural monuments. Hell. Gov. Gazzette 121 D 1980, 1417–1464.
- Ministry of Agriculture Juniperus drupacea (Europe assessment). The IUCN Red List of Threatened Species. Hell. Gov. Gazzette 1980, 1417–1464. [Google Scholar]
- Charalampopoulos, I.; Droulia, F.; Tsiros, I.X. Projecting Bioclimatic Change over the South-Eastern European Agricultural and Natural Areas via Ultrahigh-Resolution Analysis of the de Martonne Index. Atmosphere 2023, 14, 858. [Google Scholar] [CrossRef]
- Ioannidis, K.; Tomprou, I.; Panayiotopoulou, D.; Boutsios, S.; Daskalakou, E.N. Potential and Constraints on In Vitro Micropropagation of Juniperus drupacea Labill. Forests 2023, 14, 142. [Google Scholar] [CrossRef]
- Avramidou, E.V.; Korakaki, E.; Malliarou, E.; Boutsios, S. Studying the Genetic and the Epigenetic Diversity of the Endangered Species Juniperus drupacea Labill. towards Safeguarding Its Conservation in Greece. Forests 2023, 14, 1271. [Google Scholar] [CrossRef]
- Solomou, A.D.; Korakaki, E.; Avramidou, E.V.; Boutsios, S.; Oikonomidis, S.; Daskalakou, E.; Singh Bargali, S.; Solomou, A.D.; Korakaki, E.; Avramidou, E.V.; et al. Assessment of Herbaceous Plant Composition, Diversity, and Indicator Species in the Juniperus drupacea Forest Openings of the Mountain Parnonas in Greece. Sustainability 2023, 15, 13765. [Google Scholar] [CrossRef]
- Walas, Ł.; Sobierajska, K.; Ok, T.; Dönmez, A.A.; Kanoğlu, S.S.; Dagher-Kharrat, M.B.; Douaihy, B.; Romo, A.; Stephan, J.; Jasińska, A.K.; et al. Past, present, and future geographic range of an oro-Mediterranean Tertiary relict: The Juniperus drupacea case study. Reg. Environ. Chang. 2019, 19, 1507–1520. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Smith, A.B. enmSdm: Tools for Modeling Species Niches and Distributions, R Package Version 0.5.1.5; R Core Team: Cary, CA, USA, 2020. [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Clark, P.J.; Evans, F.C. Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology 1954, 35, 445–453. [Google Scholar] [CrossRef]
- Evans, J.S. spatialEco, R Package Version 1.2-0; R Core Team: Cary, CA, USA, 2019. [Google Scholar]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Hijmans, R.; Philipps, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling; R Package Version 1.1-4; R Core Team: Cary, CA, USA, 2017. [Google Scholar]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A Comprehensive, High-Resolution Database of Historical and Projected Climate Surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA—High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Liu, X. Global land projection based on plant functional types with a 1-km resolution under socio-climatic scenarios. Sci. Data 2022, 9, 125. [Google Scholar] [CrossRef]
- Hijmans, R.J. terra: Spatial Data Analysis. R Package Version 1.6-47. 2022. Available online: https://CRAN.R-project.org/package=terra (accessed on 2 October 2023).
- Cao, Y.; Wang, F.; Tseng, T.-H.; Carver, S.; Chen, X.; Zhao, J.; Yu, L.; Li, F.; Zhao, Z.; Yang, R. Identifying ecosystem service value and potential loss of wilderness areas in China to support post-2020 global biodiversity conservation. Sci. Total Environ. 2022, 846, 157348. [Google Scholar] [CrossRef]
- Lannuzel, G.; Balmot, J.; Dubos, N.; Thibault, M.; Fogliani, B.; Sanchez Mata, D. High-resolution topographic variables accurately predict the distribution of rare plant species for conservation area selection in a narrow-endemism hotspot in New Caledonia. Biodivers. Conserv. 2021, 30, 963–990. [Google Scholar] [CrossRef]
- Meineri, E.; Hylander, K. Fine-grain, large-domain climate models based on climate station and comprehensive topographic information improve microrefugia detection. Ecography 2017, 40, 1003–1013. [Google Scholar] [CrossRef]
- Tomlinson, S.; Lewandrowski, W.; Elliott, C.P.; Miller, B.P.; Turner, S.R. High-resolution distribution modeling of a threatened short-range endemic plant informed by edaphic factors. Ecol. Evol. 2020, 10, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2016, 26, 385–394. [Google Scholar] [CrossRef]
- Martin, Y.; Van Dyck, H.; Dendoncker, N.; Titeux, N. Testing instead of assuming the importance of land use change scenarios to model species distributions under climate change. Glob. Ecol. Biogeogr. 2013, 22, 1204–1216. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Trigas, P.; Tsakiri, M.; Kokkoris, I.P.; Koumoutsou, E.; Dimopoulos, P.; Tzanoudakis, D.; Iatrou, G.; Panitsa, M. Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece). Plants 2022, 11, 3548. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. ecospat: Spatial Ecology Miscellaneous Methods, R Package Version 3.2; R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling species presence-only data with random forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Flexible species distribution modelling methods perform well on spatially separated testing data. Glob. Ecol. Biogeogr. 2023, 32, 369–383. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including environmental niche information to improve IUCN Red List assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Meyer, L.; Diniz-Filho, J.A.F.; Lohmann, L.G. A comparison of hull methods for estimating species ranges and richness maps. Plant Ecol. Divers. 2017, 10, 389–401. [Google Scholar] [CrossRef]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef] [PubMed]
- Velazco, S.J.E.; Rose, M.B.; de Andrade, A.F.A.; Minoli, I.; Franklin, J. flexsdm: An r package for supporting a comprehensive and flexible species distribution modelling workflow. Methods Ecol. Evol. 2022, 13, 1661–1669. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A.J. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Raes, N.; ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Modell. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Hammer, B.; Frasco, M. metrics: Evaluation Metrics for Machine Learning; R Package Version 0.1.4; R Core Team: Cary, CA, USA, 2018. [Google Scholar]
- Márcia Barbosa, A.; Real, R.; Muñoz, A.R.; Brown, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting machine learning scores to well-calibrated probability estimates for clinical decision making. Bioinformatics 2019, 35, 2458–2465. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D. DescTools: Tools for Descriptive Statistics. R Package Version 0.99-40; R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Collart, F.; Hedenäs, L.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Intraspecific differentiation: Implications for niche and distribution modelling. J. Biogeogr. 2021, 48, 415–426. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. biomod2: Ensemble Platform for Species Distribution Modeling; R Core Team: Cary, CA, USA, 2016. [Google Scholar]
- Bouchet, P.J.; Miller, D.L.; Roberts, J.J.; Mannocci, L.; Harris, C.M.; Thomas, L. dsmextra: Extrapolation assessment tools for density surface models. Methods Ecol. Evol. 2020, 11, 1464–1469. [Google Scholar] [CrossRef]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Cañadas, A.; Chicote, C.; David, L.; Di-Méglio, N.; et al. Assessing cetacean surveys throughout the Mediterranean Sea: A gap analysis in environmental space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, D.S.; Lawler, J. Climate-niche factor analysis: A spatial approach to quantifying species vulnerability to climate change. Ecography 2019, 42, 1494–1503. [Google Scholar] [CrossRef]
- Rinnan, D.S. CENFA: Climate and Ecological Nich Factor Analysis. R Package Version 1.0.0. 2018. Available online: https://CRAN.R-project.org/package=CENFA (accessed on 2 October 2023).
- Hirzel, A.H.; Jacques, H.; Chessel, D.; Perrin, N. Ecological-Niche Factor Analysis: How to Compute Habitat-Suitability Maps without Absence Data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Petit, R.J.; Hampe, A. Some Evolutionary Consequences of Being a Tree. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 187–214. [Google Scholar] [CrossRef]
- Copernicus Emergency Management Service Fire Danger Forecast. Available online: https://effis.jrc.ec.europa.eu/about-effis/technical-background/fire-danger-forecast (accessed on 2 October 2023).
- Adaptive Greece Hub: National Hub for Climate Change Adaptation. Available online: https://geo.adaptivegreecehub.gr/ (accessed on 10 August 2023).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2021. Available online: http://qgis.osgeo.org (accessed on 2 October 2023).
- United Nations; European Commission; Food and Agricultural Organization of the United Nations; Organization for Economic Co-operation and Development; World Bank. System of Environmental-Economic Accounting 2012: Experimental Ecosystem Accounting; United Nations: New York, NY, USA, 2014; ISBN 9789210559263. [Google Scholar]
- United Nations. System of Environmental-Economic Accounting— Ecosystem Accounting (SEEA EA). White Cover Publication, Pre-Edited Text Subject to Official Editing; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Vallecillo, S.; Maes, J.; Teller, A.; Babí Almenar, J.; Barredo, J.; Trombetti, M.; Abdul Malak, D.; Paracchini, M.L.; Carré, A.; Addamo, A.; et al. EU-Wide Methodology to Map and Assess Ecosystem Condition towards a Common Approach Consistent with a Global Statistical Standard; European Commission: Luxembourg, 2022. [Google Scholar]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population genetic variability and distribution of the endangered Greek endemic Cicer graecum under climate change scenarios. AoB Plants 2020, 12, plaa007. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation genetics of four critically endangered greek endemic plants: A preliminary assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Castellari, S.; Kurnik, B. Climate Change, Impacts and Vulnerability in Europe; European Environment Agency: Copenhagen, Denmark, 2017; ISBN 9789292138356. [Google Scholar]
- Nearing, M.A.; Pruski, F.F.; O’Neal, M.R. Expected climate change impacts on soil erosion rates: A review. J. Soil Water Conserv. 2004, 59, 43–50. [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Kersebaum, K.C.; Eitzinger, J.; Hayes, M.; Hlavinka, P.; Svoboda, M.; Dubrovský, M.; Semerádová, D.; Wardlow, B.; Pokorný, E.; et al. Consequences of climate change for the soil climate in Central Europe and the central plains of the United States. Clim. Chang. 2013, 120, 405–418. [Google Scholar] [CrossRef]
- Wang, W.T.; Guo, W.Y.; Jarvie, S.; Svenning, J.C. The fate of Meconopsis species in the Tibeto-Himalayan region under future climate change. Ecol. Evol. 2021, 11, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, T.; Jin, L.; Jiang, J. Vulnerability of two Rhodiola species under climate change in the future. Biodivers. Sci. 2021, 29, 1620–1628. [Google Scholar] [CrossRef]
- Wang, W.-T.; Guo, W.-Y.; Jarvie, S.; Serra-Diaz, J.M.; Svenning, J.-C. Anthropogenic climate change increases vulnerability of Magnolia species more in Asia than in the Americas. Biol. Conserv. 2022, 265, 109425. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; de la Cruz, D.R.; Agudo, J.Á.S. Effects of the Climate Change on Peripheral Populations of Hydrophytes: A Sensitivity Analysis for European Plant Species Based on Climate Preferences. Sustainability 2021, 13, 3147. [Google Scholar] [CrossRef]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Papanikolaou, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P.; Panitsa, M. Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece. Sustainability 2022, 14, 4269. [Google Scholar] [CrossRef]
- Apostolidis, E. Dendrokedros Forest Management and Protection Study in the Area of The Holy Monastery of Malevis Parnona; YLI Environmental Projects: Athens, Greek, 2023. [Google Scholar]
- Study of the Germination Behaviour of Seeds for the Conservation of Two Native Woody Species of Greece: The Endangered Tree Cedar (Juniperus drupacea Labill.) and the Endemic Maple (Acer hyrcanum subsp. Reginae-Amaliae Orph. ex Boiss) of Parnonas (GR 2520). Available online: https://www.fria.gr/jacer.html?fbclid=IwAR0Izr_Q6AJ8PZk35HGRlE-VPc9_JsZ-AiIYPrfV9zQPCkK6k8gHn29h0bQ (accessed on 24 September 2023).
- Kokkoris, I.P.; Skuras, D.; Maniatis, Y.; Dimopoulos, P. Natura 2000 public awareness in EU: A prerequisite for successful conservation policy. Land Use Policy 2023, 125, 106482. [Google Scholar] [CrossRef]

| GCM | RCP | Period | SSP | IUCN Criterion A | IUCN Criterion B | Both IUCN Criteria |
|---|---|---|---|---|---|---|
| current | - | reference | - | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 4.5 | 2011–2040 (2020s) | 1 | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 4.5 | 2041–2070 (2050s) | 1 | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 4.5 | 2071–2100 (2080s) | 1 | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 8.5 | 2011–2040 (2020s) | 1 | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 8.5 | 2041–2070 (2050s) | 1 | Vulnerable | Least Concerned | Vulnerable |
| Ensemble | 8.5 | 2071–2100 (2080s) | 1 | Vulnerable | Least Concerned | Vulnerable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkoris, I.P.; Kougioumoutzis, K.; Charalampopoulos, I.; Apostolidis, E.; Apostolidis, I.; Strid, A.; Dimopoulos, P. Conservation Responsibility for Priority Habitats under Future Climate Conditions: A Case Study on Juniperus drupacea Forests in Greece. Land 2023, 12, 1976. https://doi.org/10.3390/land12111976
Kokkoris IP, Kougioumoutzis K, Charalampopoulos I, Apostolidis E, Apostolidis I, Strid A, Dimopoulos P. Conservation Responsibility for Priority Habitats under Future Climate Conditions: A Case Study on Juniperus drupacea Forests in Greece. Land. 2023; 12(11):1976. https://doi.org/10.3390/land12111976
Chicago/Turabian StyleKokkoris, Ioannis P., Konstantinos Kougioumoutzis, Ioannis Charalampopoulos, Ektor Apostolidis, Ilias Apostolidis, Arne Strid, and Panayotis Dimopoulos. 2023. "Conservation Responsibility for Priority Habitats under Future Climate Conditions: A Case Study on Juniperus drupacea Forests in Greece" Land 12, no. 11: 1976. https://doi.org/10.3390/land12111976
APA StyleKokkoris, I. P., Kougioumoutzis, K., Charalampopoulos, I., Apostolidis, E., Apostolidis, I., Strid, A., & Dimopoulos, P. (2023). Conservation Responsibility for Priority Habitats under Future Climate Conditions: A Case Study on Juniperus drupacea Forests in Greece. Land, 12(11), 1976. https://doi.org/10.3390/land12111976








