Interaction of Management and Spontaneous Succession Suppresses the Impact of Harmful Native Dominant Species in a 20-Year-Long Experiment
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Experimental Design and Data Collecting
2.3. Statistical Analyses
3. Results
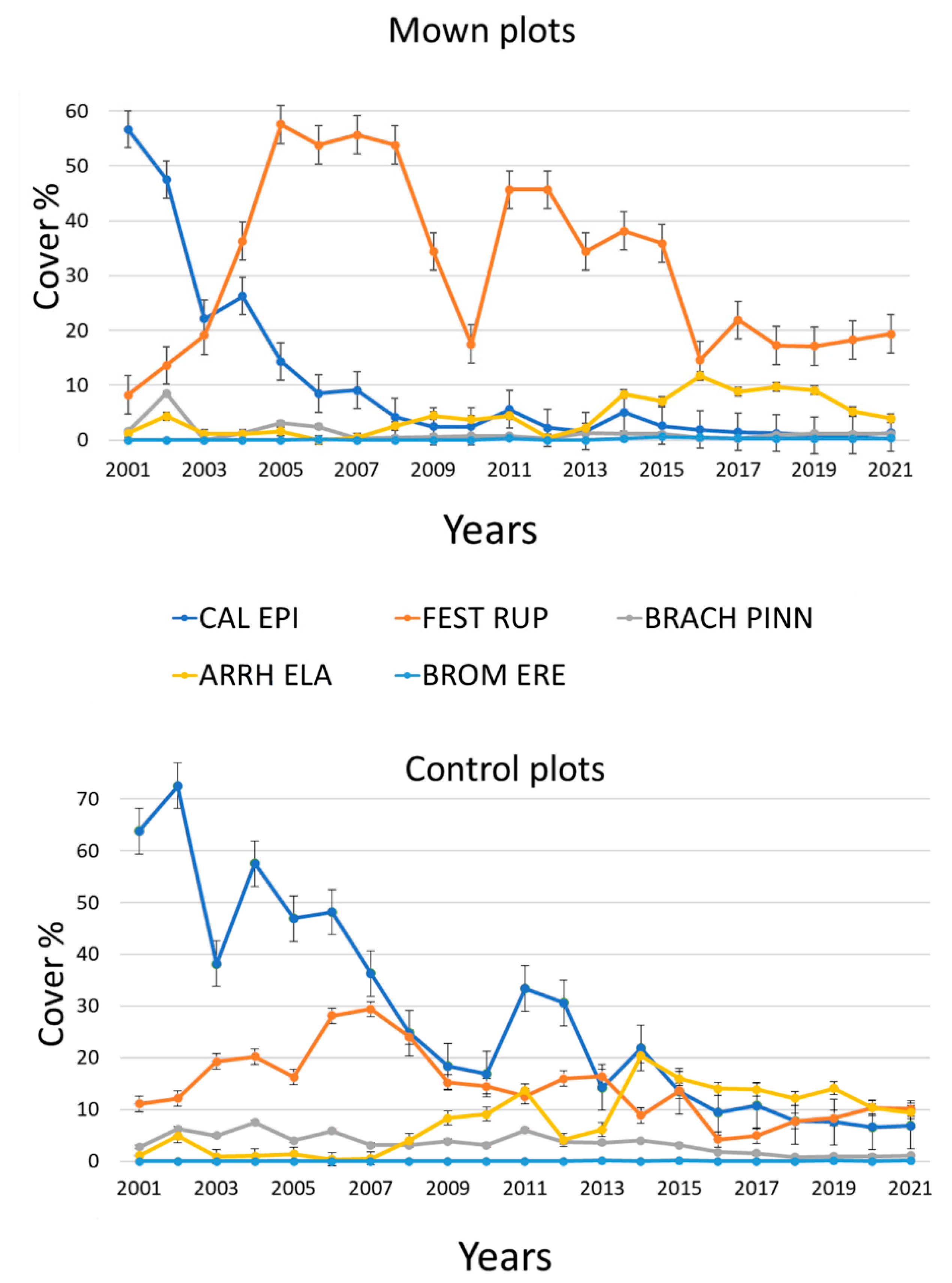
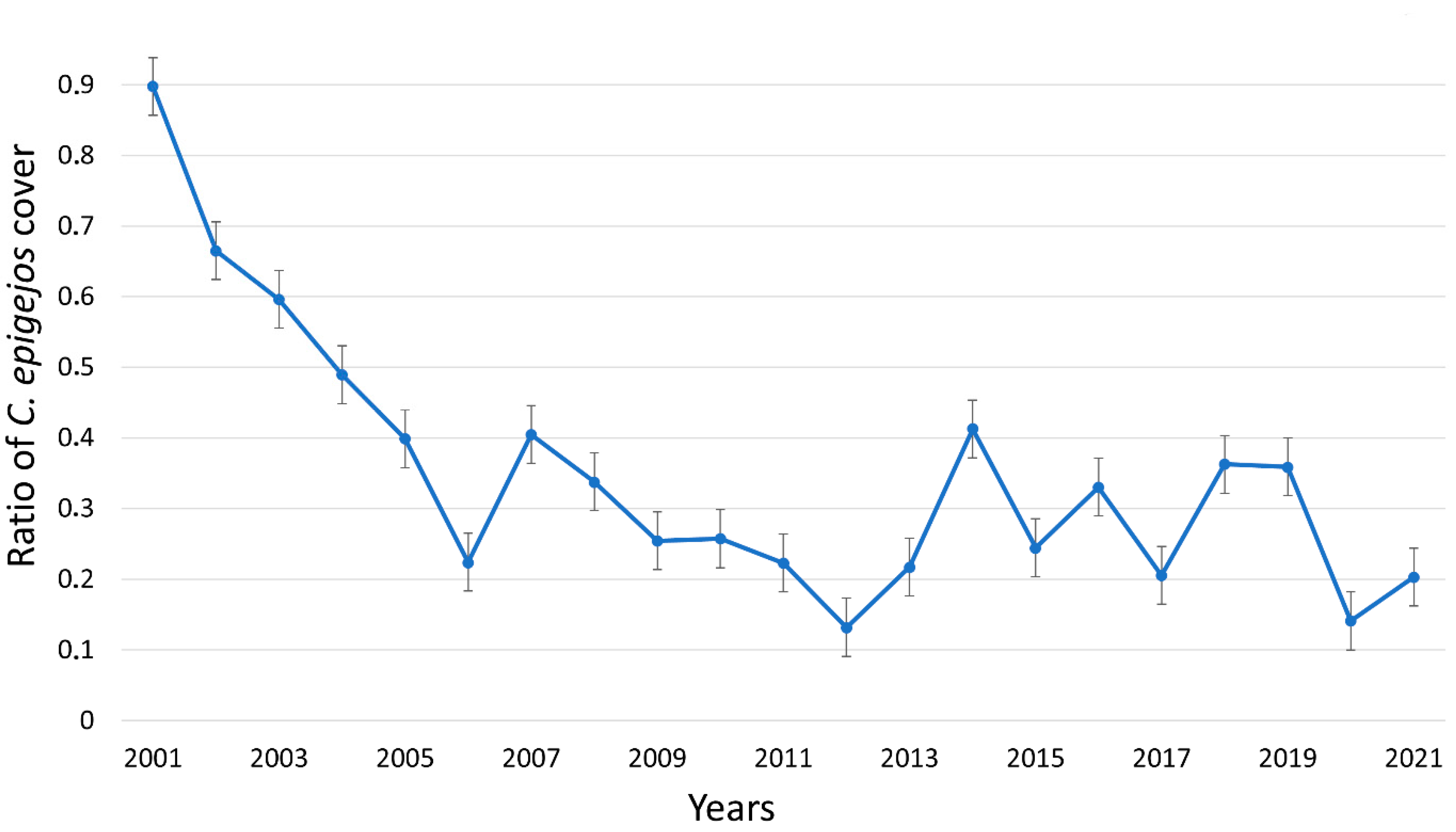
3.1. Effects of Mowing on the Cover of C. epigejos
3.2. Impact of Mowing on the Species Composition and the Cover of Subordinated Species
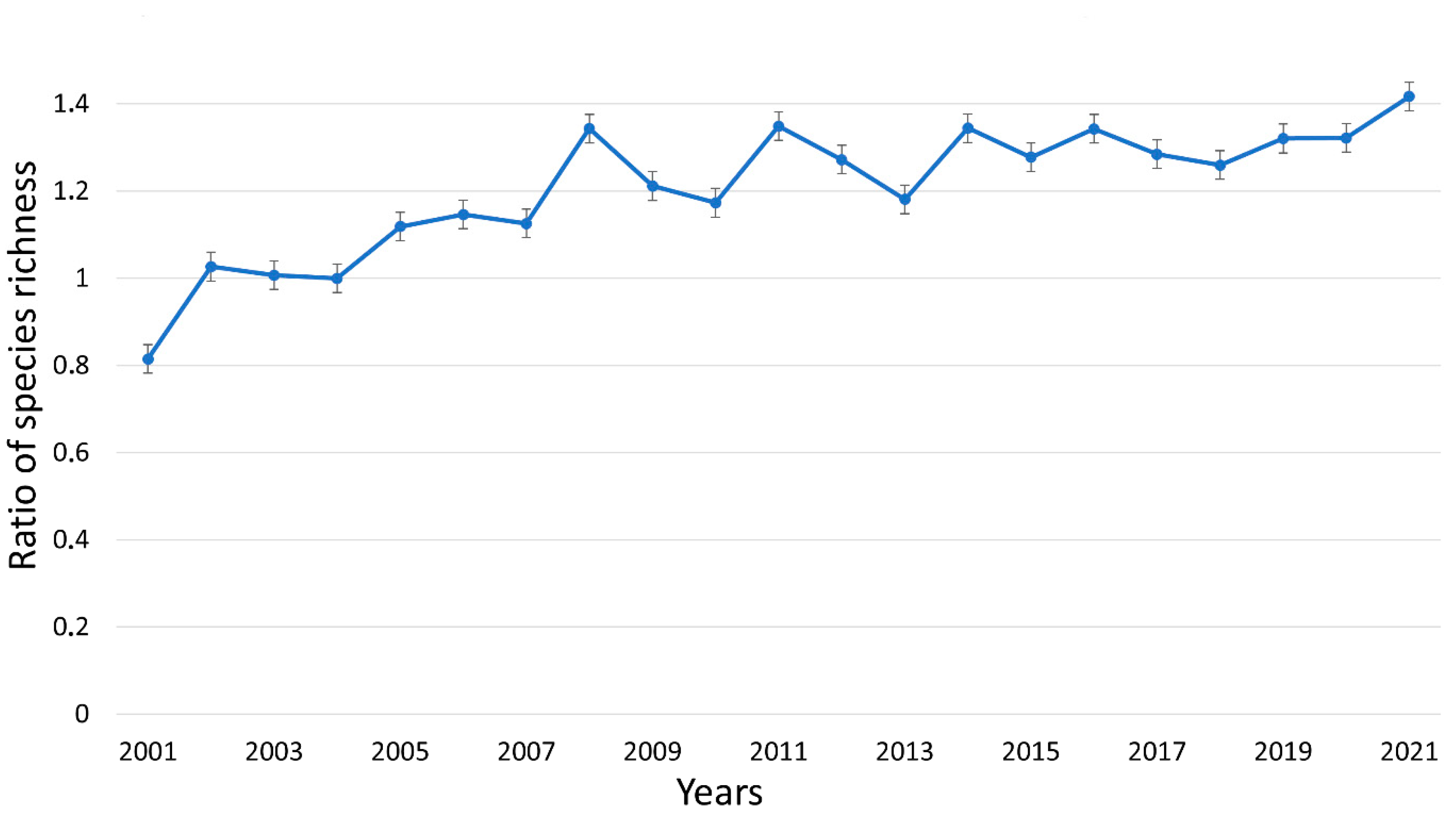
3.3. Impact of Mowing on Plant Species Richness
3.4. Impact of the Interaction between Treatment and Time
4. Discussion
4.1. Long-Term Management Needs to Suppress Native, Dominant Grasses
4.2. How Does the Mowing Treatment Affect the Performance of Other Subordinated Species and the Temporal Development of Species Richness?
4.3. Interaction between Management and Spontaneous Succession
5. Conclusions
- Over five years of conservation management (mowing twice a year) applied continuously was necessary for the effective suppression of an expanding native dominant species (C. epigejos).
- We found evidence that C. epigejos declined spontaneously in later successional stages. Although C. epigejos declined spontaneously, its period of dominance was three times longer (15 years) without management.
- Species richness increased faster during succession when plots were mowed two times per year, and after 20 years of management, species richness was 40% higher in the mown plots.
- The temporal perspective is crucial for proper assessment of the impact of harmful species (both aliens and natives).
- Long-term (20 years) in situ experiments and comprehensive botanical studies are necessary to provide a solid scientific basis for multi-objective management regimes and to achieve effective utilization of grasslands.
- Appropriate support programs and special agro-environmental management programs are necessary in order to maintain the diversity of grasslands, especially in the case of used and later abandoned agricultural lands.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Valéry, L.; Fritz, H.; Lefeuvre, J.C.; Simberloff, D. Invasive species can also be native. Trends Ecol. Evol. 2009, 24, 585. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. Native invaders. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmánek, M., Eds.; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2011. [Google Scholar]
- Hejda, M.; Sádlo, J.; Kutlvašr, J.; Petřík, P.; Vítková, M.; Vojík, M.; Pyšek, P.; Pergl, J. Impact of invasive and native dominants on species richness and diversity of plant communities. Preslia 2021, 93, 181–201. [Google Scholar] [CrossRef]
- Yazlık, A.; Ambarlı, D. Do non-native and dominant native species carry a similar risk of invasiveness? A case study for plants in Turkey. In Recent advancements in the risk screening of freshwater and terrestrial non-native species. NeoBiota 2022, 76, 53–72. [Google Scholar] [CrossRef]
- Carey, M.P.; Sanderson, B.L.; Barnas, K.A.; Olden, J.D. Native invaders—Challenges for science, management, policy, and society. Front. Ecol. Environ. 2012, 10, 373–381. [Google Scholar] [CrossRef]
- Pivello, V.R.; Vieira, M.V.; Grombone-Guaratini, M.T.; Silva Matos, D.M. Thinking about super-dominant populations of native species—Examples from Brazil. Perspect. Ecol. Conserv. 2018, 16, 74–82. [Google Scholar] [CrossRef]
- Blair, J.; Nippert, J.; Briggs, J. Grassland Ecology. The Plant Sciences, Chapter 14. In Ecology and the Environment; Monson, R.K., Ed.; Springer: New York, NY, USA, 2014; pp. 389–423. [Google Scholar] [CrossRef]
- Bartha, S. Composition, differentiation and dynamics in the forest steppe biome. In Slope Steppes, Loess Steppes and Forest Steppe Meadows in Hungary; Illyés, E., Bölöni, J., Eds.; MTA ÖBKI: Budapest, Hungary, 2007; pp. 194–211. ISBN 978-963-06-3673-5. [Google Scholar]
- Molnár, Z.; Biró, M.; Bartha, S.; Fekete, G. Past trends, present state and future prospects of Hungarian forest-steppes. In Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World; Werger, M.J.A., Staalduinen, M.A., Eds.; Springer Science & Business Media: New York, NY, USA, 2012; Volume 6, pp. 209–252. [Google Scholar]
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of Palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef]
- Török, P.; Penksza, K.; Tóth, E.; Kelemen, A.; Sonkoly, J.; Tóthmérész, B. Vegetation type and grazing intensity jointly shape grazing on grassland biodiversity. Ecol. Evol. 2018, 8, 10326–10335. [Google Scholar] [CrossRef]
- Poschlod, P.; Wallis de Vries, M.F. The historical and socioeconomic perspective of calcareous grasslands–lessons from the distant and recent past. Biol. Conserv. 2002, 104, 361–376. [Google Scholar] [CrossRef]
- Ruprecht, E.; Szabó, A.; Enyedi, M.Z.; Dengler, J. Steppe-like grasslands in Transylvania (Romania): Characterisation and influence of management on species diversity and composition. Tuexenia 2009, 29, 353–368. [Google Scholar]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in temperate European grasslands: Origin and conservation. Grassl. Sci. Eur. 2005, 10, 14. [Google Scholar]
- Simberloff, D.; Souza, L.; Nuñez, M.A.; Barrios-Garcia, M.N.; Bunn, W. The natives are restless, but not often and mostly when disturbed. Ecology 2012, 93, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Nelufule, T.; Robertson, M.P.; Wilson, J.R.U.; Faulkner, K.T. Native-alien populations—An apparent oxymoron that requires specific conservation attention. NeoBiota 2022, 74, 57–74. [Google Scholar] [CrossRef]
- Nackley, L.L.; West, A.G.; Skowno, A.L.; Bond, W.J. The nebulous ecology of native invasions. Trends Ecol. Evol. 2017, 32, 814–824. [Google Scholar] [CrossRef]
- Jendrišáková, S.; Kováčiková, Z.; Vargová, V.; Michalec, M. The impact of cattle and sheep grazing on grassland in Veľká Fatra National Park. J. Water Land Dev. 2011, 15, 83–90. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Šinžar-Sekulić, J.; Lakušić, D. Leaf anatomy of the Sesleria rigida Heuffel ex Reichenb. (Poaceae) in Serbia. Bot. Serbica 2009, 33, 51–67. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Allegrezza, M. Assessing the impact of land abandonment, nitrogen enrichment and fairy-ring fungi on plant diversity of Mediterranean grasslands. Biodivers. Conserv. 2013, 22, 2285–2304. [Google Scholar] [CrossRef]
- Tardella, F.M.; Malatesta, L.; Goia, I.G.; Catorci, A. Effects of long-term mowing on coenological composition and recovery routes of a Brachypodium rupestre-invaded community: Insight into the restoration of sub-Mediterranean productive grasslands. Rend. Lincei Sci. Fis. Nat. 2018, 29, 329–341. [Google Scholar] [CrossRef]
- Hejcman, M.; Češková, M.; Schellberg, J.; Pätzold, S. The Rengen grassland experiment: Effect of soil chemical properties on biomass production, plant species composition and species richness. Folia Geobot. 2010, 45, 125–142. [Google Scholar] [CrossRef]
- Catorci, A.; Ottaviani, G.; Ballelli, S.; Cesaretti, S. Functional differentiation of Central Apennine grasslands under mowing and grazing disturbance regimes. Pol. J. Ecol. 2011, 59, 115–128. [Google Scholar]
- Szentes, S.; Sutyinszki, Z.; Szabó, G.; Zimmermann, Z.; Járdi, I.; Házi, J.; Bartha, S.; Penksza, K. Studies on the effects of old world bluestem (Bothriochloa ischaemum (L.) Keng 1936) on species composition of grassland with microcoenological methods. Anim. Welf. Ethol. Hous. Syst. 2012, 8, 88–102. [Google Scholar]
- Tardella, F.M.; Bricca, A.; Piermarteri, K.; Postiglione, N.; Catorci, A. Context-dependent variation of SLA and plant height of a dominant, invasive tall grass (Brachypodium genuense) in subMediterranean grasslands. Flora 2017, 229, 116–123. [Google Scholar] [CrossRef]
- Rebele, F. Calamagrostis epigejos (L.) Roth auf anthropogenen Standorten–ein Überblick. Verh. Ges. Okol. 1996, 26, 753–763. [Google Scholar]
- Rebele, F.; Lehmann, C. Biological Flora of Central Europe: Calamagrostis epigejos (L.) Roth. Flora 2001, 196, 325–344. [Google Scholar] [CrossRef]
- Gloser, V.; Košvancová, M.; Gloser, J. Changes in growth parameters and content of N-storage compounds in roots and rhizomes of Calamagrostis epigejos after repeated defoliation. Biol. Bratisl. 2004, 59, 179–184. [Google Scholar]
- Prach, K.; Pyšek, P. Using spontaneous succession for restoration of human-disturbed habitats: Experience from Central Europe. Ecol. Eng. 2001, 17, 55–62. [Google Scholar] [CrossRef]
- Huhta, A.; Pasi, R.; Tuomi, J.; Laine, K. Restorative mowing on an abandoned semi-natural meadow: Short-term and predicted long-term effects. J. Veg. Sci. 2001, 12, 677–686. [Google Scholar] [CrossRef]
- Sedláková, I.; Fiala, K. Ecological degradation of alluvial meadows due to expanding Calamagrostis epigejos. Ekologia 2001, 20 (Suppl. S3), 226–333. [Google Scholar]
- Woch, W.M.; Radwańska, M.; Stanek, M.; Łopata, B.; Stefanowicz, M.A. Relationships between waste physicochemical properties, microbial activity and vegetation at coal ash and sludge disposal sites. Sci. Total Environ. 2018, 642, 264–275. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, K.M.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, M.A.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Ranđelović, D.; Jakovljević, K.; Mihailović, N.; Jovanović, S. Metal accumulation in populations of Calamagrostis epigejos (L.) Roth from diverse anthropogenically degraded sites (SE Europe, Serbia). Environ. Monitor. Assess. 2018, 190, 183. [Google Scholar] [CrossRef] [PubMed]
- Somodi, I.; Virágh, K.; Podani, J. The effect of the expansion of the clonal grass Calamagrostis epigejos on the species turnover of a semi-arid grassland. Appl. Veg. Sci. 2008, 11, 187–194. [Google Scholar] [CrossRef]
- Zhukovskaya, O.; Ulanova, N.G. Influence of brushing frequency on birch population structure after felling. Ecoscience 2006, 13, 219–225. [Google Scholar] [CrossRef]
- Csontos, P. Light ecology and regeneration on clearings of sessile oak-turkey oak forests in the Visegrád mountains, Hungary. Acta Bot. Hung. 2010, 52, 265–286. [Google Scholar] [CrossRef]
- Fiala, K.; Holub, P.; Sedláková, I.; Tůma, I.; Záhora, J.; Tesařová, M. Reasons and consequences of expansion of Calamagrostis epigejos in alluvial meadows of landscape affected by water control measures. Ekologia 2003, 22 (Suppl. S2), 242–252. [Google Scholar]
- Błónska, A.; Chmura, D.; Hutniczak, A.; Wilczek, Z.; Jarosz, J.; Besenyei, L.; Wózniak, G. The plant species composition of an abandoned meadow as an element of an ecosystem mosaic within an urban-industrial landscape. Sustainability 2022, 14, 11851. [Google Scholar] [CrossRef]
- Prach, K. Succession of vegetation on dumps from strip coal mining, N.W. Bohemia, Czechoslovakia. Folia Geobot. Phytotax 1987, 22, 339–354. [Google Scholar] [CrossRef]
- Bartha, S. Preliminary scaling for multi-species coalitions in primary succession. Abstr. Bot. 1992, 16, 31–41. [Google Scholar]
- Baasch, A.; Tischew, S.; Bruelheide, H. Twelve years of succession on sandy substrates in a post-mining landscape: A Markov chain analysis. Ecol. Appl. 2010, 20, 136–1147. [Google Scholar] [CrossRef] [PubMed]
- Virágh, K.; Horváth, A.; Bartha, S.; Somodi, I. A multi scale methodological approach novel in monitoring the effectiveness of grassland management. Commun. Ecol. 2008, 9, 237–246. [Google Scholar] [CrossRef]
- Hulten, E.; Fries, M. Atlas of North European vascular plants: North of the Tropic of Cancer; Koeltz Scientific Books: Königstein, Germany, 1986. [Google Scholar]
- Csecserits, A.; Rédei, T. Secondary succession on sandy oldfields in Hungary. Appl. Veg. Sci. 2001, 4, 63–74. [Google Scholar] [CrossRef]
- Bartha, S.; Szentes, S.; Horváth, A.; Házi, J.; Zimmermann, Z.; Molnár, C.; Dancza, I.; Margóczi, K.; Pál, R.W.; Purger, D.; et al. Impact of midsuccessional dominant species on the diversity and progress of succession in regenerating temperate grasslands. Appl. Veg. Sci. 2014, 17, 201–213. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Żołnierz, I. The effect of different restoration treatments on the vegetation of the mesic meadow degraded by the expansion of Calamagrostis epigejos. Int. J. Agric. Biol. 2019, 22, 347–354. [Google Scholar]
- Hajnáczki, S.; Pajor, F.; Péter, N.; Bodnár, A.; Penksza, K.; Póti, P. Solidago gigantea Ait. and Calamagrostis epigejos (L) Roth invasive plants as potential forage for goats. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12197. [Google Scholar] [CrossRef]
- Tesitel, J.; Mládek, J.; Horník, J.; Tesitelová, T.; Adamec, V.; Tichy, L. Suppressing competitive dominants and community restoration with native parasitic plants using the hemiparasitic Rhinanthus alectorolophus and the dominant grass Calamagrostis epigejos. J. Appl. Ecol. 2017, 54, 1487–1495. [Google Scholar] [CrossRef]
- Lepš, J. Scale- and time-dependent effects of fertilization, mowing and dominant removal on a grassland community during a 15-year experiment. J. Appl. Ecol 2014, 51, 978–987. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Chen, H.; Gong, J.; Qi, Y.; Yang, F.; Li, E. Effects of grassland management on the community structure, aboveground biomass and stability of a temperate steppe in Inner Mongolia. China J. Arid Land 2016, 8, 422–433. [Google Scholar] [CrossRef]
- Valkó, O.; Zmihorski, M.; Biurrun, I.; Loos, J.; Labadessa, R.; Venn, S. Ecology and conservation of steppes and semi-natural grasslands. Hacquetia 2016, 15, 5–14. [Google Scholar] [CrossRef]
- Maron, J.; Jefferies, R. Restoring enriched grasslands: Effects of mowing on species richness, productivity, and nitrogen retention. Ecol. Appl. 2001, 11, 1088–1100. [Google Scholar] [CrossRef]
- Marosi, S.; Somogyi, S. (Eds.) Magyarország Kistájainak Katasztere (Cadastral of Microregions of Hungary); MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 1991; pp. 379–388. [Google Scholar]
- Borhidi, A.; Kevey, B.; Lendvai, G. Plant communities of Hungary; Akadémiai Kiadó: Budapest, Hungary, 2012; 544p. [Google Scholar]
- European Environment Agency. 6250 Pannonic Loess Steppic Grasslands. Report under the Article 17 of the Habitats Directive Period 2007–2012. Available online: https://eunis.eea.europa.eu/habitats/10124#sites (accessed on 20 December 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 20 February 2018).
- Wahlman, H.; Milberg, P. Management of semi-natural grassland vegetation: Evaluation of a long-term experiment in southern Sweden. Ann. Bot. Fenn. 2002, 39, 159–166. Available online: http://www.jstor.org/stable/23726791 (accessed on 20 December 2022).
- Szary, M.; Błońska, A.; Woźniak, G.; Ziemer, B.; Roszkowska, E.; Besler, A.; Sierka, E. Changes in species composition of meadow vegetation patches dominated by Calamagrostis epigejos in response to mowing and biomass removal. In The 11th International Conference Synanthropization of Flora and Vegetation, Poznań, Poland, Book of Abstracts, Biodiversity Research and Conservation; Jackowiak, B., Ed.; Poland Biodiv. Res. Conserv.: Poznan & Obrzycko, Poland, 2014; p. 83. [Google Scholar]
- Klimeš, L.; Klimešova, J. The effects of mowing and fertilisation on carbohydrate reserves and regrowth of grasses: Do they promote plant coexistence in species-rich meadows? Evol. Ecol. 2002, 15, 363–382. [Google Scholar] [CrossRef]
- Březina, S.; Koubek, T.; Münzbergová, Z.; Herben, T. Ecological benefits of integration of Calamagrostis epigejos ramets under field conditions. Stanislav. Flora 2006, 201, 461–467. [Google Scholar] [CrossRef]
- Kavanová, M.; Gloser, V. The use of internal nitrogen stores in the rhizomatous grass Calamagrostis epigejos during regrowth after defoliation. Ann. Bot. 2005, 85, 457–463. [Google Scholar] [CrossRef]
- Házi, J.; Bartha, S.; Szentes, S.; Wichmann, B.; Penksza, K. Seminatural grassland management by long-term mowing of Calamagrostis epigejos in western Cserhát, Hungary (Management sekundärer Trockenrasen durch Langzeit-Mahd von Calamagrostis epigejos im westlichen Cserhát, Ungarn). In Steppenlebensräume Europas–Gefährdung, Erhaltungsmassnahmen und Schutz; Thüringer Ministerium für Landwirtschaft, Forsten; Baumbach, H., Pfützenreuter, S., Eds.; Umwelt und Naturschutz (TMLFUN): Erfurt, Germany, 2013; pp. 331–340. ISBN 978-3-00-044248-3. [Google Scholar]
- Michalska-Hejduk, D.; Kopéc, D. Dynamics of semi-natural vegetation with special focus on Molinion meadows after 50 years of strict protection. Pol. J. Environ. Stud. 2012, 21, 1731–1741. [Google Scholar]
- Poptcheva, K.; Schwartze, P.; Vogel, A.; Kleinebecker, T.; Hölzel, N. Changes in wet meadow vegetation after 20 years of different management in a field experiment (North-West Germany). Agric Ecosyst. Environ. 2009, 134, 108–114. [Google Scholar] [CrossRef]
- Oelmann, Y.; Broll, G.; Hölzel, N.; Kleinebecker, T.; Vogel, A.; Schwartze, P. Nutrient impoverishment and limitation of productivity after 20 years of conservation management in wet grasslands of north-western Germany. Biol. Conserv. 2009, 142, 2941–2948. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R. Effect of long-term abandonment and spring grazing on floristic and functional composition of dry grasslands in a central Apennine farmland. Pol. J. Ecol. 2013, 61, 505–518. [Google Scholar]
- Song, Y.T.; Wuyunna; Zhou, D.W. Effect of autumn cutting date on regrowth, turning green, yield and quality of Leymus chinensis grassland in Songnen Plain, Northeast China. Am. J. Plant Sci. 2018, 9, 185–195. [Google Scholar] [CrossRef]
- Házi, J.; Penksza, K.; Barczi, A.; Szentes, S.; Pápay, G. Effects of long-term mowing on biomass composition in Pannonian dry grasslands. Agron. J. 2022, 12, 1107. [Google Scholar] [CrossRef]
- Sierka, E.; Kopczynska, S. Participation of Calamagrostis epigejos (L.) Roth in plant communities of the River Bytomka valley in terms of its biomass use in power industry. Environ. Socio-Econ. Stud. 2014, 2, 38–44. [Google Scholar] [CrossRef]
- Bissels, S.; Donath, T.W.; Hölzel, N.; Otte, A. Effects of different mowing regimes on seedling recruitment in alluvial grasslands. Basic Appl. Ecol. 2006, 7, 433–442. [Google Scholar] [CrossRef]
- Fiala, K.; Tůma, I.; Holub, P. Effect of nitrogen addition and drought on aboveground biomass of expanding tall grasses Calamagrostis epigejos and Arrhenatherum elatius. Biologia 2011, 66, 275–281. [Google Scholar] [CrossRef]
- Rebele, F. Competition and coexistence of rhizomatous perennial plants along a nutrient gradient. Plant Ecol. 2000, 147, 77–94. [Google Scholar] [CrossRef]
- Házi, J.; Bartha, S.; Szentes, S.; Wichmann, B.; Penksza, K. Seminatural grassland management by mowing of Calamagrostis epigejos in Hungary. Plant Biosyst. 2011, 145, 699–707. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Maalouf, J.P.; Le Bagousse-Pinguet, Y.; Marchand, L.; Touzard, B.; Michalet, R. The interplay of stress and mowing disturbance for the intensity and importance of plant interactions in dry calcareous grasslands. Ann. Bot. 2012, 110, 821–828. [Google Scholar] [CrossRef]
- Kramberger, B.; Kaligarič, M. Semi-natural grasslands: The effect of cutting frequency on long-term changes of floristic composition. Polish J. Ecol. 2008, 56, 33–43. [Google Scholar]
- Szépligeti, M.; Kőrösi, Á.; Szentirmai, I.; Házi, J.; Bartha, D.; Bartha, S. Evaluating alternative mowing regimes for conservation management of Central European mesic hay meadows: A field experiment. Plant Biosyst. 2018, 152, 90–97. [Google Scholar] [CrossRef]
- Sheil, D.; Burslem, D.F.R.P. Defining and defending Connell’s intermediate disturbance hypothesis: A response to Fox. Trends Ecol. Evol. 2013, 28, 571–572. [Google Scholar] [CrossRef]
- Nagy, D.; Rauschert, E.S.J.; Ragan, M.; Callaway, T.H.; Filep, R.; Pal, R.W. Intense mowing management suppresses invader, but shifts competitive resistance by a native to facilitation. Restor. Ecol. 2022, 30, e13483. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Gross, E.M.; Straile, D. Release from competition and protection determine the outcome of plant interactions along a grazing gradient. Oikos 2012, 121, 95–101. [Google Scholar] [CrossRef]
- Schuhmacher, O.; Dengler, J. Das Land-Reitgras als Problemart auf Trockenrasen Handlungsempfehlungen zur Reduktion von Calamagrostis epigejos; Ergebnisse aus einem Praxisversuch—NABU: Hamburg, Germany, 2013; pp. 10–15. [Google Scholar]
- Wallis De Vries, M.F.; Poschlod, P.; Willems, J.H. Challenges for the conservation of calcareous grasslands in northwestern Europe: Integrating the requirements of flora and fauna. Biol. Conserv. 2002, 104, 265–273. [Google Scholar] [CrossRef]
- Babai, D.; Molnár, Z. Small-scale traditional management of highly species-rich grasslands in the Carpathians. Agric. Ecosyst. Environ. 2014, 182, 123–130. [Google Scholar] [CrossRef]
- Klimeš, L.; Hajek, M.; Mudrak, O.; Dancak, M.; Preislerova, Z.; Hajkova, P.; Jongepierova, I.; Klimešova, J. Effects of changes in management on resistance and resilience in three grassland communities. Appl. Veg. Sci. 2013, 16, 640–649. [Google Scholar] [CrossRef]
- Mlungele, M.; Nsikani, M.M.; Sjirk Geerts, S.; Ruwanza, S.; David Richardson, D.M. Secondary invasion and weedy native species dominance after clearing invasive alien plants in South Africa: Status quo and prognosis. S. Afr. J. Bot. 2020, 132, 338–345. [Google Scholar] [CrossRef]
- Meiners, S.J. Native and exotic plant species exhibit similar population dynamics during succession. Ecology 2007, 88, 1098–1104. [Google Scholar] [CrossRef]
- Warren, C.R. Beyond ‘Native v. Alien’: Critiques of the native/alien paradigm in the anthropocene, and their implications. Ethics Policy Environ. 2021, 1, 1–31. [Google Scholar] [CrossRef]
- Simon, T. Magyarország Edényes Flóra Határozója; Harasztok-Virágos Növények. (Vascular Plants of Hungary: Ferns-Flowering Plants); Tankönyvkiadó: Budapest, Hungary, 1992; 976p. [Google Scholar]


| MOWN | ||||||
| 2001 | 2011 | 2021 | ||||
| Mean ± SE | Mean ± SE | Mean ± SE | ||||
| Total cover absolute | 103.38 ± 2.11 | a | 117.43 ± 4.11 | b | 99.83 ± 5.00 | a |
| Cover of CALEPI absolute | 56.63 ± 4.38 | a | 5.63 ± 2.18 | b | 1.35 ± 0.59 | c |
| Cover of CALEPI relative | 0.55 ± 0.04 | a | 0.05 ± 0.01 | b | 0.01 ± 0.01 | c |
| Cover of subordinated species absolute | 46.75 ± 4.79 | a | 111.8 ± 3.39 | b | 98.48 ± 5.27 | b |
| Cover of subordinated species relative | 0.45 ± 0.04 | a | 0.95 ± 0.02 | b | 0.99 ± 0.01 | c |
| Species richness | 14.88 ± 0.95 | a | 36.75 ± 1.38 | b | 36.13 ± 1.31 | b |
| CONTROL | ||||||
| 2001 | 2011 | 2021 | ||||
| Mean ± SE | Mean ± SE | Mean ± SE | ||||
| Total cover absolute | 115.70 ± 1.69 | a | 124.80 ± 5.69 | a | 113.05 ± 3.08 | a |
| Cover of CALEPI absolute | 63.75 ± 4.30 | a | 33.38 ± 7.27 | b | 6.88 ± 2.41 | c |
| Cover of CALEPI relative | 0.55 ± 0.04 | a | 0.26 ± 0.05 | b | 0.07 ± 0.03 | c |
| Cover of subordinated species absolute | 51.95 ± 4.61 | a | 91.43 ± 7.29 | b | 106.18 ± 5.68 | b |
| Cover of subordinated species relative | 0.45 ± 0.03 | a | 0.74 ± 0.05 | b | 0.93 ± 0.02 | c |
| Species richness | 18.25 ± 1.56 | a | 27.25 ± 1.88 | b | 25.50 ± 1.16 | b |
| Treatment | F: 30.085 | p: 9.21 × 10−4 | p < 0.01 *** |
| Time | F: 39.559 | p: 1.21 × 10−47 | p < 0.01 *** |
| Treatment: Time | F: 05.493 | p: 3.68 × 10−10 | p < 0.01 *** |
| Treatment | F: 65.412 | p: 8.50 × 10−5 | p < 0.01 *** |
| Time | F: 18.923 | p: 2.01 × 10−30 | p < 0.01 *** |
| Treatment: Time | F: 08.675 | p: 3.46 × 10−16 | p < 0.01 *** |
| Treatment | F: 20.634 | p: 3.00 × 10−3 | p < 0.01 *** |
| Time | F: 25.629 | p: 3.89 × 10−37 | p < 0.01 *** |
| Treatment: Time | F: 07.114 | p: 2.36 × 10−13 | p < 0.01 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Házi, J.; Purger, D.; Penksza, K.; Bartha, S. Interaction of Management and Spontaneous Succession Suppresses the Impact of Harmful Native Dominant Species in a 20-Year-Long Experiment. Land 2023, 12, 149. https://doi.org/10.3390/land12010149
Házi J, Purger D, Penksza K, Bartha S. Interaction of Management and Spontaneous Succession Suppresses the Impact of Harmful Native Dominant Species in a 20-Year-Long Experiment. Land. 2023; 12(1):149. https://doi.org/10.3390/land12010149
Chicago/Turabian StyleHázi, Judit, Dragica Purger, Károly Penksza, and Sándor Bartha. 2023. "Interaction of Management and Spontaneous Succession Suppresses the Impact of Harmful Native Dominant Species in a 20-Year-Long Experiment" Land 12, no. 1: 149. https://doi.org/10.3390/land12010149
APA StyleHázi, J., Purger, D., Penksza, K., & Bartha, S. (2023). Interaction of Management and Spontaneous Succession Suppresses the Impact of Harmful Native Dominant Species in a 20-Year-Long Experiment. Land, 12(1), 149. https://doi.org/10.3390/land12010149






