Divergent Response of the Supply Capacity and Turnover of Inorganic Nitrogen to Pitaya Cultivation in the Subtropical Karst Region of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
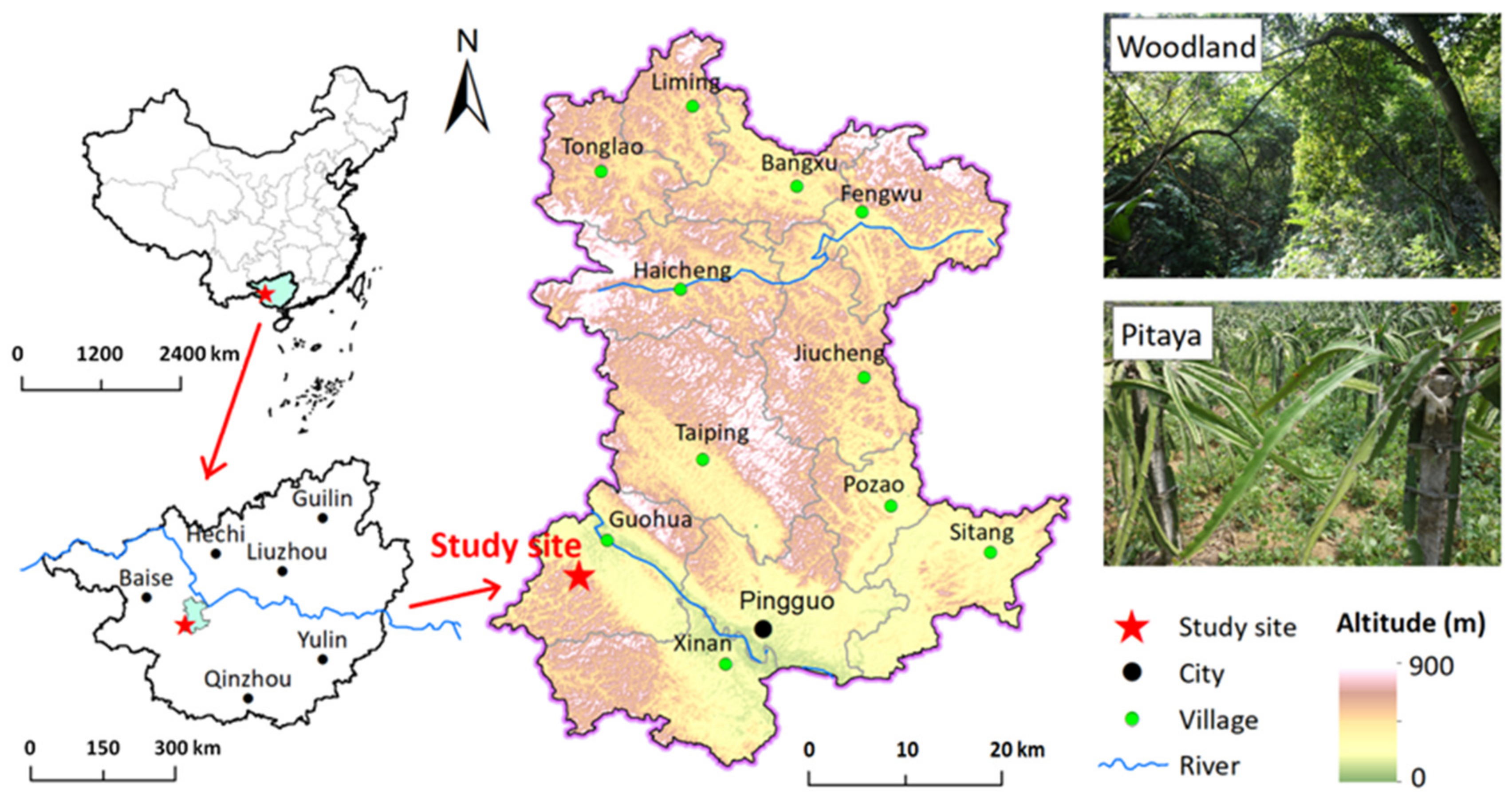
2.1. Sites and Soil Sample Collections
2.2. 15N Labelling Experiment
2.3. Analyses of Soil Properties
2.4. Determination of Archaeal amoA and Bacterial amoA Abundances
2.5. Statistical Data Analyses
3. Results
3.1. Soil Properties and AOA/AOB Abundances
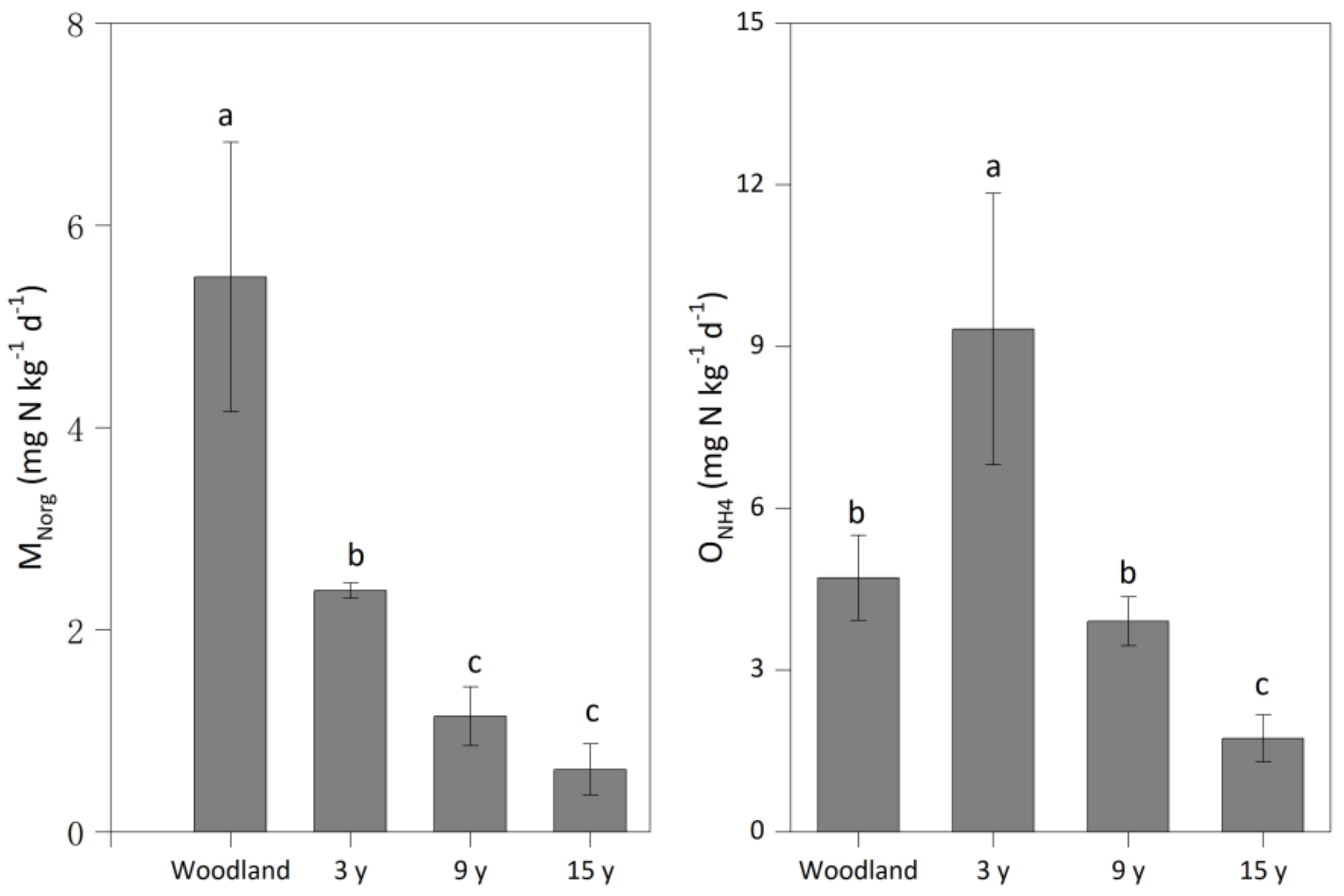
3.2. N-Cycling Rates
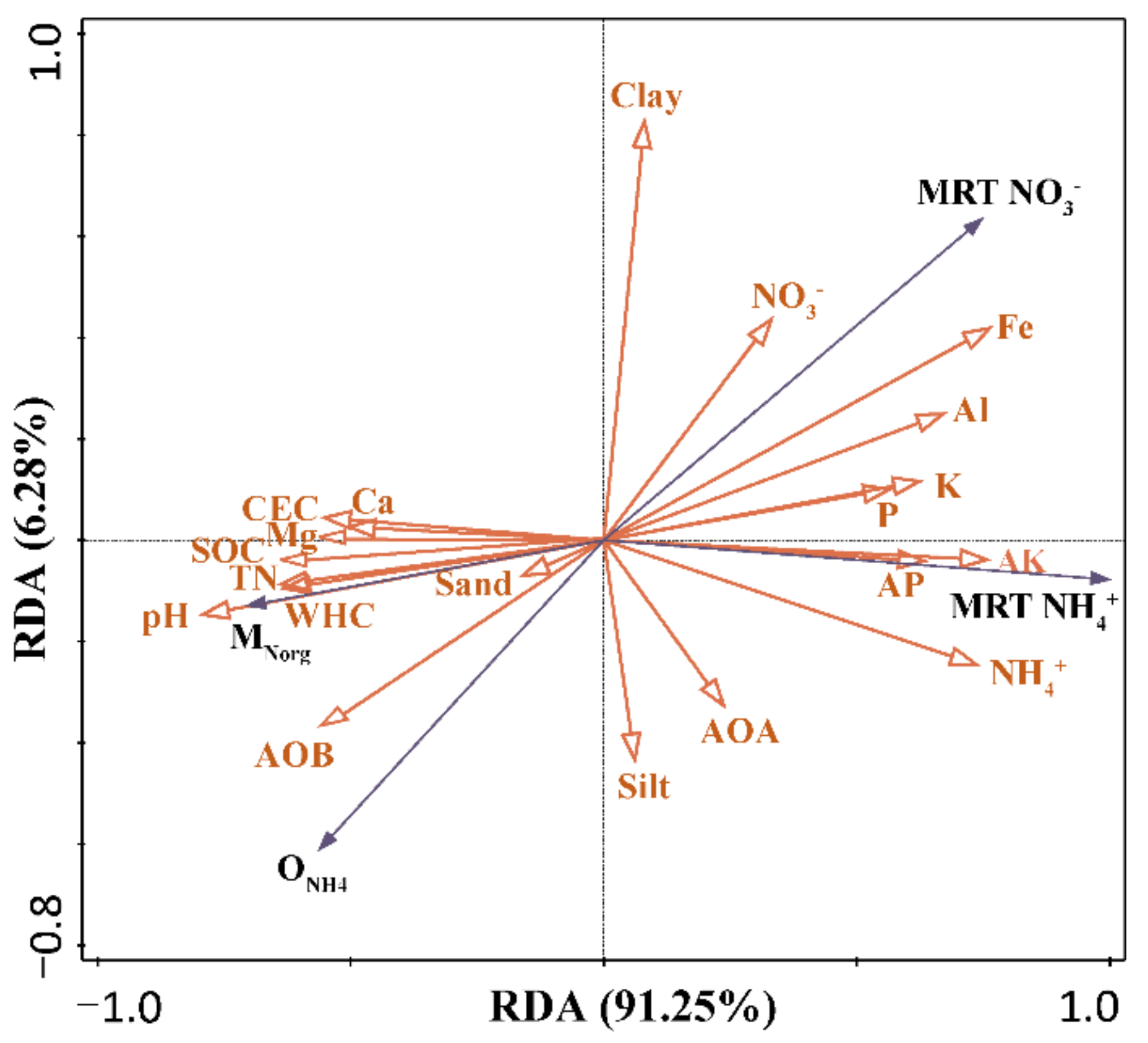
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, Q.M.; Zhang, D.F. Karst rocky desertification in Southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Cheng, F.; Lu, H.F.; Ren, H.; Zhou, L.; Zhang, L.H.; Li, J.; Lu, X.J.; Huang, D.W.; Zhao, D. Integrated emergy and economic evaluation of three typical rocky desertification control modes in karst areas of Guizhou Province, China. J. Clean Prod. 2017, 161, 1104–1128. [Google Scholar] [CrossRef]
- Fresh Plaza. 2018. Available online: https://www.freshplaza.cn/article/8812800/ (accessed on 1 February 2022).
- Luo, J.; Xu, M.; Qi, Z.; Xiong, R.; Cheng, Y.; Liu, C.L.; Wei, S.S.; Tang, H. Differential responses of the soil microbial community in two pitaya orchards with different mulch types. Sci Rep. 2019, 9, 10413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, T.T.H.; Le, T.L.; Huynh, N.; Quintela-Alonso, P. Dragon fruit: A review of health benefits and nutrients and its sustainable development under climate changes in Vietnam. Czech J. Food Sci. 2021, 39, 71–94. [Google Scholar] [CrossRef]
- Norouzi, M.; Ayoubi, S.; Jalalian, A.; Khademi, H.; Dehghani, A.A. Predicting rainfed wheat quality and quantity by artificial neural network using terrain and soil characteristics. Acta Agr. Scand B 2010, 60, 341–352. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Viggiani, R.; Venere, D.D.; Sergio, L.; Cantore, V.; Todorovic, M.; Perniola, M.; Candido, V. Impact of irrigation regime and nitrogen rate on yield, quality and water use efficiency of wild rocket under greenhouse conditions. Sci. Hortic-Amst. 2018, 229, 182–192. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in Southwest China. Plant Soil. 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Wu, M.; Ye, Y.Y.; Wang, K.L.; Li, D.J. Changes in soil nitrogen stocks following vegetation restoration in a typical karst catchment. Land Degrad. Dev. 2019, 30, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Rütting, T.; Kattge, J.; Laughlin, R.J.; Stevens, R.J. Estimation of parameters in complex 15N tracing models by Monte Carlo sampling. Soil Biol. Biochem. 2007, 39, 715–726. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.B.; Yang, H.; Zhang, J.B.; Yang, J.L.; Cao, J.H.; Bai, B.; Jiang, Z.C.; Liang, Y.M.; et al. Rapid recovery of nitrogen retention capacity in a subtropical acidic soil following afforestation. Soil Biol. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- Rütting, T.; Clough, T.J.; Müller, C.; Lieffering, M.; Newton, P.C.D. Ten years of elevated atmospheric carbon dioxide alters soil nitrogen transformations in a sheep–grazed pasture. Glob. Chang. Biol. 2010, 16, 2530–2542. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; Rousk, J.; Bååth, E. Fungal and bacterial recolonisation of acid and alkaline forest soils following artificial heat treatments. Soil Biol. Biochem. 2011, 43, 1023–1033. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Xiao, K.C.; Wang, K.L. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Li, D.J.; Yang, Y.; Chen, H.; Xiao, K.C.; Song, T.Q.; Wang, K.L. Soil gross nitrogen transformations in typical Karst and nonkarst forests, Southwest China. J. Geophys. Res Biogeo. 2017, 122, 2831–2840. [Google Scholar] [CrossRef]
- Garousi, F.; Shan, Z.J.; Ni, K.; Yang, H.; Shan, J.; Cao, J.H.; Jiang, Z.C.; Yang, J.L.; Zhu, T.B.; Müller, C. Decreased inorganic N supply capacity and turnover in calcareous soil under degraded rubber plantation in the tropical karst region. Geoderma 2021, 381, 114754. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhu, T.B.; Meng, T.Z.; Zhang, Y.C.; Yang, J.J.; Yang, W.Y.; Müller, C.; Cai, Z.C. Agricultural land use affects nitrate production and conservation in humid subtropical soils in China. Soil Biol. Biochem. 2013, 62, 107–114. [Google Scholar] [CrossRef]
- Shan, Z.J.; Yin, Z.; Yang, H.; Zuo, C.Q.; Zhu, T.B. Long-term cultivation of fruit plantations decreases mineralization and nitrification rates in calcareous soil in the karst region in Southwestern China. Forests 2020, 11, 1282. [Google Scholar] [CrossRef]
- Chu, H.Y.; Fujii, T.; Morimoto, S.; Lin, X.G.; Yagi, K. Population size and specific nitrification potential of soil ammonia–oxidizing bacteria under long–term fertilizer management. Soil Biol. Biochem. 2008, 40, 1960–1963. [Google Scholar] [CrossRef]
- Zhang, J.B.; Cai, Z.C.; Yang, W.Y.; Zhu, T.B.; Yu, Y.J.; Yan, X.Y.; Jia, Z.J. Long-term field fertilization affects soil nitrogen transformations in a rice-wheat-rotation cropping system. Plant Nutr. Soil Sci. 2012, 175, 939–946. [Google Scholar] [CrossRef]
- Yang, H.; Garousi, F.; Wang, J.; Cao, J.H.; Xu, X.L.; Zhu, T.B.; Müller, C. Land use effects on gross soil nitrogen transformations in karst desertification area. Plant Soil. 2021, 17. [Google Scholar] [CrossRef]
- Ross, D.S.; Ketterings, Q. Recommended methods for determining soil cation exchange capacity. Recomm. Soil Test. Proced. Northeast. USA 1995, 2, 62–70. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Sparks, D.L. Methods of Soil Analysis, Part 3. Chemical Methods. Madison, W.I., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 555–574. [Google Scholar]
- Aguilera, N.H.; Jackson, M.L. Iron oxide removal from soils and clays. Soil Sci. Soc. Am. J. 1953, 17, 359–364. [Google Scholar] [CrossRef]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Le, T.B.K.; Lajoie, B.R.; Dekker, J.; Laub, M.T.; Rudner, D.Z. Condensin promotes the juxtaposition of DNA flanking its loading site in Bacillus subtilis. Genes. Dev. 2015, 29, 1661–1675. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, D.; Bartholomew, W.V. Equations for following nutrient transformations in soil utilizing tracer data. Soil Sci. Soc. Am. Proc. 1954, 18, 33–34. [Google Scholar] [CrossRef]
- Corre, M.D.; Brumme, R.; Veldkamp, E.; Beese, F.O. Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany. Global Change Biol. 2007, 13, 1509–1527. [Google Scholar] [CrossRef]
- Qin, X.H.; Yang, C.; Yang, L.; Ma, E.; Meng, L.; Zhu, T.B. Response of Gross Mineralization and Nitrification Rates to Banana Cultivation Sites Converted from Natural Forest in Subtropical China. Land 2021, 10, 376. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhu, T.B.; Cai, Z.C.; Müller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil. 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Zhang, J.B.; Cai, Z.C.; Zhu, T.B.; Yang, W.Y.; Müller, C. Mechanisms for the retention of inorganic N in acidic forest soils of southern China. Sci. Rep. 2013, 3, 2342. [Google Scholar] [CrossRef] [PubMed]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions-a review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Kaiser, M.; Walter, K.; Ellerbrock, R.H.; Sommer, M. Effects of land use and mineral characteristics on the organic carbon content, and the amount and composition of Na-pyrophosphate-soluble organic matter, in subsurface soils. Eur. J. Soil Sci. 2011, 62, 226–236. [Google Scholar] [CrossRef]
- Xiao, K.C.; He, T.G.; Chen, H.; Peng, W.X.; Song, T.Q.; Wang, K.L.; Li, D.J. Impacts of vegetation restoration strategies on soil organic carbon and nitrogen dynamics in a karst area, Southwest China. Ecol. Eng. 2017, 101, 247–254. [Google Scholar] [CrossRef]
- Wan, Y.J.; Ju, X.T.; Ingwersen, J.; Schwarz, U.; Stange, C.F.; Zhang, F.S.; Streck, T. Gross nitrogen transformations and related nitrous oxide emissions in an intensively used calcareous soil. Soil Sci. Soc. Am. J. 2009, 73, 102–112. [Google Scholar] [CrossRef]
- Xiao, S.S.; Ye, Y.Y.; Xiao, D.; Chen, W.R.; Wang, K.L. Effects of tillage on soil N availability, aggregate size, and microbial biomass in a subtropical karst region. Soil Till. Res. 2019, 192, 187–195. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.J.; Xin, X.P.; Li, S.W.; Zhou, J.C.; Zhu, T.B.; Müller, C.; Cai, Z.C.; Wright, A.L. Effects of Fe oxide on N transformations in subtropical acid soils. Sci. Rep. 2015, 5, 8615. [Google Scholar] [CrossRef]
- Zhao, W.; Cai, Z.C.; Xu, Z.H. Does ammonium-based N addition influence nitrification and acidification in humid subtropical soils of China? Plant Soil 2007, 297, 213–221. [Google Scholar] [CrossRef]

| Properties | Woodland | 3-Year Plantation | 9-Year Plantation | 15-Year Plantation |
|---|---|---|---|---|
| SOC (g C kg−1) | 72.3 ± 6.64 a | 22.3 ± 2.11 b | 19.9 ± 2.62 b | 12.8 ± 1.33 c |
| TN (g N kg−1) | 6.83 ± 0.70 a | 2.87 ± 0.70 b | 2.30 ± 0.3 bc | 1.47 ± 0.13 c |
| C/N | 10.6 ± 0.14 a | 7.97 ± 1.19 b | 8.71 ± 0.87 b | 8.68 ± 0.18 b |
| pH | 6.52 ± 0.39 a | 6.47 ± 0.44 a | 6.10 ± 0.09 b | 5.57 ± 0.39 b |
| NH4+ (mg N kg−1) | 8.42 ± 5.62 a | 12.2 ± 4.82 a | 22.5± 13.2 a | 19.1 ± 8.73 a |
| NO3− (mg N kg−1) | 16.0 ± 0.81 a | 21.4 ± 2.74 a | 26.3± 14.4 a | 27.2 ± 11.8 a |
| WHC | 1.12 ± 0.11 a | 0.81 ± 0.02 b | 0.71 ± 0.06 bc | 0.68 ± 0.04 c |
| Clay (<2 µm) (%) | 53.6 ± 0.46 a | 50.9 ± 0.67 a | 52.0 ± 1.43 a | 54.4 ± 2.94 a |
| Silt (2–50 µm) (%) | 39.2 ± 2.16 a | 45.5 ± 2.08 a | 42.4 ± 4.46 a | 42.4 ± 2.82 a |
| Sand (50–2000 µm) (%) | 7.13 ± 2.06 a | 3.59 ± 1.61 a | 5.58 ± 3.21 a | 3.19 ± 0.82 b |
| CEC (cmol kg−1) | 58.1 ± 2.43 a | 24.9 ± 2.63 b | 19.6 ± 1.90 b | 20.3 ± 4.83 b |
| Available K (mg kg−1) | 92.9 ± 19.6 b | 322 ± 67.3 b | 372 ± 54.7 ab | 434 ± 31.1 a |
| Available P (mg kg−1) | 2.63 ± 0.31 b | 71.7 ± 17.1 a | 83.1 ± 13.8 a | 90.0 ± 13.7 a |
| K (g kg−1) | 3.63 ± 1.67 c | 10.7 ± 0.06 b | 10.5 ± 0.35 b | 13.2 ± 0.33 a |
| P (g kg−1) | 1.90 ± 0.32 b | 2.91 ± 0.27 a | 2.63 ± 0.24 a | 3.36 ± 0.55 a |
| Ca (g kg−1) | 56.4 ± 12.3 a | 8.00 ± 0.66 b | 6.30 ± 0.58 c | 3.18 ± 0.20 c |
| Mg (g kg−1) | 33.3 ± 6.37 a | 5.71 ± 0.34 b | 4.45 ± 0.99 b | 4.57 ± 0.75 b |
| Fe (g kg−1) | 62.3 ± 6.58 b | 69.8 ± 5.07 b | 77.0 ± 9.50 ab | 86.8 ± 4.18 a |
| Al (g kg−1) | 34.2 ± 6.22 c | 83.4 ± 4.96 b | 119 ± 14.6 a | 118 ± 14.8 a |
| Free Fe oxide (g kg−1) | 41.7 ± 2.84 b | 62.3 ± 4.57 a | 59.5 ± 7.25 a | 61.3 ± 3.80 a |
| Free Al oxide (g kg−1) | 12.8 ± 2.21 b | 15.8 ± 2.00 a | 16.5 ± 0.15 a | 17.4 ± 0.61 a |
| AOA abundance ×107 amoA gene copies (g dry soil)−1 | 6.70 ± 0.90 c | 41.9 ± 0.53 a | 23.1 ± 8.41 b | 20.4 ± 2.77 b |
| AOB abundance ×106 amoA gene copies (g dry soil)−1 | 2.54± 0.15 b | 13.6 ± 1.17 a | 3.24 ± 0.46 b | 1.88 ± 0.90 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, X.; Liu, J.; Wen, D.; Meng, L.; Zhu, T. Divergent Response of the Supply Capacity and Turnover of Inorganic Nitrogen to Pitaya Cultivation in the Subtropical Karst Region of Southwest China. Land 2022, 11, 781. https://doi.org/10.3390/land11060781
Yang L, Zhang X, Liu J, Wen D, Meng L, Zhu T. Divergent Response of the Supply Capacity and Turnover of Inorganic Nitrogen to Pitaya Cultivation in the Subtropical Karst Region of Southwest China. Land. 2022; 11(6):781. https://doi.org/10.3390/land11060781
Chicago/Turabian StyleYang, Lin, Xuebin Zhang, Jinxia Liu, Dongni Wen, Lei Meng, and Tongbin Zhu. 2022. "Divergent Response of the Supply Capacity and Turnover of Inorganic Nitrogen to Pitaya Cultivation in the Subtropical Karst Region of Southwest China" Land 11, no. 6: 781. https://doi.org/10.3390/land11060781
APA StyleYang, L., Zhang, X., Liu, J., Wen, D., Meng, L., & Zhu, T. (2022). Divergent Response of the Supply Capacity and Turnover of Inorganic Nitrogen to Pitaya Cultivation in the Subtropical Karst Region of Southwest China. Land, 11(6), 781. https://doi.org/10.3390/land11060781





