The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
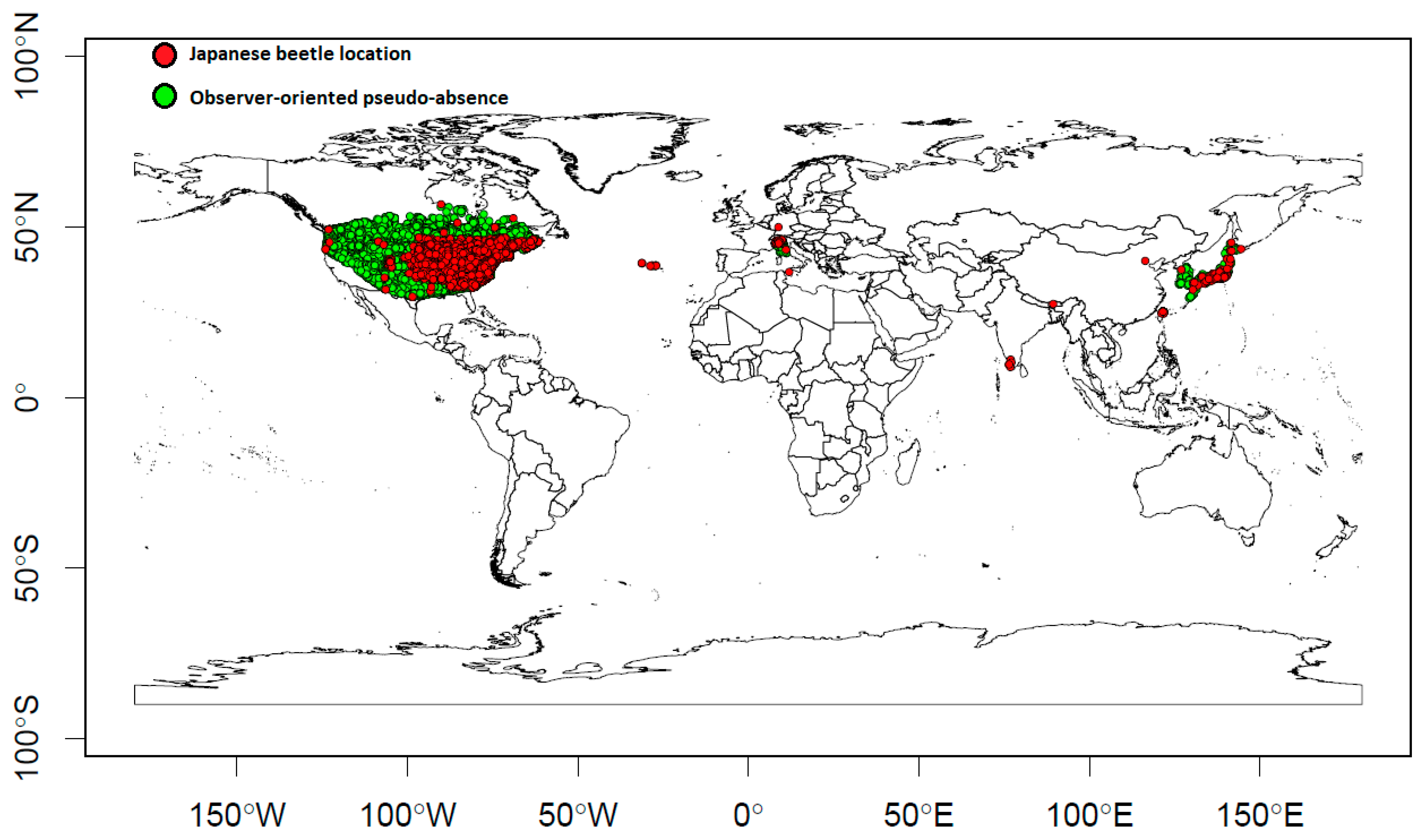
2.1. Presences and Observer-Oriented Pseudo-Absences
2.2. Predictor Variables
2.3. Species Distribution Models in INLA
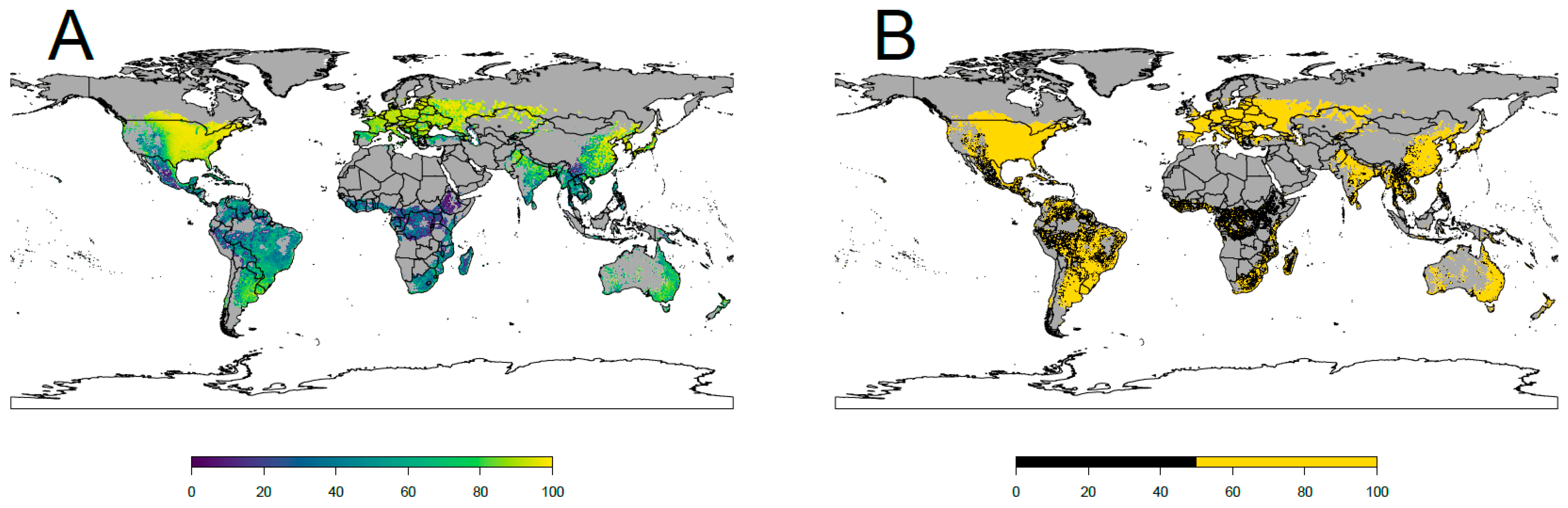
2.4. Definition of the Areas Suitable for the Colonization of the Japanese Beetle
3. Results
4. Discussion
4.1. Current Potential Distribution and Effect of Environmental Variables
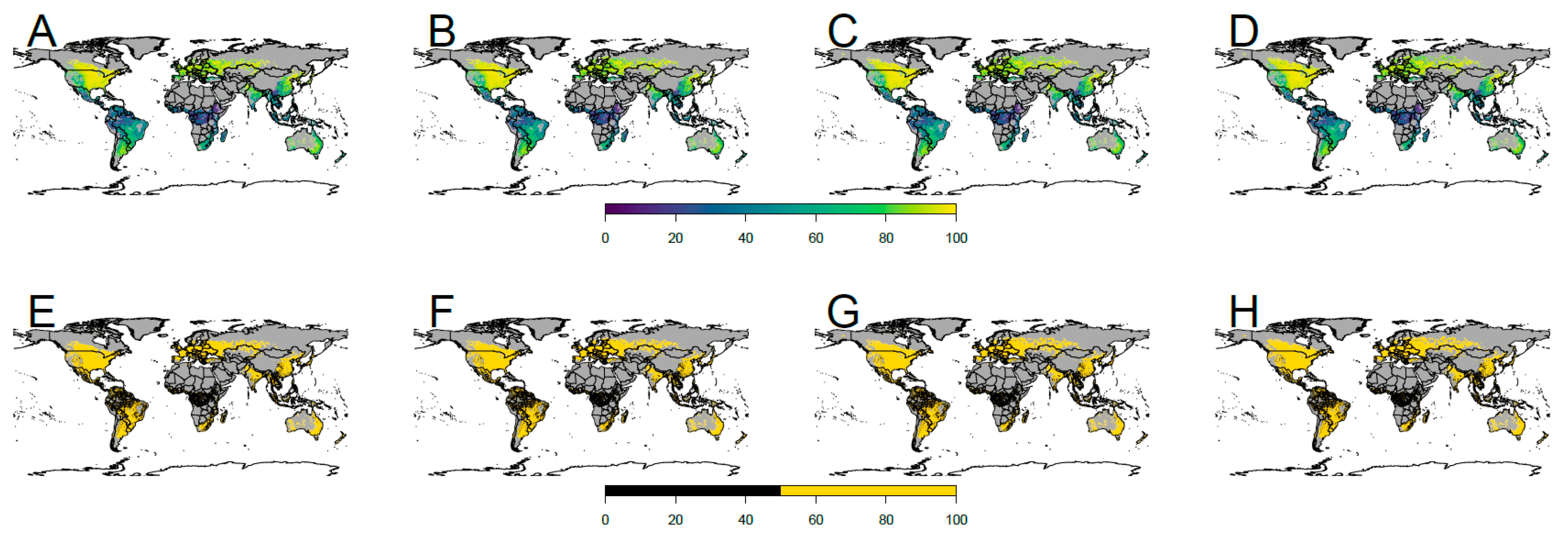
4.2. Future Potential Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung BReichard, S.; Mack, R.N.; Moyle, P.B.; Smith, M.; Andow, D.A.; Carlton, J.T. Biological invasions: Recommendations for U.S. policy and management. Ecol. Appl. 2006, 166, 2035–2054. [Google Scholar] [CrossRef] [Green Version]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Li, H.; Zhao, L. Incorporating anthropogenic variables into ecological niche modeling to predict areas of invasion of Popillia japonica. J. Pest Sci. 2017, 90, 151–160. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, J.M.; Bacher, S.; Blackburn, T.M.; Dick, J.T.A.; Essl, F.; Evans, T.; Gaertner, M.; Hulme, P.E.; Kühn, I.; Mrugała, A.; et al. Defining the Impact of Non-Native Species. Conserv. Biol. 2014, 28, 1188–1194. [Google Scholar] [CrossRef]
- Hulme, P.E. Invasion pathways at a crossroad: Policy and research challenges for managing alien species introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Dyer, E.E.; Cassey, P.; Redding, D.W.; Collen, B.; Franks, V.; Gaston, K.J.; Jones, K.; Kark, S.; Orme, C.D.L.; Blackburn, T.M. The Global Distribution and Drivers of Alien Bird Species Richness. PLoS Biol. 2017, 15, e2000942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, E.; Menchetti, M.; Zozzoli, R.; Milanesi, P. The importance of taxonomy in species distribution models at a global scale: The case of an overlooked alien squirrel facing taxonomic revision. J. Zool. 2019, 307, 43–52. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Milanesi, P.; Mori, E.; Menchetti, M. Observer-oriented approach improves species distribution models from citizen science data. Ecol. Evol. 2020, 10, 12104–12114. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.E. Biology of the Japanese Beetle; USDA Technical Bulletin; United States Department of Agriculture: Washington, DC, USA, 1972; Volume 1449, 129p.
- United States Department of Agriculture (USDA). Managing the Japanese Beetle: A Homeowner’s Handbook; APHIS 81-25-003; United States Department of Agriculture: Washington, DC, USA, 2015.
- Kistner-Thomas, E.J. The Potential Global Distribution and Voltinism of the Japanese Beetle (Coleoptera: Scarabaeidae) Under Current and Future Climates. J. Insect Sci. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- EFSAPlant Health Panel; Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Scientific Opinion on the pest categorisation of Popillia japonica. EFSA J. 2018, 16, e05438. [Google Scholar]
- iNaturalist. Available online: www.inaturalist.org (accessed on 10 February 2022).
- Barve, V.; Hart, E.; Guillou, S. Rinat: Access iNaturalist Data through APIs; R Package Version 0.1.8; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=rinat (accessed on 10 February 2022).
- Regniere, J.; Rabb, R.L.; Stinner, R.E. Popillia japonica: Simulation of temperature-dependent development of the immatures, and prediction of adult emergence. Environ. Entomol. 1981, 10, 290–296. [Google Scholar] [CrossRef]
- Calenge, C.; Fortmann-Roe, S. adehabitatHR: Home Range Estimation; R Package Version 0.4, 19; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=adehabitatHR (accessed on 10 February 2022).
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, Classification and Density Estimation Using Gaussian Finite Mixture Models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [Green Version]
- ASTER GDEM. Available online: https://www.jspacesystems.or.jp/ersdac/GDEM/E/ (accessed on 10 February 2022).
- European Space Agency Climate Change Initiative Land Cover Layers. Available online: https://www.esa-landcover-cci.org/?q=node/175 (accessed on 10 February 2022).
- Milanesi, P.; Della Rocca, F.; Robinson, R.A. Integrating dynamic environmental predictors and species occurrences: Toward true dynamic species distribution models. Ecol. Evol. 2020, 10, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Worldclim2 Dataset. Available online: https://www.worldclim.org/data/monthlywth.html (accessed on 10 February 2022).
- SEDAC 2000–2100 1-km Grid. Available online: https://sedac.ciesin.columbia.edu/data/set/popdynamics-1-km-downscaled-pop-base-year-projection-ssp-2000-2100-rev01/data-download# (accessed on 10 February 2022).
- Map of Airports in the World @ OurAirports. Available online: https://ourairports.com/world.html (accessed on 10 February 2022).
- AirLabs Data API. Available online: https://airlabs.co/ (accessed on 10 February 2022).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Landcover Projection 2050. Available online: https://www.arcgis.com/home/item.html?id=b5ee7191eda1425fa18c26532683896d (accessed on 10 February 2022).
- Worldclim2 Dataset Future Projections. Available online: https://www.worldclim.org/data/cmip6/cmip6_clim2.5m.html (accessed on 10 February 2022).
- Ihlow, F.; Courant, J.; Secondi, J.; Herrel, A.; Rebelo, R.; Measey, J.; Lillo, F.; De Villiers, F.A.; Vogt, S.; De Busschere, C.; et al. Impacts of Climate Change on the Global Invasion Potential of the African Clawed Frog Xenopus laevis. PLoS ONE 2016, 11, e0154869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Rocca, F.; Milanesi, P. Combining climate, land use change and dispersal to predict the distribution of endangered species with limited vagility. J. Biogeogr. 2020, 47, 1427–1438. [Google Scholar] [CrossRef]
- Pierce, D.W.; Barnett, T.P.; Santer, B.D.; Gleckler, P.J. Selecting global climate models for regional climate change studies. Proc. Natl. Acad. Sci. USA 2009, 106, 8441–8446. [Google Scholar] [CrossRef] [Green Version]
- Her, Y.; Yoo, S.-H.; Cho, J.; Hwang, S.; Jeong, J.; Seong, C. Uncertainty in hydrological analysis of climate change: Multi-parameter vs. multi-GCM ensemble predictions. Sci. Rep. 2019, 9, 4974. [Google Scholar] [CrossRef] [Green Version]
- CMIP Phase 6—CMIP6. Available online: https://www.wcrp-climate.org/wgcm-cmip/wgcm-cmip6 (accessed on 10 February 2022).
- Sung, H.M.; Kim, J.; Shim, S.; Seo, J.-B.; Kwon, S.-H.; Sun, M.-A.; Moon, H.; Lee, J.-H.; Lim, Y.-J.; Boo, K.-O.; et al. Climate Change Projection in the Twenty-First Century Simulated by NIMS-KMA CMIP6 Model Based on New GHGs Concentration Pathways. Asia-Pac. J. Atmos. Sci. 2021, 57, 851–862. [Google Scholar] [CrossRef]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian inference for latent Gaussian model by using integrated nested Laplace approximations (with discussion). J. R. Stat. Soc. Ser. B 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Engel, M.; Mette, T.; Falk, W. Spatial species distribution models: Using Bayes inference with INLA and SPDE to improve the tree species choice for important European tree species. For. Ecol. Manag. 2022, 507, 119983. [Google Scholar] [CrossRef]
- Blangiardo, M.; Cameletti, M.; Baio, G.; Rue, H. Spatial and spatio-temporal models with R-INLA. Spat. Spatio-Temporal Epidemiol. 2013, 4, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Minaya, J.; Cameletti, M.; Conesa, D.; Pennino, M.G. Species distribution modeling: A statistical review with focus in spatio-temporal issues. Stoch. Hydrol. Hydraul. 2018, 32, 3227–3244. [Google Scholar] [CrossRef]
- Beguin, J.; Martino, S.; Rue, H.; Cumming, S.G. Hierarchical analysis of spatially autocorrelated ecological data using integrated nested Laplace approximation. Methods Ecol. Evol. 2012, 3, 921–929. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H.; Lindström, J. An explicit link between Gaussian fields and Gaussian Markov random fields: The stochastic partial differential equation approach (with discussion). J. R. Stat. Soc. Ser. B 2011, 73, 423–498. [Google Scholar] [CrossRef] [Green Version]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package ‘Biomod2’. Species Distribution Modeling within an Ensemble Forecasting Framework. 2016. Available online: https://cran.microsoft.com/snapshot/2016-05-25/web/packages/biomod2/biomod2.pdf (accessed on 10 February 2022).
- Keller, V.; Herrando, S.; Voríšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvanová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Milanesi, P.; Herrando, S.; Pla, M.; Villero, D.; Keller, V. Towards continental bird distribution models: Environmental variables for the second European breeding bird atlas and identification of priorities for further surveys. Vogelwelt 2017, 137, 53–60. [Google Scholar]
- Ludwig, D. The Effects of Temperature on the Development of an Insect (Popillia japonica Newman). Physiol. Zool. 1928, 1, 358–389. [Google Scholar] [CrossRef]
- Korycinska, A.; Baker, R.H.A.; Eyre, D. Rapid Pest Risk Analysis (PRA) for: Popillia japonica; Defra: London, UK, 2015; p. 34.
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, A.; Bittner, T.; Reineking, B.; Beierkuhnlein, C. Can they keep up with climate change?—Integrating specific dispersal abilities of protected Odonata in species distribution modelling. Insect Conserv. Divers. 2013, 6, 93–103. [Google Scholar] [CrossRef]
- Caton, B.P.; Fang, H.; Manoukis, N.C.; Pallipparambil, G.R. Quantifying insect dispersal distances from trapping detections data to predict delimiting survey radii. J. Appl. Entomol. 2021, 146, 203–216. [Google Scholar] [CrossRef]
- Klein, M. Popillia japonica (Japanese Beetle); Invasive Species Compendium; CABI: Wallingford, UK, 2008. [Google Scholar]
- EPPO. EPPO Global Database. 2022. Available online: https://gd.eppo.int (accessed on 10 February 2022).
- CABI. Invasive Species Compendium. CAB International: Wallingford, UK, 2022. Available online: www.cabi.org/isc (accessed on 10 February 2022).
- Potter, D.A.; Held, D.W. Biology and Management of the Japanese Beetle. Annu. Rev. Entomol. 2002, 47, 175–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, T.A.; Klein, M.G. Scarabs as Pests: A Continuing Problem. Coleopt. Bull. 2006, 60, 102–119. [Google Scholar] [CrossRef]
- CFIA. Popillia japonica (Japanese Beetle); Canadian Food Inspection Agency: Ottawa, ON, Canada, 2020.
- EPPO. EPPO Standards: EPPO A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests; (PM 1/2(28)); EPPO: Paris, France, 2019. [Google Scholar]
- Dietz, H.; Edwards, P.J. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 2006, 87, 1359–1367. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Hui, C.; Ikeda, T.; Leday, G.; Richardson, D.M.; Carpintero, S.; Espadaler, X.; Gómez, C.; Guénard, B.; Hartley, S.; et al. Relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader. Proc. Natl. Acad. Sci. USA 2011, 108, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beans, C.M.; Kilkenny, F.F.; Galloway, L.F. Climate suitability and human influences combined explain the range expansion of an invasive horticultural plant. Biol. Invasions 2012, 14, 2067–2078. [Google Scholar] [CrossRef] [Green Version]
- Liebhold, A.M.; Work, T.T.; McCullough, D.G.; Cavey, J.F. Airline Baggage as a Pathway for Alien Insect Species Invading the United States. Am. Entomol. 2006, 52, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Lines, J. Chikungunya in Italy: Globalisation is to blame, not climate change. Br. Med. J. 2007, 335, 576. [Google Scholar] [CrossRef]
- McCullough, D.G.; Work, T.T.; Cavey, J.F.; Liebhold, A.M.; Marshall, D. Interceptions of Nonindigenous Plant Pests at US Ports of Entry and Border Crossings Over a 17-year Period. Biol. Invasions 2006, 8, 611–630. [Google Scholar] [CrossRef]
- Tatem, A.J.; Hay, S. Climatic similarity and biological exchange in the worldwide airline transportation network. Proc. R. Soc. B Boil. Sci. 2007, 274, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Pavesi, M.A. Popillia japonica specie aliena invasiva segnalata in Lombardia. L’Informatore Agrar. 2014, 32, 53–55. [Google Scholar]
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- Kanianska, R. Agriculture and Its Impact on Land-Use, Environment, and Ecosystem Services. In Landscape Ecology—The Influences of Land Use and Anthropogenic Impacts of Landscape Creation; Almusaed, A., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Ladd, T.L. Japanese beetle (Coleoptera: Scarabaeidae): Feeding by adults on minor host and non-host plants. J. Econ. Entomol. 1989, 82, 1616–1619. [Google Scholar] [CrossRef]
- Ladd, T.L. Influence of Sugars on the Feeding Response of Japanese Beetles (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1986, 79, 668–671. [Google Scholar] [CrossRef]
- Keathley, C.P. Determinants of Host Plant Selection in the Japanese Beetle. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 1998; 128p. [Google Scholar]
- Niziolek, O.K.; Berenbaum, M.R.; DeLucia, E.H. Impact of elevated CO2 and increased temperature on Japanese beetle herbivory. Insect Sci. 2013, 20, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, P.G.; Klein, M.G.; McCoy, E.L. Effect of Soil Moisture and Soil Texture on Oviposition by Japanese Beetle and Rose Chafer (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1992, 85, 2194–2200. [Google Scholar] [CrossRef]
- Bourke, P.A. Climatic Aspects of the Possible Establishment of the Japanese Beetle in Europe; Technical Note; World Meteorological Organization: Geneva, Switzerland, 1961; Volume 41, pp. 1–9. [Google Scholar]
- Chaney, N.W.; Roundy, J.K.; Herrera–Estrada, J.E.; Wood, E.F. High-resolution modeling of the spatial heterogeneity of soil moisture: Applications in network design. Water Resour. Res. 2015, 51, 619–638. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Zhao, W.; Wang, L.; Feng, Q.; Ding, J.; Liu, Y.; Zhang, X. Variations of deep soil moisture under different vegetation types and influencing factors in a watershed of the Loess Plateau, China. Hydrol. Earth Syst. Sci. 2016, 20, 3309–3323. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hu, Q. Groundwater influences on soil moisture and surface evaporation. J. Hydrol. 2004, 297, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Du, S.N.; Bai, G.S.; Liang, Y.L. Effects of soil moisture content and light intensity on the plant growth and leaf physiological characteristics of squash. Chin. J. Appl. Ecol. 2011, 22, 1101–1106. [Google Scholar]
- Rossato, L.; Marengo, J.A.; Angelis, C.F.; Pires, L.B.M.; Mendiondo, E.M. Impact of soil moisture over palmer drought severity index and its future projections in Brazil. Braz. J. Water Resour. 2017, 22, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hammond, R.B.; Stinner, B.R. Soybean Foliage Insects in Conservation Tillage Systems: Effects of Tillage, Previous Cropping History, and Soil Insecticide Application. Environ. Entomol. 1987, 16, 524–531. [Google Scholar] [CrossRef]
- Shanovich, H.N.; Dean, A.N.; Koch, R.L.; Hodgson, E.W. Biology and Management of Japanese Beetle (Coleoptera: Scarabaeidae) in Corn and Soybean. J. Integr. Pest Manag. 2019, 10, 9. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Everall, N.C.; Johnson, M.F.; Wilby, R.L.; Bennett, C.J. Detecting phenology change in the mayfly Ephemera danica: Responses to spatial and temporal water temperature variations. Ecol. Entomol. 2015, 40, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Petty, B.M.; Johnson, D.T.; Steinkraus, D.C. Changes in Abundance of Larvae and Adults ofPopillia japonica (Coleoptera: Scarabaeidae: Rutelinae) and Other White Grub Species in Northwest Arkansas and Their Relation to Regional Temperatures. Fla. Entomol. 2015, 98, 1006–1008. [Google Scholar] [CrossRef] [Green Version]
- Furlong, M.J.; Zalucki, M.P. Climate change and biological control: The consequences of increasing temperatures on host–parasitoid interactions. Curr. Opin. Insect Sci. 2017, 20, 39–44. [Google Scholar] [CrossRef] [Green Version]

| Variable | Unit | 2010–2020 | 2050 RCP | |||
|---|---|---|---|---|---|---|
| 2.6 | 4.5 | 7 | 8.5 | |||
| Altitude | m a.s.l. | 1.761 | 1.692 | 1.682 | 1.647 | 1.703 |
| Slope | ° | 1.548 | 1.537 | 1.506 | 1.507 | 1.542 |
| Bare areas | % | 1.991 | 2.001 | 1.816 | 1.792 | 1.854 |
| Deciduous forests | % | >3 | >3 | >3 | >3 | >3 |
| Grasslands, scrubs, shrubs | % | 1.586 | 1.621 | 1.618 | 1.547 | 1.611 |
| Needleleaf forests | % | >3 | >3 | >3 | >3 | >3 |
| Permanent snow and ice | % | >3 | >3 | >3 | >3 | >3 |
| Sparse vegetation | % | 1.329 | 1.354 | 1.379 | 1.322 | 1.385 |
| Waters | % | 1.364 | 1.256 | 1.244 | 1.209 | 1.241 |
| Wetlands | % | 1.163 | 1.155 | 1.163 | 1.141 | 1.145 |
| Croplands | % | 1.636 | 1.499 | 1.401 | 1.481 | 1.428 |
| Shannon habitat diversity index | H′ = −Σ (pi × lnpi) | 1.294 | 1.381 | 1.378 | 1.341 | 1.378 |
| Human settlements | % | 1.288 | 1.557 | 1.317 | 1.358 | 1.299 |
| Distance to airports | M | 1.487 | 1.416 | 1.474 | 1.448 | 1.451 |
| Human population density | n/km2 | 1.283 | 1.594 | 1.311 | 1.501 | 1.289 |
| Annual mean temperature | °C | >3 | >3 | >3 | >3 | >3 |
| Mean diurnal range | °C | 1.861 | 2.124 | 2.216 | 2.041 | 2.176 |
| Isothermality (BIO2/BIO7) | °C × 100 | >3 | >3 | >3 | >3 | >3 |
| Temperature seasonality | Std. Dev. × 100 | >3 | >3 | >3 | >3 | >3 |
| Max temperature of warmest month | °C | >3 | >3 | >3 | >3 | >3 |
| Min temperature of coldest month | °C | >3 | >3 | >3 | >3 | >3 |
| Temperature annual range | °C | 1.502 | 1.628 | 1.631 | 1.661 | 1.597 |
| Mean temperature of wettest quarter | °C | >3 | >3 | >3 | >3 | >3 |
| Mean temperature of driest quarter | °C | >3 | >3 | >3 | >3 | >3 |
| Mean temperature of warmest quarter | °C | >3 | >3 | >3 | >3 | >3 |
| Mean temperature of coldest quarter | °C | >3 | >3 | >3 | >3 | >3 |
| Annual precipitation | Mm | >3 | >3 | >3 | >3 | >3 |
| Precipitation of wettest month | Mm | >3 | >3 | >3 | >3 | >3 |
| Precipitation of driest month | Mm | 1.742 | 1.665 | 1.674 | 1.724 | 1.665 |
| Precipitation seasonality | Coeff. of variation | >3 | >3 | >3 | >3 | >3 |
| Precipitation of wettest quarter | Mm | >3 | >3 | >3 | >3 | >3 |
| Precipitation of driest quarter | Mm | >3 | >3 | >3 | >3 | >3 |
| Precipitation of warmest quarter | Mm | 1.976 | 2.205 | 2.198 | 2.149 | 2.142 |
| Precipitation of coldest quarter | Mm | >3 | >3 | >3 | >3 | >3 |
| Parameter | β ± S.D. |
|---|---|
| Intercept | −5.971 ± 0.938 * |
| Altitude | −1.001 ± 0.156 * |
| Bare areas | 0.065 ± 0.372 |
| Croplands | 0.928 ± 0.029 * |
| Distance to airports | −3.406 ± 0.423 * |
| Grasslands, scrubs, and shrubs | 0.029 ± 0.038 |
| Human population density | 0.059 ± 0.014 * |
| Shannon habitat diversity index | 0.208 ± 0.021 * |
| Slope | −0.081 ± 0.051 |
| Sparse vegetation | 0.537 ± 1.331 |
| Human settlements | 0.067 ± 0.006 * |
| Waters | −0.131 ± 0.022 * |
| Precipitation of driest month | 0.147 ± 0.101 |
| Precipitation of warmest quarter | 0.041 ± 0.197 |
| Mean diurnal range | −0.451 ± 0.188 * |
| Temperature annual range | 1.611 ± 0.537 * |
| Wetlands | −0.254 ± 0.041 * |
| Theta1 | −1.091 ± 0.108 |
| Theta2 | −1.351 ± 0.218 |
| DIC | 22,936.001 |
| WAIC | 22,919.409 |
| Region | km2 | Occupied Areas (%) | |
|---|---|---|---|
| Current Distribution | Suitable Areas | ||
| Europe | 62,181.10 | 10,476,600 | 0.59 |
| Asia (+Russia) | 416,362.2 (native) | 11,757,200 | 3.54 |
| North America | 5,619,197.90 | 11,091,425 | 50.66 |
| Central and South America | 0.00 | 9,376,050 | 0.00 |
| Africa | 0.00 | 1,966,700 | 0.00 |
| Australia | 0.00 | 3,302,225 | 0.00 |
| World | 6,097,741.20 | 47,970,200 | 12.71 |
| Region | RCP 2.6 | RCP 4.5 | RCP 7.0 | RCP 8.5 | |
|---|---|---|---|---|---|
| Suitable Areas (km2) | Europe | 11,154,575 | 11,911,975 | 12,305,175 | 12,595,200 |
| Asia (+Russia) | 13,133,025 | 13,828,025 | 14,240,100 | 14,543,875 | |
| North America | 14,938,800 | 16,131,275 | 16,539,050 | 17,640,250 | |
| Central and South America | 8,412,675 | 8,480,325 | 8,460,525 | 8,525,250 | |
| Africa | 2,346,125 | 2,342,275 | 2,397,400 | 2,385,500 | |
| Australia | 3,433,000 | 3,432,075 | 3,448,425 | 3,436,750 | |
| World | 53,418,200 | 56,125,950 | 57,390,675 | 59,126,825 | |
| Reachable areas (km2) | Europe | 5,638,775 | 5,724,350 | 5,749,175 | 5,774,200 |
| Asia (+Russia) | 1,377,825 | 1,373,400 | 1,377,150 | 1,376,525 | |
| North America | 14,387,900 | 15,498,550 | 15,843,575 | 16,878,550 | |
| Central and South America | 0 | 0 | 0 | 0 | |
| Africa | 0 | 0 | 0 | 0 | |
| Australia | 0 | 0 | 0 | 0 | |
| World | 21,404,500 | 22,596,300 | 22,969,900 | 24,029,275 | |
| Occupied areas (%) | Europe | 50.55 | 48.06 | 46.72 | 45.84 |
| Asia (+Russia) | 10.49 | 9.93 | 9.67 | 9.46 | |
| North America | 96.31 | 96.08 | 95.79 | 95.68 | |
| Central and South America | 0.00 | 0.00 | 0.00 | 0.00 | |
| Africa | 0.00 | 0.00 | 0.00 | 0.00 | |
| Australia | 0.00 | 0.00 | 0.00 | 0.00 | |
| World | 40.07 | 40.26 | 40.02 | 40.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rocca, F.; Milanesi, P. The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios. Land 2022, 11, 567. https://doi.org/10.3390/land11040567
Della Rocca F, Milanesi P. The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios. Land. 2022; 11(4):567. https://doi.org/10.3390/land11040567
Chicago/Turabian StyleDella Rocca, Francesca, and Pietro Milanesi. 2022. "The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios" Land 11, no. 4: 567. https://doi.org/10.3390/land11040567
APA StyleDella Rocca, F., & Milanesi, P. (2022). The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios. Land, 11(4), 567. https://doi.org/10.3390/land11040567






