Abstract
Red cabbage is known as the millennium’s functional food, which has a lot of importance in our diet because of the health-promoting ingredients present in it. The current study investigated the synergistic relationship of rhizospheric-competent microbial agents (Trichoderma harzianum, Pseudomonas fluorescens, and Bacillus subtilis) in modulating the performance of red cabbage under the field conditions of Middle Gangetic Plains, India. Growth parameters were studied at three developmental stages, viz., pre-cupping, early head formation, and maturity. Our results suggested that the dual application of T. harzianum + P. fluorescens along with the 75% recommended dose of fertilizers (RDF) increased the number of leaves (24.6), leaf area (537.2 cm2), root length (19.8 cm), and micronutrient uptake (Fe, Mn, and Cu) by head of the crop, whereas the co-inoculation of P. fluorescens and B. subtilis along with 75% RDF enhanced plant spread (39.0 cm), earliness (95.2 days), and Zn uptake. Maximum plant height (28.7 cm) and chlorophyll (SPAD, 77.3) were recorded in 100% RDF (120:60:60 kg ha−1) and the combination of T. harzianum + B. subtilis along with 75% RDF, respectively. Interestingly, consortium (T. harzianum + P. fluorescens) bio-primed plants recorded about 14% higher root length in comparison to plants receiving sole fertilizers. The regression analysis revealed a significant relationship of Fe and Mn uptake with chlorophyll (SPAD) and between Zn uptake and the earliness of the crop. The present study indicated that seedling bio-priming with the dual consortium of efficient bio-agents is a viable strategy to lessen our dependence on chemical fertilizers for improving red cabbage production.
1. Introduction
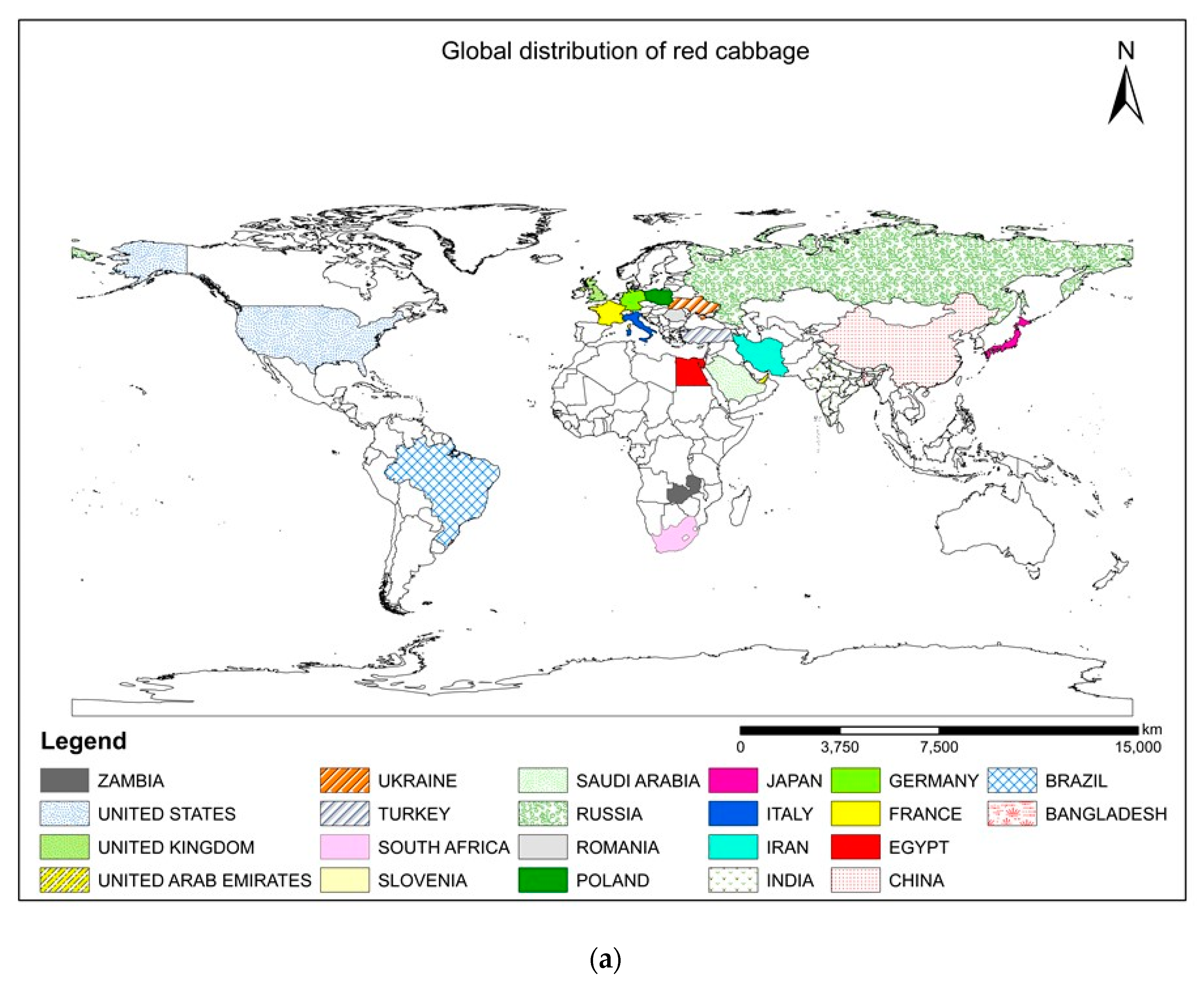
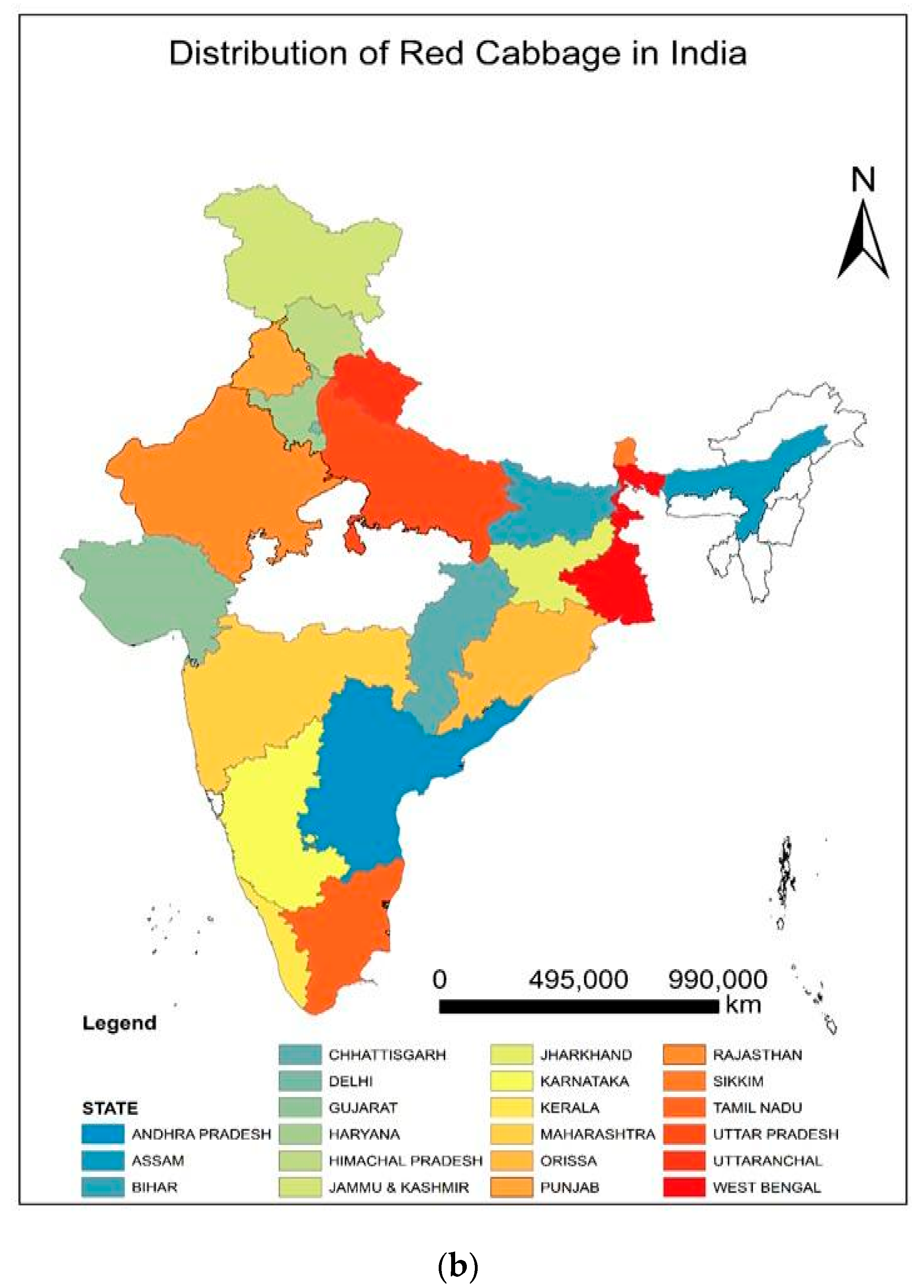
Red cabbage [Brassica oleracea L. var. capitata L. f. rubra (L.) Thell], commonly known as the modern multitasker’s dream food, has recently gained increased attention around the world. This remarkable nutritious vegetable is an excellent source of health-promoting ingredients that can boost our immune system [1]. The choice of crops is also an important armament in our arsenal against degenerative diseases that have devastated human livelihoods and crippled the global economy. The abundance of anthocyanins, carotenoids, glucosinolates, antioxidants, vitamins (C, E, A, and K), minerals (K, Ca, Mg, Fe, and Mn), and phenols in red cabbage make it a suitable supplement for preventions against oxidative stress, cardiovascular disease, cancer, obesity, and diabetes [2,3]. Therefore, it is directly consumed as microgreens (Figure 1) and salads and also cooked as a vegetable. The microgreens of this plant contain higher β-carotene (~260-fold), vitamin C (2.4−6-fold), and vitamin E (>40 times) than the matured ones [4]. Natural dyes are extracted from red cabbage due to the presence of high levels of anthocyanins (40–188 mg Cy 3-glcE 100 g−1 FW), which are suitable for coloring food products [5]. The nutritional value of red cabbage is greater than white cabbage in terms of antioxidants [6], vitamins, and minerals [7]. This will fetch farmers higher market prices and sell their products in supermarkets [8]. Although cole crops are native to the Mediterranean region, currently, red cabbage is cultivated globally [3,5] (Figure 2a). The geographical distribution maps can highlight the crop’s popularity and direct the need for suitable production strategies to maintain sustainable agriculture. Red cabbage is grown in all of Europe [2], North America, Asia, China [7], Brazil [9], South Africa [1], Japan, and many other countries of Asia and Africa [3,10,11]. Growing belts of this cruciferous leafy vegetable can be found in almost all states of India [12,13] (Figure 2b).

Figure 1.
Transition of tender immature seedlings (microgreens) of red cabbage to mature seedlings.
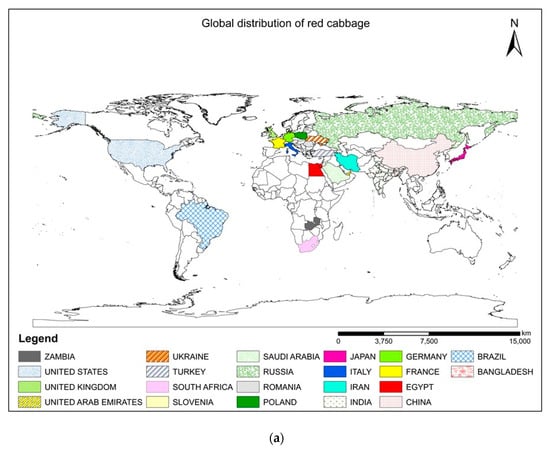
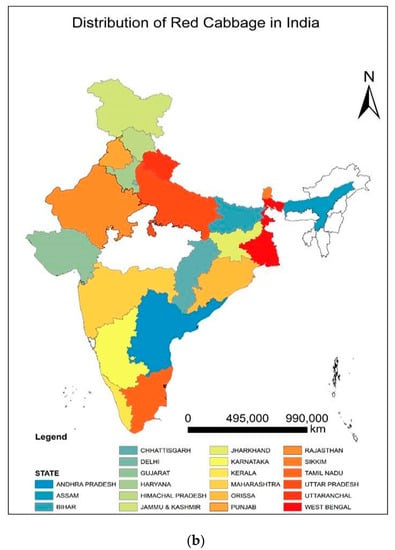
Figure 2.
(a) World map showing the major countries adapting red cabbage cultivation. (b) Different states of India promoting red cabbage cultivation.
Fertilization is an important strategy to meet the demands of the burgeoning population, which is expected to rise from the current state of 7.8 billion to 9.7 billion by 2050 [14]. Our entire dependence on these costly chemicals has severely affected the physical, chemical, and biological properties of soil all over the world [15]. In the case of nitrogen, poor management results in its loss in the form of volatilization (NH3), denitrification (NO and N2O), and leaching (NO3−). This indicates that we should search for alternative strategies or planet-friendly food production practices that could minimize environmental pollution and ensure sustainable and profitable food production hand in hand [16].
Rhizosphere modification has great relevance to soil fertility and plant nutrition for sustainable agriculture. The establishment of rhizo-microbiome will provide better nutrient supply, water absorption, and nutrient assimilation in plants with greater anchorage in soil [17,18]. Bio-priming is the best microbial delivery system because it requires a small volume of inoculums [19]. Bio-priming is a pre-sowing treatment of plants with beneficial microorganisms [20]. This technology triggers microbial colonization along with their proliferation in the rhizosphere which increases seedling vigour, nutrient and water uptake, systemic resistance to diverse stresses, and vegetative growth resulting in uniform stand [21,22]. Basically, it is a combined package covering several physiological facets and biological aspects of plant-microbe interactions. Several reports accredit plant growth promotion through modified metabolic pathways in bio-primed plants and enhanced nutrient use efficiency with changes in root architecture and other mechanisms [8,23,24]. Eligible candidates of bio-priming comrpising species of Trichoderma, Pseudomonas, Bacillus, Azotobacter, Azospirillum, Glomus, etc., have been found to enhance plant growth [25,26]. Furthermore, co-inoculation studies claimed the enhancement of synergistic effects with the application of microbial consortiums, as compared to a single inoculation of bio-agents [27,28].
According to the latest estimate by the World Health Organization (WHO), micronutrient deficiency has affected more than 2 billion people in the world [29]. Unfortunately, we have less provisions to track the micronutrient removal from the soil by crops. The proper evaluation of micronutrient status is very imperative because their deficiencies and toxicity range/limit in soil are narrow. Red cabbage is a nutrient exhaustive crop [1,8].
There are few published works on bio-primed red cabbage plants. In this backdrop, an attempt was made to examine the effect of bio-priming intervention on integrated nutrient management on growth and micronutrient uptake of red cabbage. We were also interested in monitoring the priming effect of individual bio-agent as well as the significance of a microbial consortium on the growth promotion of this exotic crop.
2. Materials and Methods
2.1. Seedling Bio-Priming
Seeds (truthful label) of red cabbage (B. oleracea, variety F1 Hybrid Red Ruby-2) were sown in a nursery (raised bed) about 1 month prior to transplanting. Pseudomonas fluorescens (OKC; GenBank accession No. JN128891) and Bacillus subtilis (BHHU100; GenBank accession No. JN099686) were grown in nutrient broth at 150 rpm, while Trichoderma harzianum (BHU P4; GenBank accession No. MH730446) was grown on a potato dextrose agar at 28 ± 2 °C. These three rhizosphere bio-agents were collected from the Department of Mycology and Plant Pathology, Institute of Agricultural Sciences, Banaras Hindu University. The process of selection was based on their plant growth-promoting traits and compatibility with each other [30]. Seedlings were handpicked when there were 5–6 leaves and then inoculated with bio-agents (liquid culture) using an adhesive agent (2% carboxymethyl cellulose). Bacterial and fungal populations were adjusted to 4 × 108 CFU mL−1 and 2 × 107 CFU mL−1, respectively. Cell suspensions and/or spore suspension were mixed in 1:1 ratio for dual consortium and 1:1:1 ratio for triple consortium.
2.2. Study Area Description
The present investigation was carried out at the Vegetable Research Farm of the Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India, during two successive rabi (winter) seasons of 2016–2017 and 2017–2018. The experimental site (25°26′ N, 82°99′ E, and 80.7 m above mean sea level) represents the Middle Gangetic Plains (agro-ecological region) of Eastern India. The soil of the experimental area is classified as Typic Ustochrept (order Inceptisol). Pre-cropping soil analysis revealed that the soil is slightly alkaline in reaction (7.58); low in organic carbon (4.04 g kg−1) and available N (203.21 kg ha−1) content; medium in available P (20.86 kg ha−1) and available K (217.73 kg ha−1) content; and sandy loam in texture [31]. The mean maximum and minimum temperatures recorded during the study period were 30.0 °C and 7.06 °C, respectively (Table 1). Meteorological data were obtained from the Department of Agronomy of our Institute.

Table 1.
Monthly meteorological data of Varanasi for the study period.
2.3. Experimental Details
Altogether, 9 treatments, viz., T1: Absolute control N:P2O5:K2O @ 0:0:0 kg ha−1; T2: Recommended dose of fertilizers (RDF) of N:P2O5:K2O @ 120:60:60 kg ha−1; T3: 75% RDF + Trichoderma harzianum; T4: 75% RDF + Pseudomonas fluorescens; T5: 75% RDF + Bacillus subtilis; T6: 75% RDF + T. harzianum + P. fluorescens; T7: 75% RDF + P. fluorescens + B. subtilis; T8: 75% RDF + T. harzianum + B. subtilis; and T9: 75% RDF + T. harzianum + P. fluorescens + B. subtilis, were laid out in a randomized block design with three replications. Seedlings (five-weeks-old) were inoculated with bio-agents and transplanted in plots (4 × 2 m2) at a spacing of 50 cm × 50 cm intervals. The full doses of P and K in the form of diammonium phosphate (DAP; 18% N, 46% P2O5) and muriate of potash (MOP; 60% K2O), respectively, were applied as basal. Nitrogen in the form of urea was applied in 3 splits, i.e., basal and top dressing at 30 and 45 days after transplanting (DAT) of red cabbage seedlings.
2.4. Biometric Observations
All data related to the growth response of cabbage were collected at different stages (Figure 3) of crop growth, viz., 30 (pre-cupping), 60 (early head formation), and 90–110 (maturity or harvesting) days after transplanting (DAT), except root length and earliness.


Figure 3.
Photographs showing the three prominent growth stages at which growth parameters were studied.
2.4.1. Plant Height
A measuring scale was used to measure the height (cm) of the plant from ground level to top surface of the highest leaf.
2.4.2. Number of Leaves
At monthly intervals, the number of leaves per plant was counted.
2.4.3. Plant Spread
The average distance (cm) between the two outermost leaves of the crop was recorded as its spread.
2.4.4. Chlorophyll (SPAD)
A portable Leaf Chlorophyll Meter or Soil Pant Analysis Development (SPAD) 502 Plus Chlorophyll Meter (Konica Minolta, Japan) was used for measuring the chlorophyll content (greenness) of leaves.
2.4.5. Leaf Area
Leaf Area Meter (Model 211, Systronics) was used to measure the area of leaves in cm2 unit.
2.4.6. Root Length
The root length was estimated by line intersect method as outlined by Tennant [32]. Roots were spread out in a box containing square grids and a thin layer of water. The number of intersections between grid lines (vertical and horizontal) and roots was counted and converted to measurements (length) using the following formula.
Root length (cm) = 11/14 × Number of intercepts × Grid unit
2.4.7. Earliness
The number of days to head maturity was calculated from the date of transplanting to the date of the formation of maximum marketable heads.
2.5. Micronutrient Uptake
The uptake of micronutrients by the head of red cabbage was determined by multiplying their respective concentration with head biomass.
The contents of micronutrients (Fe, Mn, Zn, and Cu) were estimated in an atomic absorption spectrophotometer (Model 240 FS, Agilent Technologies, Mulgrave, Australia) after the diacid digestion (HNO3:HClO4: 9:4) of plant samples.
2.6. Statistical Analysis
The experimental setup consists of nine treatments and three replications. Data presented as mean were analyzed through Statistical Package for Social Science (SPSS, version 20) software. Data were subjected to analysis of variance (ANOVA) F test with the total degrees of freedom being 26. The significance of the difference between treatment means was analyzed using Duncan’s multiple range test (DMRT) at p ≤ 0.05 significance level. Pooled data represented the average value of two years. Regression analysis was executed to find out the relationship between the chlorophyll, root length, earliness, and micronutrient uptake (Fe, Mn, Zn, and Cu).
3. Results
3.1. Effect on Plant Height
Data on plant height were periodically collected at 30 DAT, 60 DAT, and maturity (Table 2). At the early stage (30 DAT), no significant variation (p ≤ 0.05) was noticed in the height of the crop. The highest plant height of 24.1 and 24.4 cm was observed under 100% RDF (T2), and the lowest plant height of 21.6 and 21.7 cm was observed under absolute control (T1) during first and second season, respectively. During the early head formation stage (60 DAT), the tallest plants (28.7 cm) were recorded under T2, followed by the plants (28.7 cm) under 75% RDF + P. fluorescens + B. subtilis (T7). Pooled analysis at harvest revealed maximum height (30.7 cm) in T7, which was 3% higher over T2. Results were recorded in following order: T7 > T8 > T2 > T3 > T6 > T5 > T9 > T4 > T1.

Table 2.
Effect of bio-priming and fertilization on the height of red cabbage at different growth stages.
3.2. Effect on Number of Leaves
The treatments did not show any significant variation (p ≤ 0.05) in the number of leaves during the pre-cupping stage (Table 3). The maximum (16.3) and minimum (14.2) numbers of leaves at this stage were registered in 75% RDF + P. fluorescens + B. subtilis (T7) and absolute control (T1), respectively. The number of leaves was the highest during the early head formation stage (60 DAT), and it varied from 21.2 to 24.6 (pooled data). At 60 DAT, the application of 75% RDF + T. harzianum + P. fluorescens (T6) and 75% RDF + P. fluorescens + B. subtilis (T7) showed an equivalent effect on this growth parameter. Compared to 100% RDF (T2), these two bio-priming treatments recorded a 4% increment in leaf number. Again, pooled analysis revealed a similar trend between 75% RDF + T. harzianum (T3) and 75% RDF + T. harzianum + P. fluorescens + B. subtilis (T9). Results revealed the superiority of the dual consortium over the triple consortium, single-species bio-priming treatments, and non-primed treatments. Among the three priming agents, the number of leaves was highest in P. fluorescens-treated plants. The number of leaves reduced at the maturity stage. Furthermore, more leaves at this stage will be hampered in head formation. Therefore, the number of leaves was the highest (12.2) in the control plants. The lowest number of leaves (9.9) was observed in 75% RDF + T. harzianum (T3).

Table 3.
Effect of bio-priming and fertilization on the number of leaves of red cabbage at different growth stages.
3.3. Effect on Plant Spread
The application of 75% RDF + P. fluorescens + B. subtilis (T7) recorded the maximum (28.4 cm) spread of plants at 30 DAT (Table 4). Results also demonstrated that the spread of single-species and triple-species bio-primed plants were at par with each other. Among the individual bio-priming agents, P. fluorescens attained the highest value (27.4 cm). At 60 DAT, plant spread varied from 34.3 to 39.1 cm. The dual consortium attained more plant spread (Figure 4) than other treatments, and their performance was in the order of T7 > T6 > T8 (as revealed from pooled analysis). Plants spread increased by 13% in T7 as compared to the control treatment. A full dose of chemical fertilizer yielded at par results with the combined use of a reduced fertilizer and P. fluorescens. The average plant spread at maturity was 45.5 cm. The maximum (48.5 cm) spread at this stage was observed under T7 during the first season, while T6 attained maximum (48.5 cm) spread during the second season. The performance of single-species bio-priming treatments was in the following order: T4 > T3 > T5.

Table 4.
Effect of bio-priming and fertilization on spread of red cabbage at different growth stages.
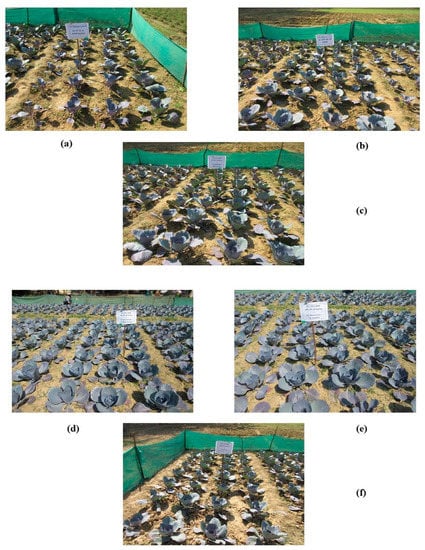
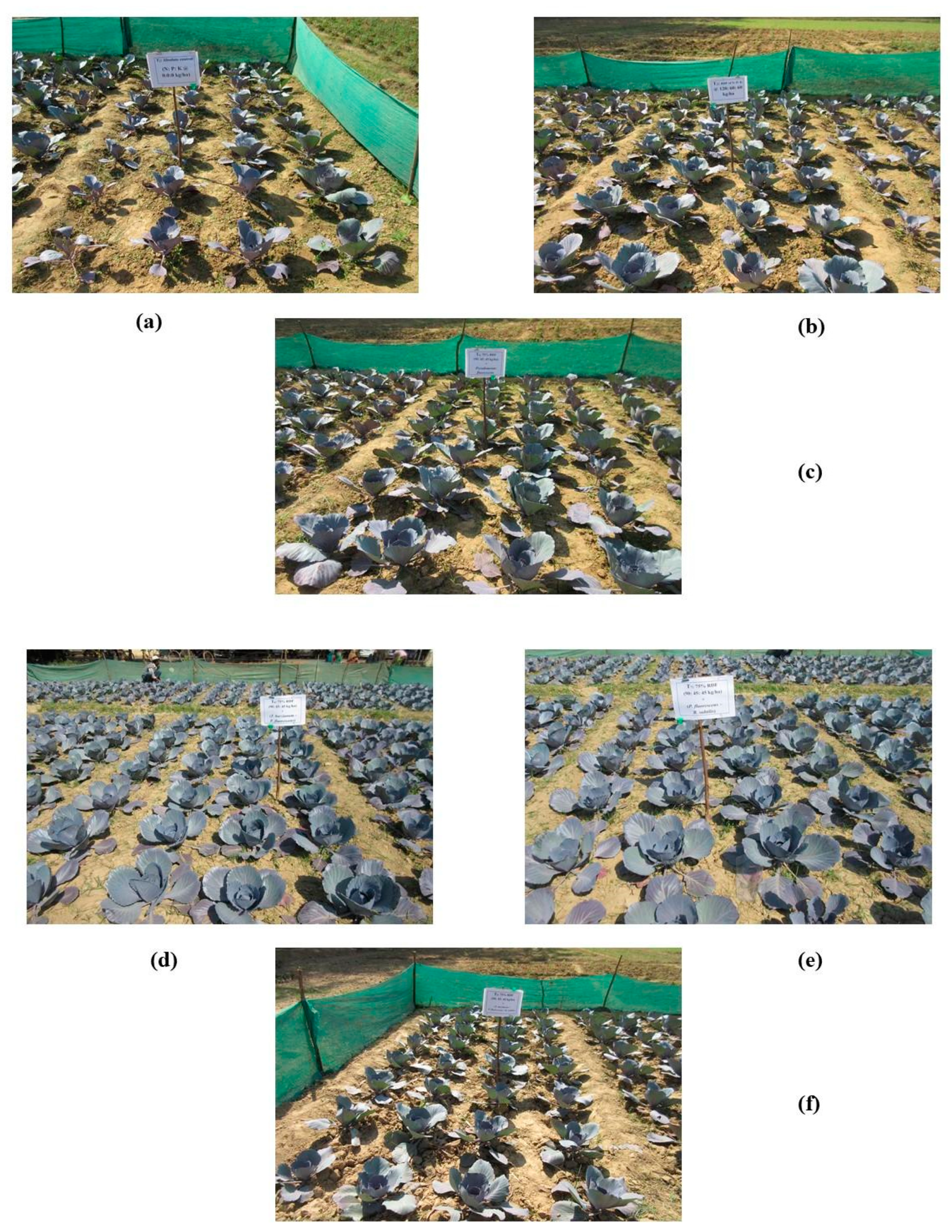
Figure 4.
Effective photographs of field experiment involving treatments; T1: Absolute control N:P2O5:K2O @ 0:0:0 kg ha−1 (a); T2: RDF of N:P2O5:K2O @ 120:60:60 kg ha−1 (b); T4: 75% RDF + Pseudomonas fluorescens (c); T6: 75% RDF + T. harzianum + P. fluorescens (d); T7: 75% RDF + P. fluorescens + B. subtilis (e); T9: 75% RDF + T. harzianum + P. fluorescens + B. subtilis (f).
3.4. Effect on Chlorophyll (SPAD)
The chlorophyll (SPAD) data of the red cabbage plant were recorded at 30 DAT, 60 DAT, and harvest (Table 5). Throughout the growth period, treatments remained at par with each other except the absolute control. At 30 DAT, the highest SPAD value (60.0) was noted in the T7 treatment (75% RDF + P. fluorescens + B. subtilis), while the lowest value (47.7) was reported in the T1 treatment (absolute control). As revealed from pooled data, the maximum value (77.3) was observed at 60 DAT under 75% RDF + T. harzianum + B. subtilis (T8), which was significantly (p ≤ 0.05) higher (23%) than the absolute control (T1). This treatment also showed the highest value (69.9) at harvest stages. It was significantly greater (21%) than compared to the absolute control. On average, our study showed the following trend: T8 > T6 > T7 > T4 > T2 > T5 > T9 > T3 > T1.

Table 5.
Effect of bio-priming and fertilization on chlorophyll (SPAD) of red cabbage at different growth stages.
3.5. Effect on Leaf Area
Data pertaining to leaf area revealed no significant (p ≤ 0.05) difference among different treatment combinations (Table 6). At 30 DAT, the leaf area ranged between 185.9 and 190.9 cm2 (pooled data). With the advancement in crop age, the leaf area increased progressively, but the rate of increment slowed after 60 DAT. Similarly to chlorophyll (SPAD) data, treatments remained at par with each other except the absolute control during the crop growth period. However, the maximum value (537.2 cm2) at 60 DAT showed a significant (p ≤ 0.05) effect of 75% RDF + T. harzianum + P. fluorescens (T6) over the absolute control (T1) and 75% RDF + T. harzianum (T3) as per the pooled analysis. At harvest, the leaf area ranged from 639.2 to 661.8 cm2 with an average value of 657.9 cm2. The application of 75% RDF + T. harzianum + B. subtilis (T8) resulted in the highest leaf area of 661.9 cm2 in the first year, while highest value of 661.8 cm2 was observed in the second year under 75% RDF + P. fluorescens + B. subtilis (T7) during the maturity of the crop. Plants bio-primed with P. fluorescens exhibited higher leaf area than other single inoculated plants at all growth stages.

Table 6.
Effect of bio-priming and fertilization on leaf area of red cabbage at different growth stages.
3.6. Effect on Root Length
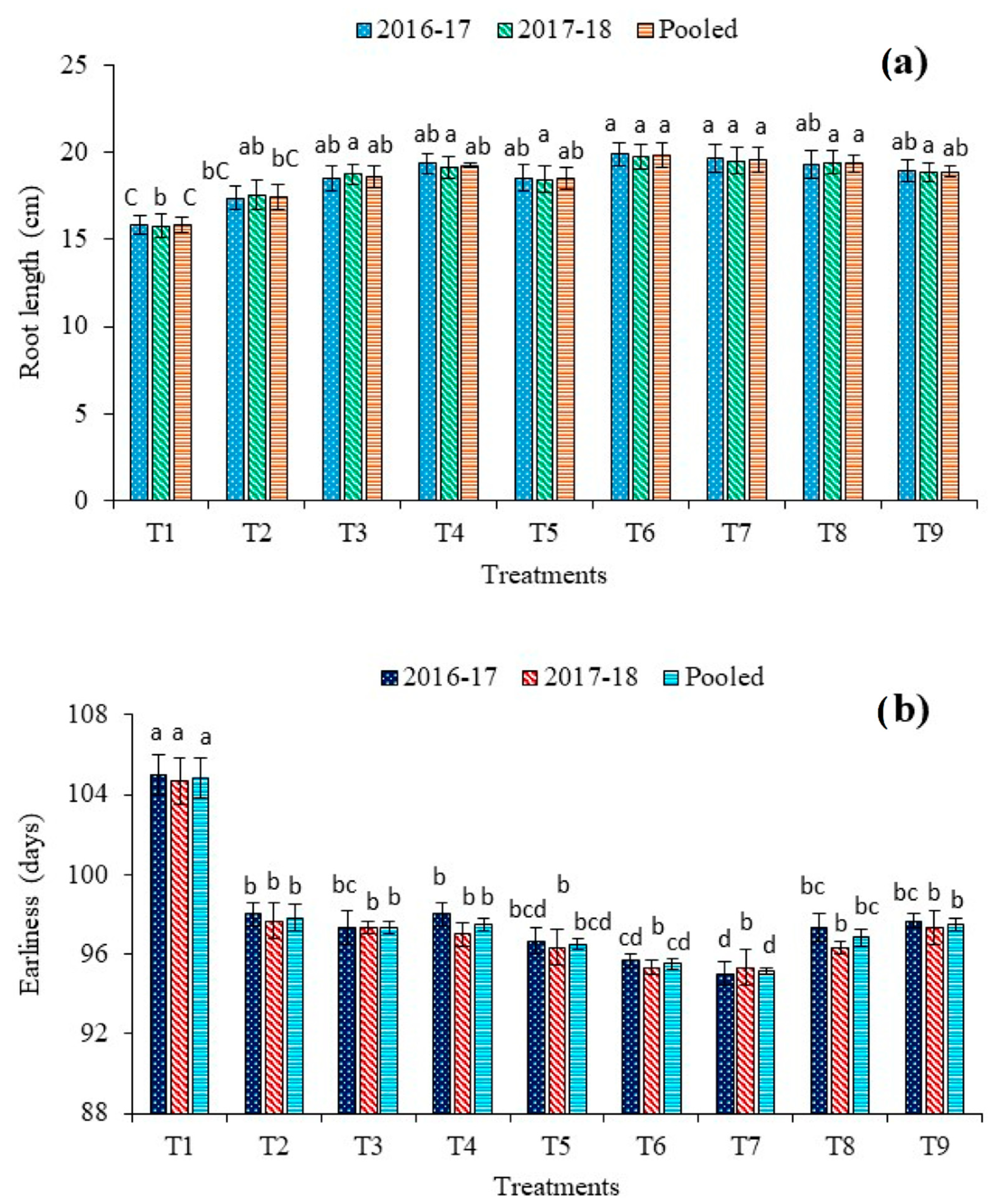
A perusal of root length data indicated a positive influence of dual microbial priming when applied along with the reduced dose of fertilizers over the application of a full dose of fertilizers and absolute control (Figure 5). The longest roots of 19.8 cm and shortest roots of 15.8 cm were found under 75% RDF + T. harzianum + P. fluorescens (T6) and absolute control (T1), respectively. In comparison to 100% RDF (T2) and 75% RDF + T. harzianum + P. fluorescens + B. subtilis (T9), about 14% and 5%, respectively, a higher length of the root was recorded in T6. However, all dual consortium treatments were at par with each other. Plants bio-primed with P. fluorescens exhibited higher root length than other single inoculated plants. The effect of triple consortium was non-significant (p ≤ 0.05) when compared with single-species and dual-species bio-priming treatments.
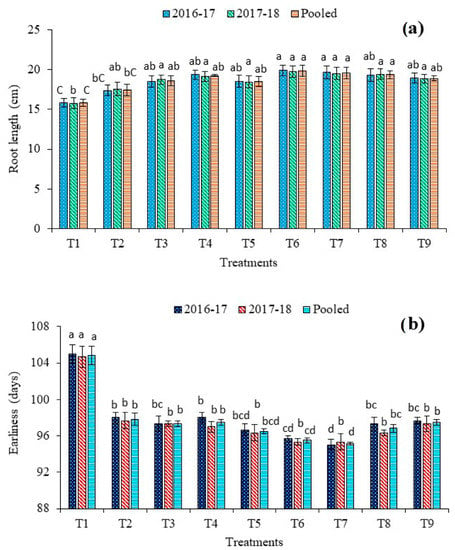
Figure 5.
Effect of bio-priming and fertilization on (a) root length and (b) earliness of red cabbage. Bars (mean ± SE; n = 3) followed by different alphabets significantly differ (p ≤ 0.05) among the treatments as per DMRT. Treatments: T1: Absolute control N:P2O5:K2O @ 0:0:0 kg ha−1; T2: RDF of N:P2O5:K2O @ 120:60:60 kg ha−1; T3: 75% RDF + Trichoderma harzianum; T4: 75% RDF + Pseudomonas fluorescens; T5: 75% RDF + Bacillus subtilis; T6: 75% RDF + T. harzianum + P. fluorescens; T7: 75% RDF + P. fluorescens + B. subtilis; T8: 75% RDF + T. harzianum + B. subtilis; T9: 75% RDF + T. harzianum + P. fluorescens + B. subtilis.
3.7. Effect on Earliness
The minimum number of days (95.2) to head maturity was taken by T7 (Figure 5b). The plants of absolute control plots (T1) significantly delayed in producing marketable heads (104.8 days). Bio-primed plants (T3–T9) demonstrated the early maturation of heads than non-primed plants (T1–T2). On the other hand, the higher dose of N increases the undue vegetative stage of growth period. A statistically equivalent effect on earliness was recorded under triple-species and single-species and bio-priming treatments.
3.8. Effect on Micronutrient Uptake
Results revealed that the application of 75% RDF + T. harzianum + P. fluorescens (T6) significantly (p ≤ 0.05) enhanced Fe uptake (Table 7) by the head of the crop over 100% RDF (T2), 75% RDF + T. harzianum + P. fluorescens + B. subtilis (T9), and absolute control (T1). The plant uptake of Mn was the highest under 75% RDF + T. harzianum + P. fluorescens (T6) for both years of the experiment. The pooled analysis confirmed the equivalent effect of dual inoculation in Mn uptake, but they were significantly (p ≤ 0.05) higher than the other treatments. The uptake of Zn varied from 2.82 to 65.49 g ha−1. The significantly lowest Zn uptake of 10.34 g ha−1 was observed in absolute control (T1) followed by 100% RDF (T2). The co-inoculation of P. fluorescens + B. subtilis along with 75% RDF (T7) showed the highest Zn uptake, but it was at par with 75% RDF + T. harzianum + P. fluorescens (T6). Similarly, Zn uptake recorded in 75% RDF + T. harzianum (T3) was at par with T. harzianum + P. fluorescens + B. subtilis (T9). Significantly highest (17.70 g ha−1) and lowest (2.11 g ha−1) Cu uptakes were obtained in 75% RDF + T. harzianum + P. fluorescens (T6) and absolute control (T1), respectively. The triple combination of bio-agents (T. harzianum + P. fluorescens + B. subtilis) showed lower micronutrient uptake than the other bio-primed plants. Among single inoculations, P. fluorescens resulted in higher uptake by the head. The micronutrient uptake followed the pattern of Fe > Zn > Mn > Cu.

Table 7.
Micronutrient uptake by head of red cabbage as influenced by bio-priming and mineral fertilization.
3.9. Regression Analysis
The statistical relationship between key growth parameters and micronutrient uptake is presented in Table 8. The Fe and Mn uptake of the crop showed strong relationships with chlorophyll (SPAD) (Y = 0.057X + 59.81, R2 = 0.94; Y = 0.450X + 59.70, R2 = 0.94). Among micronutrients, the uptake of Cu had a significant relationship with root length (Y = 0.277X + 15.27, R2 = 0.93), while the uptake of Zn had significant relationship with the earliness (Y = −0.170X + 106.06, R2 = 0.96) of the crop.

Table 8.
Relationship between key growth parameters and micronutrient uptake of red cabbage.
4. Discussion
Screening efficient microbial strains to address numerous agro-environmental challenges will help in achieving the targets of sustainable development goals. Their capacity to promote plant growth by increasing nutrient availability and acquisition has huge potential to reduce the use of chemical fertilizers in agroecosystems [8,26]. Moreover, they also serve as a good option to produce healthy foods and increase marketable head yield [30]. However, the efficacy of microbial products is often found to be low under field conditions [33]. In the current study, the effects of T. harzianum, P. fluorescens, and B. subtilis as single-species bio-primers and combination treatments (consortia) along with 75% RDF on crop growth and micronutrient uptake by red cabbage were studied.
At 60 DAT, the plant height (Table 2) was the highest in 100% RDF (T2); however, at maturity, the effect was significantly (p ≤ 0.05) higher with 75% RDF + P. fluorescens + B. subtilis (T7). Similar results in plant height was observed by Verma et al. [34] due to a combined application of chemical fertilizer and organics (Pseudomonas fluorescens + humic acid). According to Adesemoye et al. [35], 75% RDF was the optimum rate to which the addition of bacterial inoculants (Bacillus spp.) augmented the plant height of tomato. Recently, Houida et al. [36] reported the increased seedling growth of maize due to seed bio-priming. The yield of red cabbage is highly dependent on the number of leaves because they act as the main site for the assimilation of carbohydrates. At 60 DAT, dual inoculated treatments (T6–T8) were higher in the number of leaves than other treatments (Table 3). The increased absorption of nutrients in bio-primed plants triggered cell division, cell elongation, and overall metabolism in their system [21]. Our findings are in conformity with the reports of Verma et al. [34] and Thakur et al. [37]. At maturity stage, the number of leaves was reduced in bio-primed plants, which indicated a better head formation of these plants.
The plant spread was maximum with 75% RDF + P. fluorescens + B. subtilis (T7) at all three stages, i.e., pre-cupping, early head formation, and maturity (Table 4). Our observations are in partial agreement with the findings of Das et al. [12]. Chlorophyll (SPAD) was found to be highest under 75% RDF + T. harzianum + B. subtilis (T8) at all stages (30 DAT, 60 DAT, and harvest) (Table 5). Root system modulation in bio-primed plants led to a greater absorption of nutrients and the production of carbohydrates. However, the effect was not significant in comparison to plants receiving 100% RDF. Contrary to our results, Chatterjee et al. [38] observed significantly higher SPAD values in cabbage due to a combined application of inorganic fertilizers and organics (organic manure and biofertilizer) in comparison to the sole application of inorganic fertilizers. However, our results are in partial agreement with the reports of Vafadar et al. [39] who claimed positive effects of triple inoculations on chlorophyll content over single inoculations, but the effects were inferior to dual inoculations. Seed inoculation with Bacillus megaterium enhanced chlorophyll (SPAD) by 5.8% in cabbage [40].
During early head formation, leaf area (Table 6) was at its maximum with 75% RDF + T. harzianum + P. fluorescens (T6). Among single species bio-priming, P. fluorescens inoculated plants exhibited higher leaf area over other individual inoculated plants at all growth stages. A similar effect of Pantoea agglomerans over B. subtilis was reported in cabbage by Turan et al. [40]. However, significant (p ≤ 0.05) variations between bio-primed plants and 100% fertilized plants were not documented. In contrast to our findings, the significant effect of bio-priming in combination with mineral fertilizer on leaf area was reported in baby corn [19]. Furthermore, the positive effect of conjoint use of inorganic and organic biofertilization in cauliflower was also reported by Thakur et al. [37]. Thus, the improvement in morphological and physiological parameters in the host plants are a resultant of bio-priming induced seed germination, seedling vigor, activation of enzymes, root growth, nutrient uptake, etc. [41].
Root length is also a vital parameter that is directly linked to plant growth. In our investigation, higher root length (Figure 5a) was recorded in 75% RDF + T. harzianum + P. fluorescens (T6). Results also clearly suggested that bio-priming was effective in improving the root growth of red cabbage crop. The positive effect of organic and inorganic fertilizers on root length was also reported by Islam et al. [42]. Microbial agents such as Trichoderma have the ability to colonize the epidermis and outer cortical layers of the root and enhance root development for increasing the uptake of nutrients [43]. However, chemotactic signals from root exudates are also responsible for root colonization [44,45]. The significant enhanced root length of baby corn was detected with the application of 75% RDF in combination with Trichoderma viride and Glomus intraradices [19]. Increased root colonization by fungal strains (Glomus intraradices and Acaulospora laevis) in the presence of mycorrhiza helper bacteria (P. fluorescens) was recorded in broccoli plants [46]. The minimum number of days to head maturity (earliness) was recorded with 75% RDF + P. fluorescens + B. subtilis (T7) (Figure 5b). Our results are in agreement with the observation of Kumari et al. [47] and Thakur et al. [37], who noticed a significant reduction in the number of days to maturity of cabbage head and cauliflower curd, respectively, with the use of bio-inoculants.
Results showed that bio-priming not only promoted the growth (Figure 4) of red cabbage but also influenced micronutrient uptake (Fe, Mn, Zn, and Cu) by the head (Table 7). The plant uptake of Fe, Mn, and Cu was the highest with 75% RDF + T. harzianum + P. fluorescens (T6), while the highest Zn uptake was observed with 75% RDF + P. fluorescens + B. subtilis (T7). The micronutrient bioavailability to plants is a consequence of microbial activities including siderophore production, exudation of organic ligands and organic acids, rhizospheric acidification, etc. [48,49]. The bacterial inoculation of Pseudomonas spp. and Bacillus spp. showed increased uptake of Fe, Mn, Zn, and Cu by maize plants [50]. Modifications in root architecture, viz., higher as well as longer secondary root hairs induced by qid74 gene in T. harzianum-treated cucumber plants, facilitated the enhanced uptake of macronutrients and micronutrients as a resultant of the increment in total absorptive surface and, consequently, led to better plant biomass [51]. The solubility of Zn increased due to the secretion of organic acids (citrate and malate) by rhizospheric microbes [52]. Diffusible metabolites produced by T. harzianum 1295-22 (T-22) have the ability to solubilize Fe, Mn, Zn, and Cu [53]. The increased uptake of micronutrients in bio-primed plants indicated the existence of positive interactions among the growth promotion, root colonization, and micronutrient uptake. The Fe and Mn uptake of the crop showed strong relationships (Table 8) with chlorophyll (SPAD). The micronutrients Fe and Mn are important for photosynthesis, carbohydrate metabolism, phytohormone synthesis, and chlorophyll formation [49,54,55]. Cu and Zn uptakes showed a significant relationship with root length and earliness, respectively. Zinc is essential for the synthesis of carbohydrates, protein, and phytohormones [50,52,56].
5. Conclusions
The present investigation confirms that bio-priming in combination with mineral fertilizer triggered growth promotion in red cabbage. Dual inoculations of T. harzianum and P. fluorescens followed by P. fluorescens and B. subtilis had synergistic activities and were found to be the most effective bio-priming agents in the enhancement of growth and the micronutrient uptake of red cabbage. The formulations of novel biofertilizers will have a significant impact for the production of exotic crops under sustainable agriculture. Furthermore, improving nutrient use efficiency in crops by using pragmatic solutions such as bio-priming reduces fossil fuel consumption (fertilizers, pesticides, etc.) and promotes the fundamental goals of sustainable agriculture. Future studies should also target to determine the root colonization pattern of bio-agents under the rhizosphere engineering of exotic crops.
Author Contributions
Conceptualization, D.S. and A.R.; method, D.S.; software, D.S., S.D. and R.D.; validation, D.S., A.R. and H.P.P.; writing—original draft preparation, D.S. and A.R.; writing—review and editing, D.S., A.R., H.P.P., S.D., S.A. and R.D.; supervision, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
D.S. is thankful to BHU-UGC, India for financial support in form of field experiments and laboratory facilities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets are available at [30].
Acknowledgments
We thank H.B. Singh (Department of Mycology and Plant Pathology, BHU) for providing the microbial agents and laboratory facilities. We also acknowledge the help received from field workers and support from the Head, Department of Soil Science and Agricultural Chemistry, BHU, regarding laboratory facilities and instruments during the research. Deepranjan Sarkar is grateful to BHU for providing financial assistance during the PhD programme. This project was supported by Researchers Supporting Project Number (RSP-2022R7) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Phahlane, C.J.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Development, yield, and antioxidant content in red cabbage as affected by plant density and nitrogen rate. Int. J. Veg. Sci. 2018, 24, 160–168. [Google Scholar] [CrossRef]
- Draghici, G.A.; Alexandra, L.M.; Aurica–Breica, B.; Nica, D.; Alda, S.; Liana, A.; Gogoasa, I.; Gergen, I.; Despina-Maria, B. Red cabbage, millennium’s functional food. J. Hortic. For. Biotechnol. 2013, 17, 52–55. [Google Scholar]
- Song, H.; Yi, H.; Lee, M.; Han, C.T.; Lee, J.; Kim, H.; Park, J.I.; Nou, I.S.; Kim, S.J.; Hur, Y. Purple Brassica oleracea var. capitata F. rubra is due to the loss of BoMYBL2–1 expression. BMC Plant Biol. 2018, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kołton, A. Phenolic compounds as the major antioxidants in red cabbage. Folia Hortic. 2010, 22, 19–24. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A. Red cabbage as potential functional food in the present perspective. Int. J. Bioresour. Sci. 2017, 4, 7–8. [Google Scholar] [CrossRef][Green Version]
- Sarkar, D.; Sankar, A.; Devika, O.S.; Singh, S.; Parihar, M.; Rakshit, A.; Sayyed, R.Z.; Gafur, A.; Ansari, M.J.; Danish, S.; et al. Optimizing nutrient use efficiency, productivity, energetics, and economics of red cabbage following mineral fertilization and biopriming with compatible rhizosphere microbes. Sci. Rep. 2021, 11, 15680. [Google Scholar] [CrossRef]
- Galvão, A.C.; Souza, P.P.; Robazza, W.S.; França, C.A.L. Capacity of solutions involving organic acids in the extraction of the anthocyanins present in jabuticaba skins (Myrciaria cauliflora) and red cabbage leaves (Brassica oleracea). J. Food Sci. Technol. 2020, 57, 3995–4002. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Baldoni, G. Factors influencing anthocyanin content in red cabbage (Brassica oleracea var capitata L. f. rubra (L) Thell). J. Sci. Food Agric. 2002, 82, 1504–1509. [Google Scholar] [CrossRef]
- Fruits and Vegetables. Red Cabbage—Origin and Production—Frutas. Available online: https://www.frutas-hortalizas.com/Vegetables/Origin-production-Red-Cabbage.html (accessed on 1 October 2020).
- Das, R.; Thapa, U.; Mandal, A.R.; Lyngdoh, Y.A.; Kulshreshtha, S.K.; Debnath, S. Response of red cabbage (Brassica oleracea var. capitata f. rubra) to the integrated use of chemical fertilizers, biofertilizers and boron. Appl. Biol. Res. 2014, 16, 110–113. [Google Scholar] [CrossRef]
- Manasa, S.; Mukunda, L.; Sadarunnisa, S.; Rajasekharam, T. Response of red cabbage (Brassica oleracea L. var. capitata f. rubra) to different levels of plant and row spacing. Intl. J. Curr. Microbiol. App. Sci. 2017, 6, 1684–1689. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakesh, S.; Ganguly, S.; Rakshit, A. Management of increasing soil pollution in the ecosystem. Adv. Res. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Dubey, P.K.; Singh, A.; Chaurasia, R.; Pandey, K.K.; Bundela, A.K.; Singh, G.S.; Abhilash, P.C. Animal manures and plant residue-based amendments for sustainable rice-wheat production and soil fertility improvement in eastern Uttar Pradesh, North India. Ecol. Eng. 2022, 177, 106551. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Sharma, P.; Chaturvedi, P.; Chandra, R.; Kumar, S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere 2022, 295, 133823. [Google Scholar] [CrossRef]
- Yadav, R.S.; Singh, V.; Pal, S.; Meena, S.K.; Meena, V.S.; Sarma, B.K.; Singh, H.B.; Rakshit, A. Seed bio-priming of baby corn emerged as a viable strategy for reducing mineral fertilizer use and increasing productivity. Sci. Hortic. 2018, 241, 93–99. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Shadangi, S.; Mohapatra, P.K.D.; Panneerselvam, P. Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef]
- Sarkar, D.; Pal, S.; Singh, H.B.; Yadav, R.S.; Rakshit, A. Harnessing bio-priming for integrated resource management under changing climate. In Advances in PGPR Research; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; CAB International: Croydon, UK, 2017; pp. 349–363. [Google Scholar]
- Meena, S.K.; Rakshit, A.; Singh, H.B.; Meena, V.S. Effect of nitrogen levels and seed bio-priming on root infection, growth and yield attributes of wheat in varied soil type. Biocatal. Agric. Biotechnol. 2017, 12, 172–178. [Google Scholar] [CrossRef]
- Meena, S.K.; Rakshit, A.; Meena, V.S. Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal. Agric. Biotechnol. 2016, 6, 68–75. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Shukla, R.; Sahu, P.; Prabha, R.; Gupta, A.; Sarma, B.K.; Gupta, V.K. Stage-dependent concomitant microbial fortification improves soil nutrient status, plant growth, antioxidative defense system and gene expression in rice. Microbiol. Res. 2020, 239, 126538. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Sunita, K.; Pal, S.; Singh, A.; Singh, H.B. Bio-priming mediated nutrient use efficiency of crop species. In Nutrient Use Efficiency: From Basics to Advances; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: New Delhi, India, 2015; pp. 181–191. [Google Scholar]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant. Stress. 2022, 3, 100052. [Google Scholar] [CrossRef]
- Meena, K.K.; Mesapogu, S.; Kumar, M.; Yandigeri, M.S.; Singh, G.; Saxena, A.K. Co-inoculation of the endophytic fungus Piriformospora indica with the phosphate-solubilising bacterium Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol. Fertil. Soils 2010, 46, 169–174. [Google Scholar] [CrossRef]
- Gavilanes, F.Z.; Andrade, D.S.; Zucareli, C.; Horácio, E.H.; Yunes, J.S.; Barbosa, A.P.; Alves, L.A.R.; Cruzatti, L.G.; Maddela, N.R.; de Fátima Guimarães, M. Co-inoculation of Anabaena cylindrica with Azospirillum brasilense increases grain yield of maize hybrids. Rhizosphere 2020, 15, 100224. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Micronutrient Deficiency. 2017. Available online: https://ourworldindata.org/micronutrient-deficiency (accessed on 1 October 2020).
- Sarkar, D.; Rakshit, A. Bio-priming in combination with mineral fertilizer improves nutritional quality and yield of red cabbage under Middle Gangetic Plains, India. Sci. Hortic. 2021, 283, 110075. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A. Amalgamation of farmers’ bio-priming knowledge in integrated nutrient management for sustainable management of red cabbage soil under Middle Gangetic Plains, India. Environ. Manag. 2022; accepted. [Google Scholar]
- Tennant, D. A test of a modified line intersect method of estimating root length. J. Ecol. 1975, 63, 995–1001. [Google Scholar] [CrossRef]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Verma, R.; Maurya, B.R.; Meena, V.S. Integrated effect of bio-organics with chemical fertilizer on growth, yield and quality of cabbage (Brassica oleracea var. capitata). Indian J. Agric. Sci. 2014, 84, 914–919. [Google Scholar]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Houida, S.; Yakkou, L.; Kaya, L.O.; Bilen, S.; Fadil, M.; Raouane, M.; El Harti, A.; Amghar, S. Biopriming of Maize seeds with plant growth-promoting bacteria isolated from the earthworm Aporrectodea molleri: Effect on seed germination and seedling growth. Lett. Appl. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.; Kumar, P.; Mohit. Studies on conjoint application of nutrient sources and PGPR on growth, yield, quality, and economics of cauliflower (Brassica oleracea var. botrytis L.). J. Plant Nutr. 2018, 41, 1862–1867. [Google Scholar] [CrossRef]
- Chatterjee, R.; Jana, J.C.; Paul, P.K. Enhancement of head yield and quality of cabbage (Brassica oleracea) by combining different sources of nutrients. Indian J. Agric. Sci. 2012, 82, 324–328. [Google Scholar]
- Vafadar, F.; Amooaghaie, R.; Otroshy, M. Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J. Plant Interact. 2014, 9, 128–136. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Yildirim, E.; Güneş, A.; Karagöz, K.; Kotan, R.; Dursun, A. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk. J. Agric. For. 2014, 38, 327–333. [Google Scholar] [CrossRef]
- Lahrizi, Y.; Oukaltouma, K.; Mouradi, M.; Farissi, M.; Qaddoury, A.; Bouizgaren, A.; Ghoulam, C. Seed biopriming with osmo-tolerant rhizobacteria enhances the tolerance of alfalfa (Medicago sativa L.)-rhizobia symbiosis to water deficit. Appl. Ecol. Environ. Res. 2021, 19, 563–580. [Google Scholar] [CrossRef]
- Islam, M.A.; Ferdous, G.; Akter, A.; Hossain, M.M.; Nandwani, D. Effect of organic, inorganic fertilizers and plant spacing on the growth and yield of cabbage. Agriculture 2017, 7, 31. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Pathan, S.I.; Větrovský, T.; Giagnoni, L.; Datta, R.; Baldrian, P.; Nannipieri, P.; Renella, G. Microbial expression profiles in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 2018, 433, 401–413. [Google Scholar] [CrossRef]
- Holátko, J.; Brtnický, M.; Kučerík, J.; Kotianová, M.; Elbl, J.; Kintl, A.; Kynický, J.; Benada, O.; Datta, R.; Jansa, J. Glomalin—Truths, myths, and the future of this elusive soil glycoprotein. Soil Biol. Biochem. 2021, 153, 108116. [Google Scholar] [CrossRef]
- Tanwar, A.; Aggarwal, A.; Parkash, V. Effect of bioinoculants and superphosphate fertilizer on the growth and yield of broccoli (Brassica oleracea L. var. italica Plenck). New Zealand J. Crop. Hortic. Sci. 2014, 42, 288–302. [Google Scholar] [CrossRef]
- Kumari, C.; Mankar, A.; Karuna, K.; Solankey, S.S.; Singh, V.K. Effect of different levels of nitrogen and microbial inoculants on yield and quality of cabbage (Brassica oleracea var capitata) cv Pride of India. Indian J. Agric. Sci. 2015, 85, 515–518. [Google Scholar]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Singh, D.; Prasanna, R. Potential of microbes in the biofortification of Zn and Fe in dietary food grains. A review. Agron. Sustain. Dev. 2020, 40, 15. [Google Scholar] [CrossRef]
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ozturk, L.; Asif, M.; Siddique, K.H. Zinc nutrition in wheat-based cropping systems. Plant Soil 2018, 422, 283–315. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Rahi, A.A.; Anjum, M.A.; Iqbal Mirza, J.; Ahmad Ali, S.; Marfo, T.D.; Fahad, S.; Danish, S.; Datta, R. Yield enhancement and better micronutrients uptake in tomato fruit through potassium humate combined with micronutrients mixture. Agriculture 2021, 11, 357. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.M.; Brestic, M.; El Sabagh, A.; et al. Biofertilizer-based zinc application enhances maize growth, gas exchange attributes, and yield in zinc-deficient soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).