Abstract
Forest plantations have significantly more potential for carbon storage than non-forested areas. In this study, the amount of carbon stored in the biomass (trees, shrubs, herb, litter, and deadwood) and soil of 25-year-old plantations with P. nigra and P. abies species was measured and compared with the non-planted adjacent area (control) in a mountainous region of northern Iran. The results show that the amount of carbon stored in the biomass of P. nigra and P. abies plantations was 4.4 and 3.3 times higher than the value of the control (4.59 C Mg ha−1), respectively. In addition, the amount of carbon stored in soil was 1.5 and 1.2 times higher than the value at the control site (47.91 C Mg ha−1), respectively. Of the total carbon stored in the biomass of plantations, the highest level was observed in trees (86.5–88.5%), followed by shrubs (4.6–6.5%), litter (2.7–2.8%), the herbaceous layer (1.8–2.5%), and deadwood (1.7–2.4%), while 45.5%, 34.6%, 10.8%, 5.8%, and 3.3% of the total carbon stored in the biomass of the control site were in shrubs, trees, the herbaceous layer, litter, and deadwood, respectively. The soil carbon sequestration rate (SCSR) in soil depths of 0–10 and 10–20 cm was 0.46 and 0.44 C Mg ha−1 yr−1 in the P. nigra plantation and 0.15 and 0.23 C Mg ha−1 yr−1 in the P. abies plantation, respectively. According to the results, we conclude that the restoration of the landscape by tree plantation has a substantially determining impact on the acceleration of carbon sequestration.
1. Introduction
One of the major challenges on a global level that is environment-related is climate change [1]. It is highly likely that increased concentrations of greenhouse gases (GHGs) in the atmosphere are contributing to an increase in global warming. Global warming is directly affecting ecosystems and their biodiversity in different parts of the world [2,3]. In particular, CO2 concentrations have become a problem related to global warming due to the fact that CO2 is a critical GHGs component [1]. Forests are seen as a mitigative strategy to reduce global warming. Plantation forests play a crucial role in forest management due to their high productivity and large contribution to carbon sequestration. Increasing global carbon sequestration through enlargement of the proportion of planting forests on non-forested lands on the planet has been suggested to be an effective measure to lessen elevated concentrations of atmospheric carbon dioxide [4,5,6,7].
Plant biomass forms an important carbon stock in many ecosystems. Shrubs and trees can accumulate more than hundreds of tons ha−1 of carbon over their lifespan [2]. Among terrestrial ecosystems, forests have the highest level of potential to combat climate change due to the extensive amount of wood and fertile soil [4,5]. Almost one third of the Earth’s surface is covered by forests. These complex ecosystems have up to 80% of the total above-ground terrestrial C and 40% of the below-ground C [6]. Forests have the potential to store 20 to 50 times more carbon than barren lands [7], acting as a sink of carbon.
Large-size trees are important components of forest ecosystems, which are fundamental in the C cycle, storing large amounts of carbon in their tissues thanks to photosynthesis [8,9,10,11,12]. Forests are also important in regulating atmospheric CO2 levels [11]. Artificial forest plantations have two main purposes: timber production and ecological restoration. They account for 7% of the global forest area and positively affect the global C cycle [13]. These particular forests play an important role in C sequestration thanks to their high rate of growth [14].
Forests positively affect soil physicochemical properties and soil communities (animal and microbial communities) [15,16,17]. Soil contains the world’s largest terrestrial active C pool; for this reason, it is considered fundamental in the global C cycle [18]. The assessed amount of organic C stored in soils is about 1100–1600 petagrams (Pg), more than twice the C in living vegetation (560 Pg) or in the atmosphere (750 Pg) [19]. Jackson et al. [20] examined the impact on carbon sequestration of a plantation in a region of the United States with an annual rainfall of about 230 to 660 mm and found that the total ecosystem carbon storage increased from 2.9 to 10.1 Mg C ha−1. In addition, a study by Zou and Bashkin [21] showed that afforestation with eucalyptus trees in degraded lands resulted in carbon sequestration of about 3 tons ha−1 year−1 in a 25 cm layer of soil.
The forests in Iran cover about 12.4 million ha, 7.3% of the national surface, and the forest plantations represent approximately 944,000 ha, with a share of broadleaves and conifers at 61% and 39%, respectively [22]. Given that Iran is located in arid and semi-arid zones and has poor forest cover (less than 10%), plantations play a crucial role in mitigating wood demand pressure on natural forests. The purposes of plantations are wood production, the protection of biological diversity, and soil and water conservation in watershed basins. The fixation of sand dunes by vegetation is another important reason for tree plantation in arid and semi-arid regions of the country in order to combat desertification.
Black pine (Pinus nigra) and Norway spruce (Picea abies) are non-native coniferous species planted at different sites and under different climate conditions in Iran. These plantations were established to increase soil and water, biodiversity, and landscape protection. Forestation and reforestation remain the most effective strategies to combat climate change [2,23] and are also the most commonly applied [24,25]. The specific objectives of our study were to determine the (1) plant biomass by category (tree, shrub, grass, deadwood, and litter) and (2) vegetation and soil C storage in plantations with two species of conifers (P. nigra and P. abies) and at natural sites in a mountainous region in northern Iran. Therefore, the aim of this research was to estimate the differences in afforestation with conifers and the degraded natural sites to evaluate the performance of management (plantation) and the alternative of abandonment.
2. Materials and Methods
2.1. Study Area
This research was conducted on the northern slopes of the Alborz mountain range in the Ardebil province, northern Iran (latitude 38°26′51″ N to 38°27′11″ N, longitude 47°36′10″ E to 47°36′57″ E). The average annual rainfall is 350 mm. The average temperature of the hottest and coldest months is 15.5 and 3.6 °C, respectively. The average annual humidity value is 51.4% to 67.3% in summer and 83.1% in winter. The climate is cold mid-arid (aridity index, I = 16.1) according to the De Martonne climate classification [26]. Most days of the year have a lot of wind, and most of the precipitation is in the form of snow. The number of frost days is relatively high (130 frost days per year). The soil of the area is relatively shallow with a loamy texture and the bedrock is typically limestone. The main type of vegetation in the area is grassland (pasture) with scattered shrubs and short trees. Caucasian oak (Quercus macranthera Meyer) and Oriental hornbeam (Carpinus orientalis Miller) are the main tree species in this area. The main woody species of shrubs and short trees include [27]: Corylus avellana L. (Corylaceae), Rosa canina L. (Rosaceae), Mespilus germanica L. (Fagaceae), Pyrus syriacus Boiss. (Rosaceae), Prunus spinosa L. (Rosaceae), Rubus hirtus Waldst and Kit. (Rosaceae), Prunus divaricate Ledeb. (Rosaceae), Malus orientalis Ugl. (Rosaceae), Sorbus orientalis Schon. (Rosaceae), Viburnum lantana L. (Caperifoliaceae), Sorbus torminalis (L.) Crantz. (Rosaceae), Crataegus melanocarpa M.B. (Rosaceae), Cratageus mdyeri A. Pojark (Rosaceae), Ilex spinigera Leos. (Aquifoliaceae), Salix eegyptiaca L. (Salicaceae), and Lonicera coucasica Pall. (Caprifoliaceae).
Plantations with the black pine (Pinus nigra J.F. Arnold) and Norway spruce (Picea abies (L.) H. Karst.) tree species, each in an area of 20 hectares, were planted in 1997 at altitudes of 1500 to 1700 m in this area. These plantation areas were protected by barbed wire fences against animal grazing and human disturbance. During this period, neither timber nor firewood was extracted from the plantations [27]. Detailed characteristics of the study site are shown in Table 1.

Table 1.
Description of geographical characteristics of the study sites.
2.2. Study Design
Three sites, including the Pinus nigra and Picea abies plantations, were selected, and adjacent natural habitats were used as a control (Table 1). In order to collect plant biomass data, a systematic plot sampling method with a random starting point was used. The dimensions of the network were 100 m by 200 m and the area of each plot was 400 m2 (20 m by 20 m). There were 10 plots at each site. Each plot was divided into 4 10 m × 10 m quadrats, and each quadrat was further divided into 25 sub quadrats (2 m × 2 m).
2.3. Tree Biomass
The biomass of the whole tree was calculated by summing the biomass of the trunk, branch, leaf, and root.
2.3.1. Above-Ground Biomass (AGB)
AGB is mainly estimated in forest stands by the allometric method with high accuracy. The method proposed by the FAO, which is a faster and easier-to-use method than the allometric method, is also used to estimate AGB in plantations. In order to increase the accuracy when estimating the AGB value and to evaluate the accuracy of the FAO method, AGB in this study was estimated using both methods, as described in the following paragraphs.
Allometric Method
The diameter at breast height (DBH) and height (h) of trees were measured by a dendrometric caliper and clinometer, respectively, in each plot. Two trees were selected randomly in each plot (20 trees at each site) to estimate the biomass of the tree leaf, branch, trunk, and root. The number of branches (Bn) of each tree was counted and one branch was randomly taken from the mid-point of the stem as a sample of the tree crown. Then, all the leaves of the sample branch were collected and weighed. Sample branches were cut into 30-cm pieces and weighed. The fresh mass of the tree leaf was estimated by multiplying the fresh mass of the leaves of the sample branch by the number of branches. Additionally, the fresh mass of the tree branches was estimated by multiplying the fresh mass of the sample branch by the number of branches. For the estimation of the fresh mass of the tree trunk, the first tree trunk volume (TV, m3) was calculated from Equation (1).
where DBH is the diameter at breast height in m, h is the tree height in m, and f is a constant stem form factor. The calculated values for f were 0.3925 for P. nigra, 0.4163 for P. abies, 0.3640 for Q. macranthera, and 0.3520 for C. orientalis [28].
Approximately 500 g of fresh samples of tree leaves and branches was randomly collected for moisture determination. In addition, one core sample was collected from each sample tree trunk at 1.30 m from ground level for basic density determination. Cylindrical samples of wood were collected using a drill with cylindrical coring and subsequently processed in the laboratory following the procedures described in Lo Monaco et al. [29]. For splinted or irregular fresh samples, the volume was calculated with the water-displacement method [28]. Wood dry mass was determined by a gravimetric method after drying to constant mass in an oven at a temperature of 103 ± 2 °C [29].
The total biomass of leaves and branches was calculated through multiplying the fresh mass by the dry/wet ratio. The biomass of the trunk was calculated through multiplying the trunk volume by the wood basic density. Allometric equations between the tree component biomass and the independent variable (squared DBH multiplied by the tree height (D2H)) were developed using curve fitting. The optimum equations were selected to calculate the tree component biomass at the plantation sites.
FAO Method
For the estimation of the AGB of trees, the model developed by the FAO Forest Resources Assessment was used, applying Equation (2).
where AGB is the above-ground biomass in Mg/ha, VOB is the volume over bark (m3/ha), WD is the wood density (kg m−3), and BEF is the biomass expansion factor (the ratio of above-ground oven-dry biomass of trees to oven-dry biomass of the inventoried volume).
AGB = VOB × WD × BEF
2.3.2. Below-Ground Biomass (BGB)
Due to the fact that measuring the root biomass of trees is destructive, time consuming, and costly, as well as the fact that the roots have a highly variable distribution in the soil, many studies use a conservative and cautious fitting method of trunk-to-root ratio [30] to estimate the root biomass. The BGB of trees was calculated by multiplying the AGB of trees by a default value (DV) of 0.2.
2.4. Understory Biomass
All understory vegetation (shrub and herb) was harvested from two sub quadrats randomly located in each quadrat. Shrubs were separated into leaves, branches, and roots; herbs were separated into above-ground and below-ground parts [31].
The fresh mass of each component (leaves, branches, and roots) was measured to the nearest 1.0 g by using an electronic balance. About 500 g of fresh samples of shrub and herb components was randomly collected for moisture determination. Samples were dried at 65 °C until they reached a constant mass [31]. The total biomass of leaves and branches was calculated through multiplying the fresh mass by the dry/wet ratio.
2.5. Deadwood Biomas
The volume of deadwood (DW) was estimated in two components: standing (standing dead tree, snag) and downed (log or branch). Every snag with a minimum DBH of 5 cm and every piece of downed woody debris with a minimum diameter of 5 cm at the base (wider end) was included for measurement in each plot. The biomass of snags was estimated by allometric equations as described for live trees. Each snag and piece of downed DW encountered was assigned to a decay class based on the observed extent of decomposition. These ratings varied on a scale from 1 to 5, with 1 being sound wood and 5 being highly friable wood with little structural integrity remaining [32]. Within each plot, one piece of downed DW representing each decay class present was sampled by a handsaw. Samples were dried at 103 °C until they reached a constant weight. For estimation of the biomass of downed DW, the volume of each piece was calculated by Huber’s equation for regular samples using Equation (3), and by the water displacement method for irregular samples; then, the volume of DW was multiplied by the basic density of downed DW.
where V is the volume (m3), dm is the diameter under bark at the middle of the stump or short snag (m), and L is the height of the stump or short snag (m).
2.6. Litter Biomass
All litter was collected from three sub quadrats randomly located in each quadrat. About 500 g of fresh samples of litter was randomly collected for moisture determination. Samples were dried at 65 °C until they reached a constant mass. The total biomass of litter was calculated through multiplying the fresh weight by the dry/wet ratio.
2.7. Carbon Stock in above-Ground Biomass (AGB), below-Ground Biomass (BGB), Litter, Deadwood (DW), and Soil
2.7.1. Carbon Stock in AGB, BGB, Litter, and DW
Considering that the allometric method is probably more accurate than the FAO method in estimating the AGB value, to estimate the carbon stock in AGB, the AGB value calculated by the allometric method was used.
In order to obtain accurate estimates of stand-level C sequestration in living tree biomass, litter, and DW, we multiplied the stand-level estimates of dry mass by the appropriate conversion factors. We estimated the C sequestration in living woody biomass by multiplying stem, branch, and root dry mass by 0.531 [33]. Leaf dry mass was multiplied by 0.47 to estimate the C in leaves [34]. Estimates of DW dry mass were multiplied by 0.50 to estimate the C stored in DW. The C in whole-tree and DW biomass was summed to estimate the total C sequestration (Mg C ha−1) in both living and dead biomass. Litter dry mass was multiplied by 0.37 to estimate the C in litter as recommended by the protocol of the Intergovernmental Panel on Climate Change [2].
2.7.2. Carbon Stock in Soil
Ten soil samples were randomly collected for each plot (20 m × 20 m) at depths of 0–10 cm and 10–20 cm using a soil corer (5 cm in diameter). Then, the 10 soil samples from the same layer in each plot (respectively for each plantation-type plot and control plot) were mixed for a more representative sample for the measurement of soil C stock. In addition, soil cores (5 cm in height, 5 cm in diameter) with two replications of each plot were sampled for bulk density measurement. Soil core samples were oven dried for 24 h at 105 °C. Soil organic carbon (OC) was determined by the Walkley and Black method [35].
Soil C storage was calculated from the OC multiplied by the bulk density and the thickness of the soil layer using Equation (4).
where SCS is the soil carbon stock (Mg ha−1), BD is the soil bulk density (g cm−3), OC is the soil organic carbon (%), and e is the soil depth (m).
SCS = 100 × BD × OC × e
The soil C sequestration rate was calculated by subtracting the SC storage of the control site from that of the plantation and then dividing the result by the stand age using Equation (5).
where SCSR is the soil carbon sequestration rate in the plantation (Mg C ha−1 yr−1), SCSP is the soil carbon storage in the plantation (Mg C ha−1), SCSC is the soil carbon storage in the control area (Mg C ha−1), and t is the plantation age (year).
SCSR = (SCSP − SCSC)/t
2.8. Statistical Analysis
Statistical analysis was performed by the software SPSS, ver. 19.0 (SPSS Inc., Chicago, IL, USA). ANOVA analyses were used to determine the statistically significant differences between species for the biomass and C storage; multiple comparisons were carried out by Duncan’s test, with differences at the p < 0.05 significance level.
3. Results
The tree density and live and deadwood volume in the plantation stands were significantly higher than in the control stand (Table 2). The density and volume of live trees and snags in the P. nigra plantation were significantly higher than the values in the P. abies plantation.

Table 2.
Structural characteristics (mean ± SD) of the study stands.
The wood basic densities of natural broadleaved trees were significantly higher than those of coniferous plantation species (Table 3). The wood basic density value decreased with increasing decay stage in all tree species.

Table 3.
Wood basic density (mean ± SD, g cm−3) of trees species and decay classes.
All calculated allometric equations had a good coefficient of determination (R2). In particular, only one R2 value (i.e., the trunk component of C. orientalis) is below 0.7; all the other R2 values are above 0.7 (Table 4). All R2 values were significant at α = 0.01.

Table 4.
Allometric equations for different tree species and components. D is the DBH in cm, H is the tree height in m, B is the biomass of the tree component in kg tree−1, and SEE is the standard error of the estimate.
The individual tree biomass of P. nigra and P. abies was significantly higher than that of Q. macranthera and C. orientalis, and the individual tree biomass of Q. macranthera was significantly higher than that of C. orientalis, while the difference in the individual tree biomass means between P. nigra and P. abies was not significant (Table 5). The leaf, branch, trunk, and root biomass followed the order P. nigra followed by P. abies followed by Q. macranthera followed by C. orientalis. The biomass of the tree component followed the order trunk followed by branch followed by root followed by leaf in all tree species. It is worth noting that the amount of branch biomass accounted for about 32% to 39% of the total tree biomass in the whole species. Only about 3.1% to 5.5% of the trees’ biomass was leaf biomass.

Table 5.
Biomass (mean ± SD, kg tree−1) of tree components. Values in brackets are the percentage of the tree component biomass to the whole tree biomass.
The results indicate that the total biomass in the P. nigra plantation was significantly higher than that in the P. abies plantation, and the total biomass in the P. abies plantation was significantly higher than that in the control area (Table 6). The biomass of trees, litter, and DW in the plantations was significantly higher than in the control, while the biomass of shrub and herb layers in the control was significantly higher than in the plantations.

Table 6.
Biomass (mean ± SD, Mg ha−1) of trees, shrubs, herbs, litter, and deadwood (DW) by the allometric method.
The AGB value of trees, estimated by the FAO model, was the highest in the P. nigra plantation, followed by the P. abies plantation, followed by the control area (Table 7).

Table 7.
The above-ground biomass (AGB) of trees (mean ± SD) estimated by the FAO model in the study sites.
The amount of C storage was the highest in the trunks of trees, followed by branches, roots, and leaves at all studied sites (Figure 1). The amount of C storage in all components of the trees was at the highest level in the P. nigra plantation, followed by P. abies plantation, followed by the control area. The amount of C storage in the shrub components was at the highest level in the control area, followed by the P. abies plantation, followed by the P. nigra plantation (Figure 2). The highest amount of C storage was detected in the branches of shrubs, followed by roots and then by leaves.
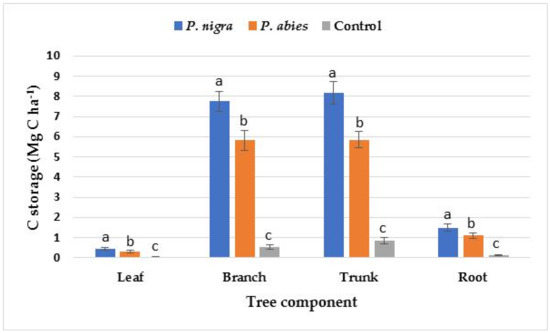
Figure 1.
C storage in tree components at the study sites (significant differences among the means at different sites are indicated with lowercase letters by Duncan’s test at α = 0.05; the bars indicate the standard error of the estimate).
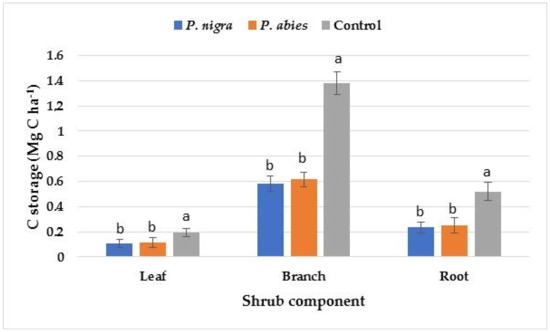
Figure 2.
C storage in shrub components at the study sites. Significant differences among the means at different sites are indicated with lowercase letters by Duncan’s test at α = 0.05; the bars indicate the standard error of the estimate.
The amount of C storage observed in the herbal layer was similar to that in the shrub layer. Both the above-ground biomass (AGB) and the below-ground biomass (BGB) were the highest in the control area followed by the P. abies plantation and then the P. nigra plantation (Figure 3).
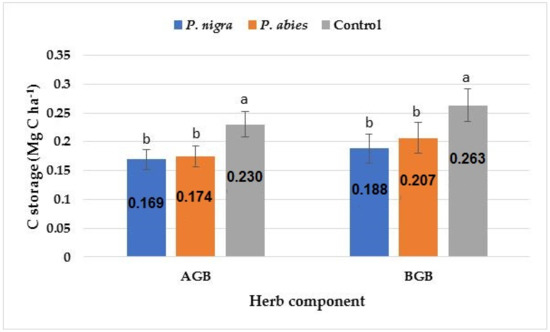
Figure 3.
C storage in herb components at the study sites. Significant differences among the means at different sites are indicated with lowercase letters by Duncan’s test at α = 0.05; the bars indicate the standard error of the estimate.
The amount of C storage in litter and deadwood was at the highest level in the P. nigra plantation followed by the P. abies plantation and the control area (Figure 4). The amount of C storage in the litter was higher than that in the deadwood at all sites.
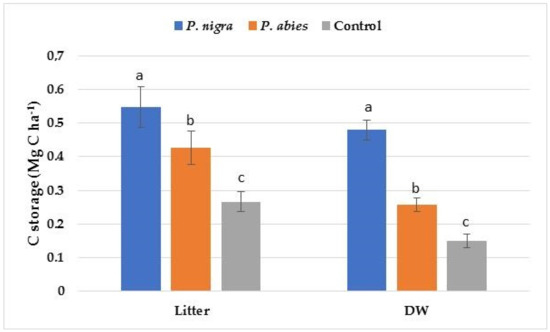
Figure 4.
C storage in litter and deadwood (DW) at the study sites. Significant differences among the means at different sites are indicated with lowercase letters by Duncan’s test at α = 0.05; the bars indicate the standard error of the estimate.
The volume of deadwood in the P. nigra plantation was higher than that in the P. abies plantation in all decay classes except DC1 (Table 8). The biomass and C storage of deadwood in the P. nigra plantation were higher than those in the P. abies plantation in all decay classes. The lowest values of the volume, biomass, and C storage of deadwood were observed in the control area in all decay classes.

Table 8.
Volume, biomass, and C storage of deadwood (DW) at the study sites.
The bulk density (BD) at the upper soil layer (0–10 cm) in the control area was significantly higher than that in the plantations, while at the lower soil layer (10–20 cm) the BD was not significantly different between the plantations and the control (Table 9). The highest organic C (OC) value at both soil depths was detected in the P. nigra plantation followed by the P. abies plantation and the control area. The soil C storage (SCS) value in the P. nigra plantation was significantly higher than that in the P. abies plantation, and the SCS value in the P. abies plantation was significantly higher than that in the control area. The soil carbon sequestration rate (SCSR) at the depths of 0–10 cm and 10–20 cm in the P. nigra plantation was about three and two times higher than in the P. abies plantation, respectively (Table 9).

Table 9.
Soil C storage (SCS) and soil carbon sequestration rate (SCSR) (mean ± SD) in different soil depth classes at the study sites. BD, soil bulk density; OC, soil organic C.
Considering the total carbon storage, for above-ground biomass, both in the plant components and in the deadwood, and below-ground biomass down to the 20 cm soil depth, the highest value was found in the P. nigra plantation followed by the P. abies plantation and then the control area (Figure 5).
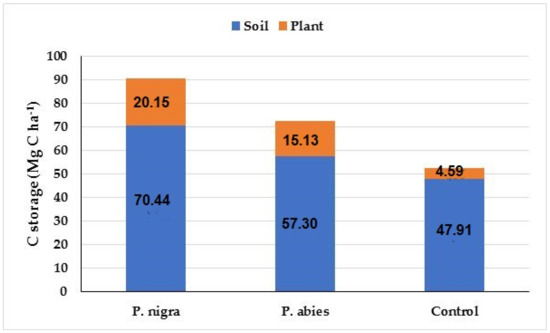
Figure 5.
C storage in plants (trees + shrubs + herb + litter + DW) and soil (depth: 0–20 cm) at the study sites.
4. Discussion
The plantations set up in the last century were not made for the purpose of compensating for climate change and carbon sequestration [36,37,38], but the results of the current study show that the plantations with the P. nigra and P. abies tree species increased the carbon storage of the biomass and soil as compared with the control area.
The AGB of trees was estimated by both the allometric method and the FAO method. The AGB estimated by the FAO method was 3.2% lower in the P. nigra plantation (30.89 Mg ha−1 by the allometric method and 29.90 Mg ha−1 by the FAO method) and 10.7% lower in the P. abies plantation (22.65 Mg ha−1 by the allometric method and 20.23 by the FAO method) than the allometric estimate, while the AGB estimated by the FAO method in the control area was 6.9% higher (2.75 Mg ha−1 by the allometric method and 2.94 Mg ha−1 by the FAO method) than the value that was estimated by the allometric method.
Our results indicate that the volume of deadwood in the plantations was greater than the volume in the control area. The deadwood accounted for 7.46% of the standing volume and 2.67% of the stand’s carbon storage in the P. nigra plantation, and 6.98% of the standing volume and 1.93% of the stand’s carbon storage in the P. abies plantation. These results are in line with the results of previous research showing that the volume of deadwood in forests is directly related to the forest standing volume [29]. Lo Monaco et al. [29] also stated that the volume and dynamics of deadwood are related to forest vegetation, and that deadwood plays an important role in storing atmospheric carbon in oak and pine forests. Therefore, it is predicted that with the aging of these plantations, the standing volume and consequently the volume of deadwood and the amount of carbon stored in them will increase in the coming years.
Our results and those of others provide insights into how the species of trees used in plantations influence carbon stores. Previous studies have demonstrated that plantations with different coniferous tree species increase the carbon storage within the ecosystem [14,31]. The amount of carbon stored in both the biomass and soil of the P. nigra plantation was higher than in the P. abies plantation. Furthermore, Gao et al. [31] studied the carbon storage in biomass, litter, and soil of different plantations in a semiarid temperate region of northwest China and reported the ecosystem C storage to be as follows: Picea crassifolia (469 C Mg ha−1) followed by Larix gmelinii (375 C Mg ha−1), Populus simonii (330 C Mg ha−1), and Pinus tabuliformis (281 C Mg ha−1), 59.5–91.1% of which was in the soil, and the highest soil C was stored in the Picea crassifolia plantation (411 C Mg ha−1). Yen et al. [14] observed that the C sequestration in plantations was differentiated by species. In fact, in four different coniferous species, they found that the mean C sequestration in Japanese cedar (Cryptomeria japonica) (4.03 Mg ha−1 yr−1) and Taiwania (Taiwania cryptomerioides) (3.52 Mg ha−1 yr−1) was higher than in Chinese fir (1.79 Mg ha−1 yr−1) and Taiwanese red cypress (2.36 Mg ha−1 yr−1).
In forests, terrestrial C is stored in different pools, but trees and soil are the main pools as they store more C than the others. Our results reveal that the share of soil in the carbon storage was higher than the share of biomass. About 78% to 79% of the ecosystem’s carbon was stored in the soil of plantations and 21% to 22% was stored in the biomass of the plantations, while in non-plantation areas (the control area) the soil share of the ecosystem’s carbon storage was 91.3%. The results show that the amount of carbon storage in the upper soil layer (0–10 cm) was higher than that in the lower soil layer (10–20 cm) in both plantations and the control area. Usually, the largest amount of carbon accumulates in the surface layer of the soil and the amount of carbon decreases with increasing soil depth, similar to this study’s findings, as reported by Paul and Clark [39]. Accordingly, soil carbon storage is an important part of the carbon storage in terrestrial ecosystems, which has a large impact on the CO2 in the atmosphere (about 75% of the atmospheric carbon is stored in the soil) [39,40].
The role of the forest in C sequestration has ecological, environmental, social, and economic value and is related to other ecosystem services [37], such as educational, aesthetic, and cultural heritage value, recreation, and tourism. Plantation is considered to be an effective strategy to prevent soil erosion and degradation and to promote the restoration of degraded ecosystems. Forest plantation renaturalization should be considered to promote the forest ecosystem’s evolution towards a natural forest system in order to protect biodiversity and improve wildlife habitat [41]. On the other hand, the process of naturalization of degraded forests may not have much influence on the C stores for a considerable amount of time. Guedes et al. [42] demonstrated that the total ecosystem C stocks in the Miombo woodlands (116 Mg ha−1) were significantly lower than in plantation stands with P. taeda (363 Mg ha−1) and Eucalyptus grandis (407 Mg ha−1). Nevertheless, Chen et al. [43], who studied the carbon stock density in planted as compared to natural Pinus massoniana forests in sub-tropical China, found that carbon stock densities ranged from 78 to 210 Mg ha−1 and from 97 to 177 Mg ha−1, respectively. The fixation of large amounts of carbon is regarded as an important environmental contribution of forests, and this characteristic has global implications [8,9,10,11,41,42,43,44,45]. Soto-Cervantes et al. [45] reported that the average carbon sequestration per year is 0.30 kg for each tree in a mixed Pinus durangensis and Pinus cooperi plantation. In accordance with the results of this study, Justine et al. [39] studied the biomass stock and carbon sequestration in a chronosequence of Pinus massoniana plantations and reported that the total ecosystem carbon storage varied with stand age, ranging from 169.90 C Mg ha−1 in the five-year plantation to 326.46 C Mg ha−1 in the 42-year plantation, of which 80.29% came from the mineral soil carbon and 19.71% came from the vegetation. Likewise, Sharma et al. [44] estimated a carbon stock of 108 Mg ha−1 in the Chir Pine (Pinus roxburghii Sarg.) plantation in central Nepal.
As found in another study [46], close-to-nature plantation management could mitigate climate change by improving the plantation’s carbon sequestration capacity. In addition to the benefits that forest ecosystems generally provide for society, forest plantations can capture significant amounts of GHGs and CO2. Large-scale afforestation could significantly change the ground cover, the soil quality, and the water conservation function, which all constitute fundamental issues in semi-arid mountain ecosystems [47,48,49].
One of the most important issues that should be considered is the time dependence of the forest carbon store. In the current study, the age of forest stands was limited, and the current results pertain to the limited range of forest ages. Therefore, further research is needed to elucidate the carbon storage in forest stands that are older in age.
5. Conclusions
Afforestation (forest plantation) is an excellent alternative to barren lands to mitigate high atmospheric concentrations of CO2 and, at the same time, reduce global warming. The results of this study show that 25-year-old plantation ecosystems (vegetation and soils) with P. nigra and P. abies species stored 1.73 and 1.38 times more carbon as compared with adjacent non-planted (control) areas, respectively. Soil conservation and an increase in the biodiversity of plant and animal species are other positive consequences of these plantations. Plantations with tree species that are resistant to harsh mountain climates with non-timber production purposes constitute a suitable and sustainable solution to reducing atmospheric carbon and global warming. According to the results, we conclude that the restoration of landscapes by tree plantation has a substantial and favorable impact on the acceleration of carbon sequestration. Forest plantation, even with atypical species, can in fact be considered a strategy that promotes the evolution to more stable natural forest systems that provide multiple ecosystem services.
Author Contributions
Conceptualization, R.P., F.T. and M.J.; Data curation, R.P., F.T., H.R. and A.R.K.; Formal analysis, F.T., H.R. and A.R.K.; Investigation, F.T., H.R. and A.R.K.; Methodology, R.P., F.T., A.R.K. and A.L.M.; Supervision, R.P. and F.T.; Validation, R.P. and M.J.; Writing—original draft, F.T., H.R., A.R.K. and A.L.M.; Writing—review & editing, R.P., F.T., M.J. and A.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Due to privacy restrictions, the data are only available on request from the corresponding author.
Acknowledgments
This work was financially supported by the Italian Ministry for Education, University and Research (MIUR) (Law 232/2016, Italian University Departments of excellence—UNITUS-DAFNE WP3).
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. IPCC expert meeting on climate change, food, and agriculture. In Land Use, Land-Use Change, and Forestry; Special Report of the IPCC; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015; Available online: http://www.ipcc.ch/pdf/supportingmaterial/FoodEM_MeetingReport_FINAL.pdfIPCC (accessed on 1 February 2022).
- IPCC. Default biomass conversion and expansion factors. IPCC guidelines for national greenhouse gas inventories e Agriculture, Forestry and Other Land Use. In Intergovernmental Panel on Climate Change; The Institute for Global Environmental Strategies for the IPCC: Kanagawa, Japan, 2006. [Google Scholar]
- Wan, J.Z.; Wang, C.J.; Qu, H.; Liu, R.; Zhang, Z.X. Vulnerability of forest vegetation to anthropogenic climate change in China. Sci. Total Environ. 2018, 621, 1633–1641. [Google Scholar] [CrossRef]
- Sharma, C.M.; Gairola, S.; Baduni, N.P.; Ghildiyal, S.K.; Suyal, S. Variation in carbon stocks on different slope aspects in seven major types of temperate region of Garhwal Himalaya, India. J. Biosci. 2011, 36, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Danquah, J.A.; Appiah, M.; Pappinen, A. The effect of African mahogany species on soil chemical properties in degraded dry semi-deciduous forest ecosystems in Ghana. Int. J. Agric. Biol. 2012, 14, 321–328. [Google Scholar]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.A. Aboveground Forest Biomass and the Global Carbon Balance. Glob. Chang. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Wood Plants; McGraw Hill: New York, NY, USA, 1979. [Google Scholar]
- Lamlom, S.; Savidge, R. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass-Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Yen, T.M. Culm height development, biomass accumulation and carbon storage in an initial growth stage for a fast-growing moso bamboo (Phyllostachy pubescens). Bot. Stud. 2016, 57, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, T.M.; Wang, C.T. Assessing carbon storage and carbon sequestration for natural forests, man-made forests, and bamboo forests in Taiwan. Int. J. Sustain. Dev. World Ecol. 2013, 20, 455–460. [Google Scholar] [CrossRef]
- Yosef, B.A.; Eshetu, Z.; Garedew, E.; Kassa, H. Carbon stock potentials of woodlands in north western lowlands of Ethiopia. J. Sustain. For. 2019, 38, 629–650. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment How Are the World’s Forests Changing? 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; pp. 1–54. [Google Scholar]
- Yen, T.M.; Huang, K.L.; Li, L.E.; Wang, C.H. Assessing carbon sequestration in plantation forests of important conifers based on the system of permanent sample plots across Taiwan. J. Sustain. For. 2020, 39, 392–406. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Kozlowski, T. Physiological ecology of natural regeneration of harvested and disturbed forest stands: Implications for forest management. For. Ecol. Manag. 2002, 158, 195–221. [Google Scholar] [CrossRef]
- Lal, R. Global soil erosion by water and carbon dynamics. In Soils and Globle Change; Lal, R., Kimble, J., Levine, E., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Sundquist, E.T. The Global Carbon Dioxide Budget. Science 1993, 259, 934–941. [Google Scholar] [CrossRef]
- Jackson, R.B.; Banner, J.L.; Jobbágy, E.G.; Pockman, W.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef]
- Zou, X.; Bashkin, M. Soil carbon accretion and earthworm recovery following revegetation in abandoned sugarcane fields. Soil Biol. Biochem. 1998, 30, 825–830. [Google Scholar] [CrossRef]
- FAO. FAO Global Forest Resources Assessment Program: Islamic Republic of Iran; Country FAO: Rome, Italy.
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Clemente, A.S.; Werner, C.; Maguas, C.; Cabral, M.S.; Martins-Loucao, M.A.; Correia, O. Restoration of a Limestone Quarry: Effect of Soil Amendments on the Establishment of Native Mediterranean Sclerophyllous Shrubs. Restor. Ecol. 2004, 12, 20–28. [Google Scholar] [CrossRef]
- Kou, M.; Garcia-Fayos, P.; Hu, S.; Jiao, J. The effect of Robinia pseudoacacia afforestation on soil and vegetation properties in the Loess Plateau (China): A chronosequence approach. For. Ecol. Manag. 2016, 375, 146–158. [Google Scholar] [CrossRef]
- De Martonne, E. Aréisme et Indice D’aridité; Comptes Rendus de L’Academy of Science: Paris, France, 1926; pp. 1395–1398. [Google Scholar]
- Tavankar, F.; Rafie, H.; Latterini, F.; Nikooy, M.; Senfett, M.; Behjou, F.K.; Maleki, M. Growth parameters of Pinus nigra J.F. Arnold and Picea abies (L.) H. Karst. plantations and their impact on understory woody plants in above-timberline mountain areas in the north of Iran. J. For. Sci. 2018, 64, 416–426. [Google Scholar] [CrossRef]
- Lo Monaco, A.; Luziatelli, G.; Latterini, F.; Tavankar, F.; Picchio, R. Structure and Dynamics of Deadwood in Pine and Oak Stands and their Role in CO2 Sequestration in Lowland Forests of Central Italy. Forests 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Lo Monaco, A.; Todaro, L.; Sarlatto, M.; Spina, R.; Calienno, L.; Picchio, R. Effect of moisture on physical parameters of timber from Turkey oak (Quercus cerris L.) coppice in Central Italy. For. Stud. China 2011, 13, 276–284. [Google Scholar] [CrossRef]
- Mac Dicken, K.G. A Guide to Monitoring Carbon Storage in Forestry and Agro forestry Projects; Forest Carbon Monitoring Program; Winrock International Institute for Agricultural Development: North Little Rock, AR, USA, 1997. [Google Scholar]
- Gao, Y.; Cheng, J.; Ma, Z.; Zhao, Y.; Su, J. Carbon storage in biomass, litter, and soil of different plantations in a semiarid temperate region of northwest China. Ann. For. Sci. 2014, 71, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Behjou, F.K.; Lo Monaco, A.; Tavankar, F.; Venanzi, R.; Nikooy, M.; Mederski, P.S.; Picchio, R. Coarse Woody Debris Variability Due to Human Accessibility to Forest. Forests 2018, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Birdsey, R.A. Carbon Storage and Accumulation in United States Forest Ecosystems; General Technical Report GTR-WO-59; USDA Forest Service: Washington, DC, USA, 1992. [Google Scholar]
- Hamilton, J.G.; DeLucia, E.H.; George, K.; Naidu, S.L.; Finzi, A.; Schlesinger, W.H. Forest carbon balance under elevated CO2. Oecologia 2002, 131, 250–260. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Taylor, A.R.; Wang, J.R.; Chen, H.Y. Carbon storage in a chronosequence of red spruce (Picea rubens) forests in central Nova Scotia, Canada. Can. J. For. Res. 2007, 37, 2260–2269. [Google Scholar] [CrossRef]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Khan, M.N.; Zhao, Y. Biomass Stock and Carbon Sequestration in a Chronosequence of Pinus massoniana Plantations in the Upper Reaches of the Yangtze River. Forests 2015, 6, 3665–3682. [Google Scholar] [CrossRef] [Green Version]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Harrison, K.G.; Broecker, W.S.; Bonani, G. The Effect of Changing Land Use on Soil Radiocarbon. Science 1993, 262, 725–726. [Google Scholar] [CrossRef]
- Picchio, R.; Mercurio, R.; Venanzi, R.; Gratani, L.; Giallonardo, T.; Lo Monaco, A.; Frattaroli, A.R. Strip Clear-Cutting Application and Logging Typologies for Renaturalization of Pine Afforestation—A Case Study. Forests 2018, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Guedes, B.S.; Olsson, B.A.; Egnell, G.; Sitoe, A.A.; Karltun, E. Plantations of Pinus and Eucalyptus replacing degraded mountain miombo woodlands in Mozambique significantly increase carbon sequestration. Glob. Ecol. Conserv. 2018, 14, 00401. [Google Scholar] [CrossRef]
- Chen, L.C.; Liang, M.J.; Wang, S.L. Carbon stock density in planted versus natural Pinus massoniana forests in sub-tropical China. Ann. For. Sci. 2016, 73, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.P.; Bhatta, S.P.; Khatri, G.B.; Pajiyar, A.; Joshi, D.K. Estimation of Carbon Stock in the Chir Pine (Pinus roxburghii Sarg.) Plantation Forest of Kathmandu Valley, Central Nepal. J. For. Environ. Sci. 2020, 36, 37–46. [Google Scholar] [CrossRef]
- Soto-Cervantes, J.A.; Carrillo-Parra, A.; Rodríguez-Laguna, R.; Corral-Rivas, J.J.; Pompa-García, M.; Dominguez-Calleros, P.A. Survival, growth and carbon content in a forest plantation established after a clear-cutting in Durango, Mexico. PeerJ 2020, 8, e9506. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Han, H.; Shi, Z.; Yang, X. Biomass Accumulation and Carbon Sequestration in an Age-Sequence of Mongolian Pine Plantations in Horqin Sandy Land, China. Forests 2019, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.S. Soil Organic Matter: A Link between Forest Management and Productivity. In Carbon Forms and Functions in Forest Soils; Soil Science Society of America: Madison, WI, USA, 2006; pp. 419–435. [Google Scholar]
- Noormets, A.; McNulty, S.G.; Domec, J.; Gavazzi, M.; Sun, G.; King, J.S. The role of harvest residue in rotation cycle carbon balance in loblolly pine plantations. Respiration partitioning approach. Glob. Chang. Biol. 2012, 18, 3186–3201. [Google Scholar] [CrossRef]
- He, J.; Dai, Q.; Xu, F.; Peng, X.; Yan, Y. Variability in Carbon Stocks across a Chronosequence of Masson Pine Plantations and the Trade-Off between Plant and Soil Systems. Forests 2021, 12, 1342. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).