Seasonal Variations in the Particulate Matter Accumulation and Leaf Traits of 24 Plant Species in Urban Green Space
Abstract
:1. Introduction
2. Materials and Methods
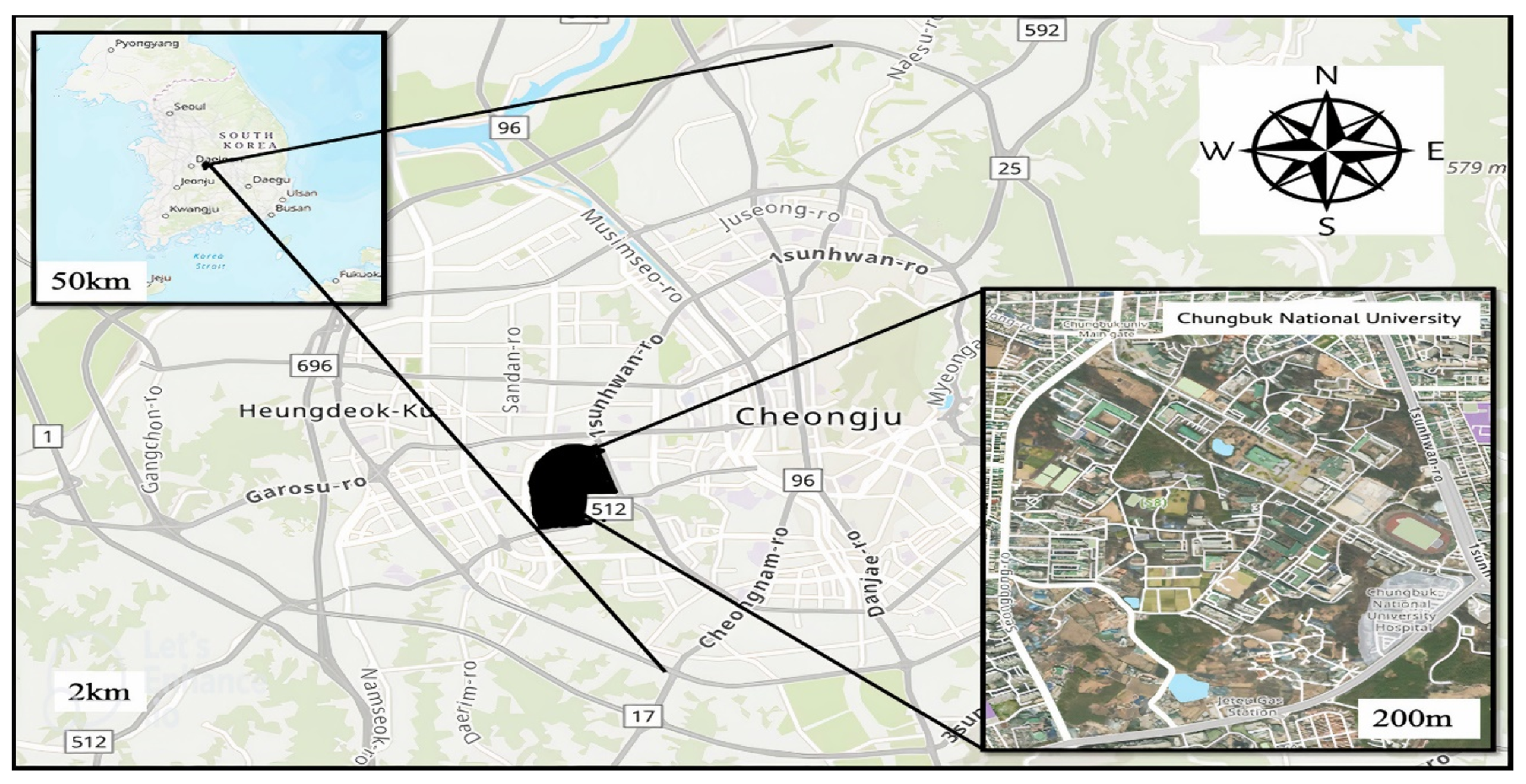
2.1. Study Area and Sample Collection
2.2. Leaf Surface PM (sPM) and Epicuticular Wax (wPM) Analysis
2.3. Analysis of Leaf Traits
2.3.1. Chlorophyll (Chl a, Chl b, Total Chlorophyll (Tchl))
2.3.2. Specific Leaf Area (SLA)
2.3.3. Leaf Extract pH (pH)
2.3.4. Relative Water Content (RWC)
3. Statistical Analysis
4. Results and Discussion
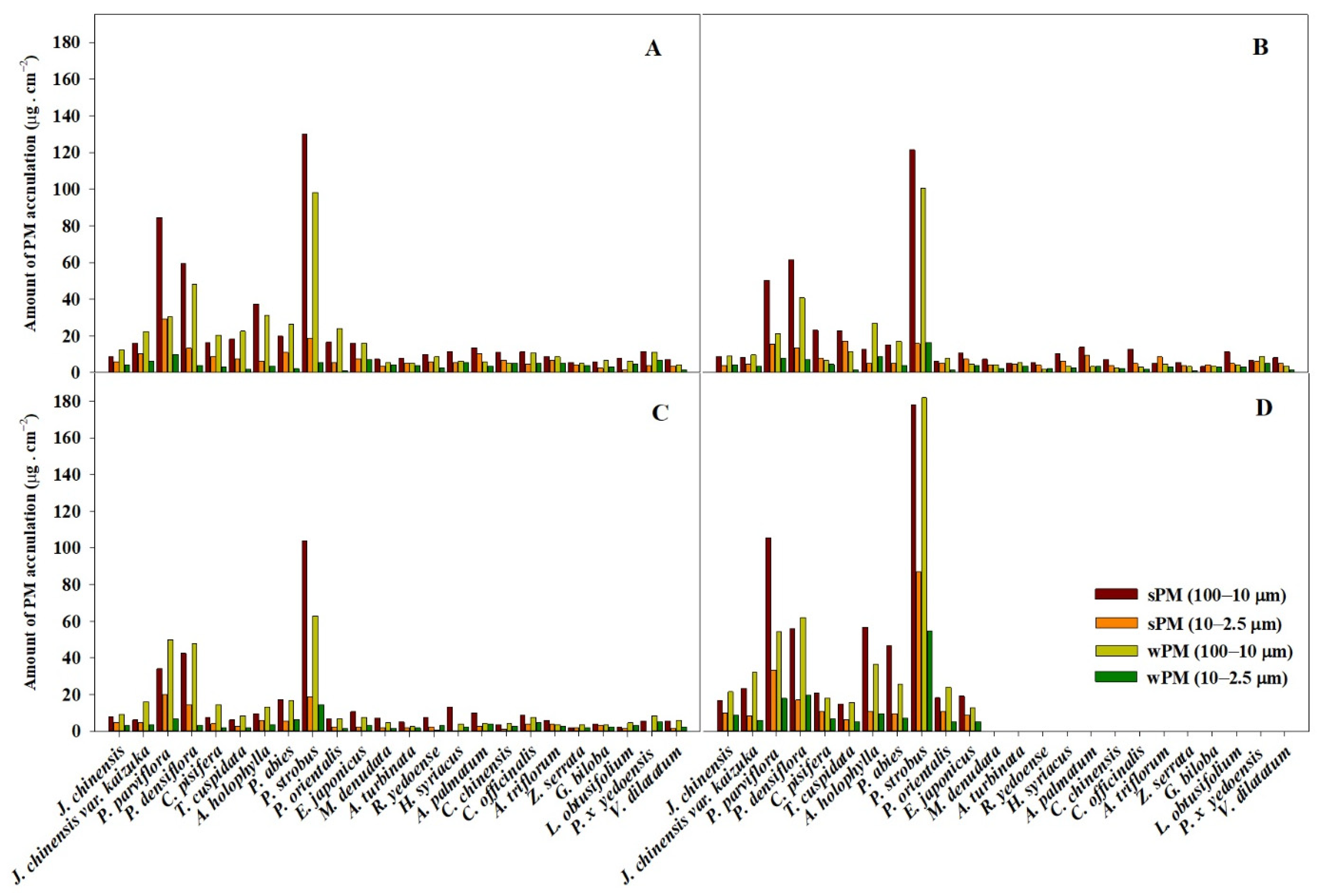
4.1. PM Accumulation of Plant Species
4.2. The Leaf Traits of 24 Plant Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doreswamy; Harishkumar, K.S.; Yogesh, K.M.; Ibrahim, G. Forecasting air pollution particulate matter (PM2.5) using machine learning regression models. Procedia Comput. Sci. 2020, 171, 2057–2066. [Google Scholar] [CrossRef]
- WHO. WHO’s global air-quality guidelines. Lancet 2006, 368, 1302. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef]
- Kwon, K.-J.; Urrintuya, O.; Kim, S.-Y.; Yang, J.-C.; Sung, J.-W.; Park, B.-J. Removal potential of particulate matter of 12 woody plant species for landscape planting. J. People Plants Environ. 2020, 23, 647–654. [Google Scholar] [CrossRef]
- Bui, H.-T.; Ousuren, U.; Kwon, K.-J.; Kim, S.-Y.; Yang, J.-C.; Jeong, N.-R.; Park, B.-J. Assessment of air pollution tolerance and particulate matter accumulation of 11 woody plant species. Atmosphere 2021, 12, 1067. [Google Scholar] [CrossRef]
- Bui, H.-T.; Odsuren, U.; Jeong, M.; Seo, J.-W.; Kim, S.-Y.; Park, B.-J. Evaluation of the air pollution tolerance index of 12 plant species growing in environments with different air pollution levels. J. People Plants Environ. 2022, 25, 23–31. [Google Scholar] [CrossRef]
- Bui, H.-T.; Odsuren, U.; Kim, S.-Y.; Park, B.-J. Particulate matter accumulation and leaf traits of ten woody species growing with different air pollution conditions in Cheongju City, South Korea. Atmosphere 2022, 13, 1351. [Google Scholar] [CrossRef]
- Wróblewska, K.; Jeong, B.R. Effectiveness of plants and green infrastructure utilization in ambient particulate matter removal. Envrion. Sci. Eur. 2021, 33, 110. [Google Scholar] [CrossRef]
- Zhang, W.K.; Wang, B.; Niu, X. Study on the adsorption capacities for airborne particulates of landscape plant in different polluted regions in Beijing (China). Int. J. Envrion. Res. Public Health 2015, 12, 9623–9638. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Niu, X. Relationship between leaf surface characteristics and particle capturing capacities of different tree species in Beijing. Forests 2017, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.G.; Bealey, W.J.; Fowler, D.; Dragosits, U.; Skiba, U.; Smith, R.I.; Donovan, R.G.; Brett, H.E.; Hewitt, C.N.; Nemitz, E. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos. Environ. 2007, 41, 8455–8467. [Google Scholar] [CrossRef]
- Mo, L.; Ma, Z.; Xu, Y.; Sun, F.; Lun, X.; Liu, X.; Chen, J.; Yu, X. Assessing the capacity of plant species to accumulate particulate matter in Beijing, China. PLoS ONE 2015, 10, e0140664. [Google Scholar] [CrossRef] [PubMed]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilisation in waxes—A 3-year study. Int. J. Phytoremediation 2013, 15, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Meng, H.; Zhang, T. How does leaf surface micromorphology of different trees impact their ability to capture particulate matter? Forests 2018, 9, 681. [Google Scholar] [CrossRef] [Green Version]
- Leonard, R.J.; McArthur, C.; Hochuli, D.F. Particulate matter deposition on roadside plants and the importance of leaf trait combination. Urban For. Urban Green. 2016, 20, 249–253. [Google Scholar] [CrossRef]
- Przybysz, A.; Popek, R.; Gawrońska, H.; Grab, K.; Łoskot, K.; Wrochna, M.; Gawroński, S.W. Efficiency of photosynthetic apparatus of plants grown in sites differing in level of particulate matter. Acta Sci. Pol. Hortorum Cultus 2014, 13, 17–30. [Google Scholar]
- Wang, H.; Shi, H.; Wang, Y. Effects of weather, time, and pollution level on the amount of particulate matter deposited on leaves of Ligustrum lucidum. Sci. World J. 2015, 2015, 935942. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in tree species ability to capture and retain airborne fine particulate matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef] [Green Version]
- Popek, R.; Haynes, A.; Przybysz, A.; Robinson, S.A. How much does weather matter? Effects of rain and wind on PM accumulation by four species of Australian native trees. Atmosphere 2019, 10, 633. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.K.; Prasad, S.; Pana, S.; Obaidullah, S.M.; Pandey, V.; Singh, H. Effects of dust load on the leaf attributes of the tree species growing along the roadside. Environ. Monit. Assess. 2013, 185, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Yu, H.; Chen, M.; Piao, Z.; Dang, J.; Sui, Y. Effects of particle matters on plant: A review. Phyton 2019, 88, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Farmer, A.M. The effects of dust on vegetation—A review. Environ. Pollut. 1993, 79, 63–75. [Google Scholar] [CrossRef]
- Mulenga, C.; Clarke, C.; Meincken, M. Physiological and growth responses to pollutant-induced biochemical changes in plants: A review. Pollution, Pollution 2020, 6, 827–848. [Google Scholar] [CrossRef]
- He, C.; Qie, K.; Pott, R. Reduction of urban traffic-related particulate matter-leaf trait matters. Environ. Sci. Pollut. Res. 2020, 27, 5825–5844. [Google Scholar] [CrossRef] [PubMed]
- Panda, L.R.L.; Aggarwal, R.K.; Bhardwaj, D.R. A review on air pollution tolerance index (APTI) and anticipated performance index (API). Curr. World Environ. 2018, 13, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Popek, R.; Przybysz, A.; Gawrońska, H.; Klamkowski, K.; Gawroński, S.W. Impact of particulate matter accumulation on the photosynthetic apparatus of roadside woody plants growing in the urban conditions. Ecotoxicol. Environ. Saf. 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Shannigrahi, A.S.; Fukushima, T.; Sharma, R.C. Anticipated air pollution tolerance of some plant species considered for green belt development in and around an industrial/urban area in India: An overview. Int. J. Environ. Stud. 2004, 61, 125–137. [Google Scholar] [CrossRef]
- Ter, S.; Chettri, M.K.; Shakya, K. Air pollution tolerance index of some tree species of Pashupati and Budhanilkantha area, Kathmandu. Amrit Res. J. 2020, 1, 20–28. [Google Scholar] [CrossRef]
- Dzierzanowski, K.; Popek, R.; Gawrońska, H.; Saebø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Singh, S.; Rao, D.; Agrawal, M.; Pandey, J.; Naryan, D. Air pollution tolerance index of plants. J. Environ. Manag. 1991, 32, 45–55. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Xu, X.; Yu, X.; Mo, L.; Xu, Y.; Bao, L.; Lun, X. Atmospheric particulate matter accumulation on trees: A comparison of boles, branches and leaves. J. Clean. Prod. 2019, 226, 349–356. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, B.; Dole, J.M.; Shahid, M.; Ziaf, K. Evaluation of the air pollution tolerance index of ornamentals growing in an industrial area compared to a less polluted area. Hortic. Environ. Biotechnol. 2019, 60, 595–602. [Google Scholar] [CrossRef]
- He, C.; Qiu, K.; Alahnad, A.; Pott, R. Particulate matter capturing capacity of roadside evergreen vegetation during the winter season. Urban For. Urban Green. 2020, 48, 126510. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob. Chang. Biol. 2000, 8, 995–1003. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Bao, L.; Mo, L.; Yu, X.; Fan, D.; Lun, X. Influence of rainfall duration and intensity on particulate matter removal from plant leaves. Sci. Total Environ. 2017, 609, 11–16. [Google Scholar] [CrossRef]
- Jin, E.J.; Yoon, J.H.; Bae, E.J.; Jeong, B.R.; Yong, S.H.; Choi, M.S. Particulate matter removal ability of ten evergreen trees planted in Korea urban greening. Forests 2021, 12, 438. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Chen, Q. Potential of thirteen urban greening plants to capture particulate matter on leaf surfaces across three levels of ambient atmospheric pollution. Int. J. Environ. Res. Public Health 2019, 16, 402. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, Z.; Zou, S.; Liu, H.; Hai, X.; Wang, S.; Duan, J.; Xi, B.; Yan, G.; Zhang, S.; et al. An investigation of the leaf retention capacity, efficiency and mechanism for atmospheric particulate matter of five greening tree species in Beijing, China. Sci. Total Environ. 2018, 616–617, 417–426. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, G.; An, H.; Yin, W.; Xia, X. Quantifying the particulate matter accumulation on leaf surfaces of urban plants in Beijing, China. Atmos. Pollut. Res. 2017, 8, 836–842. [Google Scholar] [CrossRef]
- Han, D.; Shen, H.; Duan, W.; Chen, L. A review on particulate matter removal capacity by urban forests at different scales. Urban For. Urban Green. 2020, 48, 126565. [Google Scholar] [CrossRef]
- Chen, G.; Lin, L.; Zhang, Y.; Ma, K. Net particulate matter removal ability and efficiency of ten plant species in Beijing. Urban For. Urban Green. 2021, 63, 127230. [Google Scholar] [CrossRef]
- Popek, R.; Łukowsk, A.; Karolewski, P. Particulate matter accumulation—Further differences between native Prunus padus and non-native P. serotina. Dendrobiology 2017, 78, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Gautnam, M. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 2007, 28, 127–132. [Google Scholar] [PubMed]
- Popek, R.; Łukowski, A.; Grabowski, M. Influence of particulate matter accumulation on photosynthetic apparatus of Physocarpus opulifolius and Sorbaria sorbifolia. Pol. J. Environ. Stud. 2018, 27, 2391–2396. [Google Scholar] [CrossRef]
- Giri, S.; Shrivastava, D.; Deshmukh, K.; Dubey, P. Effect of air pollution on chlorophyll content of leaves. Curr. Agric. Res. J. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Ghafari, S.; Kaviani, B.; Sedaghathoor, S.; Allahyari, M.S. Assessment of air pollution tolerance index (APTI) for some ornamental woody species in green space of humid temperature region (Rasht, Iran). Environ. Dev. Sustain. 2020, 23, 1579–1600. [Google Scholar] [CrossRef]
- Rai, P.K.; Panda, L.L.S.; Chutia, B.M.; Singh, M.M. Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and non industrial area (Aizawl) of India: An ecomanagement approach. Afr. J. Environ. Sci. Technol. 2013, 7, 944–948. [Google Scholar] [CrossRef]
- Wuytack, T.; Wuyts, K.; Van Dongen, S.; Baeten, L.; Kardel, F.; Verheyen, K.; Samson, R. The effect of air pollution and other environmental stressors on leaf fluctuating asymmetry and specific leaf area of Salix ablba L. Environ. Pollut. 2011, 159, 2405–2411. [Google Scholar] [CrossRef]

| Species | Family | Habit | Type |
|---|---|---|---|
| Juniperus chinensis L. | Cupressaceae | Tree | Evergreen |
| Juniperus chinensis var. kaizuka Hort. | Cupressaceae | Tree | Evergreen |
| Pinus parviflora Siebold & Zucc. | Pinaceae | Tree | Evergreen |
| Pinus densiflora Siebold & Zucc. | Pinaceae | Tree | Evergreen |
| Chamaecyparis pisifera Siebold & Zucc. | Cupressaceae | Tree | Evergreen |
| Taxus cuspidata Siebold & Zucc. | Taxaceae | Tree | Evergreen |
| Abies holophylla Maxim. | Pinaceae | Tree | Evergreen |
| Picea abies (L.) H.Karst. | Pinaceae | Tree | Evergreen |
| Pinus strobus L. | Pinaceae | Tree | Evergreen |
| Platycladus orientalis (L.) Franco | Cupressaceae | Tree | Evergreen |
| Euonymus japonica Thunb. | Celastraceae | Shrub | Evergreen |
| Magnolia denudata Desr. | Magnoliaceae | Tree | Deciduous |
| Aesculus turbinata Blume | Hippocastanaceae | Tree | Deciduous |
| Rhododendron yedoense Maxim | Ericaceae | Shrub | Deciduous |
| Hibiscus syriacus L. | Malvaceae | Shrub | Deciduous |
| Acer palmatum Thunb. | Aceraceae | Tree | Deciduous |
| Cercis chinensis Bunge | Fabaceae | Shrub | Deciduous |
| Cornus officinalis Siebold&Zucc. | Cornaceae | Tree | Deciduous |
| Acer triflorum Kom. | Aceraceae | Tree | Deciduous |
| Zelkova serrata (Thunb.) Makino | Ulmaceae | Tree | Deciduous |
| Ginkgo biloba L. | Ginkgoaceae | Tree | Deciduous |
| Ligustrum obtusifolium Siebold & Zucc. | Oleaceae | Shrub | Deciduous |
| Prunus x yedoensis Matsum. | Rosaceae | Tree | Deciduous |
| Viburnum dilatatum Thunb. | Adoxaceae | Shrub | Deciduous |
| Species | Total sPM (µg·cm−2) | Total wPM (µg·cm−2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| J. chinensis | 14.5 ± 4.1 | 12.5 ± 2.1 | 12.6 ± 1.7 | 26.5 ± 1.1 | 16.6 ± 0.9 | 13.6 ± 3.4 | 12.2 ± 4.7 | 30.3 ± 1.2 |
| J. chinensis var. kaizuka | 26.5 ± 1.6 | 12.8 ± 1.1 | 10.9 ± 1.5 | 31.3 ± 0.2 | 28.7 ± 1.8 | 13.3 ± 2.8 | 19.4 ± 6.8 | 38.1 ± 1.3 |
| P. parviflora | 113.9 ± 26.4 | 65.6 ± 7.1 | 54.1 ± 11.8 | 138.8 ± 7.5 | 40.4 ± 7.5 | 29.3 ± 6.6 | 56.8 ± 8.7 | 71.8 ± 8.4 |
| P. densiflora | 72.9 ± 2.7 | 75.1 ± 11.4 | 56.8 ± 6.0 | 72.7 ± 6.8 | 52.1 ± 5.6 | 47.8 ± 1.7 | 51.0 ± 10.2 | 81.3 ± 3.2 |
| C. pisifera | 24.8 ± 2.7 | 31.1 ± 7.5 | 11.5 ± 0.6 | 31.5 ± 0.9 | 23.5 ± 1.3 | 11.3 ± 0.6 | 16.5 ± 9.5 | 24.8 ± 3.2 |
| T. cuspidata | 25.7 ± 4.5 | 40.1 ± 5.0 | 9.0 ± 0.5 | 21.0 ± 0.7 | 24.3 ± 2.3 | 12.8 ± 1.3 | 10.4 ± 0.2 | 20.7 ± 0.5 |
| A. holophylla | 43.8 ± 3.6 | 17.5 ± 3.4 | 15.5 ± 0.3 | 67.5 ± 10.8 | 34.7 ± 11.1 | 35.3 ± 3.8 | 16.6 ± 2.5 | 46.0 ± 3.4 |
| P. abies | 30.7 ± 1.2 | 20.1 ± 0.5 | 22.7 ± 2.2 | 55.8 ± 4.9 | 28.4 ± 4.3 | 20.8 ± 0.7 | 23.3 ± 1.8 | 32.7 ± 3.3 |
| P. strobus | 148.4 ± 23.0 | 137.0 ± 31.8 | 122.8 ± 25.8 | 264.4 ± 31.1 | 103.6 ± 22.2 | 116.7 ± 5.4 | 77.2 ± 27.5 | 236.5 ± 23.1 |
| P. orientalis | 22.0 ± 2.3 | 11.6 ± 1.1 | 8.9 ± 0.9 | 28.7 ± 2.1 | 25.3 ± 1.3 | 9.3 ± 1.4 | 8.5 ± 0.8 | 28.8 ± 1.7 |
| E. japonicus | 23.7 ± 3.4 | 17.9 ± 0.3 | 12.9 ± 1.0 | 27.7 ± 3.2 | 22.9 ± 5.2 | 8.7 ± 0.5 | 10.7 ± 1.4 | 17.7 ± 1.6 |
| M. denudata | 10.9 ± 1.0 | 11.5 ± 0.8 | 9.0 ± 0.4 | - | 9.8 ± 0.7 | 6.7 ± 0.4 | 6.2 ± 0.8 | - |
| A. turbinata | 12.9 ± 0.8 | 9.9 ± 0.7 | 6.8 ± 1.1 | - | 9.2 ± 1.0 | 9.0 ± 0.5 | 4.5 ± 0.5 | - |
| R. yedoense | 16.0 ± 2.3 | 9.8 ± 0.2 | 9.8 ± 0.7 | - | 11.0 ± 0.2 | 4.1 ± 0.9 | 3.8 ± 0.7 | - |
| H. syriacus | 16.9 ± 0.4 | 16.6 ± 1.2 | 13.6 ± 1.9 | - | 12.0 ± 0.1 | 6.0 ± 0.3 | 6.3 ± 1.9 | - |
| A. palmatum | 23.8 ± 2.4 | 23.2 ± 9.9 | 12.6 ± 0.7 | - | 9.2 ± 0.6 | 6.6 ± 1.3 | 8.1 ± 1.3 | - |
| C. chinensis | 17.6 ± 2.1 | 10.8 ± 0.0 | 4.8 ± 0.7 | - | 10.3 ± 0.6 | 5.1 ± 0.1 | 7.3 ± 2.2 | - |
| C. officinalis | 16.2 ± 0.6 | 17.6 ± 0.9 | 12.7 ± 0.4 | - | 15.8 ± 1.3 | 5.2 ± 0.4 | 12.2 ± 1.2 | - |
| A. triflorum | 15.3 ± 0.8 | 13.5 ± 1.3 | 10.0 ± 0.1 | - | 13.5 ± 6.3 | 8.0 ± 2.1 | 6.3 ± 1.1 | - |
| Z. serrata | 10.0 ± 0.5 | 9.6 ± 0.6 | 3.7 ± 0.2 | - | 8.9 ± 06 | 4.3 ± 1.0 | 5.5 ± 0.0 | - |
| G. biloba | 8.3 ± 1.1 | 7.7 ± 2.4 | 7.2 ± 0.3 | - | 9.5 ± 1.5 | 6.3 ± 0.8 | 5.8 ± 0.5 | - |
| L. obtusifolium | 9.4 ± 0.9 | 16.2 ± 2.8 | 4.0 ± 0.3 | - | 11.1 ± 2.0 | 7.2 ± 0.5 | 7.7 ± 0.5 | - |
| P. × yedoensis | 15.4 ± 0.6 | 13.0 ± 1.0 | 5.7 ± 1.6 | - | 17.5 ± 2.9 | 13.8 ± 1.8 | 13.4 ± 1.4 | - |
| V. dilatatum | 10.6 ± 1.3 | 13.3 ± 1.5 | 7.0 ± 1.3 | - | 5.7 ± 1.1 | 4.9 ± 0.6 | 8.1 ± 1.1 | - |
| Seasons | Chl a (mg·g−1 FW) | Chl b (mg·g−1 FW) | TChl (mg·g−1 FW) | RWC (%) | pH | SLA (cm2·g−1) | |
|---|---|---|---|---|---|---|---|
| J. chinensis | Spring | 0.056 ± 0.001 | 0.024 ± 0.001 | 0.080 ± 0.001 | 74.66 ± 1.15 | 5.08 ± 0.05 | 19.06 ± 0.60 |
| Summer | 0.090 ± 0.003 | 0.030 ± 0.003 | 0.124 ± 0.004 | 73.45 ± 0.15 | 5.04 ± 0.02 | 24.87 ± 0.51 | |
| Autumn | 0.049 ± 0.001 | 0.020 ± 0.000 | 0.069 ± 0.002 | 79.28 ± 0.18 | 4.88 ± 0.03 | 20.97 ± 0.65 | |
| Winter | 0.066 ± 0.002 | 0.027 ± 0.002 | 0.094 ± 0.004 | 74.14 ± 0.58 | 5.70 ± 0.01 | 16.25 ± 0.32 | |
| J. chinensis var. kaizuka | Spring | 0.052 ± 0.003 | 0.022 ± 0.002 | 0.078 ± 0.006 | 78.65 ± 0.04 | 4.75 ± 0.01 | 19.72 ± 0.96 |
| Summer | 0.058 ± 0.001 | 0.026 ± 0.000 | 0.084 ± 0.001 | 77.93 ± 0.99 | 4.75 ± 0.01 | 18.77 ± 0.48 | |
| Autumn | 0.053 ± 0.002 | 0.023 ± 0.001 | 0.076 ± 0.004 | 91.87 ± 0.59 | 4.93 ± 0.05 | 19.40 ± 0.16 | |
| Winter | 0.055 ± 0.001 | 0.023 ± 0.002 | 0.078 ± 0.002 | 83.36 ± 1.12 | 5.77 ± 0.08 | 17.02 ± 0.60 | |
| P. parviflora | Spring | 0.189 ± 0.007 | 0.060 ± 0.008 | 0.249 ± 0.015 | 71.99 ± 1.08 | 5.00 ± 0.03 | 9.65 ± 1.36 |
| Summer | 0.117 ± 0.000 | 0.048 ± 0.000 | 0.166 ± 0.001 | 79.69 ± 1.47 | 5.09 ± 0.05 | 9.06 ± 0.97 | |
| Autumn | 0.119 ± 0.001 | 0.045 ± 0.000 | 0.165 ± 0.002 | 81.71 ± 0.49 | 4.93 ± 0.02 | 13.12 ± 0.70 | |
| Winter | 0.088 ± 0.000 | 0.039 ± 0.000 | 0.128 ± 0.000 | 76.85 ± 0.28 | 5.85 ± 0.04 | 9.02 ± 0.30 | |
| P. densiflora | Spring | 0.178 ± 0.004 | 0.075 ± 0.001 | 0.250 ± 0.007 | 77.58 ± 1.13 | 4.62 ± 0.03 | 14.34 ± 0.52 |
| Summer | 0.122 ± 0.001 | 0.047 ± 0.001 | 0.184 ± 0.014 | 86.58 ± 1.26 | 4.59 ± 0.02 | 11.11 ± 0.67 | |
| Autumn | 0.106 ± 0.001 | 0.041 ± 0.001 | 0.146 ± 0.001 | 96.52 ± 1.10 | 4.85 ± 0.01 | 13.08 ± 1.24 | |
| Winter | 0.075 ± 0.004 | 0.032 ± 0.003 | 0.108 ± 0.007 | 93.19 ± 0.50 | 6.07 ± 0.05 | 9.23 ± 0.12 | |
| C. pisifera | Spring | 0.157 ± 0.003 | 0.059 ± 0.001 | 0.216 ± 0.004 | 81.78 ± 0.80 | 4.90 ± 0.05 | 39.54 ± 2.07 |
| Summer | 0.139 ± 0.007 | 0.056 ± 0.002 | 0.195 ± 0.009 | 80.96 ± 0.53 | 4.94 ± 0.03 | 58.19 ± 0.28 | |
| Autumn | 0.131 ± 0.001 | 0.051 ± 0.001 | 0.183 ± 0.001 | 84.92 ± 0.31 | 5.03 ± 0.01 | 42.99 ± 0.35 | |
| Winter | 0.089 ± 0.001 | 0.038 ± 0.001 | 0.127 ± 0.001 | 80.65 ± 1.66 | 6.04 ± 0.10 | 32.61 ± 0.43 | |
| T. cuspidata | Spring | 0.124 ± 0.020 | 0.053 ± 0.008 | 0.140 ± 0.024 | 75.52 ± 0.54 | 5.21 ± 0.14 | 63.01 ± 1.89 |
| Summer | 0.105 ± 0.005 | 0.045 ± 0.002 | 0.141 ± 0.006 | 77.25 ± 0.98 | 4.84 ± 0.07 | 55.18 ± 1.69 | |
| Autumn | 0.111 ± 0.003 | 0.042 ± 0.001 | 0.148 ± 0.005 | 79.31 ± 0.36 | 5.24 ± 0.01 | 58.30 ± 2.69 | |
| Winter | 0.105 ± 0.004 | 0.051 ± 0.002 | 0.156 ± 0.005 | 73.02 ± 1.14 | 5.69 ± 0.04 | 42.38 ± 0.77 | |
| A. holophylla | Spring | 0.105 ± 0.009 | 0.044 ± 0.003 | 0.135 ± 0.011 | 74.88 ± 1.25 | 4.74 ± 0.03 | 28.94 ± 0.58 |
| Summer | 0.113 ± 0.001 | 0.042 ± 0.000 | 0.156 ± 0.001 | 73.96 ± 0.24 | 4.74 ± 0.03 | 33.90 ± 1.49 | |
| Autumn | 0.114 ± 0.004 | 0.042 ± 0.001 | 0.157 ± 0.005 | 88.70 ± 0.57 | 4.93 ± 0.02 | 27.12 ± 0.59 | |
| Winter | 0.062 ± 0.002 | 0.030 ± 0.001 | 0.092 ± 0.002 | 77.56 ± 1.18 | 6.28 ± 0.07 | 28.10 ± 0.55 | |
| P. abies | Spring | 0.103 ± 0.004 | 0.042 ± 0.003 | 0.128 ± 0.002 | 79.12 ± 1.07 | 4.19 ± 0.09 | 27.62 ± 1.04 |
| Summer | 0.122 ± 0.003 | 0.046 ± 0.001 | 0.168 ± 0.004 | 73.40 ± 0.35 | 4.19 ± 0.09 | 31.79 ± 0.93 | |
| Autumn | 0.087 ± 0.001 | 0.033 ± 0.001 | 0.120 ± 0.002 | 83.60 ± 0.81 | 5.06 ± 0.04 | 33.57 ± 0.41 | |
| Winter | 0.101 ± 0.000 | 0.042 ± 0.002 | 0.142 ± 0.002 | 80.702 ± 0.35 | 5.84 ± 0.07 | 21.70 ± 0.41 | |
| P. strobus | Spring | 0.094 ± 0.007 | 0.037 ± 0.003 | 0.147 ± 0.012 | 62.41 ± 0.53 | 4.84 ± 0.04 | 7.80 ± 0.86 |
| Summer | 0.083 ± 0.001 | 0.035 ± 0.001 | 0.120 ± 0.003 | 72.78 ± 0.31 | 4.84 ± 0.04 | 3.78 ± 0.91 | |
| Autumn | 0.101 ± 0.004 | 0.042 ± 0.002 | 0.143 ± 0.006 | 73.44 ± 0.77 | 5.05 ± 0.02 | 1.27 ± 0.11 | |
| Winter | 0.084 ± 0.002 | 0.040 ± 0.000 | 0.124 ± 0.002 | 78.97 ± 0.46 | 5.54 ± 0.03 | 4.85 ± 0.52 | |
| P. orientalis | Spring | 0.094 ± 0.002 | 0.040 ± 0.001 | 0.139 ± 0.003 | 78.17 ± 2.58 | 4.84 ± 0.02 | 49.33 ± 1.98 |
| Summer | 0.105 ± 0.002 | 0.045 ± 0.001 | 0.150 ± 0.002 | 76.43 ± 0.20 | 4.84 ± 0.02 | 46.32 ± 1.88 | |
| Autumn | 0.096 ± 0.003 | 0.039 ± 0.001 | 0.135 ± 0.003 | 85.22 ± 1.06 | 5.38 ± 0.02 | 51.76 ± 0.49 | |
| Winter | 0.065 ± 0.005 | 0.029 ± 0.002 | 0.094 ± 0.008 | 75.84 ± 1.28 | 6.16 ± 0.15 | 39.36 ± 1.72 | |
| E. japonica | Spring | 0.048 ± 0.003 | 0.024 ± 0.001 | 0.072 ± 0.002 | 67.96 ± 1.40 | 5.16 ± 0.01 | 81.74 ± 1.51 |
| Summer | 0.071 ± 0.004 | 0.033 ± 0.001 | 0.103 ± 0.004 | 70.75 ± 0.85 | 5.16 ± 0.01 | 82.66 ± 4.59 | |
| Autumn | 0.039 ± 0.002 | 0.018 ± 0.001 | 0.057 ± 0.002 | 74.41 ± 0.59 | 5.31 ± 0.03 | 91.05 ± 2.82 | |
| Winter | 0.044 ± 0.006 | 0.021 ± 0.003 | 0.065 ± 0.009 | 60.96 ± 1.07 | 6.14 ± 0.03 | 77.15 ± 1.44 | |
| M. denudata | Spring | 0.096 ± 0.005 | 0.038 ± 0.004 | 0.145 ± 0.014 | 67.29 ± 0.85 | 5.47 ± 0.01 | 179.51 ± 7.27 |
| Summer | 0.111 ± 0.003 | 0.046 ± 0.002 | 0.156 ± 0.004 | 77.87 ± 0.31 | 5.47 ± 0.01 | 168.66 ± 6.71 | |
| Autumn | 0.086 ± 0.002 | 0.036 ± 0.001 | 0.122 ± 0.003 | 84.80 ± 0.13 | 6.14 ± 0.03 | 177.88 ± 10.6 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| A. turbinata | Spring | 0.214 ± 0.015 | 0.043 ± 0.005 | 0.271 ± 0.021 | 69.42 ± 0.35 | 5.17 ± 0.03 | 131.94 ± 2.31 |
| Summer | 0.172 ± 0.000 | 0.073 ± 0.001 | 0.245 ± 0.001 | 72.26 ± 0.32 | 5.17 ± 0.03 | 145.68 ± 6.54 | |
| Autumn | 0.122 ± 0.003 | 0.047 ± 0.001 | 0.172 ± 0.005 | 80.75 ± 1.09 | 5.56 ± 0.05 | 123.81 ± 2.56 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| R. yedoense | Spring | 0.119 ± 0.026 | 0.029 ± 0.012 | 0.148 ± 0.037 | 77.55 ± 0.41 | 5.21 ± 0.04 | 165.65 ± 1.17 |
| Summer | 0.165 ± 0.006 | 0.076 ± 0.005 | 0.261 ± 0.018 | 87.68 ± 1.78 | 5.18 ± 0.07 | 163.55 ± 2.48 | |
| Autumn | 0.165 ± 0.004 | 0.066 ± 0.001 | 0.230 ± 0.006 | 86.44 ± 0.40 | 5.47 ± 0.03 | 151.35 ± 1.82 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| H. syriacus | Spring | 0.128 ± 0.013 | 0.026 ± 0.004 | 0.154 ± 0.013 | 80.34 ± 0.65 | 5.63 ± 0.03 | 160.35 ± 4.51 |
| Summer | 0.173 ± 0.012 | 0.071 ± 0.006 | 0.244 ± 0.017 | 78.41 ± 0.79 | 5.63 ± 0.03 | 147.46 ± 4.12 | |
| Autumn | 0.157 ± 0.002 | 0.071 ± 0.002 | 0.228 ± 0.003 | 81.45 ± 0.88 | 6.15 ± 0.02 | 151.61 ± 3.38 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| A. palmatum | Spring | 0.075 ± 0.007 | 0.026 ± 0.012 | 0.070 ± 0.007 | 85.26 ± 0.19 | 4.71 ± 0.02 | 152.04 ± 5.05 |
| Summer | 0.164 ± 0.015 | 0.071 ± 0.002 | 0.242 ± 0.012 | 91.30 ± 0.27 | 4.71 ± 0.02 | 193.06 ± 5.44 | |
| Autumn | 0.168 ± 0.003 | 0.068 ± 0.001 | 0.237 ± 0.003 | 92.00 ± 0.47 | 4.99 ± 0.09 | 201.17 ± 6.55 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| C. chinensis | Spring | 0.193 ± 0.008 | 0.054 ± 0.007 | 0.269 ± 0.015 | 61.45 ± 1.75 | 4.31 ± 0.05 | 159.38 ± 13.53 |
| Summer | 0.191 ± 0.004 | 0.085 ± 0.002 | 0.274 ± 0.006 | 65.69 ± 0.34 | 4.42 ± 0.06 | 156.05 ± 17.76 | |
| Autumn | 0.166 ± 0.000 | 0.070 ± 0.001 | 0.234 ± 0.001 | 75.35 ± 0.50 | 5.44 ± 0.01 | 253.01 ± 21.21 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| C. officinalis | Spring | 0.127 ± 0.029 | 0.055 ± 0.014 | 0.182 ± 0.042 | 64.92 ± 0.16 | 5.84 ± 0.02 | 156.65 ± 2.40 |
| Summer | 0.150 ± 0.005 | 0.065 ± 0.001 | 0.209 ± 0.003 | 73.81 ± 0.77 | 5.84 ± 0.02 | 210.40 ± 21.70 | |
| Autumn | 0.081 ± 0.006 | 0.036 ± 0.002 | 0.117 ± 0.008 | 73.75 ± 0.86 | 6.25 ± 0.04 | 121.68 ± 3.91 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| A. triflorum | Spring | 0.194 ± 0.019 | 0.070 ± 0.015 | 0.287 ± 0.035 | 68.34 ± 4.52 | 4.41 ± 0.02 | 214.34 ± 7.11 |
| Summer | 0.217 ± 0.023 | 0.100 ± 0.009 | 0.317 ± 0.031 | 75.56 ± 0.85 | 4.39 ± 0.04 | 238.45 ± 9.92 | |
| Autumn | 0.152 ± 0.005 | 0.063 ± 0.002 | 0.212 ± 0.008 | 71.01 ± 0.31 | 5.81 ± 0.01 | 205.55 ± 8.00 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| Z. serrata | Spring | 0.177 ± 0.005 | 0.061 ± 0.002 | 0.237 ± 0.006 | 52.93 ± 3.92 | 5.15 ± 0.01 | 203.19 ± 0.53 |
| Summer | 0.192 ± 0.018 | 0.078 ± 0.006 | 0.269 ± 0.023 | 61.44 ± 0.93 | 5.15 ± 0.01 | 174.44 ± 2.83 | |
| Autumn | 0.233 ± 0.030 | 0.102 ± 0.010 | 0.374 ± 0.035 | 56.92 ± 0.40 | 5.86 ± 0.02 | 224.08 ± 3.65 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| G. biloba | Spring | 0.093 ± 0.003 | 0.029 ± 0.002 | 0.122 ± 0.003 | 75.00 ± 0.06 | 5.00 ± 0.09 | 129.80 ± 5.15 |
| Summer | 0.074 ± 0.004 | 0.034 ± 0.003 | 0.107 ± 0.006 | 72.13 ± 0.05 | 5.00 ± 0.09 | 131.70 ± 1.51 | |
| Autumn | 0.053 ± 0.002 | 0.024 ± 0.000 | 0.074 ± 0.001 | 80.03 ± 0.67 | 5.69 ± 0.03 | 156.01 ± 1.22 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| L. obtusifolium | Spring | 0.203 ± 0.005 | 0.070 ± 0.003 | 0.257 ± 0.017 | 69.74 ± 2.66 | 5.14 ± 0.02 | 122.36 ± 2.48 |
| Summer | 0.174 ± 0.004 | 0.063 ± 0.002 | 0.237 ± 0.005 | 66.93 ± 0.71 | 5.14 ± 0.02 | 157.87 ± 13.67 | |
| Autumn | 0.156 ± 0.011 | 0.065 ± 0.003 | 0.240 ± 0.013 | 72.35 ± 3.70 | 5.34 ± 0.02 | 207.37 ± 3.50 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| P. × yedoensis | Spring | 0.137 ± 0.002 | 0.049 ± 0.002 | 0.186 ± 0.004 | 75.81 ± 0.22 | 5.03 ± 0.00 | 95.33 ± 1.98 |
| Summer | 0.134 ± 0.005 | 0.055 ± 0.004 | 0.189 ± 0.008 | 76.55 ± 0.80 | 5.03 ± 0.00 | 110.75 ± 3.71 | |
| Autumn | 0.124 ± 0.019 | 0.042 ± 0.001 | 0.146 ± 0.004 | 81.83 ± 0.43 | 5.38 ± 0.01 | 120.18 ± 3.51 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |
| V. dilatatum | Spring | 0.164 ± 0.021 | 0.039 ± 0.006 | 0.203 ± 0.028 | 46.36 ± 1.82 | 5.60 ± 0.01 | 216.28 ± 11.07 |
| Summer | 0.212 ± 0.010 | 0.091 ± 0.004 | 0.303 ± 0.014 | 64.15 ± 1.48 | 5.60 ± 0.01 | 251.32 ± 1.95 | |
| Autumn | 0.146 ± 0.007 | 0.063 ± 0.003 | 0.209 ± 0.010 | 62.67 ± 0.78 | 5.68 ± 0.01 | 302.54 ± 4.60 | |
| Winter | 0 | 0 | 0 | 0 | 0 | 0 |
| Species (F23,192) | Seasons (F3,192) | Species × Season (F69,192) | |
|---|---|---|---|
| sPM (10–100) | 155.44 *** | 20.46 *** | 5.77 *** |
| sPM (2.5–10) | 149.94 *** | 47.48 *** | 38.04 *** |
| wPM (10–100) | 229.74 *** | 34.54 *** | 13.88 *** |
| wPM (2.5–10) | 27.95 *** | 9.71 *** | 9.39 *** |
| Chl a | 47.32 *** | 750.56 *** | 23.66 *** |
| Chl b | 29.11 *** | 498.36 *** | 20.88 *** |
| TChl | 48.89 *** | 747.12 *** | 27.2 *** |
| RWC | 576.54 *** | 8009.28 *** | 297.33 *** |
| pH | 882.41 *** | 19134.2 *** | 1415.78 *** |
| SLA | 465.06 *** | 2098.27 *** | 84.38 ** |
| PM Size | Chl a | Chl b | TChl | RWC | pH | SLA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | sPM (10–100) | −0.012 | 0.106 | 0.046 | −0.037 | −0.204 | −0.554 | *** | |||||
| sPM (2.5–10) | 0.035 | 0.110 | 0.069 | 0.141 | −0.284 | * | −0.548 | *** | |||||
| wPM (10–100) | −0.148 | 0.066 | −0.070 | 0.047 | −0.269 | * | −0.633 | *** | |||||
| wPM (2.5–10) | 0.087 | 0.095 | 0.127 | −0.007 | 0.016 | −0.154 | |||||||
| Summer | sPM (10–100) | −0.285 | * | −0.295 | * | −0.266 | * | 0.155 | −0.148 | −0.484 | *** | ||
| sPM (2.5–10) | −0.196 | −0.168 | −0.183 | 0.314 | * | −0.200 | −0.381 | *** | |||||
| wPM (10–100) | −0.344 | ** | −0.359 | ** | −0.332 | ** | 0.062 | −0.244 | * | −0.537 | *** | ||
| wPM (2.5–10) | −0.306 | ** | −0.329 | ** | −0.300 | * | 0.138 | −0.165 | −0.471 | *** | |||
| Autumn | sPM (10–100) | −0.123 | −0.136 | −0.131 | 0.055 | −0.313 | ** | −0.445 | *** | ||||
| sPM (2.5–10) | −0.175 | −0.210 | −0.181 | 0.182 | −0.479 | *** | −0.579 | *** | |||||
| wPM (10–100) | −0.177 | −0.214 | −0.189 | 0.190 | −0.477 | *** | −0.577 | *** | |||||
| wPM (2.5–10) | −0.134 | −0.139 | −0.137 | −0.025 | −0.258 | * | −0.331 | ** | |||||
| Winter | sPM (10–100) | 0.231 | 0.287 | 0.252 | 0.200 | −0.360 | −0.559 | *** | |||||
| sPM (2.5–10) | 0.177 | 0.250 | 0.202 | 0.107 | −0.459 | −0.472 | ** | ||||||
| wPM (10–100) | 0.142 | 0.209 | 0.165 | 0.269 | −0.423 | −0.545 | *** | ||||||
| wPM (2.5–10) | 0.149 | *** | 0.217 | *** | 0.172 | *** | 0.214 | −0.413 | −0.492 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, H.-T.; Odsuren, U.; Kim, S.-Y.; Park, B.-J. Seasonal Variations in the Particulate Matter Accumulation and Leaf Traits of 24 Plant Species in Urban Green Space. Land 2022, 11, 1981. https://doi.org/10.3390/land11111981
Bui H-T, Odsuren U, Kim S-Y, Park B-J. Seasonal Variations in the Particulate Matter Accumulation and Leaf Traits of 24 Plant Species in Urban Green Space. Land. 2022; 11(11):1981. https://doi.org/10.3390/land11111981
Chicago/Turabian StyleBui, Huong-Thi, Uuriintuya Odsuren, Sang-Yong Kim, and Bong-Ju Park. 2022. "Seasonal Variations in the Particulate Matter Accumulation and Leaf Traits of 24 Plant Species in Urban Green Space" Land 11, no. 11: 1981. https://doi.org/10.3390/land11111981
APA StyleBui, H.-T., Odsuren, U., Kim, S.-Y., & Park, B.-J. (2022). Seasonal Variations in the Particulate Matter Accumulation and Leaf Traits of 24 Plant Species in Urban Green Space. Land, 11(11), 1981. https://doi.org/10.3390/land11111981







