Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
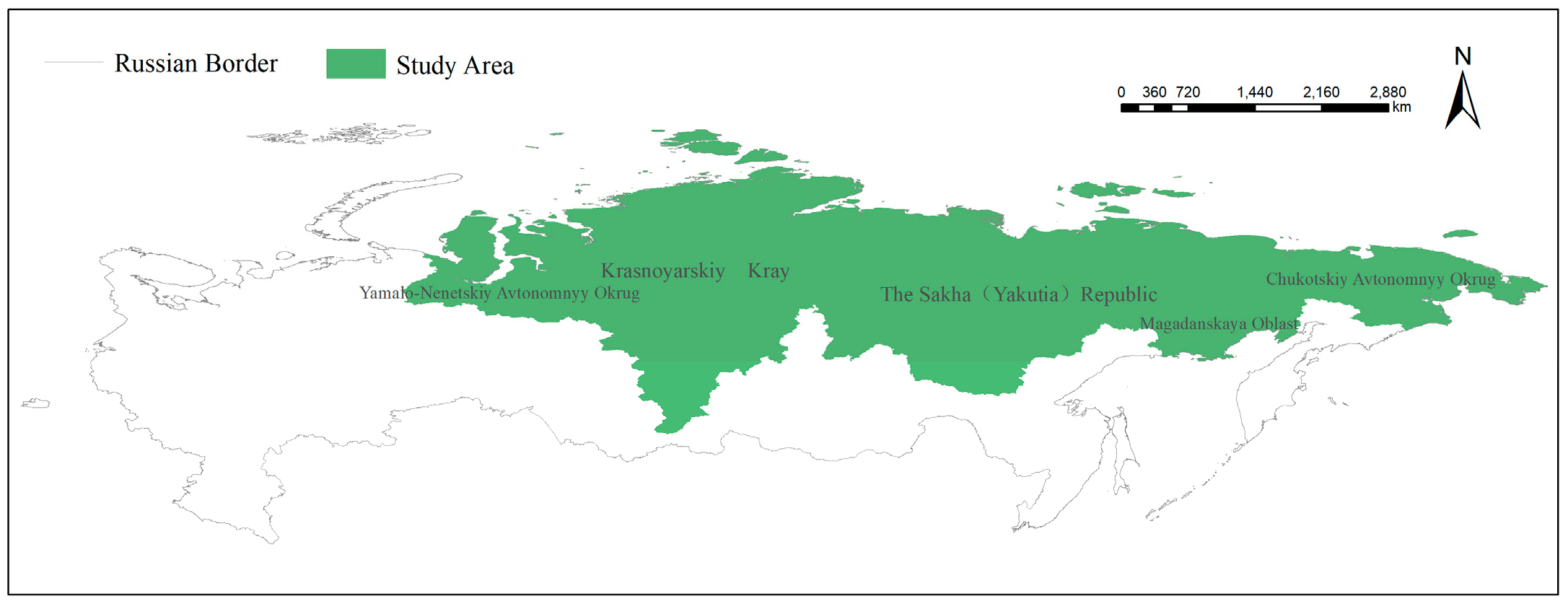
2.1. Study Area
2.2. Data Collection
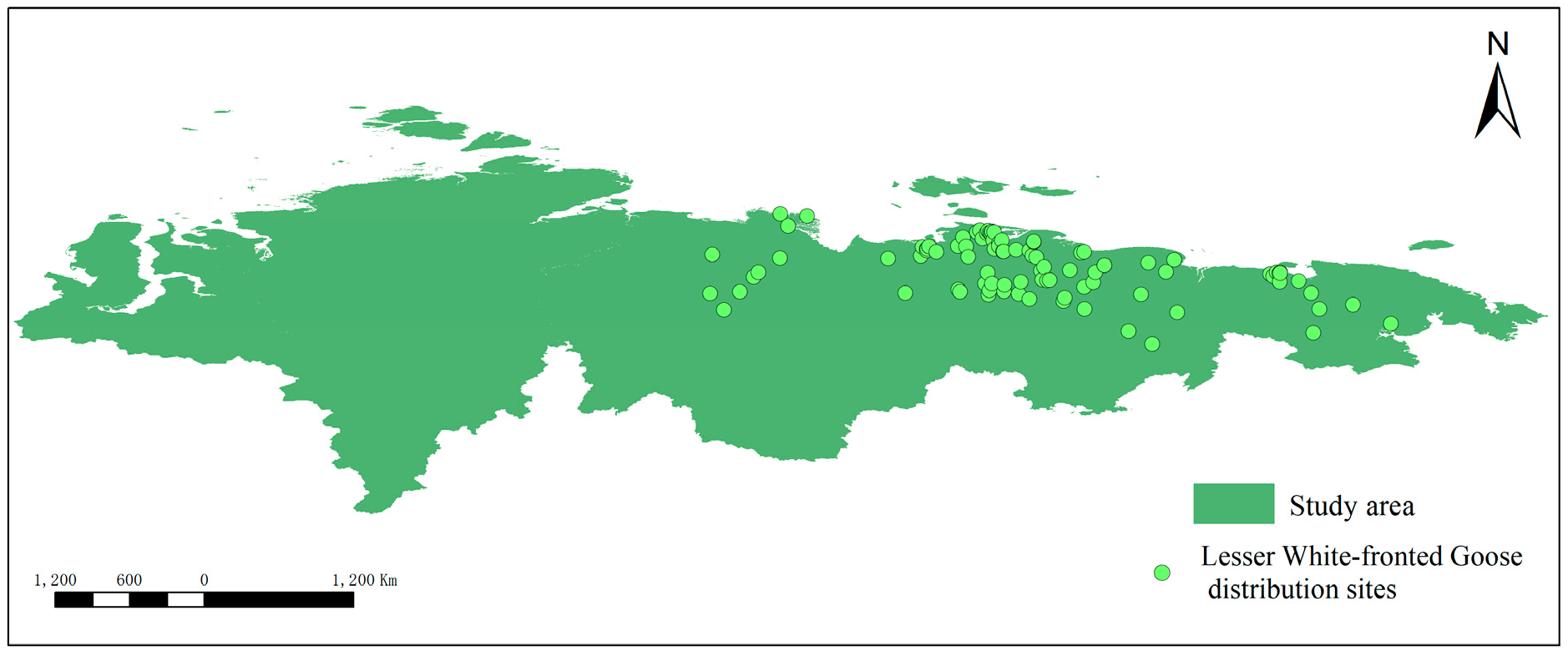
2.2.1. Species Distribution Data
2.2.2. Boundaries of the Protected Areas
2.2.3. Climatic Data
2.3. Maxent Modeling and Evaluation
2.3.1. Maximum Entropy
2.3.2. Bioclimatic Variables
2.3.3. Conservation Area Gap Analysis
2.3.4. Comparison between the Current Distribution and Future Projections
3. Results
3.1. Distribution of Lesser White-Fronted Goose
3.2. Model Evaluation
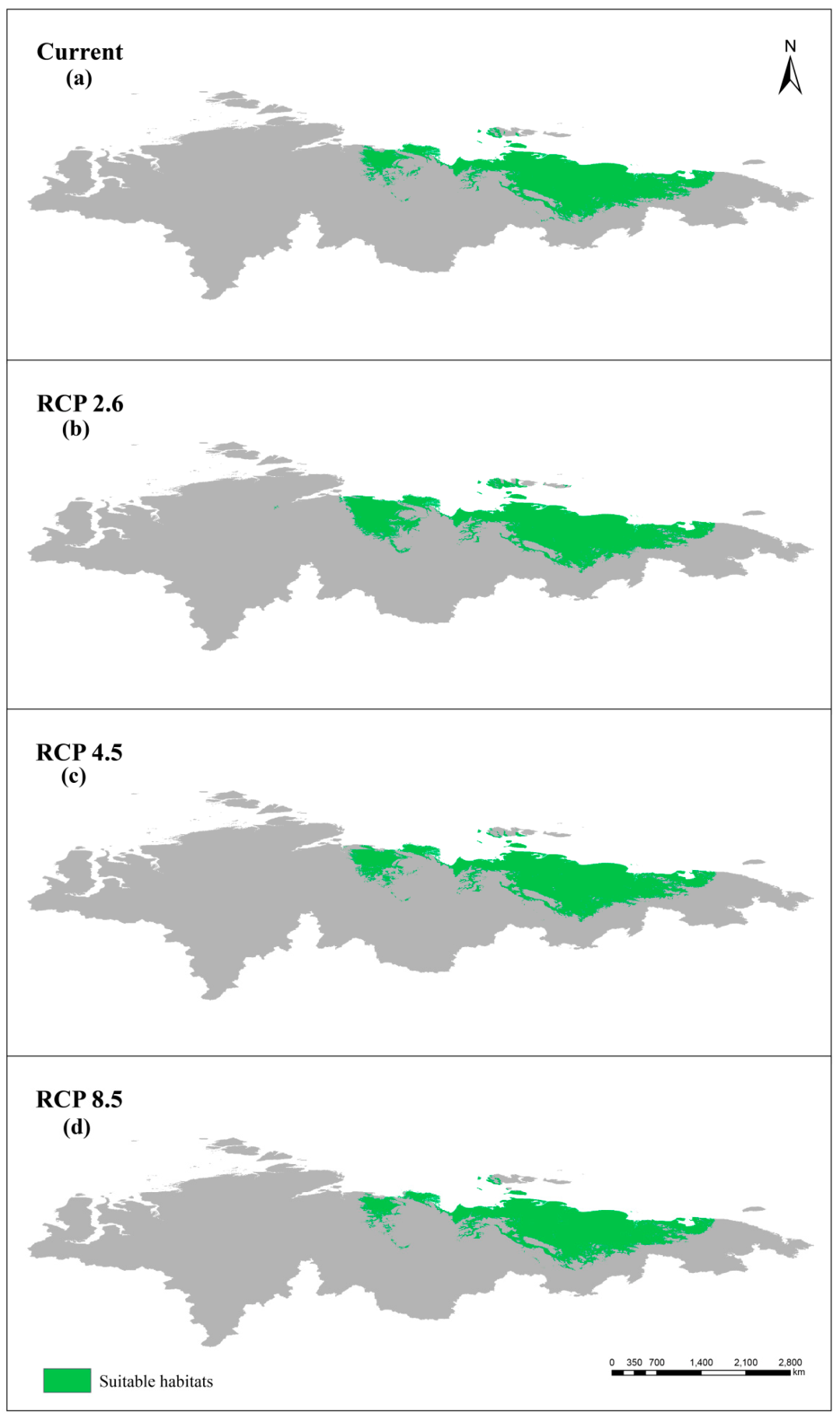
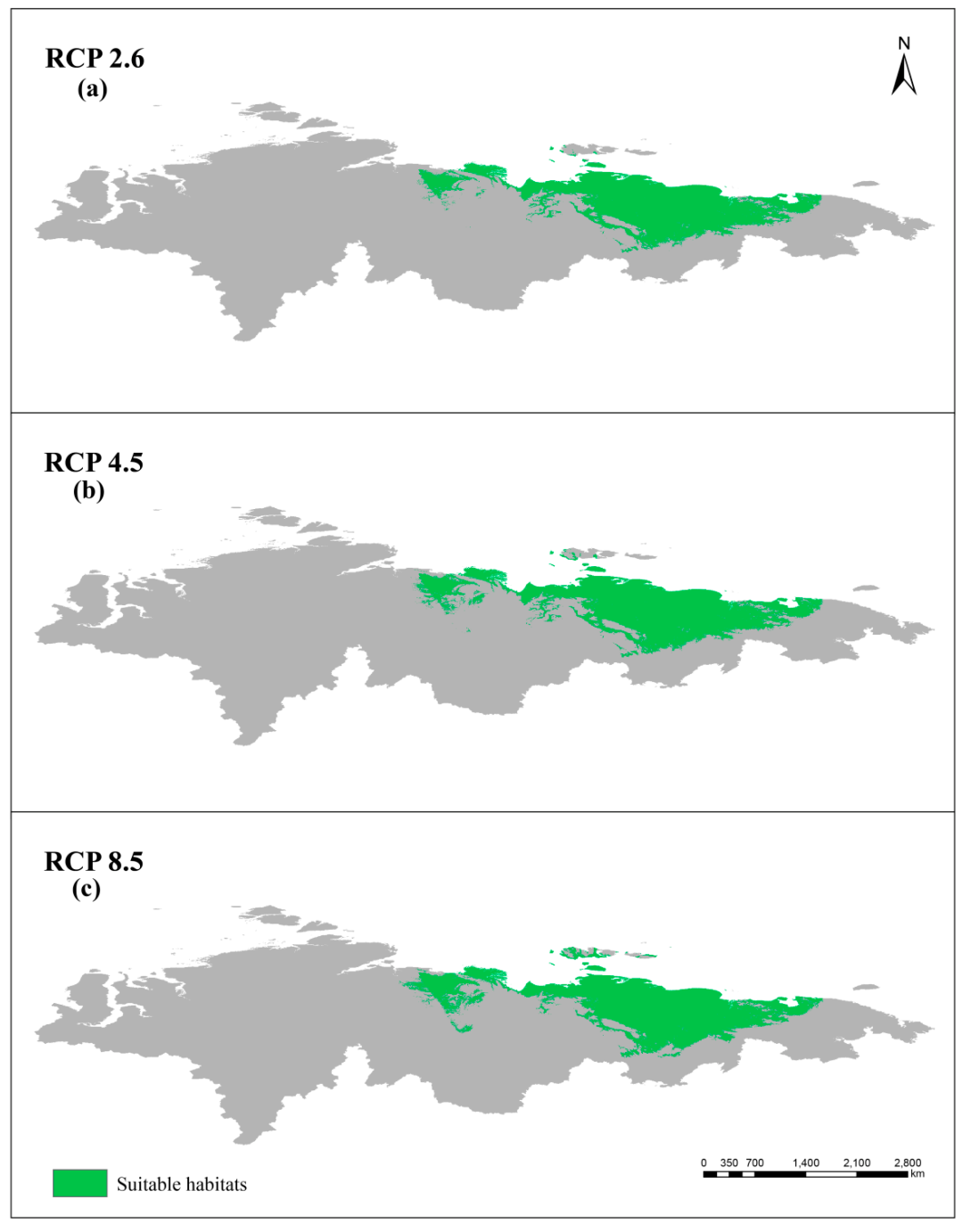
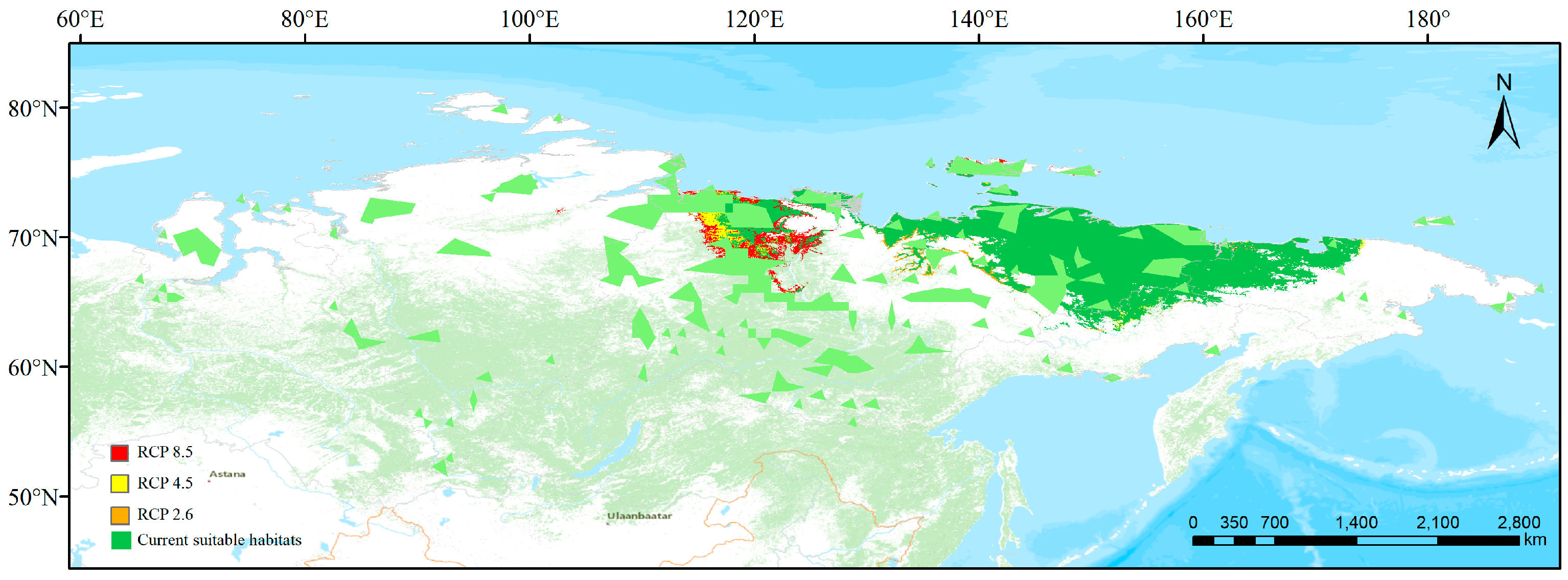
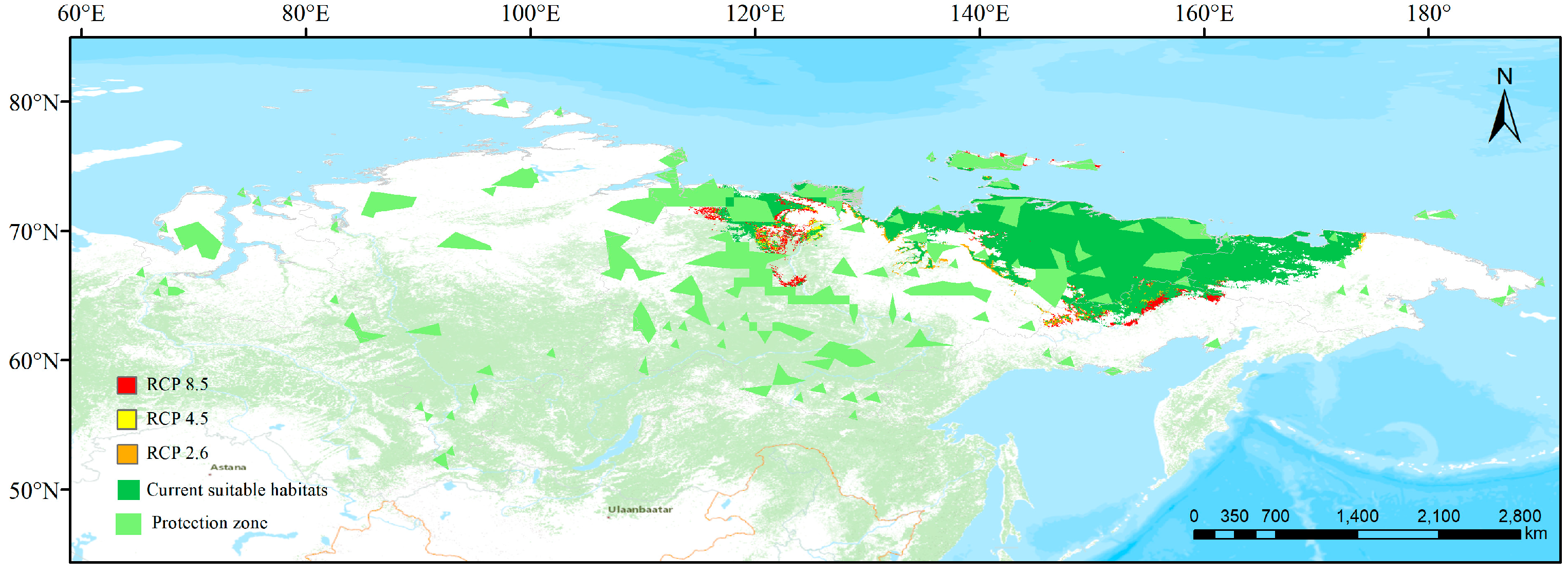
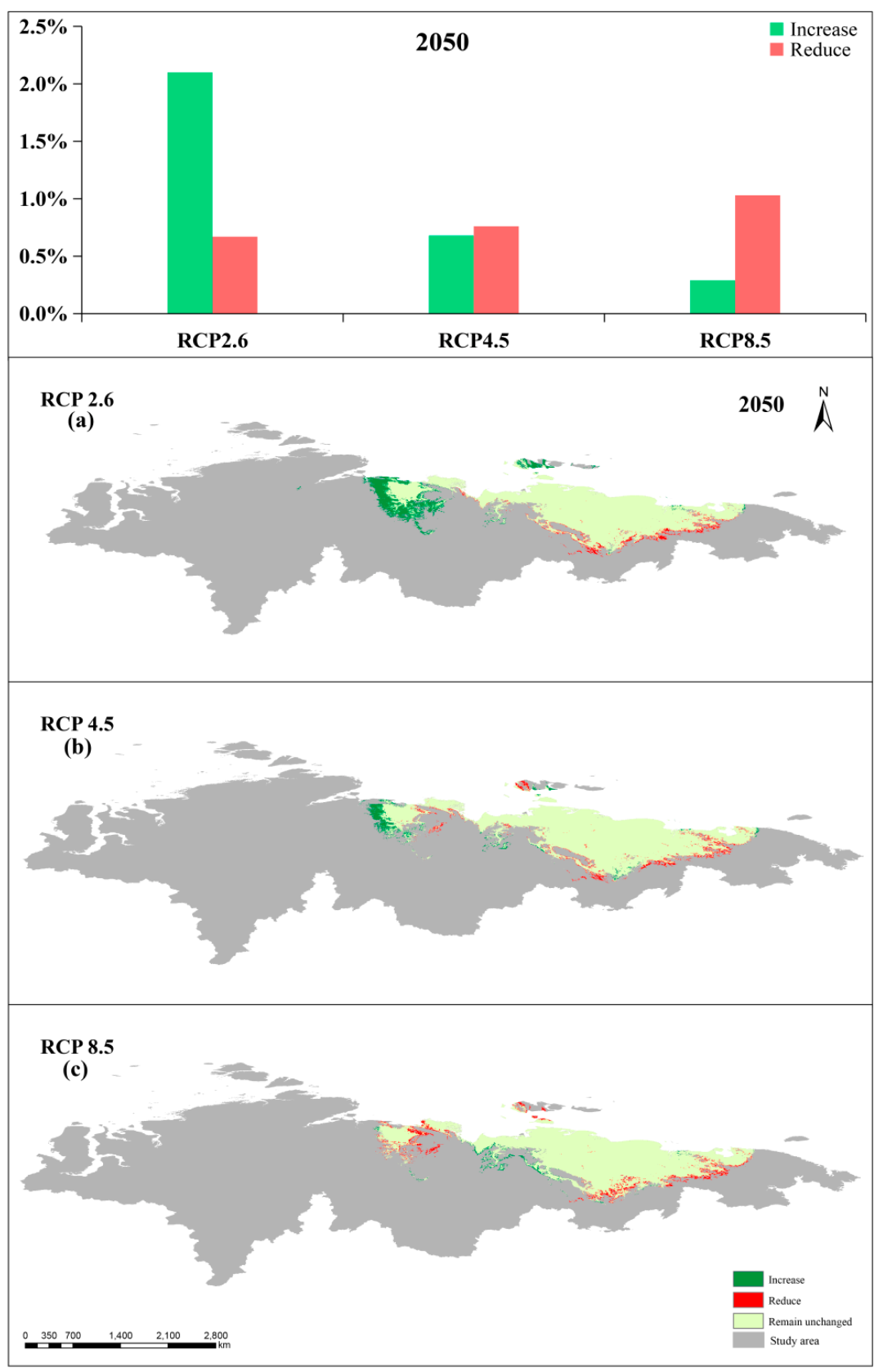
3.3. Simulated Suitable Habitats and Associated Shifts under Different Scenarios
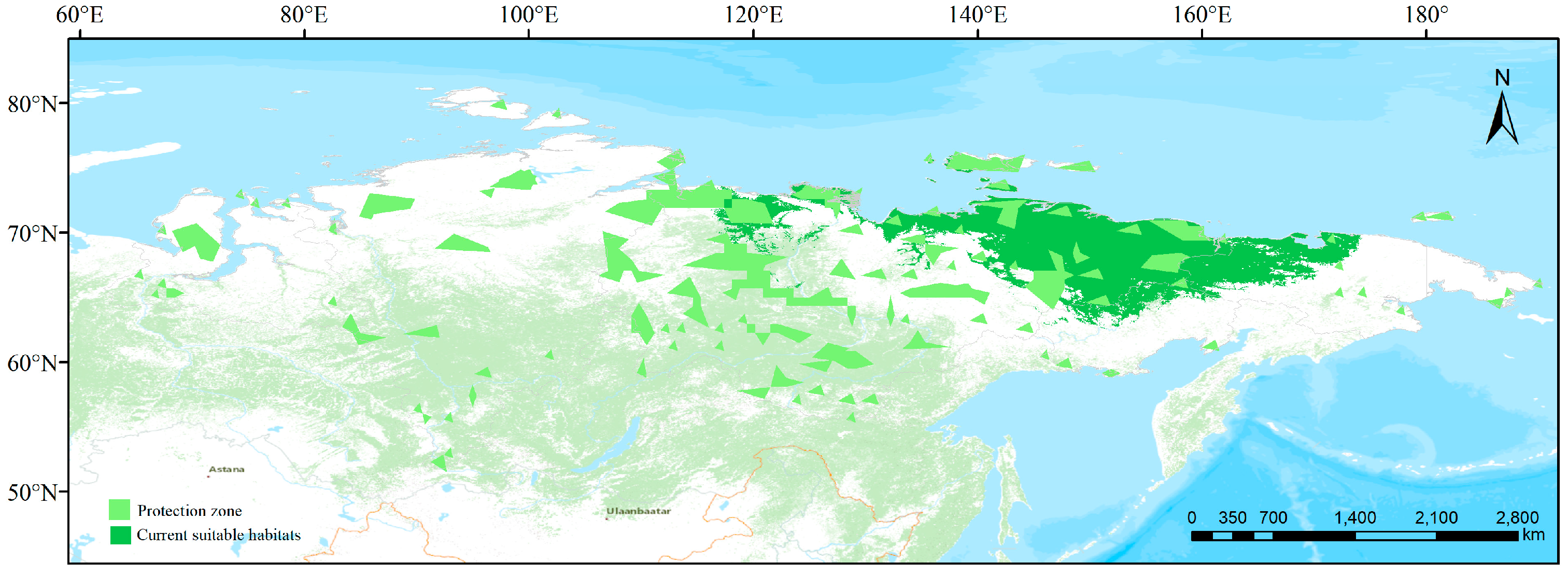
3.4. Conservation Gaps
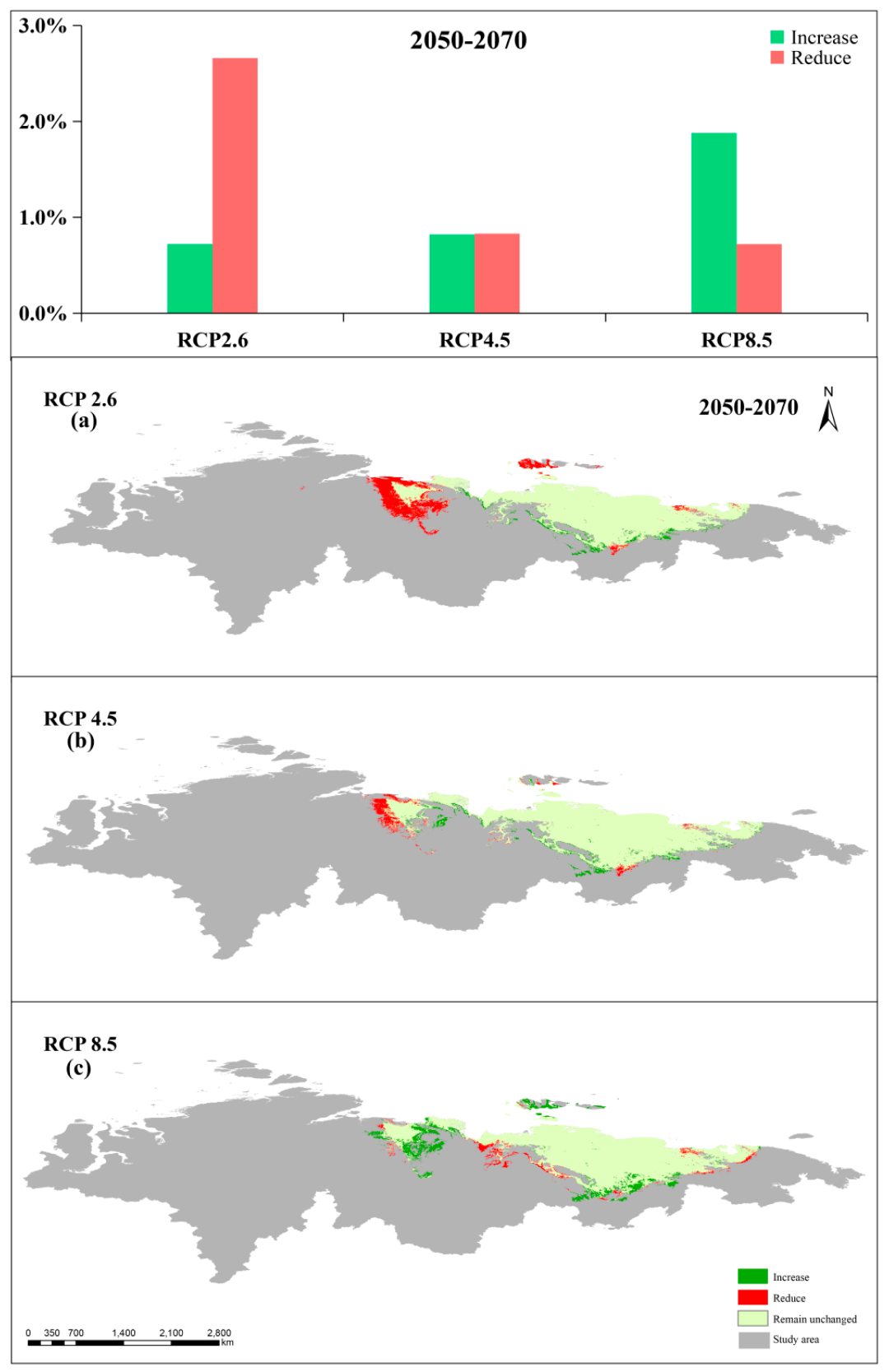
3.5. Comparison between Present and Future Distributions
4. Discussion
4.1. Research Innovation and Limitations
4.2. Distribution of Future Breeding Grounds and Protection Gaps
4.3. Challenges and Conservation Strategies of Lesser White-fronted Goose
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Wang, E.; Sun, R. Climate adaptation of metabolic heat production in four small bird species in China. J. Zool. 2005, 51, 24–30. [Google Scholar]
- Shi, J.; Li, D.; Xiao, W. The impact of climate change on birds: The significance of long-term research. Zool. Res. 2006, 27, 637–646. [Google Scholar]
- Li, T.; Ma, Z.; Qiang, W.; Liu, H.; Tang, D.; Ying, W.; Zhou, X. Impacts of Climate Change on Waterbirds and Their Responses. Chin. J. Wildl. 2017, 38, 529–534. [Google Scholar]
- Beale, C.M.; Baker, N.E.; Brewer, M.J.; Lennon, J.J. Protected area networks and savannah bird biodiversity in the face of climate change and land degradation. Ecol. Lett. 2013, 16, 1061–1068. [Google Scholar] [CrossRef]
- Sorte, F.A.L.; Iii, F.R.T. Poleward shifts in winter ranges of north american birds. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef]
- Steen, V.; Powell, A.N. Potential Effects of Climate Change on the Distribution of Waterbirds in the Prairie Pothole Region, U.S.A. Waterbirds 2012, 35, 217–229. [Google Scholar] [CrossRef]
- Zhi-Ying, O.U. Progress in Studies on Plant Responses to Elevated CO2. J. Trop. Subtrop. Bot. 2003, 11, 190–196. [Google Scholar]
- Bai, J.H.; Ouyang, H.; Yang, Z.F.; Cui, B.S.; Cui, L.J.; Wang, Q.J. Changes in Wetland Landscape Patterns: A Review. Prog. Geogr. 2005, 24, 36–45. [Google Scholar]
- Bellisario, B.; Cerfolli, F.; Nascetti, G. Climate effects on the distribution of wetland habitats and connectivity in networks of migratory waterbirds. Acta Oecol. 2014, 58, 5–11. [Google Scholar] [CrossRef]
- Stralberg, D.; Bayne, E.M.; Cumming, S.G.; Sólymos, P.; Song, S.J.; Schmiegelow, F.K. Conservation of future boreal forest bird communities considering lags in vegetation response to climate change: A modified refugia approach. Divers. Distrib. 2015, 21, 1112–1128. [Google Scholar] [CrossRef]
- Prince, Z. Climate change in our backyards: The reshuffling of North America’s winter bird communities. Glob. Chang. Biol. 2015, 21, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.E.; Rehfisch, M.M. Shifting nonbreeding distributions of migratory fauna in relation to climatic change. Glob. Chang. Biol. 2005, 11, 31–38. [Google Scholar] [CrossRef]
- Visser, M.E.; Perdeck, A.C.; van Balen, J.H.; Both, C. Climate change leads to decreasing bird migration distances. Glob. Chang. Biol. 2009, 15, 1859–1865. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.D.; Lennon, J.J. Birds extend their ranges northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Brommer, J.E. Extent of recent polewards range margin shifts in Finnish birds depends on their body mass and feeding ecology. Ornis Fenn. 2008, 85, 109–117. [Google Scholar]
- Peterson, A.T. Projected climate change effects on Rocky Mountain and Great Plains birds: Generalities of biodiversity consequences. Glob. Chang. Biol. 2003, 9, 647–655. [Google Scholar] [CrossRef]
- Virkkala, R.; Heikkinen, R.K.; Leikola, N.; Luoto, M. Projected large-scale range reductions of northern-boreal land bird species due to climate change. Biol. Conserv. 2008, 141, 1343–1353. [Google Scholar] [CrossRef]
- Doswald, N. Potential Effects of Climate Change on the Distribution and Migration of European Breeding Migratory Birds; Durham University: Durham, UK, 2009. [Google Scholar]
- Hu, J.; Hu, H.; Jiang, Z. The impacts of climate change on the wintering distribution of an endangered migratory bird. Oecologia 2010, 164, 555–565. [Google Scholar] [CrossRef]
- Maggini, R.; Lehmann, A.; Kery, M.; Schmid, H.; Beniston, M.; Jenni, L.; Zbinden, N. Are Swiss birds tracking climate change? Detecting elevation shifts using response curve shapes. Ecol. Model. 2011, 222, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Buermann, W.; Chaves, J.; Dudley, R.; Mcguire, J.A.; Smith, T.; Altshuler, D.L. Projected changes in elevational distributionand flight performance of montane Neotropical hummingbirdsin response to climate change. Glob. Chang. Biol. 2011, 17, 1671–1680. [Google Scholar] [CrossRef]
- Pavel, S.T. List of wader species of Chukchi, northern far east of Russia: Their banding and migratory links. Stilt 2003, 44, 29–43. [Google Scholar]
- Jones, T.; Martin, K.; Barov, B.; Nagy, S. International single species action plan for the conservation of the Western Palearctic population of the Lesser White-fronted Goose Anser erythropus. J. AEWA Tech. Ser. 2008, 36, 1–130. [Google Scholar]
- BirdLife International. Anser Erythropus. The IUCN Red List of Threatened Species 2018: E.T 22679886A132300164. 2018. Available online: https://www.iucnredlist.org/species/22679886/132300164 (accessed on 21 May 2022).
- Guan, L. Effects of Changing Hydrological Conditions on Food Resources and Distribution of Geese in Dongting Lake; Beijing Forestry University: Beijing, China, 2015. [Google Scholar]
- Lysenko, I.; Zöckler, C. Water Birds on the Edge: First Circumpolar Assessment of Climate Chage Impact of Arctic Breeding Water Birds; WCMC Biodiversity Series; World Conservation Monitoring Center: Cambridge, UK, 2000; pp. 44–60. [Google Scholar]
- Lei, J. Response of Migratory Geese to Habitat Change and Conservation Management Measures in East Asia-Australasia; Beijing Forestry University: Beijing, China, 2019. [Google Scholar]
- Wang, X.; Fox, A.D.; Cong, P.; Barter, M.; Cao, L. Changes in the distribution and abundance of wintering Lesser White-fronted Geese Anser erythropus in eastern China. Bird Conserv. Int. 2012, 22, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, S.; Amano, T.; Ushiyama, K. Creating a potential distribution map for Greater White-fronted Geese wintering in Japan. Ornithol. Sci. 2013, 12, 117–125. [Google Scholar] [CrossRef]
- Li, X.; Si, Y.; Ji, L.; Gong, P. Dynamic response of East Asian Greater White-fronted Geese to changes of environment during migration: Use of multi-temporal species distribution model. Ecol. Model. 2017, 360, 70–79. [Google Scholar] [CrossRef]
- Ruokonen, M.; Kvist, L.; Aarvak, T.; Markkola, J.; Morozov, V.V.; Øien, I.J.; Syroechkovsky, E.E.; Tolvanen, P.; Lumme, J. Population Genetic Structure and Conservation of the Lesser White-Fronted Goose Anser erythropus. Conserv. Genet. 2004, 5, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Morozov, V.V. Status, distribution and trends of the lesser white-fronted goose (Anser erythropus) population in Russia. Bull. Goose Study Group East. Eur. North. Asia 1995, 1, 131–144, (In Russian with English summary). [Google Scholar]
- Morozov, V.; Syroechkovski, E., Jr. Lesser White-fronted Goose on the verge of the Millenium. Casarca 2002, 8, 233–276, (In Russian with English summary). [Google Scholar]
- Egorov, N.; Okhlopkov, I. New data on nesting of the white-fronted goose (Anser erythropus) from Yakutia. Zool. Zhurnal 2007, 86, 1482–1485, (In Russian with English summary). [Google Scholar]
- Solovieva, D.; Sergey, V. Lesser White-Fronted Goose Anser erythropus: Good news about the breeding population in west Chukotka, Russia. Wildfowl 2011, 61, 110–120. [Google Scholar]
- Degtyarev, A.G.; Perfilyev, V.I. The Lesser White-fronted Goose (Anser erythropus) in Yakutia. Casarca 1996, 2, 113–124. [Google Scholar]
- Tian, H.; Solovyeva, D.; Danilo, V.G.; Vartanyan, S.; Wen, L.; Lei, J.; Zeng, Q. Combining modern tracking data and historical records improves understanding of the summer habitats of the Eastern Lesser White-fronted Goose Anser erythropus. Ecol. Evol. 2021, 11, 4126–4139. [Google Scholar] [CrossRef]
- Zwaan, D.R.D.; Scridel, D.; Altamirano, T.A.; Gokhale, P.; Kumar, R.S.; Sevillano-Ríos, S.; Barras, A.G.; Arredondo-Amezcua, L.; Asefa, A.; Carrillo, R.A.; et al. GABB: A global dataset of alpine breeding birds and their ecological traits. Sci. Data 2022, 9, 627. [Google Scholar] [CrossRef]
- Marchant, J.H.; Musgrove, A.J. Review of European flyways of the Lesser White-fronted Goose Anser erythropus; BTO Research Report; BTO: Nieuwegein, The Netherlands, 2011. [Google Scholar]
- Artiukhov, A.I.; Syroechkovski, E.E., Jr. New data on distribution of Lesser White-fronted Goose in the Abyi Lowland (Eastern Yakutia). Casarka 1999, 5, 136–143, (In Russian with English summary). [Google Scholar]
- Tian, Z.; Jiang, D. Analysis of climate simulation capability of CCSM4 for East Asia and China with different resolutions. Atmos. Sci. 2013, 37, 171–186. [Google Scholar]
- Vuuren, D.; Stehfest, E.; Elzen, M.; Kram, T.; van Vliet, J.; Deetman, S.; van Ruijven, B. RCP2.6: Exploring the possibility to keep global mean temperature increase below 2 °C. Clim. Chang. 2011, 109, 95. [Google Scholar] [CrossRef]
- Clarke, L.E.; Edmonds, J.A.; Jacoby, H.D.; Pitcher, H.; Reilly, J.; Richels, R. Scenarios of Greenhouse Gas Emissions and Atmospheric Concentrations; U.S. Climate Change Science Program: Washington, DC, USA, 2007; Volume 2, pp. 64–81. [Google Scholar]
- Aaheim, A.; Godal, F.O. Special Issue: Multi-Greenhouse Gas Mitigation and Climate Policy Costs Savings of a Flexible Multi-Gas Climate Policy. Energy J. 2006, 27, 485–501. [Google Scholar]
- Wise, M.; Calvin, K.; Thomson, A.; Clarke, L.; Bond-Lamberty, B.; Sands, R.; Smith, S.J.; Janetos, A.; Edmonds, J. Implications of Limiting CO2 Concentrations for Land Use and Energy. Science 2009, 324, 1183–1186. [Google Scholar] [CrossRef]
- Fujino, J.; Nair, R.; Kainuma, M.; Masui, T.; Matsuoka, Y. Multi-gas Mitigation Analysis on Stabilization Scenarios Using Aim Global Model. Energy J. 2006, 27, 343–353. [Google Scholar] [CrossRef]
- Hijioka, Y.; Matsuoka, Y.; Nishimoto, H.; Masui, T. Global GHG Emission Scenarios under GHG Concentration Stabilization Targets. J. Glob. Environ. Eng. 2008, 13, 97–108. [Google Scholar]
- Riahi, K.; Grübler, A.; Nakicenovic, N. Scenarios of long-term socio-economic and environmental development under climate stabilization. Technol. Forecast Soc. Chang. 2007, 74, 887–935. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.; Dai, L. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodiv. Sci. 2007, 15, 365–372. [Google Scholar] [CrossRef]
- Rong, L.I.; Jiao, Y.M.; Liu, X.; Yuan, I.O. Suitability evaluation and corridor design of habitats for Green Peafowl based on MaxEnt Model. Chin. J. Ecol. 2019, 38, 919–926. [Google Scholar]
- Jennings, M.D. Gap analysis: Concepts, methods, and recent results. Landsc. Ecol. 2000, 15, 5–20. [Google Scholar] [CrossRef]
- Liu, J.; Lü, X. Study on the spatial pattern of wetland bird richness and hotspots in Sanjiang Plain. Acta Ecol. Sin. 2011, 31, 5894–5902. [Google Scholar]
- Xiao, H.; Zhao, J.; Jiang, F.; Zeng, H.; Ecology, D.O.; Sciences, C. GAP Analysis and Regional Biodiversity Conservation. Acta Sci. Nat. Univ. Pekin. 2006, 42, 153. [Google Scholar]
- Rodrigues, A.; Akçakaya, H.R.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Yan, X. Global gap analysis: Priority regions for expanding the global protected-area network. BioScience 2004, 54, 1092–1100. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions. 2006. Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 25 October 2022).
- Hodges, J.I.; Eldridge, W.D. Aerial surveys of eiders and other waterbirds on the eastern Arctic coast of Russia. Wildfowl 2001, 52, 127–142. [Google Scholar]
- Krechmar, A.; Kondratiev, A. Anseriformes of Northeast Asia; North-Eastern Scientific Centre, Far-Eastern Branch of the Russian Academy of Sciences: Magadan, Russia, 2006. [Google Scholar]
- Pozdnyakov, V.I. Status and breeding ecology of Bewick’s swans in the Lena Delta, Yakutia, Northern Asia. Waterbirds 2002, 25, 95–99. [Google Scholar]
- Yasue, M. The Breeding Ecology and Potential Impacts of Habitat Change on the Malaysian Plover Charadrius Peronii in the Gulf of Thailand; University of Victoria (Canada): Victoria, BC, Canada, 2007. [Google Scholar]
- Bruner, A.G.; Gullison, R.E.; Rice, R.E.; Fonseca, G.A.B. Effectiveness of parks in protecting tropical biodiversity. Science 2001, 291, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Soares-Filhoa, B.; Moutinhob, P.; Nepstadb, D.; Anderson, A.; Rodrigues, H.; Garcia, R.; Maretti, C. Role of Brazilian Amazon protected areas in climate change mitigation. Proc. Natl. Acad. Sci. USA 2010, 107, 10821–10826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garden, J.G.; O’Donnell, T.; Catterall, C.P. Changing habitat areas and static reserves: Challenges to species protection under climate change. Landsc. Ecol. 2015, 30, 1959–1973. [Google Scholar] [CrossRef]
- Guan, L.; Lei, J.; Zuo, A.; Zhang, H.; Lei, G.; Wen, L. Optimizing the timing of water level recession for conservation of wintering geese in Dongting Lake, China. Ecol. Eng. J. Ecotechnol. 2016, 88, 90–98. [Google Scholar] [CrossRef]
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press on Demand: Oxford, UK, 2003. [Google Scholar]
- Pasquale, G.D.; Saracino, A.; Bosso, L.; Russo, D.; Moroni, A.; Bonanomi, G.; Allevato, E. Coastal Pine-Oak glacial refugia in the Mediterranean basin: A biogeographic approach based on charcoal analysis and spatial modelling. Forests 2020, 11, 673. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, S.; Capinha, C.; Weterings, R.; Gao, T. Using species distribution model to predict the impact of climate change on the potential distribution of Japanese whiting Sillago japonica. Ecol. Indic. 2019, 104, 33–340. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Prowse, T.D.; Wrona, F.J.; Reist, J.D.; Hobbie, J.E.; Lévesque, L.M.; Vincent, W.F. General features of the Arctic relevant to climate change in freshwater ecosystems. AMBIO A J. Hum. Environ. 2006, 35, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Wrona, F.J.; Johansson, M.; Culp, J.M.; Jenkins, A.; Mård, J.; Myers-Smith, I.H.; Prowse, T.D.; Vincent, W.F.; Wookey, P.A. Transitions in Arctic ecosystems: Ecological implications of a changing hydrological regime. J. Geophys. Res. Biogeosci. 2016, 121, 650–674. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.M.; Lyon, S.W.; Destouni, G. Thermokarst lake, hydrological flow and water balance indicators of permafrost change in Western Siberia. J. Hydrol. 2012, 464–465, 459–466. [Google Scholar] [CrossRef]
- Merow, C.; Allen, J.M.; Aiello-Lammens, M.; Silander, J.A., Jr. Improving niche and range estimates with Maxent and point process models by integrating spatially explicit information. Glob. Ecol. Biogeogr. 2016, 25, 1022–1036. [Google Scholar] [CrossRef]
- Lei, J.; Jia, Y.; Wang, Y.; Lei, G.; Lu, C.; Saintilan, N.; Wen, L. Behavioural plasticity and trophic niche shift: How wintering geese respond to habitat alteration. Freshw. Biol. 2019, 64, 1183–1195. [Google Scholar] [CrossRef]
- Government of the Russian Federation. Environmental Protection Report of the Russian Federation [EB/OL]. Available online: http://www.mnr.gov.ru/regulatory/list.php?part=1101 (accessed on 1 August 2018).
- Yu, Y.; Persherev; Wang, F. Russia’s ecological protection framework: System of special nature reserves. Wildlife 2007, 28, 39–41. [Google Scholar]
- Ellis, C.J. Lichen epiphyte diversity: A species, community and traitbased review. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Degtyaryev, V.G.; Sleptsov, S.M.; Pshennikov, A.E. Piscivory in eastern population of Siberian Crane (Grus leucogeranus). Zool. Zhurnal 2013, 92, 588. [Google Scholar] [CrossRef]
- Bysykatova, I.P.; Krapu, G.L.; Germogenov, N.I.; Buhl, D.A. Distribution, Densities, and Ecology of Siberian Cranes in the Khroma River Region of Northern Yakutia in Northeastern Russia. 2016. Available online: chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1377&context=nacwgproc (accessed on 25 October 2022).
- Andreev, A. Summary of Wetlands International Asian Waterfowl Census results for Perennou et al. 1994. Unpublished. [Google Scholar]

| ID | Mark Time | Receipt Date | Terminus ad Quem | Tracking Time (Date) | Number of Times of Summer Observations |
|---|---|---|---|---|---|
| BFUL041 | 20 November 2016 | 23 November 2016 | 16 April 2018 | 509 | 1 |
| BFUL044 | 30 November 2016 | 2 December 2016 | 9 June 2018 | 554 | 1 |
| BFUL050 | 25 November 2016 | 27 November 2016 | 19 May 2018 | 538 | 1 |
| BFUL057 | 30 November 2016 | 2 December 2016 | 17 July 2018 | 592 | 1 |
| BFUL059 | 30 November 2016 | 2 December 2016 | 29 December 2017 | 392 | 1 |
| BFUL065 | 5 December 2016 | 7 December 2016 | 5 September 2017 | 272 | 1 |
| BFUL068 | 15 December 2016 | 16 December 2016 | 28 May 2018 | 528 | 1 |
| BFUL051 | 25 November 2016 | 28 November 2016 | 25 December2018 | 757 | 2 |
| BFUL061 | 30 November 2016 | 2 December 2016 | 12 May 2019 | 891 | 2 |
| BFUL074 | 15 January 2017 | 19 January 2017 | 14 May 2019 | 845 | 2 |
| BFUL062 | 8 December 2016 | 11 December 2016 | 27 November 2017 | 1081 | 3 |
| Number | Type | Variable | Units |
|---|---|---|---|
| Bio1 | Bioclimatic | Annual Mean Temperature | °C |
| Bio2 | Bioclimatic | Mean Diurnal Range (mean of monthly (maxtemp-min temp)) | °C |
| Bio3 | Bioclimatic | Isothermality (BIO2/BIO7) (* 100) (“*” is the multiplication sign.) | °C |
| Bio4 | Bioclimatic | Temperature Seasonality (standard deviation * 100) | °C |
| Bio5 | Bioclimatic | Max Temperature of Warmest Month | °C |
| Bio6 | Bioclimatic | Min Temperature of Coldest Month | °C |
| Bio7 | Bioclimatic | Temperature Annual Range (BIO5-BIO6) | °C |
| Bio8 | Bioclimatic | Mean Temperature of Wettest Quarter | °C |
| Bio9 | Bioclimatic | Mean Temperature of Driest Quarter | °C |
| Bio10 | Bioclimatic | Mean Temperature of Warmest Quarter | °C |
| Bio11 | Bioclimatic | Mean Temperature of Coldest Quarter | °C |
| Bio12 | Bioclimatic | Annual Precipitation | mm |
| Bio13 | Bioclimatic | Precipitation of Wettest Month | mm |
| Bio14 | Bioclimatic | Precipitation of Driest Month | mm |
| Bio15 | Bioclimatic | Precipitation Seasonality (Coefficient of Variation) | - |
| Bio16 | Bioclimatic | Precipitation of Wettest Quarter | mm |
| Bio17 | Bioclimatic | Precipitation of Driest Quarter | mm |
| Bio18 | Bioclimatic | Precipitation of Warmest Quarter | mm |
| Bio19 | Bioclimatic | Precipitation of Coldest Quarter | mm |
| Period | Climate Scenario | Habitat Area (km2) | Protected Habitat Area (km2) | Unprotected Habitat Area (km2) | Protection Rate (%) |
|---|---|---|---|---|---|
| Current | 1,063,667 | 34,280 | 1,029,386 | 3.22% | |
| 2050 | RCP2.6 | 1,161,775 | 34,280 | 1,127,495 | 2.95% |
| RCP4.5 | 1,057,336 | 34,280 | 1,023,056 | 3.24% | |
| RCP8.5 | 1,009,603 | 34,280 | 975,322 | 3.40% | |
| 2070 | RCP2.6 | 1,019,544 | 34,280 | 985,264 | 3.36% |
| RCP4.5 | 1,057,137 | 34,280 | 1,022,857 | 3.24% | |
| RCP8.5 | 1,094,531 | 34,280 | 1,060,250 | 3.13% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Lei, J.; Wu, E.; Lu, C.; Jia, Y.; Zeng, Q.; Lei, G. Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change. Land 2022, 11, 1946. https://doi.org/10.3390/land11111946
Fan R, Lei J, Wu E, Lu C, Jia Y, Zeng Q, Lei G. Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change. Land. 2022; 11(11):1946. https://doi.org/10.3390/land11111946
Chicago/Turabian StyleFan, Rong, Jialin Lei, Entao Wu, Cai Lu, Yifei Jia, Qing Zeng, and Guangchun Lei. 2022. "Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change" Land 11, no. 11: 1946. https://doi.org/10.3390/land11111946
APA StyleFan, R., Lei, J., Wu, E., Lu, C., Jia, Y., Zeng, Q., & Lei, G. (2022). Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change. Land, 11(11), 1946. https://doi.org/10.3390/land11111946







