Hydrogeochemistry of Shallow Groundwater in a Karst Aquifer System of Bijie City, Guizhou Province
Abstract
:1. Introduction
2. Geology and Hydrogeology Setting
2.1. Geographical Conditions
2.2. Geological and Hydrogeological Setting
3. Materials and Methods
3.1. Sampling and Analysis Methods
3.2. The Saturation Indices (SIs) of Minerals
3.3. Principal Component Analysis (PCA)
4. Results
4.1. Groundwater Chemistry
4.2. Deuterium and Oxygen Isotopes
4.3. PCA of Hydrochemistry
5. Discussion
5.1. Rock Weathering
5.1.1. Carbonate and Gypsum Dissolution
5.1.2. K-feldspar and Mica Minerals Dissolution
5.1.3. Halite Minerals Dissolution
5.2. Cation Exchange
5.3. Anthropogenic Activities
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pu, T.; He, Y.; Zhang, T.; Wu, J.; Zhu, G.; Chang, L. Isotopic and geochemical evolution of ground and river waters in a karst dominated geological setting: A case study from Lijiang basin, South-Asia monsoon region. Appl. Geochem. 2013, 33, 199–212. [Google Scholar] [CrossRef]
- Adinolfi Falcone, R.; Falgiani, A.; Parisse, B.; Petitta, M.; Spizzico, M.; Tallini, M. Chemical and isotopic (δ18O‰, δ2H‰, δ13C‰, 222Rn) multi-tracing for groundwater conceptual model of carbonate aquifer (Gran Sasso INFN underground laboratory—central Italy). J. Hydrol. 2008, 357, 368–388. [Google Scholar] [CrossRef]
- Barberá, J.A.; Andreo, B. Hydrogeological processes in a fluviokarstic area inferred from the analysis of natural hydrogeochemical tracers. The case study of eastern Serranía de Ronda (S Spain). J. Hydrol. 2015, 523, 500–514. [Google Scholar]
- Bhat, N.A.; Jeelani, G.; Bhat, M.Y. Hydrogeochemical assessment of groundwater in karst environments, Bringi watershed, Kashmir Himalayas, India. Curr. Sci. 2014, 106, 1000–1007. [Google Scholar]
- Calijuri, M.L.; do Couto, d.E.A.; Santiago, d.A.F.; Camargo, d.R.A.; e Silva, M.D.F.M. Evaluation of the Influence of Natural and Antrhopogenic Processes on Water Quality in Karstic Region. Water Air Soil Poll. 2012, 223, 2157–2168. [Google Scholar] [CrossRef]
- López-Chicano, M.; Bouamama, M.; Vallejos, A.; Pulido-Bosch, A. Factors which determine the hydrogeochemical behaviour of karstic springs. A case study from the Betic Cordilleras, Spain. Appl. Geochem. 2001, 16, 1179–1192. [Google Scholar] [CrossRef]
- Han, Y.; Wang, G.C.; Cravotta, C.A., III; Hu, W.Y.; Bian, Y.Y.; Zhang, Z.W.; Liu, Y.Y. Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China. Hydrol. Process. 2012, 27, 2247–2257. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, D.; Zhang, G.; He, R. Impact of land use change on groundwater quality in a typical karst watershed of southwest China: A case study of the Xiaojiang watershed, Yunnan Province. Hydrogeol. J. 2008, 16, 727–735. [Google Scholar] [CrossRef]
- Lang, Y.-C.; Liu, C.-Q.; Zhao, Z.-Q.; Li, S.-L.; Han, G.-L. Geochemistry of surface and ground water in Guiyang, China: Water/rock interaction and pollution in a karst hydrological system. Appl. Geochem. 2006, 21, 887–903. [Google Scholar] [CrossRef]
- Re, V.; Sacchi, E.; Mas-Pla, J.; Menció, A.; El Amrani, N. Identifying the effects of human pressure on groundwater quality to support water management strategies in coastal regions: A multi-tracer and statistical approach (Bou-Areg region, Morocco). Sci. Total Environ. 2014, 500–501, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ma, R.; Wang, Y.; Ma, T.; Liu, Y. Using isotopic, hydrogeochemical-tracer and temperature data to characterize recharge and flow paths in a complex karst groundwater flow system in northern China. Hydrogeol. J. 2016, 24, 1393–1412. [Google Scholar] [CrossRef]
- Wu, P.; Tang, C.; Zhu, L.; Liu, C.; Cha, X.; Tao, X. Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China. Hydrol. Process. 2009, 23, 2012–2022. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Galazoulas, E.; Petalas, C.; Tsihrintzis, V. Evaluation of multivariate statistical methods for the identification of groundwater facies, in a multilayered coastal aquifer. Adv. Res. Aquat. Environ. 2011, 1, 315–322. [Google Scholar]
- Love, D.; Hallbauer, D.; Amos, A.; Hranova, R. Factor analysis as a tool in groundwater quality management: two southern African case studies. Phys. Chem. Earth Pt. A/B/C 2004, 29, 1135–1143. [Google Scholar] [CrossRef]
- Menció, A.; Folch, A.; Mas-Pla, J. Identifying key parameters to differentiate groundwater flow systems using multifactorial analysis. J. Hydrol. 2012, 472–473, 301–313. [Google Scholar] [CrossRef]
- Blake, S.; Henry, T.; Murray, J.; Flood, R.; Muller, M.R.; Jones, A.G.; Rath, V. Compositional multivariate statistical analysis of thermal groundwater provenance: A hydrogeochemical case study from Ireland. Appl. Geochem. 2016, 75, 171–188. [Google Scholar] [CrossRef]
- Cortes, J.E.; Muñoz, L.F.; Gonzalez, C.A.; Niño, J.E.; Polo, A.; Suspes, A.; Siachoque, S.C.; Hernández, A.; Trujillo, H. Hydrogeochemistry of the formation waters in the San Francisco field, UMV basin, Colombia-A multivariate statistical approach. J. Hydrol. 2016, 539, 113–124. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology, 3rd ed.; John Willey & Sons: New Jersey, NJ, USA, 2002. [Google Scholar]
- Hadj Ammar, F.; Chkir, N.; Zouari, K.; Hamelin, B.; Deschamps, P.; Aigoun, A. Hydro-geochemical processes in the Complexe Terminal aquifer of southern Tunisia: An integrated investigation based on geochemical and multivariate statistical methods. J. Afr. Earth Sci. 2014, 100, 81–95. [Google Scholar] [CrossRef]
- Helstrup, T.; Jørgensen, N.; Banoeng-Yakubo, B. Investigation of hydrochemical characteristics of groundwater from the Cretaceous-Eocene limestone aquifer in southern Ghana and southern Togo using hierarchical cluster analysis. Hydrogeol. J. 2007, 15, 977–989. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, H.; Jia, Y.; Cao, Y.; Hu, C. Principal component analysis and hierarchical cluster analyses of arsenic groundwater geochemistry in the Hetao basin, Inner Mongolia. Chem. Erde-Geochem. 2015, 75, 197–205. [Google Scholar] [CrossRef]
- Koh, D.-C.; Chae, G.-T.; Ryu, J.-S.; Lee, S.-G.; Ko, K.-S. Occurrence and mobility of major and trace elements in groundwater from pristine volcanic aquifers in Jeju Island, Korea. Appl. Geochem. 2016, 65, 87–102. [Google Scholar] [CrossRef]
- Moore, P.J.; Martin, J.B.; Screaton, E.J. Geochemical and statistical evidence of recharge, mixing, and controls on spring discharge in an eogenetic karst aquifer. J. Hydrol. 2009, 376, 443–455. [Google Scholar] [CrossRef]
- Omo-Irabor, O.O.; Olobaniyi, S.B.; Oduyemi, K.; Akunna, J. Surface and groundwater water quality assessment using multivariate analytical methods: A case study of the Western Niger Delta, Nigeria. Phys. Chem. Earth, Pt. A/B/C 2008, 33, 666–673. [Google Scholar] [CrossRef]
- Salifu, A.; Petrusevski, B.; Ghebremichael, K.; Buamah, R.; Amy, G. Multivariate statistical analysis for fluoride occurrence in groundwater in the Northern region of Ghana. J. Contam. Hydrol. 2012, 140–141, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Voutsis, N.; Kelepertzis, E.; Tziritis, E.; Kelepertsis, A. Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. J. Geochem. Explor. 2015, 159, 79–92. [Google Scholar] [CrossRef]
- Zghibi, A.; Merzougui, A.; Zouhri, L.; Tarhouni, J. Understanding groundwater chemistry using multivariate statistics techniques to the study of contamination in the Korba unconfined aquifer system of Cap-Bon (North-east of Tunisia). J. Afr. Earth Sci. 2014, 89, 1–15. [Google Scholar] [CrossRef]
- Owen, D.D.R.; Cox, M.E. Hydrochemical evolution within a large alluvial groundwater resource overlying a shallow coal seam gas reservoir. Sci. Total Environ. 2015, 523, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Tang, Y.; Li, P.; Yuan, J.; liu, X.; Li, F. Investigation Reports of 1:50,000 Hydrogeology and Environmental Geology Survey in the Wumeng Mountain; Chengdu Center, China Geological Survey: Chengdu, China, 2015. (In Chinese) [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater, and Pollution; Balkema: Rotterdam, The Netherlands, 1996. [Google Scholar]
- Epstein, S.; Mayeda, K. Variation of 18O content of waters from natural sources. Geochim. Cosmochim. Ac. 1953, 4, 213–224. [Google Scholar] [CrossRef]
- Coleman, M.L.; Shepherd, T.J.; Durham, J.J.; Rouse, J.E.; Moore, G.R. Reduction of water with zinc for hydrogen isotope analysis. Anal. Chem. 1982, 54, 993–995. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (version 2): A Computer Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations; Water-resources Investigations Report 99–4259; US Geological Survey, Earth Science Information Center, Open-File Reports Section: Denver, CO, USA, 1999. [Google Scholar]
- Kim, J.-H.; Kim, R.-H.; Lee, J.; Cheong, T.-J.; Yum, B.-W.; Chang, H.-W. Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol. Process. 2005, 19, 1261–1276. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Statistic IBM S. IBM SPSS STATISTIC Program, version 19; IBM Corporation: Armonk, NY, USA, 2011. [Google Scholar]
- Li, P.-Y.; Wu, J.-H.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 1–17. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 3rd ed.; World Health Organization (WHO): Geneva, Switzerland, 2008. [Google Scholar]
- Yuan, J. Hydrogeochemistry of the geothermal systems in coastal areas of Guangdong Province, south China. Wuhan: China Univ. Geosci. 2013, 74–78. (In Chinese) [Google Scholar]
- Liu, W.; Wang, S.; Luo, W. The response of epikarst spring to precipitation and its impications in karst peak-cluster region of Libo Country, Guizhou Province, China. Geochimica 2011, 40, 487–496. (In Chinese) [Google Scholar]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yuan, D.; Yuan, W.; Kuang, Y.; Jia, P.; He, Q. Formations of groundwater hydrogeochemistry in a karst system during storm events as revealed by PCA. Chinese. Sci. Bull. 2010, 55, 788–797. (In Chinese) [Google Scholar] [CrossRef]
- Yang, P.; Yuan, D.; Ye, X.; Xie, S.; Chen, X.; Liu, Z. Sources and migration path of chemical compositions in a karst groundwater system during rainfall events. Chinese. Sci. Bull. 2013, 58, 1755–1763. (In Chinese) [Google Scholar] [CrossRef]
- Chen, K.; Jiao, J.J.; Huang, J.; Huang, R. Multivariate statistical evaluation of trace elements in groundwater in a coastal area in Shenzhen, China. Environ. Pollut. 2007, 147, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Sun, J.; Zhang, Y.; Chen, Z.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci.Total Environ. 2013, 463–464, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Deng, W.; He, Y.; Ramsis, S. Hydrochemical characteristics and evolution laws of groundwater in Songnen Plain, Northeast China. Adv. Water Sci. 2006, 17, 20–28. (In Chinese) [Google Scholar]
- Charfi, S.; Zouari, K.; Feki, S.; Mami, E. Study of variation in groundwater quality in a coastal aquifer in north-eastern Tunisia using multivariate factor analysis. Quatern. Int. 2013, 302, 199–209. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.-W.; Wan, L.; Han, G.; Guo, H. Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin. J. Hydrol. 2015, 527, 433–441. [Google Scholar] [CrossRef]

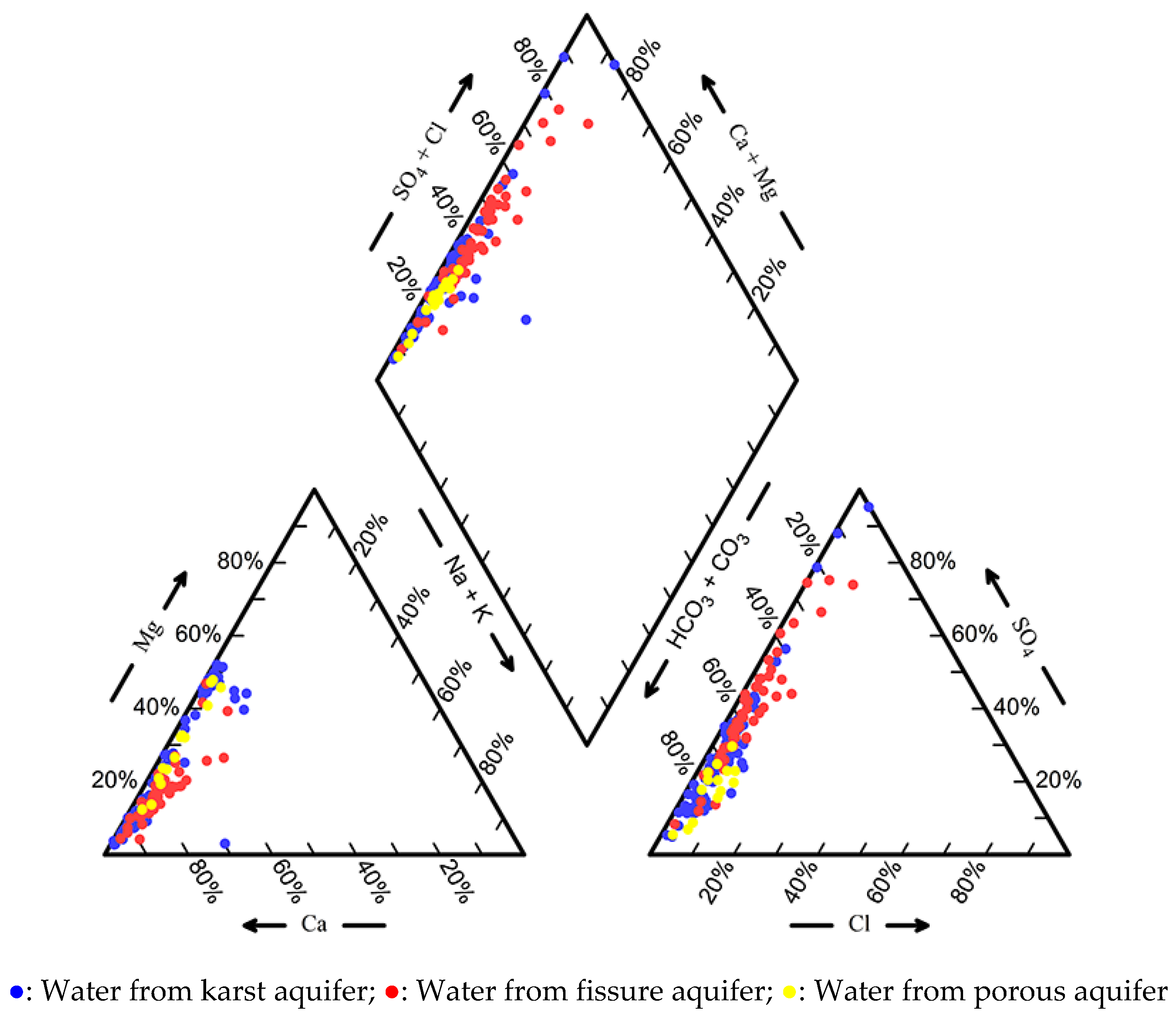
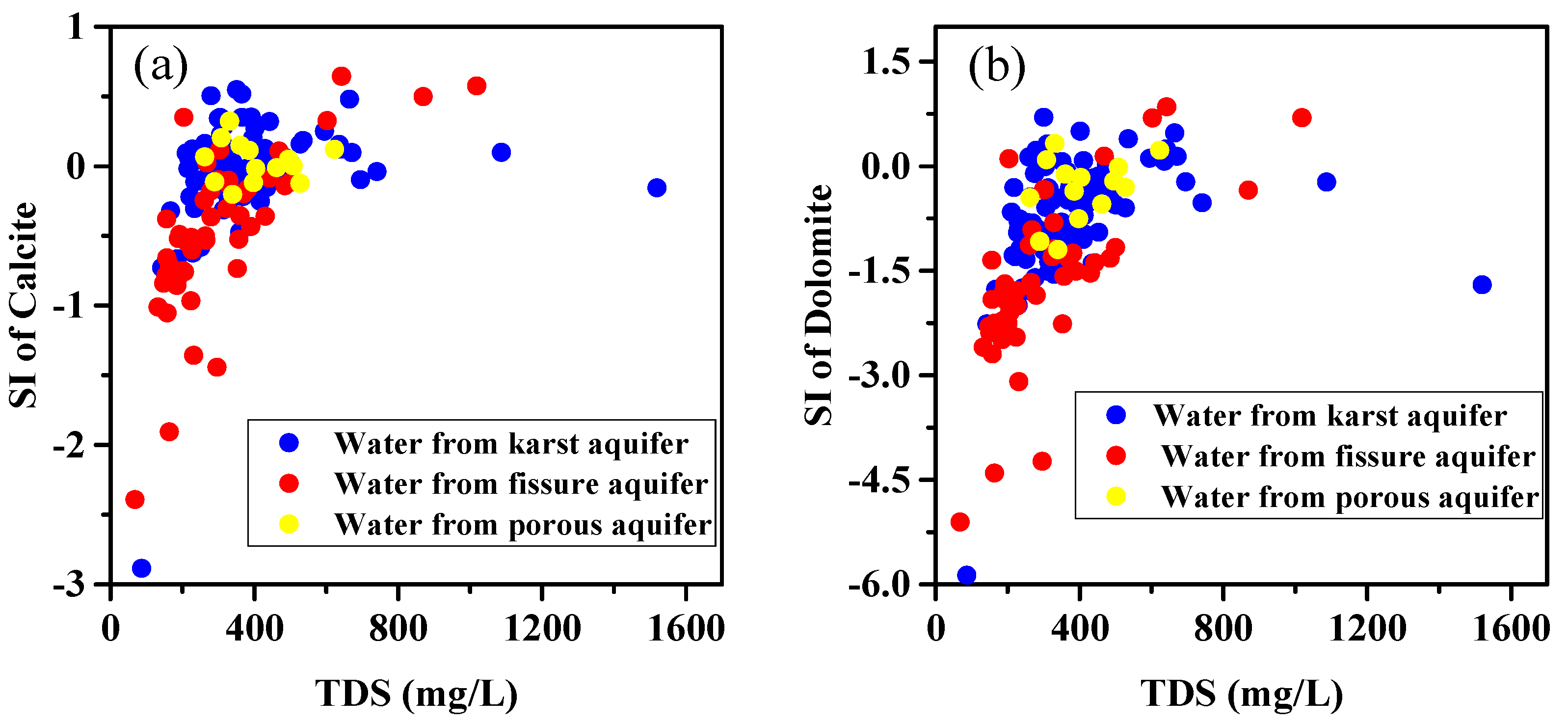
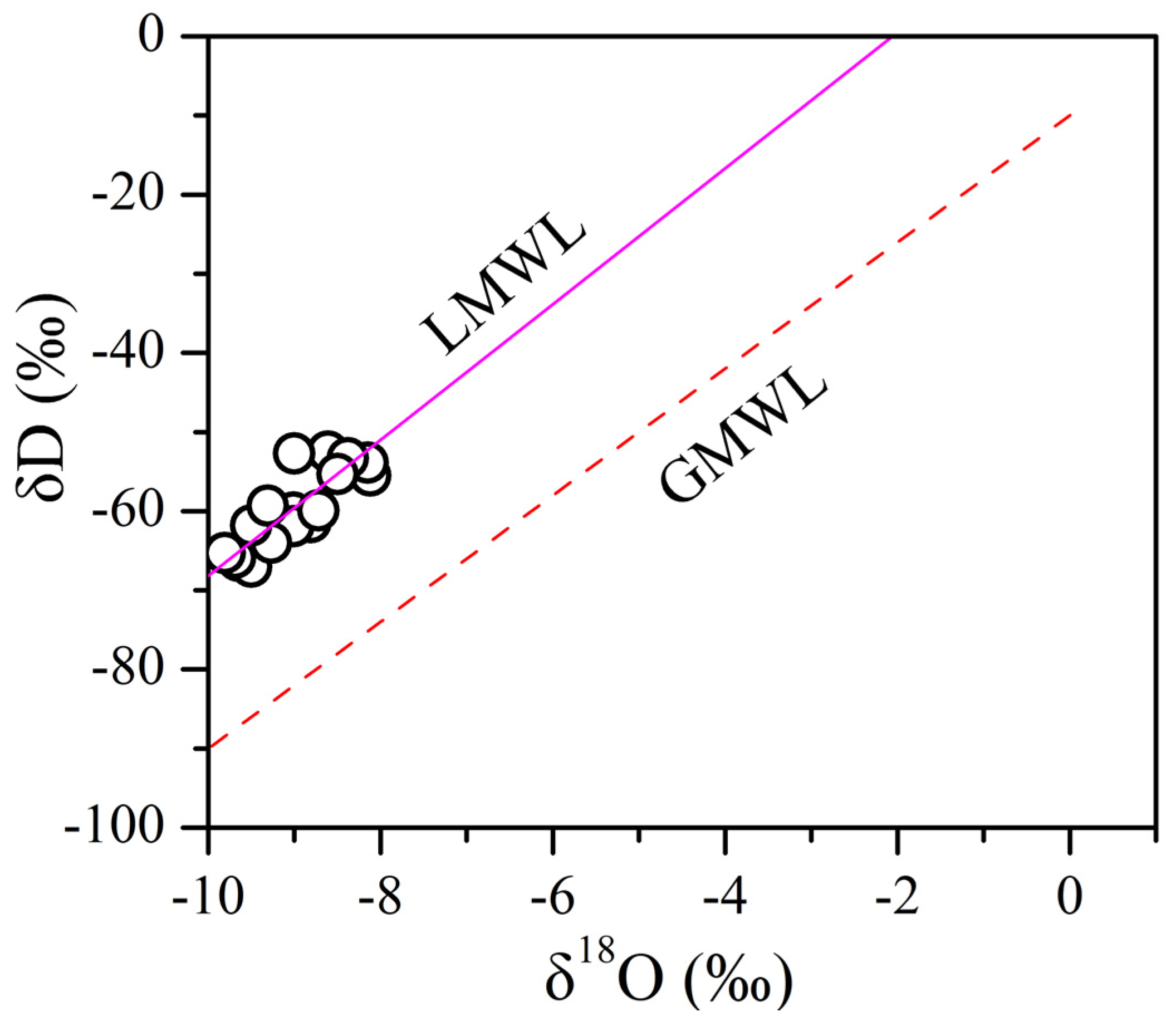
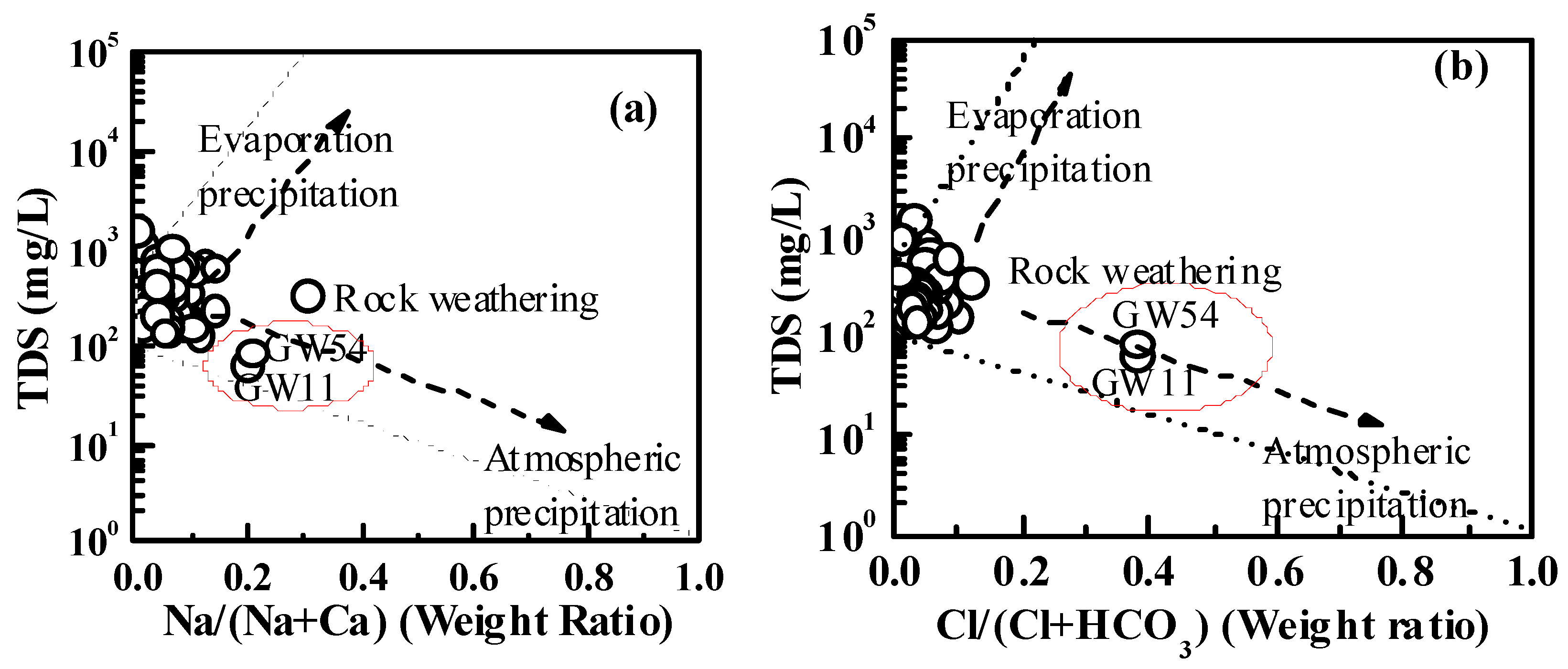
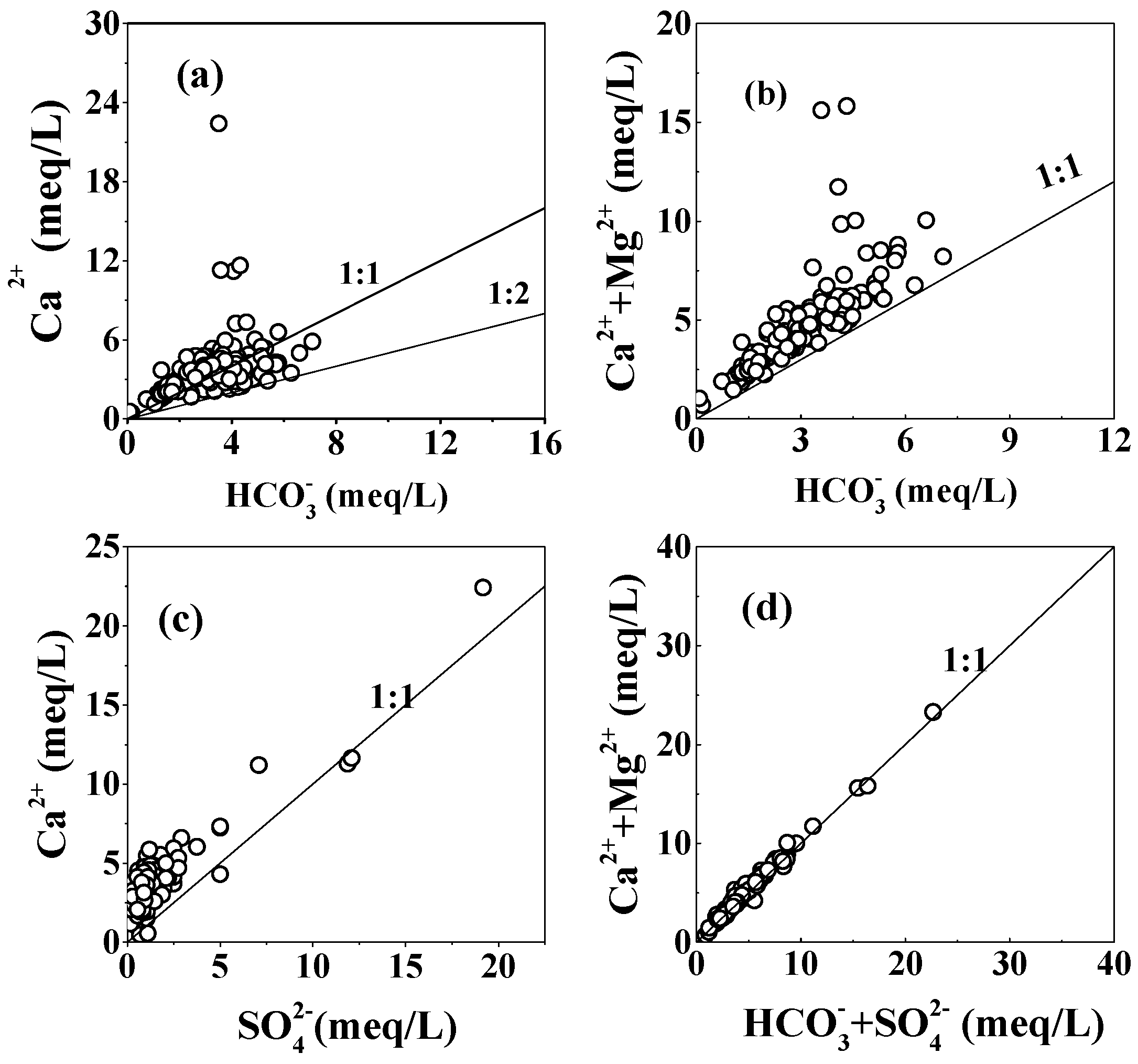
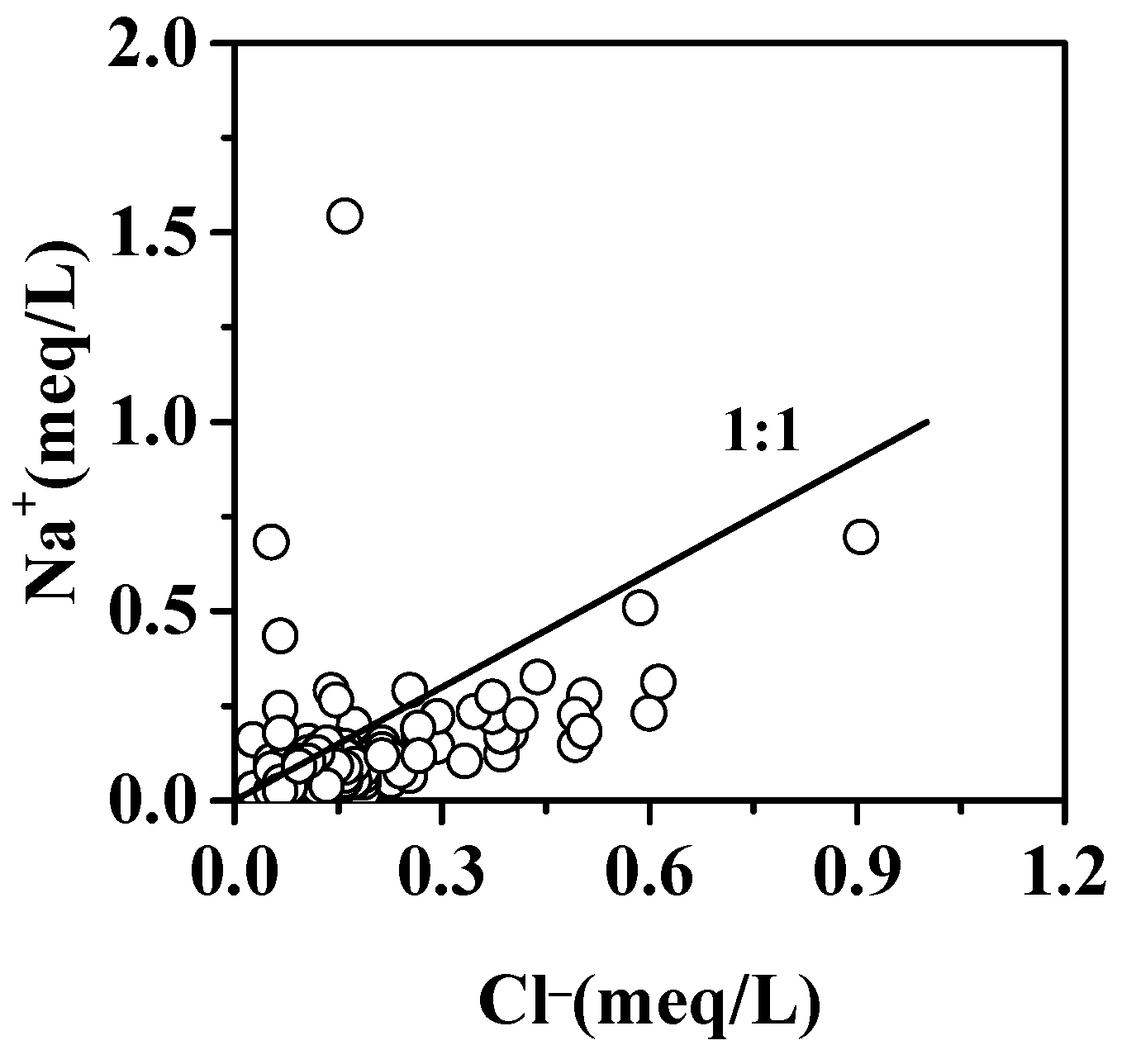
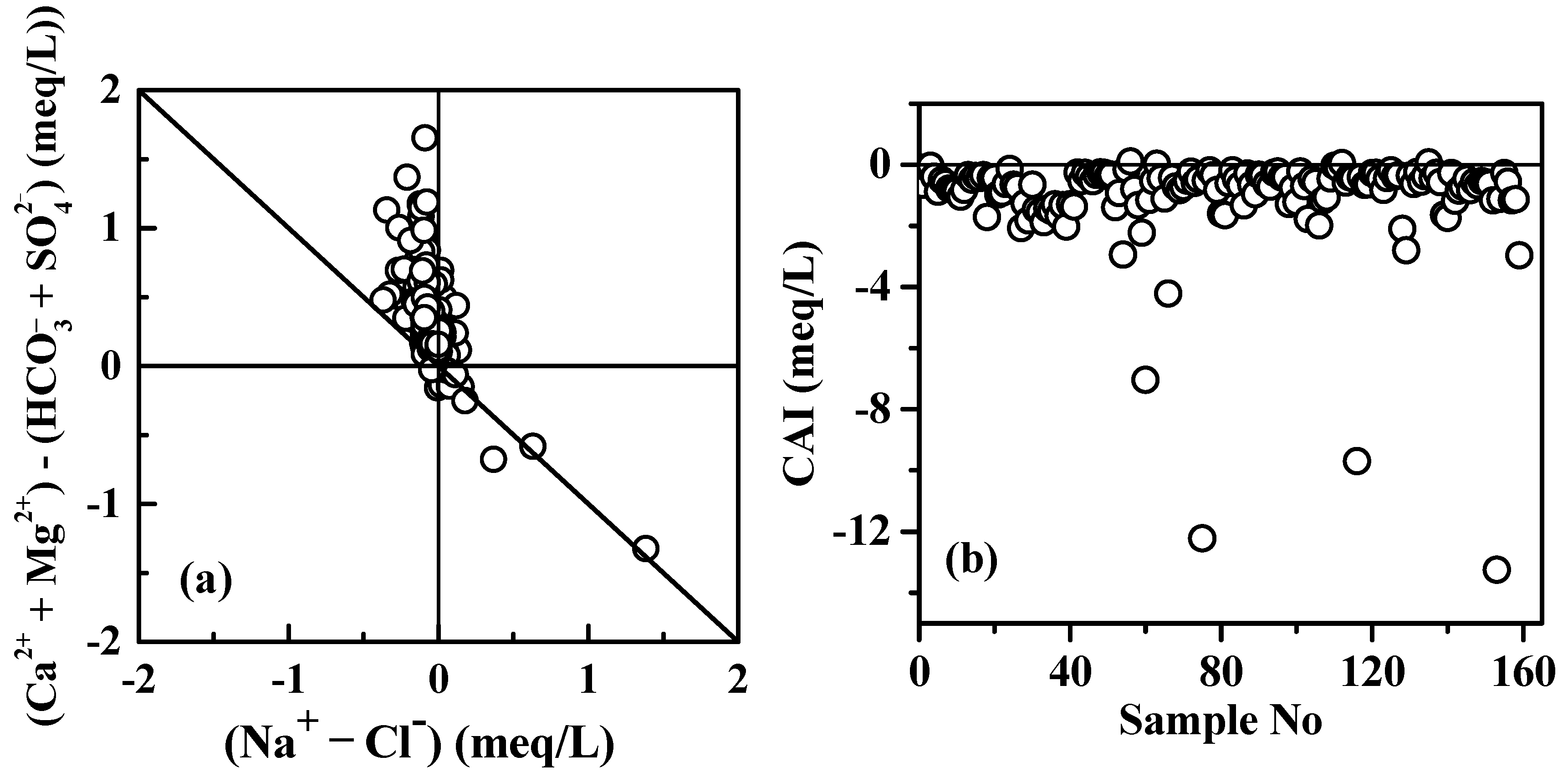
| Parameters | Water from karst Aquifer (N = 93) | Water from Fissured Aquifer (N = 52) | Water from Porous Aquifer (N = 14) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Stdev | Min | Max | Mean | Stdev | Min | Max | Mean | Stdev | |
| T | 12.5 | 19.1 | 15.1 | 1.3 | 13.2 | 28.4 | 15.2 | 2.8 | 13.9 | 16.9 | 15.1 | 0.9 |
| pH | 6.73 | 8.02 | 7.42 | 0.22 | 6.40 | 7.80 | 7.27 | 0.27 | 7.10 | 7.90 | 7.32 | 0.19 |
| K+ | 0.30 | 24.70 | 1.84 | 3.37 | 0.20 | 14.90 | 1.33 | 2.12 | 1.00 | 4.80 | 2.06 | 0.99 |
| Na+ | 0.00 | 35.50 | 2.61 | 4.17 | 0.60 | 15.70 | 3.25 | 2.38 | 1.10 | 6.40 | 3.10 | 1.70 |
| Ca2+ | 11.21 | 448.30 | 80.98 | 47.28 | 9.48 | 232.77 | 65.60 | 41.37 | 55.18 | 109.49 | 80.76 | 13.16 |
| Mg2+ | 1.05 | 60.64 | 15.47 | 14.03 | 2.09 | 50.19 | 9.19 | 9.40 | 7.32 | 46.53 | 23.34 | 12.66 |
| Cl− | 0.95 | 32.16 | 6.18 | 4.50 | 1.89 | 14.66 | 4.60 | 3.19 | 5.20 | 21.28 | 11.18 | 5.82 |
| SO42− | 10.00 | 920.00 | 72.79 | 110.13 | 8.00 | 580.00 | 75.75 | 91.25 | 14.00 | 80.00 | 47.20 | 21.16 |
| HCO3− | 4.97 | 432.48 | 208.20 | 68.35 | 9.94 | 298.26 | 136.90 | 67.59 | 173.99 | 382.77 | 278.02 | 59.03 |
| NO3− | 0.00 | 104.00 | 23.14 | 19.86 | 0.00 | 50.00 | 16.29 | 12.31 | 6.00 | 48.00 | 17.50 | 11.26 |
| TDS | 86.62 | 1519.78 | 378.00 | 187.47 | 67.61 | 1018.30 | 301.04 | 178.31 | 261.70 | 623.43 | 406.74 | 103.82 |
| TH | 51.67 | 1162.47 | 265.92 | 134.82 | 34.42 | 789.93 | 201.65 | 127.76 | 206.67 | 398.25 | 297.77 | 58.22 |
| CO2(g) | −3.26 | −1.47 | −2.19 | 0.29 | −3.14 | −1.57 | −2.26 | 0.29 | −2.69 | −1.60 | −1.96 | 0.26 |
| SIcalcite | −2.89 | 0.50 | −0.06 | 0.38 | −2.39 | 0.50 | −0.49 | 0.56 | −0.20 | 0.32 | 0.03 | 0.14 |
| SIdolomite | −5.87 | 0.70 | −0.77 | 0.84 | −5.10 | 0.10 | −1.67 | 1.15 | −1.20 | 0.33 | −0.33 | 0.45 |
| SIgypsum | −2.53 | −0.22 | −1.83 | 0.38 | −2.69 | −0.59 | −1.88 | 0.44 | −2.44 | −1.70 | −1.92 | 0.26 |
| SIanhydrite | −3.12 | −1.41 | −2.24 | 0.38 | −3.08 | −1.00 | −2.29 | 0.44 | −2.87 | −2.09 | −2.33 | 0.26 |
| SIaragonite | −0.88 | 0.40 | −0.21 | 0.38 | −2.54 | 0.49 | −0.64 | 0.56 | −0.35 | 0.17 | −0.12 | 0.14 |
| SIhalite | −10.71 | −7.87 | −9.60 | 0.53 | −10.20 | −8.70 | −9.51 | 0.35 | −9.79 | −8.51 | −9.14 | 0.45 |
| Parameters | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | TH | TDS |
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | 1.00 | |||||||||
| Na+ | 0.46 | 1.00 | ||||||||
| Ca2+ | 0.27 | 0.17 | 1.00 | |||||||
| Mg2+ | 0.53 | 0.12 | 0.25 | 1.00 | ||||||
| Cl− | 0.62 | 0.39 | 0.41 | 0.25 | 1.00 | |||||
| SO42− | 0.24 | 0.40 | 0.66 | 0.23 | 0.16 | 1.00 | ||||
| HCO3− | 0.38 | −0.01 | 0.72 | 0.58 | 0.41 | 0.19 | 1.00 | |||
| NO3− | 0.14 | −0.01 | 0.26 | −0.05 | 0.54 | −0.05 | 0.20 | 1.00 | ||
| TH | 0.45 | 0.14 | 0.90 | 0.63 | 0.43 | 0.61 | 0.84 | 0.17 | 1.00 | |
| TDS | 0.48 | 0.21 | 0.84 | 0.59 | 0.45 | 0.62 | 0.77 | 0.21 | 0.94 | 1.00 |
| Variables | Principal Components | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| Ca2+ | 0.934 | 0.128 | 0.245 | 0.036 |
| SO42− | 0.803 | −0.094 | −0.187 | 0.447 |
| TH | 0.847 | 0.499 | 0.147 | 0.029 |
| TDS | 0.816 | 0.469 | 0.170 | 0.107 |
| Mg2+ | 0.232 | 0.884 | −0.110 | 0.072 |
| K+ | 0.083 | 0.656 | 0.261 | 0.575 |
| HCO3− | 0.590 | 0.639 | 0.262 | −0.212 |
| NO3− | 0.108 | −0.092 | 0.913 | −0.073 |
| Cl− | 0.174 | 0.319 | 0.748 | 0.412 |
| Na+ | 0.110 | 0.030 | 0.042 | 0.915 |
| Eigenvalue | 4.934 | 1.488 | 1.331 | 1.132 |
| Explained variance (%) | 49.34 | 14.88 | 13.31 | 11.32 |
| Cumulative % of variance | 49.34 | 64.22 | 77.53 | 88.85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Xu, F.; Deng, G.; Tang, Y.; Li, P. Hydrogeochemistry of Shallow Groundwater in a Karst Aquifer System of Bijie City, Guizhou Province. Water 2017, 9, 625. https://doi.org/10.3390/w9080625
Yuan J, Xu F, Deng G, Tang Y, Li P. Hydrogeochemistry of Shallow Groundwater in a Karst Aquifer System of Bijie City, Guizhou Province. Water. 2017; 9(8):625. https://doi.org/10.3390/w9080625
Chicago/Turabian StyleYuan, Jianfei, Fen Xu, Guoshi Deng, Yeqi Tang, and Pengyue Li. 2017. "Hydrogeochemistry of Shallow Groundwater in a Karst Aquifer System of Bijie City, Guizhou Province" Water 9, no. 8: 625. https://doi.org/10.3390/w9080625
APA StyleYuan, J., Xu, F., Deng, G., Tang, Y., & Li, P. (2017). Hydrogeochemistry of Shallow Groundwater in a Karst Aquifer System of Bijie City, Guizhou Province. Water, 9(8), 625. https://doi.org/10.3390/w9080625





