Interspecific Relationship and Ecological Requirements of Two Potentially Harmful Cyanobacteria in a Deep South-Alpine Lake (L. Iseo, I)
Abstract
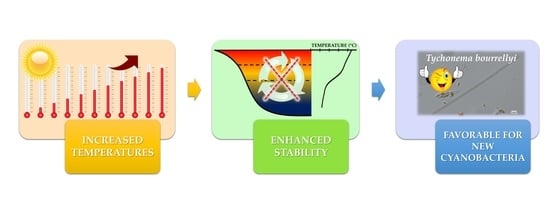
1. Introduction
2. Materials and Methods
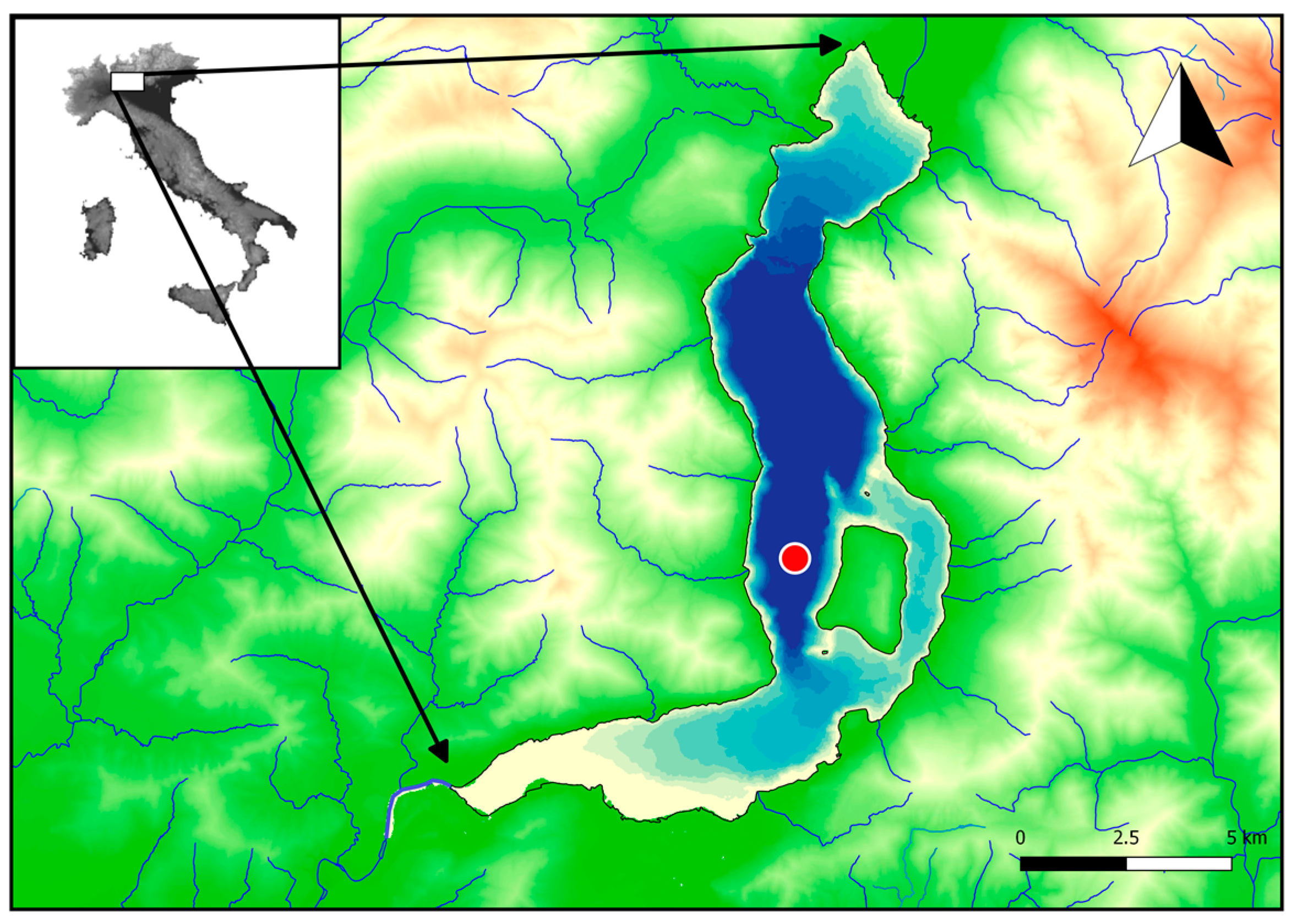
2.1. Study Area
2.2. Sampling, Field Measurements, and Laboratory Analyses
2.3. Statistical Analyses
3. Results
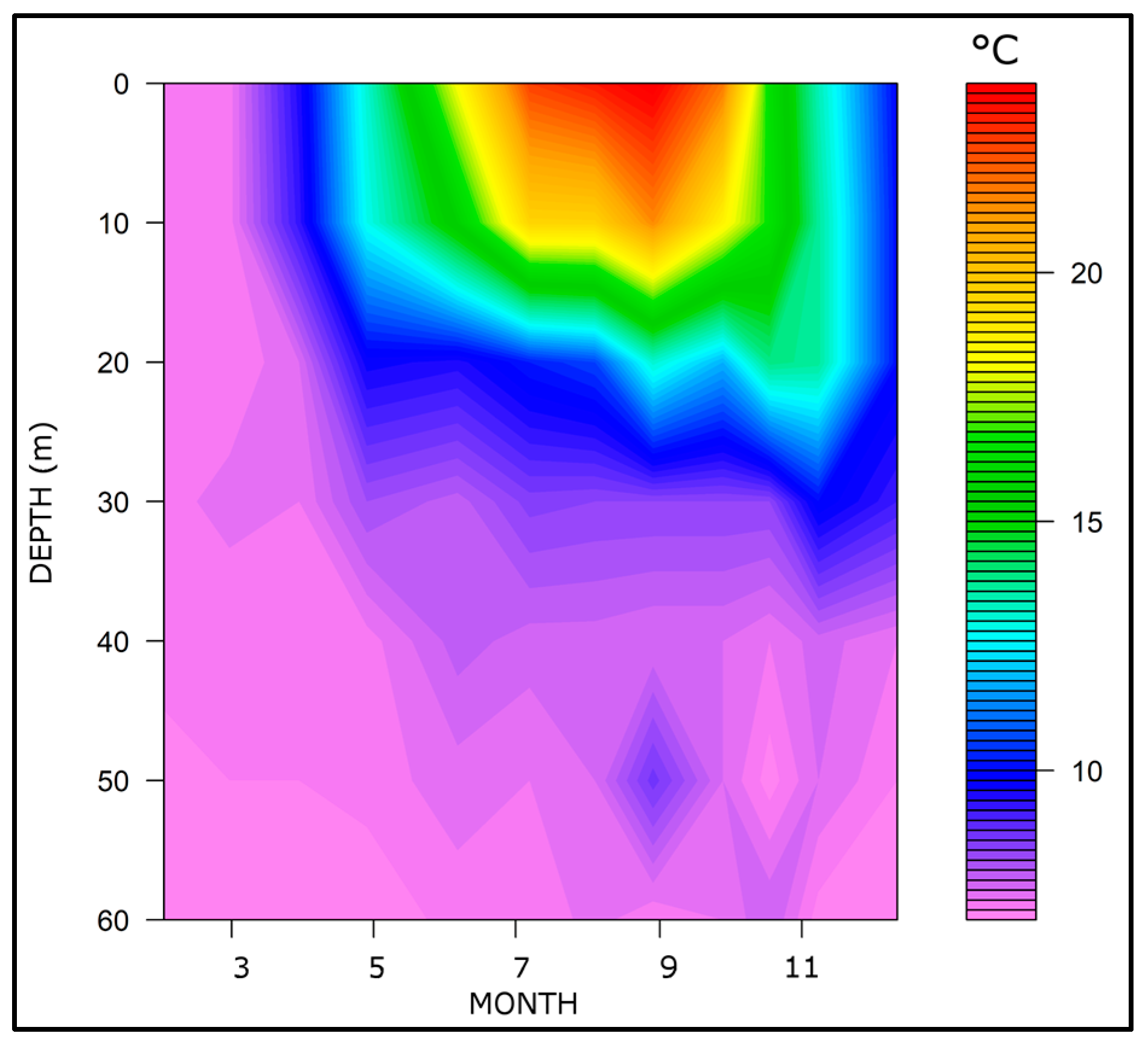
3.1. Thermal Regime and Water Stability
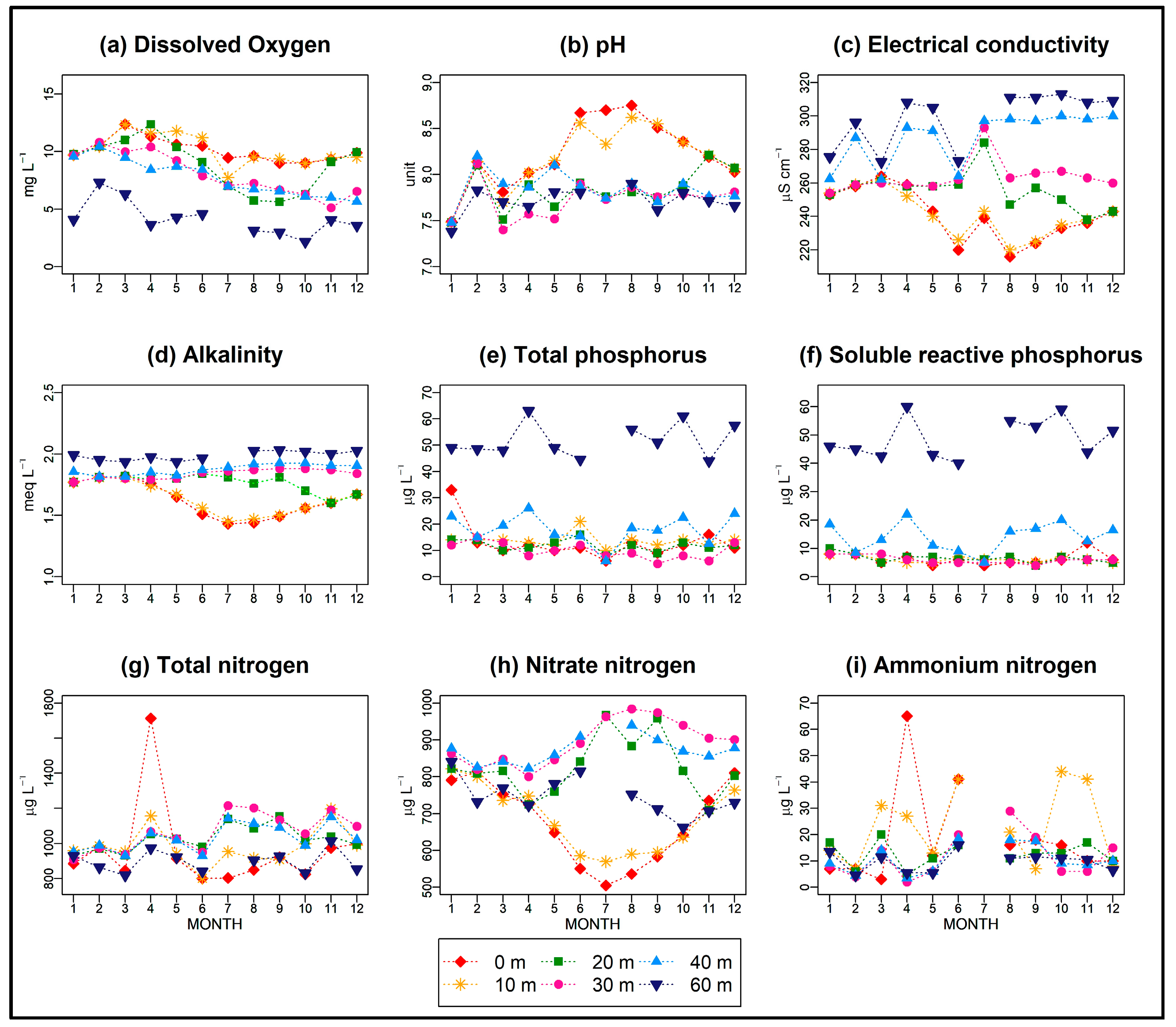
3.2. Chemical Characteristics
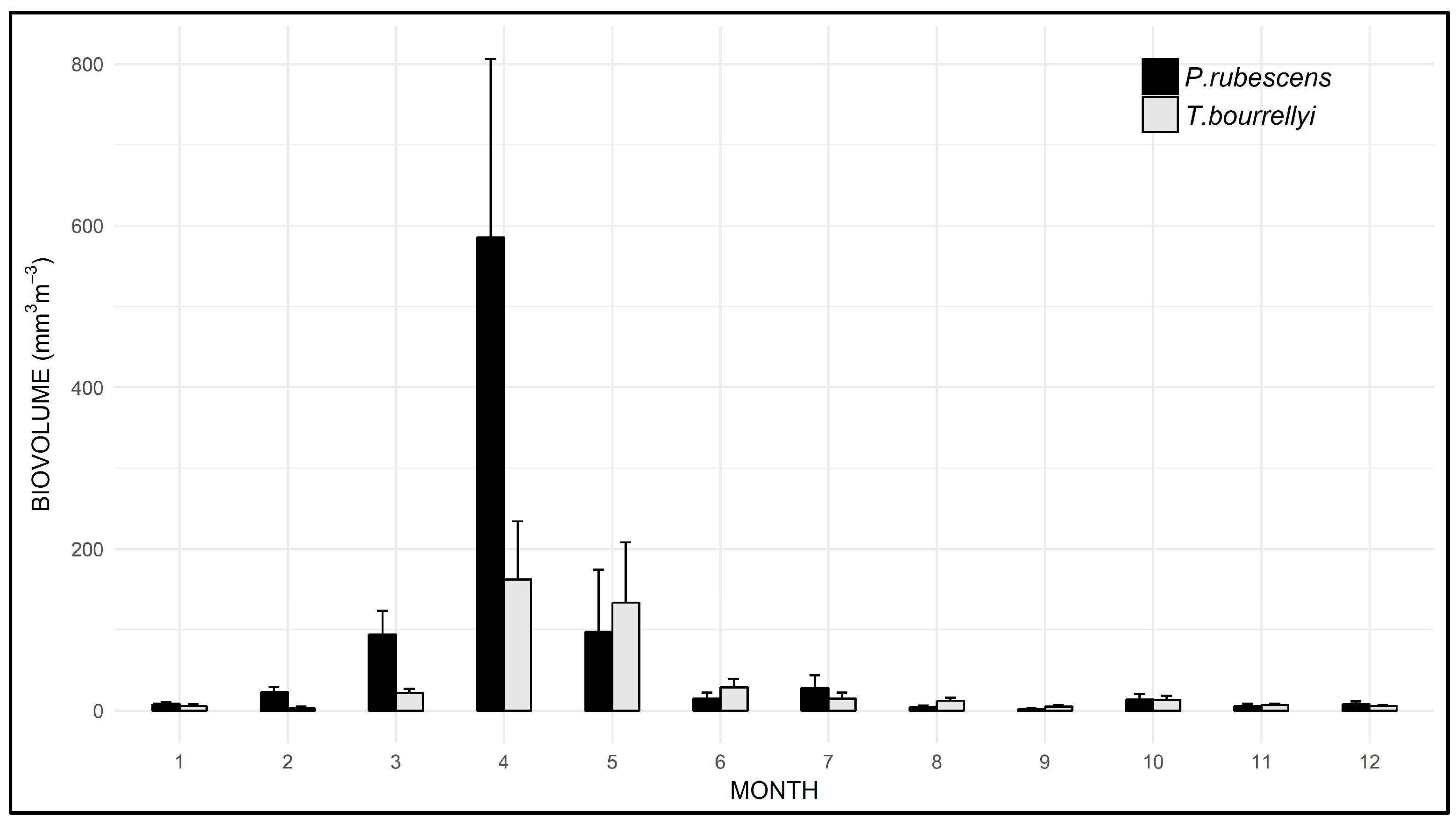
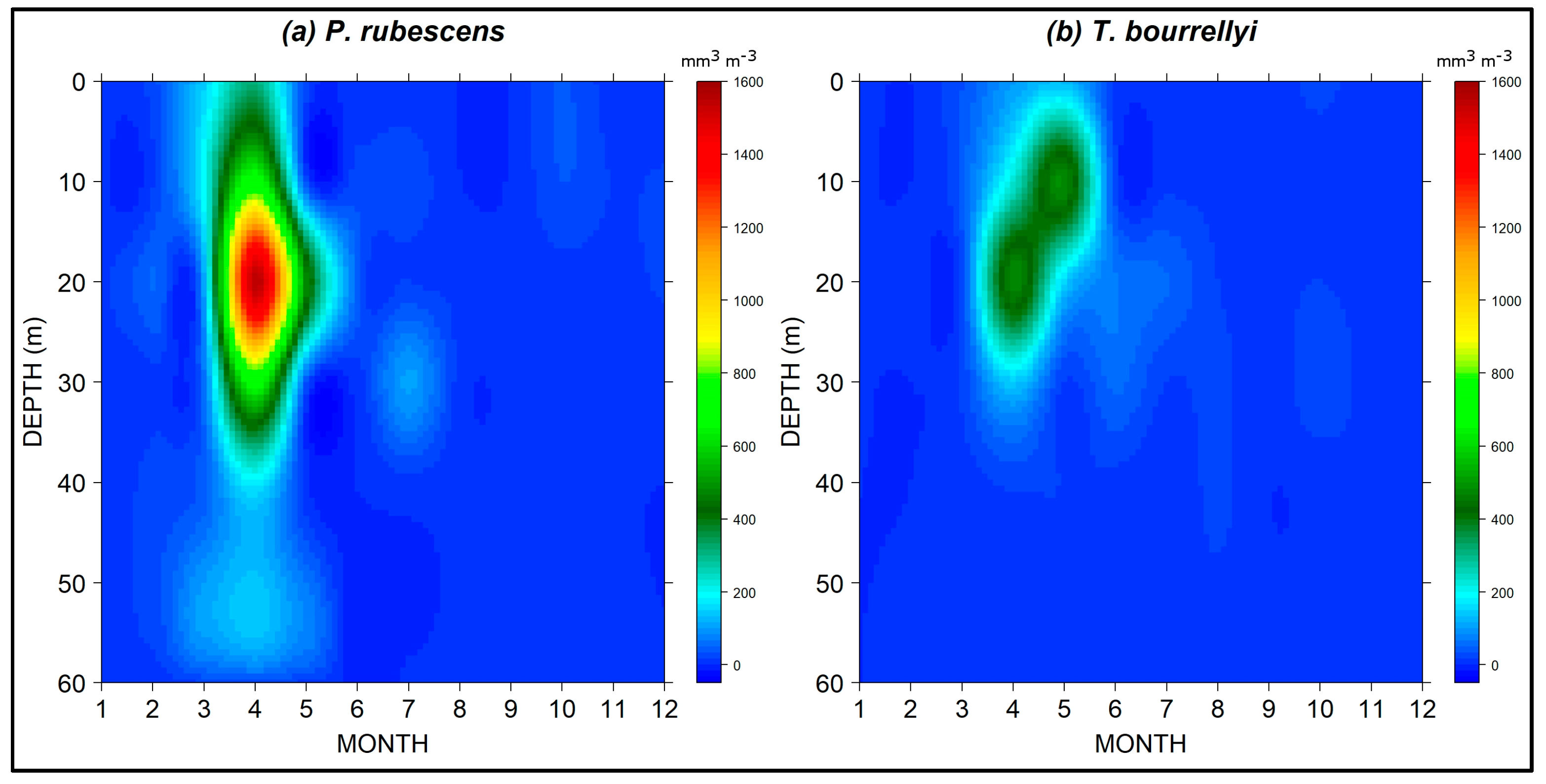
3.3. Seasonal and Spatial Dynamics of P. rubescens and T. bourrellyi
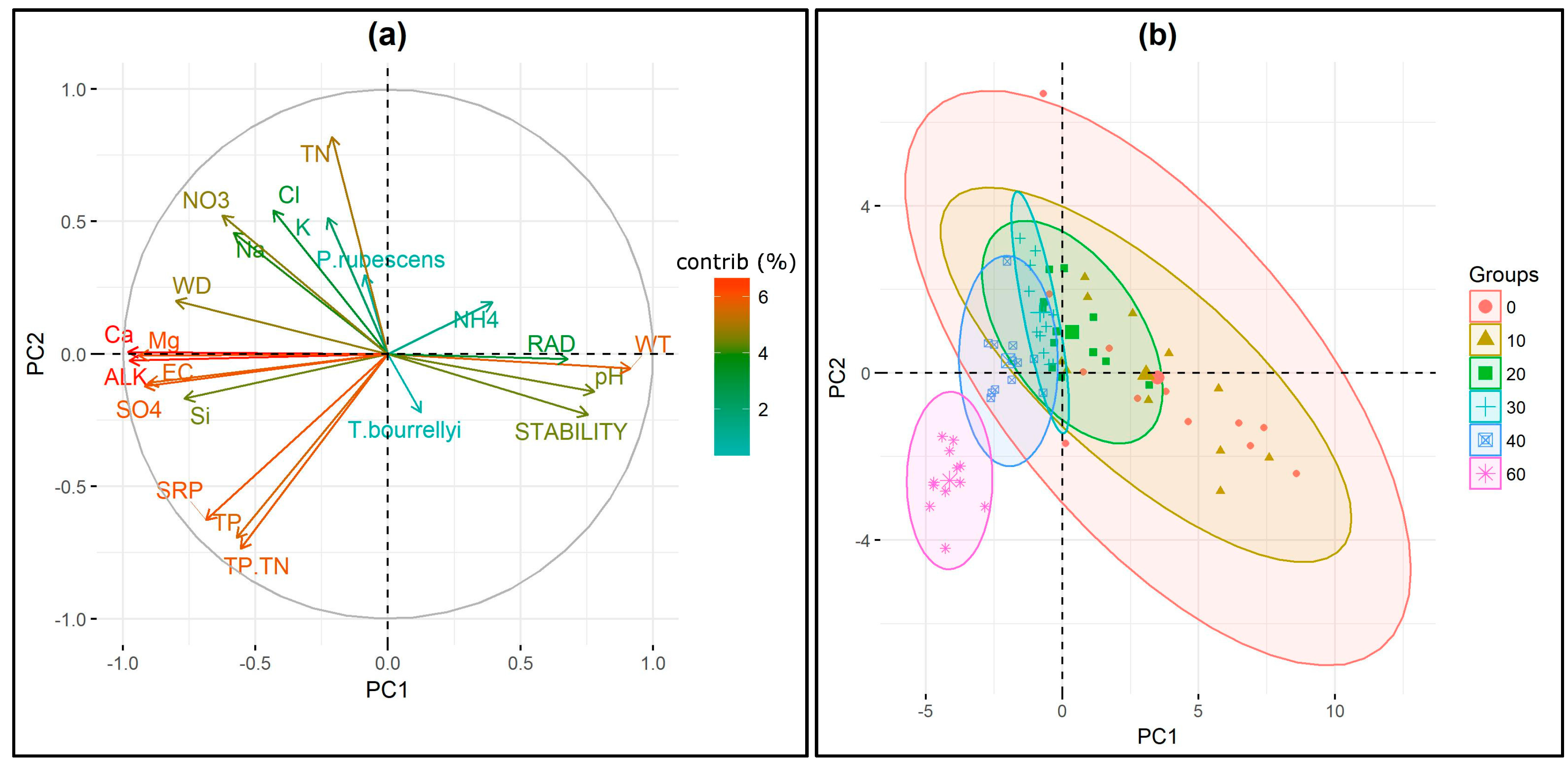
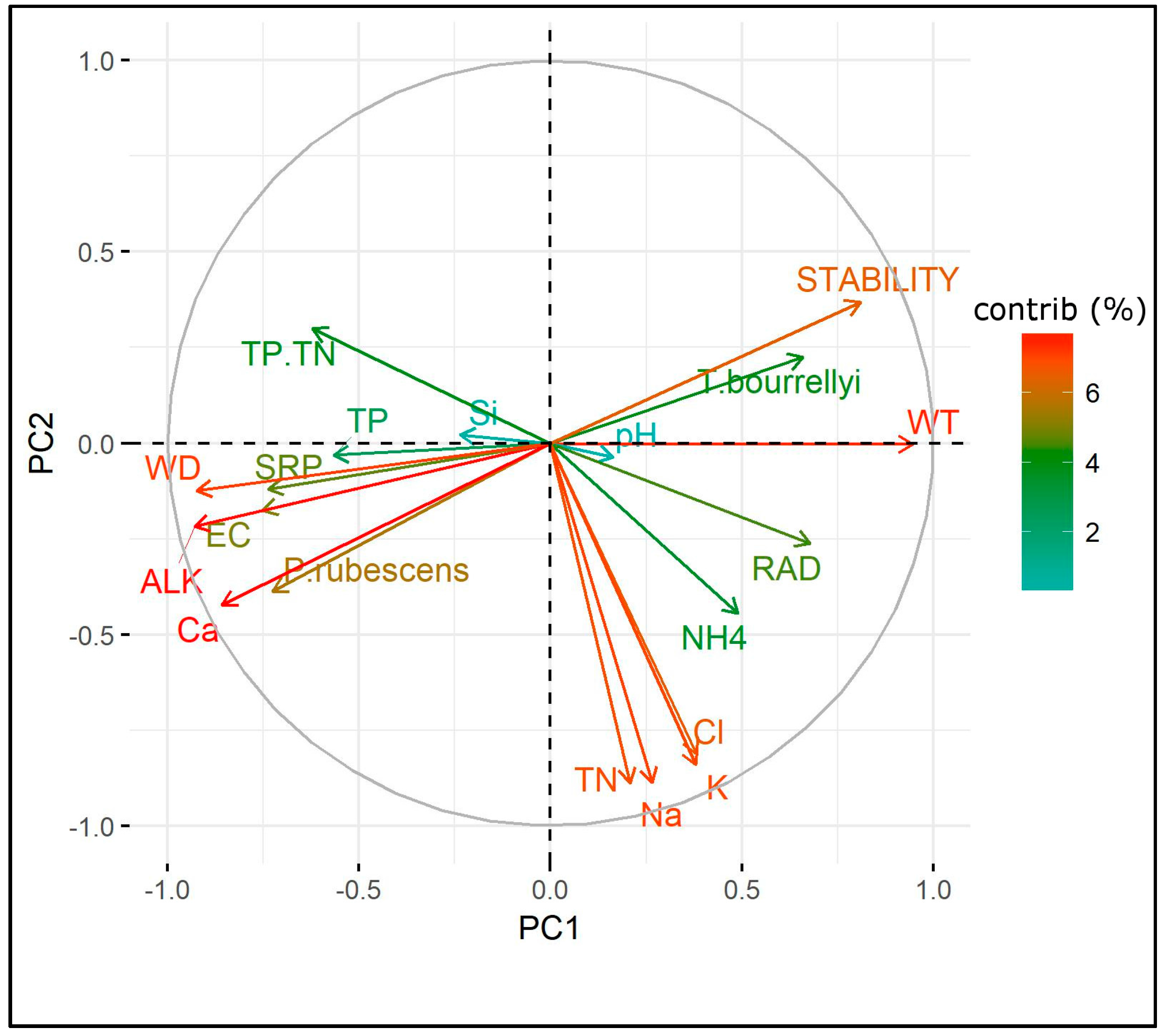
3.4. Ecological Requirements of P. rubescens and T. bourrellyi
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paerl, H.W. Controlling cyanobacterial harmful blooms in freshwater ecosystems. Microb. Biotechnol. 2017, 10, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Holland, H.D.; Wang, P.L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Capelli, C.; Cerasino, L.; Ballot, A.; Dietrich, D.R.; Sivonen, K.; Salmaso, N. Anatoxin-a producing Tychonema (Cyanobacteria) in European waterbodies. Water Res. 2015, 69, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, N.; Cerasino, L.; Boscaini, A.; Capelli, C. Planktic Tychonema (Cyanobacteria) in the large lakes south of the alps: Phylogenetic assessment and toxigenic potential. FEMS Microbiol. Ecol. 2016, 92, fiw155. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate Change Effects on Runoff, Catchment Phosphorus Loading and Lake Ecological State, and Potential Adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Duan, H.; Shi, X.; Yu, Y.; Kong, F. Contributions of meteorology to the phenology of cyanobacterial blooms: Implications for future climate change. Water Res. 2012, 46, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Joyner, J.J.; Joyner, A.R.; Arthur, K.; Paul, V.; O’Neil, J.M.; Heil, C.A. Co-occurrence of dinoflagellate and cyanobacterial harmful algal blooms in southwest Florida coastal waters: Dual nutrient (N and P) input controls. Mar. Ecol. Prog. Ser. 2008, 371, 143–153. [Google Scholar] [CrossRef]
- Sukenik, A.; Hadas, O.; Kaplan, A.; Quesada, A. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes—Physiological, regional, and global driving forces. Front. Microbiol. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Meriluoto, J.; Blaha, L.; Bojadzija, G.; Bormans, M.; Brient, L.; Codd, G.A.; Drobac, D.; Faassen, E.J.; Fastner, J.; Hiskia, A.; et al. Toxic cyanobacteria and cyanotoxins in European waters—Recent progress achieved through the CYANOCOST Action and challenges for further research. Adv. Oceanogr. Limnol. 2017, 8, 161–178. [Google Scholar] [CrossRef]
- Manganelli, M.; Stefanelli, M.; Vichi, S.; Andreani, P.; Nascetti, G.; Scialanca, F.; Scardala, S.; Testai, E.; Funari, E. Cyanobacteria biennal dynamic in a volcanic mesotrophic lake in central Italy: Strategies to prevent dangerous human exposures to cyanotoxins. Toxicon 2016, 115, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Gallina, N.; Beniston, M.; Jacquet, S. Estimating future cyanobacterial occurrence and importance in lakes: A case study with Planktothrix rubescens in Lake Geneva. Aquat. Sci. 2017, 79, 249–263. [Google Scholar] [CrossRef]
- Garibaldi, L.; Anzani, A.; Marieni, A.; Leoni, B.; Mosello, R. Studies on the phytoplankton of the deep subalpine Lake Iseo. J. Limnol. 2003, 62, 177–189. [Google Scholar] [CrossRef]
- Leoni, B.; Marti, C.L.; Imberger, J.; Garibaldi, L. Summer spatial variations in phytoplankton composition and biomass in surface waters of a warm-temperate, deep, oligo-holomictic lake: Lake Iseo, Italy. Inland Waters 2014, 4, 303–310. [Google Scholar] [CrossRef]
- Morabito, G.; Ruggiu, D.; Panzani, P. Recent dynamics (1995–1999) of the phytoplankton assemblages in Lago Maggiore as a basic tool for defining association patterns in the Italian deep lakes. J. Limnol. 2002, 61, 129–145. [Google Scholar] [CrossRef]
- Salmaso, N. Seasonal variation in the composition and rate of change of the phytoplankton community in a deep subalpine lake (Lake Garda, Northern Italy). An application of nonmetric multidimensional scaling and cluster analysis. Hydrobiologia 1996, 337, 49–68. [Google Scholar] [CrossRef]
- Salmaso, N.; Mosello, R. Limnological research in the deep southern subalpine lakes: Synthesis, directions and perspectives. Adv. Oceanogr. Limnol. 2010, 1, 29–66. [Google Scholar] [CrossRef]
- Ruggiu, D.; Morabito, G.; Panzani, P.; Pugnetti, A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy). Hydrobiologia 1998, 369, 243–257. [Google Scholar] [CrossRef]
- Ambrosetti, W.; Barbanti, L.; Mosello, R.; Pugnetti, A. Limnological studies on the deep southern Alpine lakes Maggiore, Lugano, Como, Iseo and Garda. Mem. Ist. Ital. Idrobiol. 1992, 50, 117–146. [Google Scholar]
- Manca, M.; Bertoni, R. Seventy five years of limnology at the Istituto Italiano di Idrobiologia in Pallanza. J. Limnol. 2014, 73, 5–19. [Google Scholar] [CrossRef]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Arch. Hydrobiol. 1988, 80, 327–472. [Google Scholar]
- Shao, J.; Peng, L.; Luo, S.; Yu, G.; Gu, J.-dong; Lin, S.; Li, R. First report on the allelopathic effect of Tychonema bourrellyi (Cyanobacteria) against Microcystis aeruginosa (Cyanobacteria). J. Appl. Phycol. 2013, 25, 1567–1573. [Google Scholar] [CrossRef]
- Salmaso, N.; Morabito, G.; Mosello, R.; Garibaldi, L.; Simona, M.; Buzzi, F.; Ruggiu, D. A synoptic study of phytoplankton in the deep lakes south of the Alps (lakes Garda, Iseo, Como, Lugano and Maggiore). J. Limnol. 2003, 62, 207–227. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Dokulil, M.T. Impact of climate warming on European inland waters. Inl. Waters 2014, 4, 27–40. [Google Scholar] [CrossRef]
- Mosello, R.; Ambrosetti, W.; Arisci, S.; Bettinetti, R.; Buzzi, F.; Calderoni, A.; Carrara, E.; De Bernardi, R.; Galassi, S.; Garibaldi, L.; et al. Evoluzione recente della qualità delle acque dei laghi profondi sudalpini (Maggiore, Lugano, Como, Iseo e Garda) in risposta alle pressioni antropiche e alle variazioni climatiche. Biol. Ambient. 2010, 24, 167–177. [Google Scholar]
- Rogora, M.; Buzzi, F.; Dresti, C.; Leoni, B.; Lepori, F.; Mosello, R.; Patelli, M.; Salmaso, N. Climatic effects on vertical mixing and deep-water oxygenation in the deep subalpine lakes in Italy. Hydrobiologia 2017. accepted for publication. [Google Scholar]
- Leoni, B.; Garibaldi, L.; Gulati, R.D. How does interannual trophic variability caused by vertical water mixing affect reproduction and population density of the Daphnia longispina group in Lake Iseo, a deep stratified lake in Italy? Inland Waters 2014, 4, 193–203. [Google Scholar] [CrossRef]
- Marti, C.M.; Imberger, J.; Garibaldi, L.; Leoni, B. Using time scales to characterize phytoplankton assemblages in a deep subalpine lake during the thermal stratification period: Lake Iseo, Italy. Water Resour. Res. 2015, 52, 1762–1780. [Google Scholar] [CrossRef]
- Pilotti, M.; Valerio, G.; Leoni, B. Data set for hydrodynamic lake model calibration: A deep prealpine case. Water Resour. Res. 2013, 49, 7159–7163. [Google Scholar] [CrossRef]
- Valerio, G.; Pilotti, M.; Barontini, S.; Leoni, B. Sensitivity of the multiannual thermal dynamics of a deep pre-alpine lake to climatic change. Hydrol. Process. 2015, 29, 767–779. [Google Scholar] [CrossRef]
- Leoni, B. Zooplankton predators and preys: Body size and stable isotope to investigate the pelagic food web in a deep lake (Lake Iseo, Northern Italy). J. Limnol. 2017, 76, 85–93. [Google Scholar] [CrossRef]
- Graham, M.H.; Mitchell, B.G. Obtaining absorption spectra from individual macroalgal spores using microphotometry. Hydrobiologia 1999, 398/399, 231–239. [Google Scholar] [CrossRef]
- Komárek, J.; Albertano, P. Cell structure of a planktic cyanoprokaryote, Tychonema bourrellyi. Algol. Stud. 1994, 75, 157–166. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; ISBN 0875532357. [Google Scholar]
- Salmaso, N. Factors affecting the seasonality and distribution of cyanobacteria and chlorophytes: A case study from the large lakes south of the Alps, with special reference to Lake Garda. Hydrobiologia 2000, 438, 43–63. [Google Scholar] [CrossRef]
- Winslow, L.; Read, J.; Woolway, R.; Brentrup, J.; Leach, T.; Zwart, J. rLakeAnalyzer: Lake Physics Tools. 2017. Available online: https://cran.r-project.org/web/packages/rLakeAnalyzer/rLakeAnalyzer.pdf (accessed on 17 December 2017).
- Imberger, J.; Patterson, J.C. Physical Limnology. Adv. Appl. Mech. 1989, 27, 303–475. [Google Scholar] [CrossRef]
- Jollife, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data, 1st ed.; Springer: New York, NY, USA, 2007; ISBN 0-387-45967-7. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre; Legendre, P. Numerical Ecology with R, 1st ed.; Springer: New York, NY, USA, 2011; ISBN 9788578110796. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017; ISBN 3_900051_00_3. [Google Scholar]
- Kraemer, B.M.; Anneville, O.; Chandra, S.; Dix, M.; Kuusisto, E.; Livingstone, D.M.; Rimmer, A.; Schladow, S.G.; Silow, E.; Sitoki, L.M.; et al. Morphometry and average temperature affect lake stratification responses to climate change. Geophys. Res. Lett. 2015, 42, 4981–4988. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, B.; Yu, Y.; Yang, Z.; Shi, X.; Kong, F. Effects of temperature fluctuation on the development of cyanobacterial dominance in spring: Implication of future climate change. Hydrobiologia 2016, 763, 135–146. [Google Scholar] [CrossRef]
- Wood, S.A.; Borges, H.; Puddick, J.; Biessy, L.; Atalah, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P. Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: Insights into potential effects of climate change. Hydrobiologia 2017, 785, 71–89. [Google Scholar] [CrossRef]
- Gallina, N.; Anneville, O.; Beniston, M. Impacts of extreme air temperatures on cyanobacteria in five deep peri-alpine lakes. J. Limnol. 2011, 70, 186–196. [Google Scholar] [CrossRef]
- Havens, K.; Paerl, H.; Phlips, E.; Zhu, M.; Beaver, J.; Srifa, A. Extreme weather events and climate variability provide a lens to how shallow lakes may respond to climate change. Water 2016, 8, 229. [Google Scholar] [CrossRef]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Watanabe, M.M.; Otsuka, S.; Mahakahant, A.; Yongmanitchai, W.; Nopartnaraporn, N.; Liu, Y.; Day, J.G. Taxonomic revision of water-bloom-forming species of oscillatorioid cyanobacteria. Int. J. Syst. Evol. Microbiol. 2002, 52, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Bright, D.I.; Walsby, A.E. The relationship between critical pressure and width of gas vesicles in isolates of Planktothrix rubescens from Lake Zurich. Microbiology 1999, 145, 2769–2775. [Google Scholar] [CrossRef] [PubMed]
- Garneau, M.È.; Posch, T.; Pernthaler, J. Seasonal patterns of microcystin-producing and non-producing Planktothrix rubescens genotypes in a deep pre-alpine lake. Harmful Algae 2015, 50, 21–31. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Pancrace, C.; Barny, M.-A.; Ueoka, R.; Calteau, A.; Scalvenzi, T.; Pédron, J.; Barbe, V.; Piel, J.; Humbert, J.-F.; Gugger, M. Insights into the Planktothrix genus: Genomic and metabolic comparison of benthic and planktic strains. Sci. Rep. 2017, 7, 41181. [Google Scholar] [CrossRef] [PubMed]
- Ganf, G.G.; Heaney, S.I.; Corry, J. Light-Absorption and Pigment Content in Natural-Populations and Cultures of a Non-Gas Vacuolate Cyanobacterium Oscillatoria bourrellyi (=Tychonema bourrellyi). J. Plankton Res. 1991, 13, 1101–1121. [Google Scholar] [CrossRef]
- Li, L.; Chesson, P. The Effects of Dynamical Rates on Species Coexistence in a Variable Environment: The Paradox of the Plankton Revisited. Am. Nat. 2016, 188, E46–E58. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.E. The Paradox of the Plankton. Am. Nat. 1961, 95, 137–145. [Google Scholar] [CrossRef]
- Jacquet, S.; Briand, J.F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vinçon-Leite, B.; Paolini, G.; Druart, J.C.; Anneville, O.; et al. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Abundance and toxicity of Planktothrix rubescens in the pre-alpine Lake Ammersee, Germany. Harmful Algae 2009, 8, 329–342. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. Hydrobiologia 2012, 698, 29–46. [Google Scholar] [CrossRef]
- Savichtcheva, O.; Debroas, D.; Perga, M.E.; Arnaud, F.; Villar, C.; Lyautey, E.; Kirkham, A.; Chardon, C.; Alric, B.; Domaizon, I. Effects of nutrients and warming on Planktothrix dynamics and diversity: A palaeolimnological view based on sedimentary DNA and RNA. Freshw. Biol. 2015, 60, 31–49. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Salmaso, N.; Buzzi, L.; Cerasino, L.; Garibaldi, L.; Leoni, B.; Manca, M.; Morabito, G.; Rogora, M.; Simona, M. Influenza delle fluttuazioni climatiche sui grandi laghi a sud delle Alpi: Implicazioni nel contesto del riscaldamento globale. Biol. Ambient. 2014, 28, 17–32. [Google Scholar]
- O’Reilly, C.M.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; Mcintyre, P.B.; Kraemer, B.M. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef]
- Pareeth, S.; Bresciani, M.; Buzzi, F.; Leoni, B.; Lepori, F.; Ludovisi, A.; Morabito, G.; Adrian, R.; Neteler, M.; Salmaso, N. Warming trends of perialpine lakes from homogenised time series of historical satellite and in-situ data. Sci. Total Environ. 2017, 578, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Posch, T.; Köster, O.; Salcher, M.M.; Pernthaler, J. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat. Clim. Chang. 2012, 2, 809–813. [Google Scholar] [CrossRef]
- Salmaso, N.; Morabito, G.; Garibaldi, L.; Mosello, R. Trophic development of the deep lakes south of the Alps: A comparative analysis. Arch. Hydrobiol. 2007, 170, 177–196. [Google Scholar] [CrossRef]
- Leoni, B.; Nava, V.; Patelli, M. Relationship among climate variability, Cladocera phenology and the pelagic food web in deep lakes in different trophic states. Mar. Freshw. Res. 2017. accepted for publication. [Google Scholar]
- Walsby, A.E.; Schanz, F. Light-dependent growth rate determines changes in the population of Planktothrix rubescens over the annual cycle in lake Zürich, Switzerland. New Phytol. 2002, 154, 671–687. [Google Scholar] [CrossRef]
- Mantzouki, E.; Visser, P.M.; Bormans, M.; Ibelings, B.W. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol. 2016, 50, 333–350. [Google Scholar] [CrossRef]
- Walsby, A.E.; Avery, A.; Schanz, F. The critical pressures of gas vesicles in Planktothrix rubescens in relation to the depth of winter mixing in Lake Zurich, Switzerland. J. Plankton Res. 1998, 20, 1357–1375. [Google Scholar] [CrossRef]
- D’Alelio, D.; Gandolfi, A.; Boscaini, A.; Flaim, G.; Tolotti, M.; Salmaso, N. Planktothrix populations in subalpine lakes: Selection for strains with strong gas vesicles as a function of lake depth, morphometry and circulation. Freshw. Biol. 2011, 56, 1481–1493. [Google Scholar] [CrossRef]
- Walsby, A.E.; Bleything, A. The Dimensions of Cyanobacterial Gas Vesicles in Relation to Their Efficiency in Providing Buoyancy and Withstanding Pressure. J. Gen. Microbiol. 1988, 134, 2635–2645. [Google Scholar] [CrossRef]
- Hayes, P.K.; Walsby, A.E. The inverse correlation between width and strength of gas vesicles in cyanobacteria. Br. Phycol. J. 1986, 21, 191–197. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Alex Elliott, J.; Frassl, M.A. Predictive utility of trait-separated phytoplankton groups: A robust approach to modeling population dynamics. J. Great Lakes Res. 2014, 40, 143–150. [Google Scholar] [CrossRef]
- Yankova, Y.; Villiger, J.; Pernthaler, J.; Schanz, F.; Posch, T. Prolongation, deepening and warming of the metalimnion change habitat conditions of the harmful filamentous cyanobacterium Planktothrix rubescens in a prealpine lake. Hydrobiologia 2016, 776, 125–138. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava, V.; Patelli, M.; Soler, V.; Leoni, B. Interspecific Relationship and Ecological Requirements of Two Potentially Harmful Cyanobacteria in a Deep South-Alpine Lake (L. Iseo, I). Water 2017, 9, 993. https://doi.org/10.3390/w9120993
Nava V, Patelli M, Soler V, Leoni B. Interspecific Relationship and Ecological Requirements of Two Potentially Harmful Cyanobacteria in a Deep South-Alpine Lake (L. Iseo, I). Water. 2017; 9(12):993. https://doi.org/10.3390/w9120993
Chicago/Turabian StyleNava, Veronica, Martina Patelli, Valentina Soler, and Barbara Leoni. 2017. "Interspecific Relationship and Ecological Requirements of Two Potentially Harmful Cyanobacteria in a Deep South-Alpine Lake (L. Iseo, I)" Water 9, no. 12: 993. https://doi.org/10.3390/w9120993
APA StyleNava, V., Patelli, M., Soler, V., & Leoni, B. (2017). Interspecific Relationship and Ecological Requirements of Two Potentially Harmful Cyanobacteria in a Deep South-Alpine Lake (L. Iseo, I). Water, 9(12), 993. https://doi.org/10.3390/w9120993