Long-Term Comparison of Disinfection By-Product Formation Potential in a Full Scale Treatment Plant Utilizing a Multi-Coagulant Drinking Water Treatment Scheme
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Experimental Methods
2.3. Analytical Methods
2.3.1. PARAFAC Analysis
2.3.2. Modeling Procedures
3. Results and Discussion
3.1. Source Water Characteristics and Dissolved Organic Carbon Removal
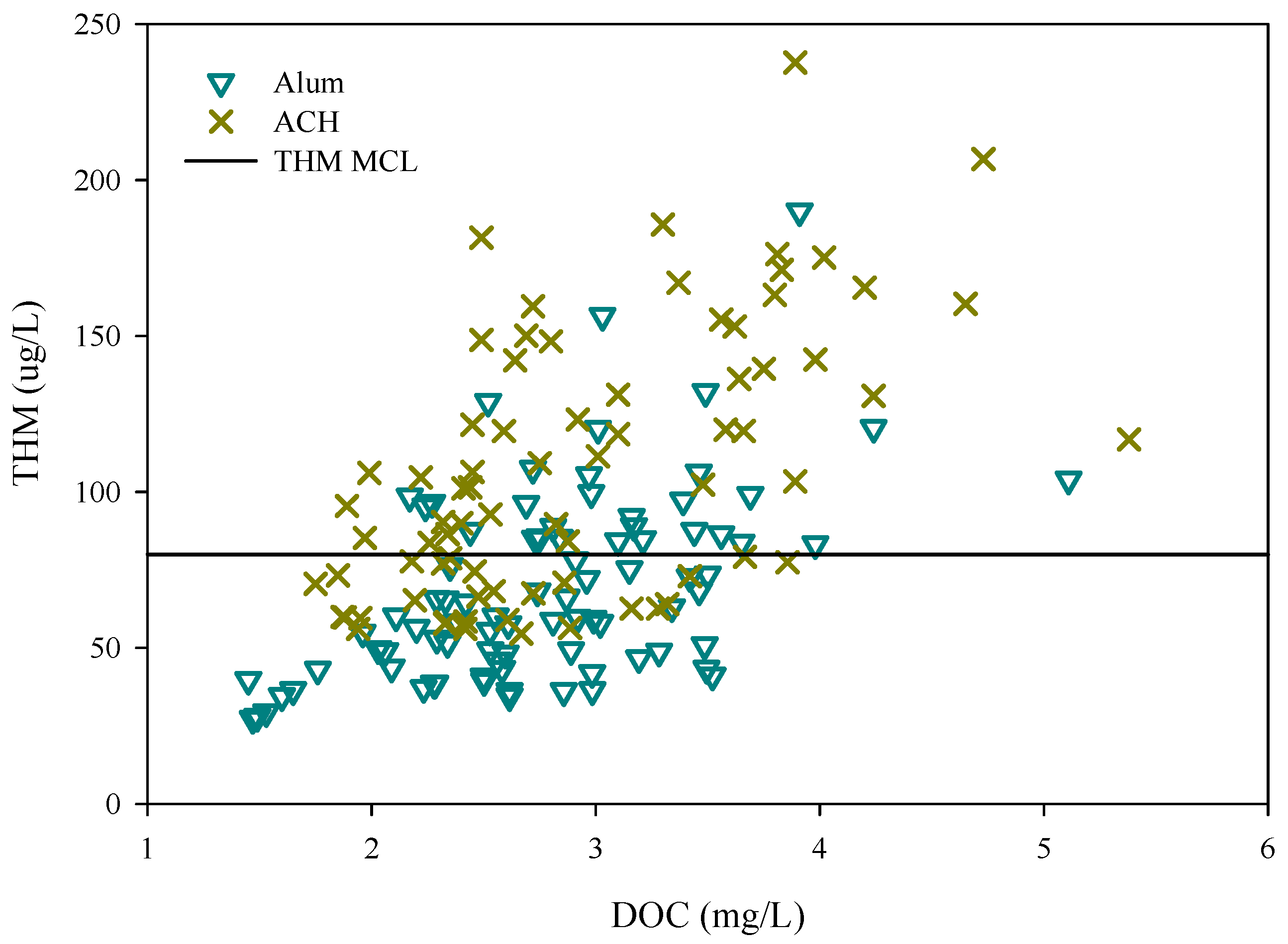
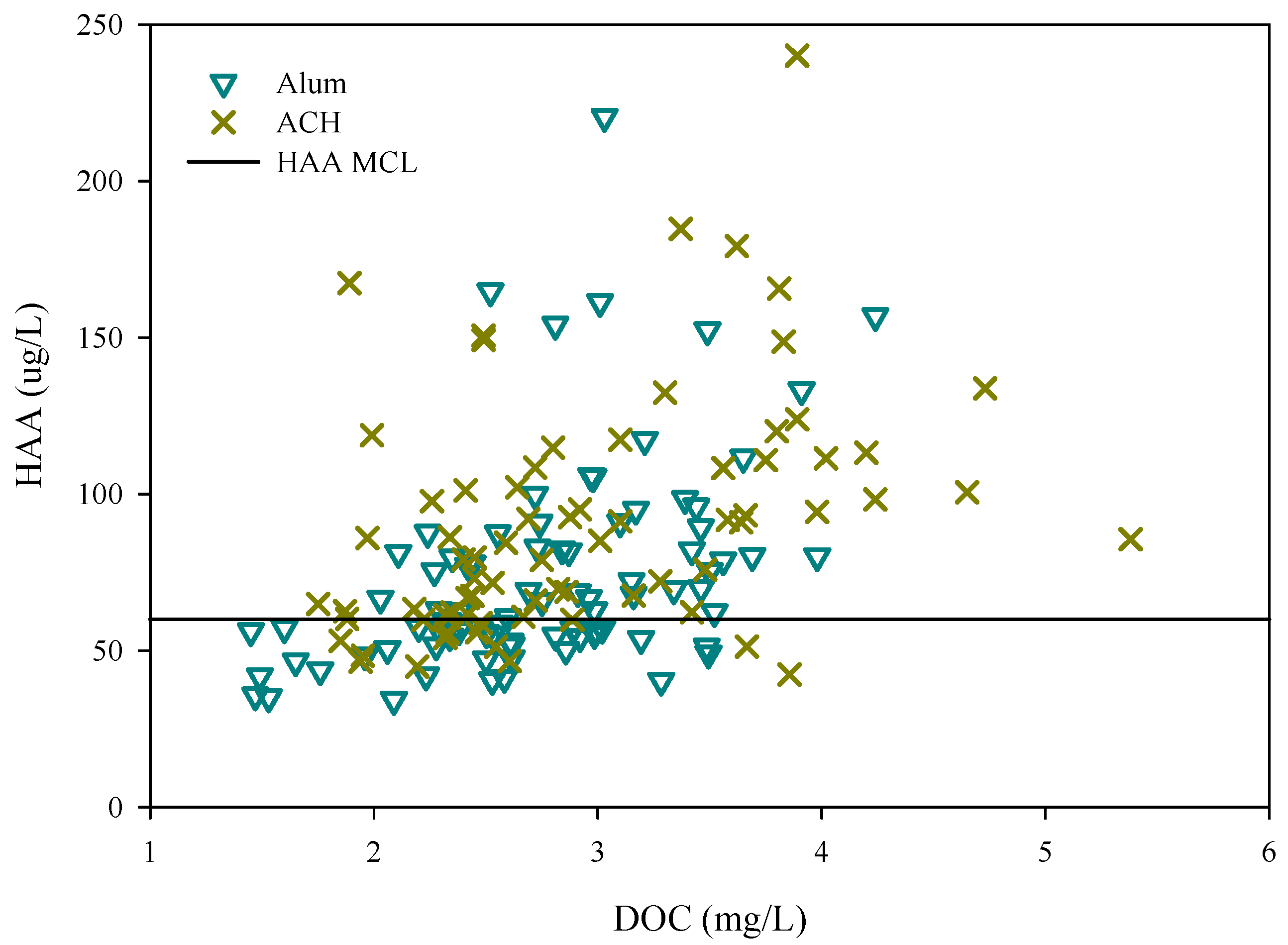
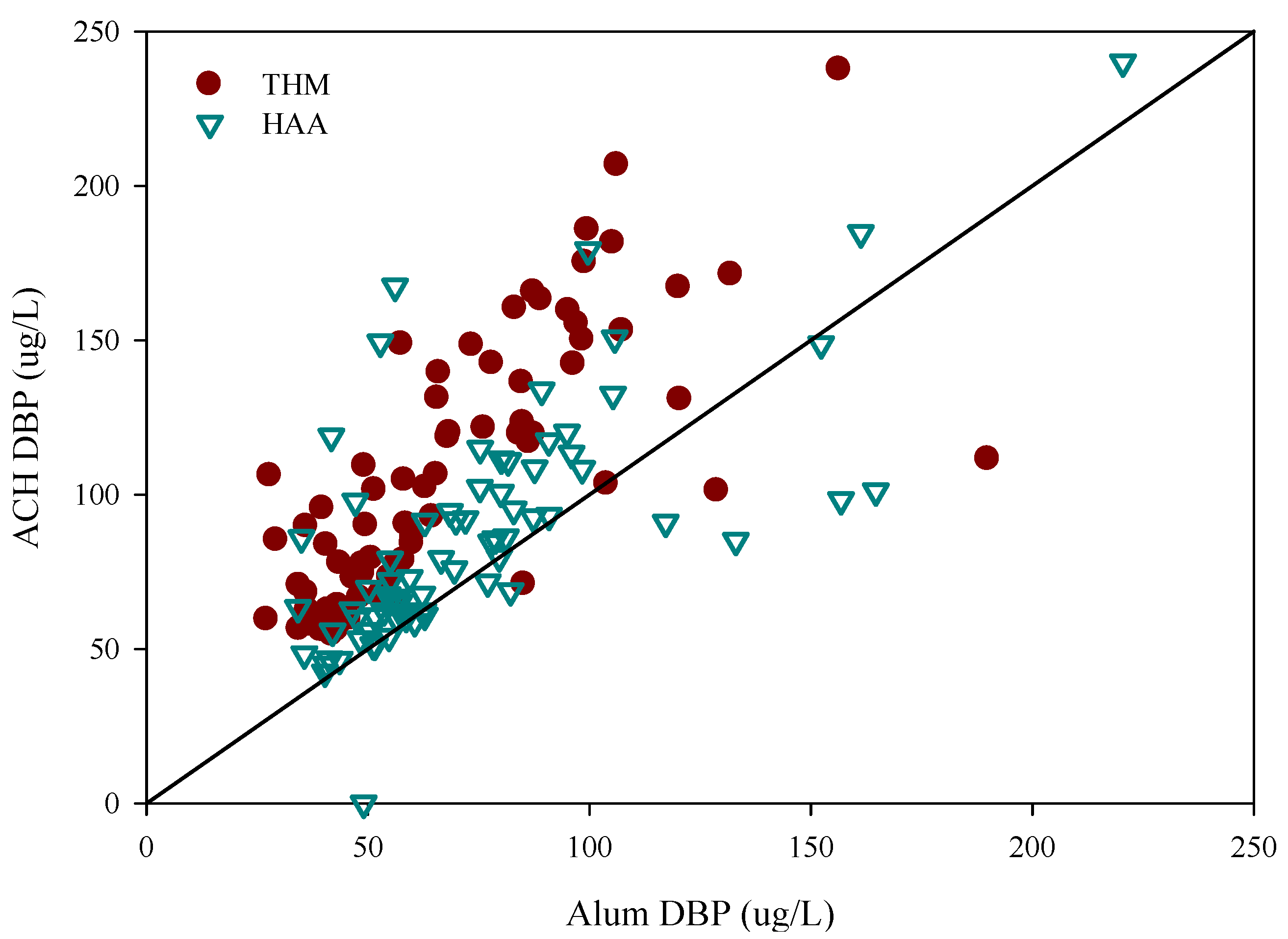
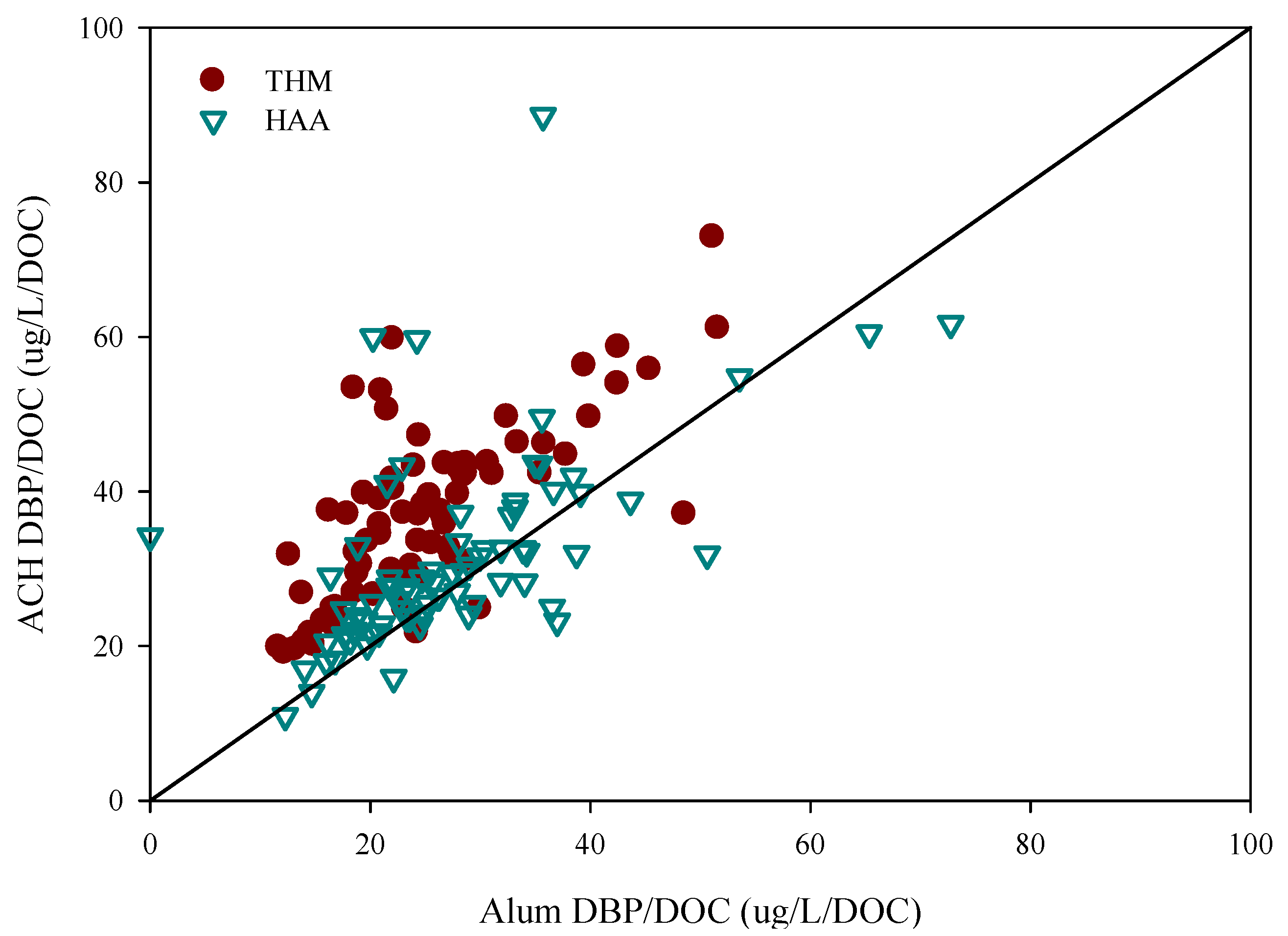
3.2. Disinfection By-Product Formation Potential
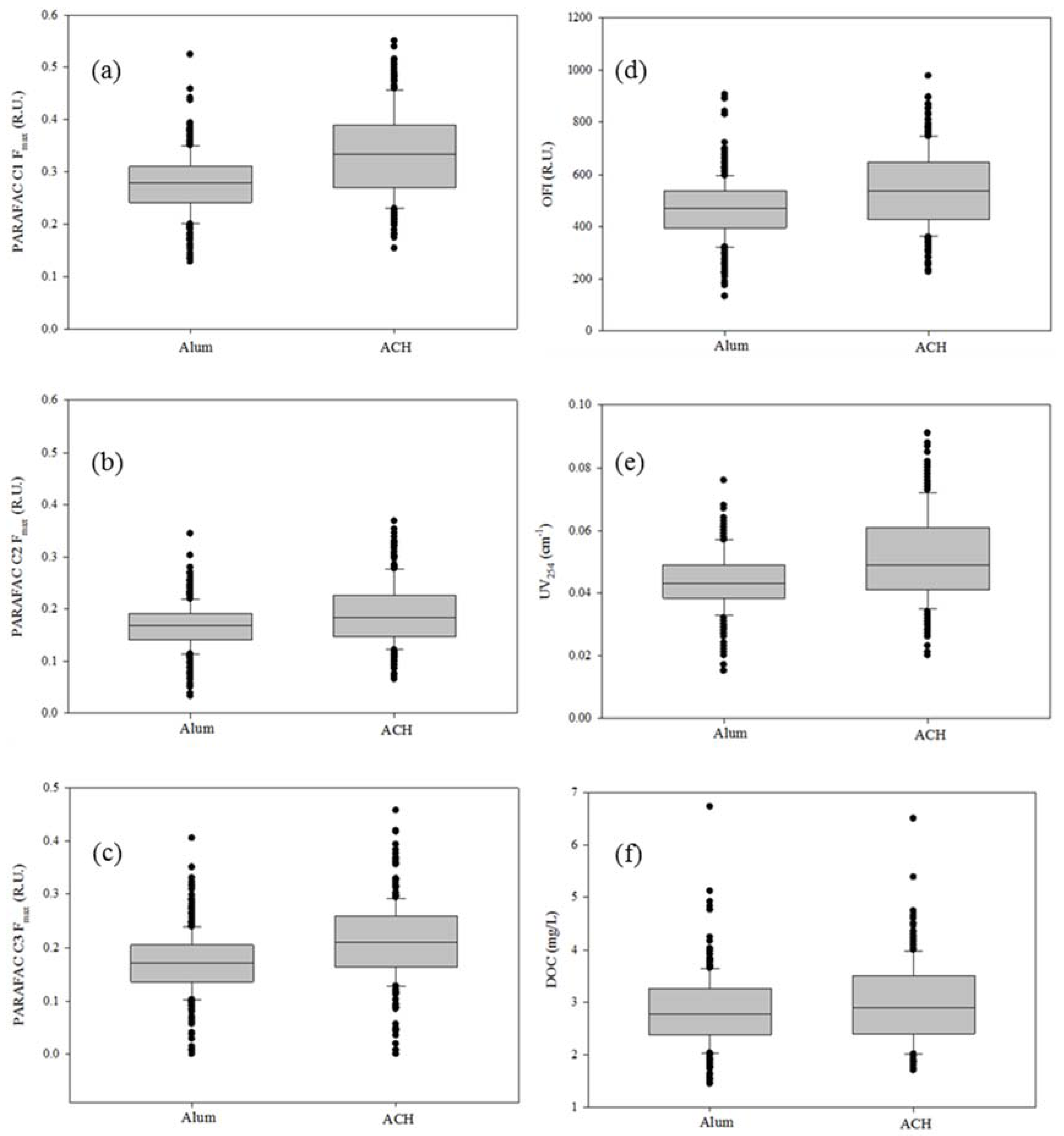
3.3. Fluorescence and DOC Analysis
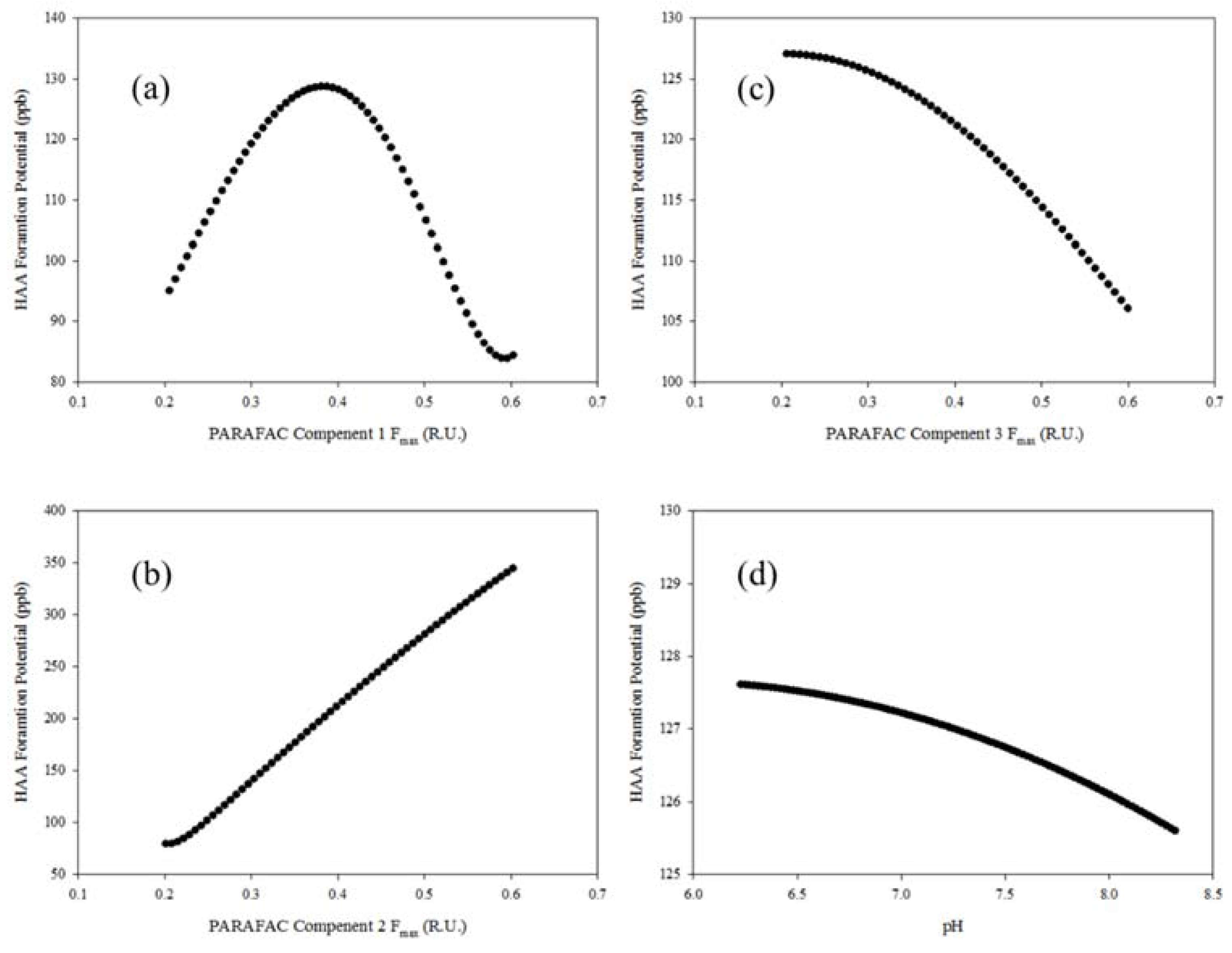
3.4. Monte Carlo Simulations of Disinfection By-Product Formation Potential Modelling
4. Summary
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Environmental Protection Agency. National primary drinking water regulations: Stage 2 disinfectants and disinfection by-products rule. Fed. Regist. 2006, 71, 387–493. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.J. Formation of haloforms during chlorination of natural waters. Water Treat. Exam. 1974, 23, 234–243. [Google Scholar]
- Leenheer, J.A.; Croué, J.-P. Peer reviewed: Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, J.; Sikorska, E. Characterization of natural organic matter fractions by high pressure size-exclusion chromatography, specific UV absorbance and total luminescence spectroscopy. Pol. J. Environ. Stud. 2005, 15, 145–153. [Google Scholar]
- Archer, D.A.; Singer, P.C. An evaluation of the relationship between: SUVA and NOM coagulation using the ICR database. J. Am. Water Works Assoc. 2006, 98, 110–123. [Google Scholar]
- Bose, P.; Reckhow, D.A. The effect of ozonation on natural organic matter removal by alum coagulation. Water Res. 2007, 41, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; Demarco, J.; Owen, D.M.; Randtke, S.J. Selected processes for removing NOM: An overview: Natural organic matter. J. Am. Water Works Assoc. 1995, 87, 64–71. [Google Scholar]
- Owen, D.M.; Amy, G.L.; Chowdhary, Z.K. Characterization of Natural Organic Matter and Its Relationship to Treatability; AWWARF: Denver, CO, USA, 1993. [Google Scholar]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, J.D.; Thruston, A.D., Jr. Occurrence of a New Generation of Disinfection Byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Ates, N.; Kaplan, S.S.; Sahinkaya, E.; Kitis, M.; Dilek, F.B.; Yetis, U. Occurrence of disinfection by-products in low DOC surface waters in Turkey. J. Hazard. Mater. 2007, 142, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Bieroza, M.; Baker, A.; Bridgeman, J. Relating freshwater organic matter fluorescence to organic carbon removal efficiency in drinking water treatment. Sci. Total Environ. 2009, 407, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Peiris, R.H.; Halle, C.; Budman, H.; Moresoli, C.; Peldszus, S.; Huck, P.M.; Legge, R.L. Indentifying fouling events in a membrane-based drinking water treatment process using principal component analysis of fluorescence excitation-emission matrices. Water Res. 2010, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking natural organic matter (NOM) in a drinking water treatment using fluorescence excitation-emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andreson, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursors organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidfond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic carbon in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC, Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Holbrook, D.R.; Yen, J.H.; Grizzard, T.J. Characterizing natural organic material from Occoquan Watershed (Northern Virginia, US) using fluorescence spectroscopy and PARAFAC. Sci. Total Environ. 2006, 361, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Baghoth, S.A.; Dignum, M.; Grefta, A.; Kroesbergen, J. Characterization of NOM in a drinking water treatment process train with no disinfectant residual. Water Sci. Technol. Water Supply 2009, 9, 379–386. [Google Scholar] [CrossRef]
- Johnstone, D.W.; Sanchez, N.P.; Miller, C.M. Parallel Factor Analysis of Excitation-Emission Matrices to Assess Drinking Water Disinfection Byproduct Formation during a Peak Formation Period. Environ. Eng. Sci. 2009, 26, 1551–1559. [Google Scholar] [CrossRef]
- Pifer, A.D.; Fairey, J.L. Improving UV254 using fluorescence-PARAFAC analysisand asymmetric flow-field flow fractionation for assessing disinfection byproduct formation and control. Water Res. 2012, 46, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.W.; Miller, C.M. Fluorescence Excitation–Emission Matrix Regional Transformation and Chlorine Consumption to Predict Trihalomethane and Haloacetic Acid Formation. Environ. Eng. Sci. 2009, 26, 1163–1170. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Yue, Q.; Ren, H. Effect of second coagulant addition on coagulation efficiency, floc particles and residual Al for humic acid treatment by Al13 polymer and polyaluminum chloride (PACl). J. Hazard. Mater. 2012, 215–216, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Duan, J. Hydrolyzing metal salts as coagulants. Pure Appl. Chem. 2001, 73, 2017–2026. [Google Scholar] [CrossRef]
- Bell-Ajy, K.; Abbaszadegan, M.; Ibrahim, E.; Verges, D.; LeChevallier, M. Conventional and optimized coagulation for NOM removal. J. Am. Water Works Assoc. 2000, 92, 44–58. [Google Scholar]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, B.A.; Sheu, H.; Ahmed, T.M.T.; Mentick, J. Polyaluminum chloride an alum coagulation of clay-fulvic acid suspensions. J. Am. Water Works Assoc. 1985, 77, 74–80. [Google Scholar]
- Van Benschoten, J.E.; Edzwald, J.K. Chemical aspects of coagulation using aluminum salts—I, Hydrolytic reactions of alum and polyaluminum chloride. Water Res. 1990, 24, 1519–1526. [Google Scholar] [CrossRef]
- Edwards, M. Predicting DOC removal during enhanced coagulation. J. Am. Water Works Assoc. 1997, 89, 78–89. [Google Scholar]
- Sanchez, N.P.; Skeriotis, A.T.; Miller, C.M. Assessment of Dissolved Organic Matter Fluorescence PARAFAC components before and after coagulation-filtration in a full scale water treatment plant. Water Res. 2013, 47, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-liquid Extraction, Derivatization and Gas Chromatography with Electron Capture Detection; U.S. EPA Method 552.2; National Exposure Research Laboratory Office of Research and Development U.S. EPA: Cincinnati, OH, USA, 1995.
- Xie, Y.; Reckhow, D.A.; Springborg, D.C. Analyzing HAAs and ketoacids without diazomethane. J. Am. Water Works Assoc. 1998, 90, 131–138. [Google Scholar]
- U.S. EPA. Determination of Chlorinated Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-liquid Extraction and Gas Chromatography with Electron-Capture Detection; U.S. EPA Method 551.1; National Exposure Research Laboratory Office of Research and Development U.S. EPA: Cincinnati, OH, USA, 1995.
- Anderson, C.M.; Bro, R. Practical aspects of PARAFAC modeling of fluorescence excitation emission data. J. Chemom. 2003, 17, 200–215. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanoue, E. Chemical characterization of protein-like fluorescence in DOM in relation to aromatic amino acids. Mar. Chem. 2003, 82, 255–271. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Rinnan, Å.; Barsberg, S.; Møller, J.K.S. Stabilizing the PARAFAC decomposition of fluorescence spectra by insertion of zeroes outside the data area. Chemom. Intell. Lab. Syst. 2004, 71, 97–106. [Google Scholar] [CrossRef]
- Harshman, R.A.; Lundy, M.E. The PARAFAC Model for Three-Way Factor Analysis and Multidimensional; Law, H.G., Snyder, C.W., Hattie, J.A., McDonald, R.P., Eds.; Research Methods for Multimode Data Analysis, Praeger: New York, NY, USA, 1984. [Google Scholar]
- Tucker, L. Some mathematical notes on three-mode factor analysis. Psychometrika 1966, 31, 279–311. [Google Scholar] [CrossRef] [PubMed]
- Bro, R.; Kiers, H.A. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Ged, E.C.; Chadik, P.A.; Boyer, T.H. Predictive capability of chlorination disinfection byproducts models. J. Environ. Manag. 2014, 149, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.P.; Skeriotis, A.T.; Miller, C.M. A PARAFAC-based Long-Term Assessment of DOM in a Multi-Coagulant Drinking Water Treatment Scheme. Environ. Sci. Technol. 2015, 48, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Beggs, K.; Summers, S.; McKnight, D. Characterizing chlorine oxidation of dissolved organic matter and disinfection by-product formation with fluorescence spectroscopy and parallel factor analysis. J. Geophys. Res. 2009, 114, G04001. [Google Scholar] [CrossRef]
- Peleto, N.M.; Armour, J.; Andrews, A.C. Statistical significance testing of parallel pilot-scale coagulation optimization study to compare aluminum sulfate and polyaluminum chloride performance. J. Water Supply Res. Technol. AQUA 2014, 63, 532–540. [Google Scholar] [CrossRef]
- Liang, L.; Singer, P.C. Factors Influencing the Formation of and Relative Distribution of Haloacetic Acids and Trihalomethanes in Drinking Water. Environ. Sci. Technol. 2003, 37, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
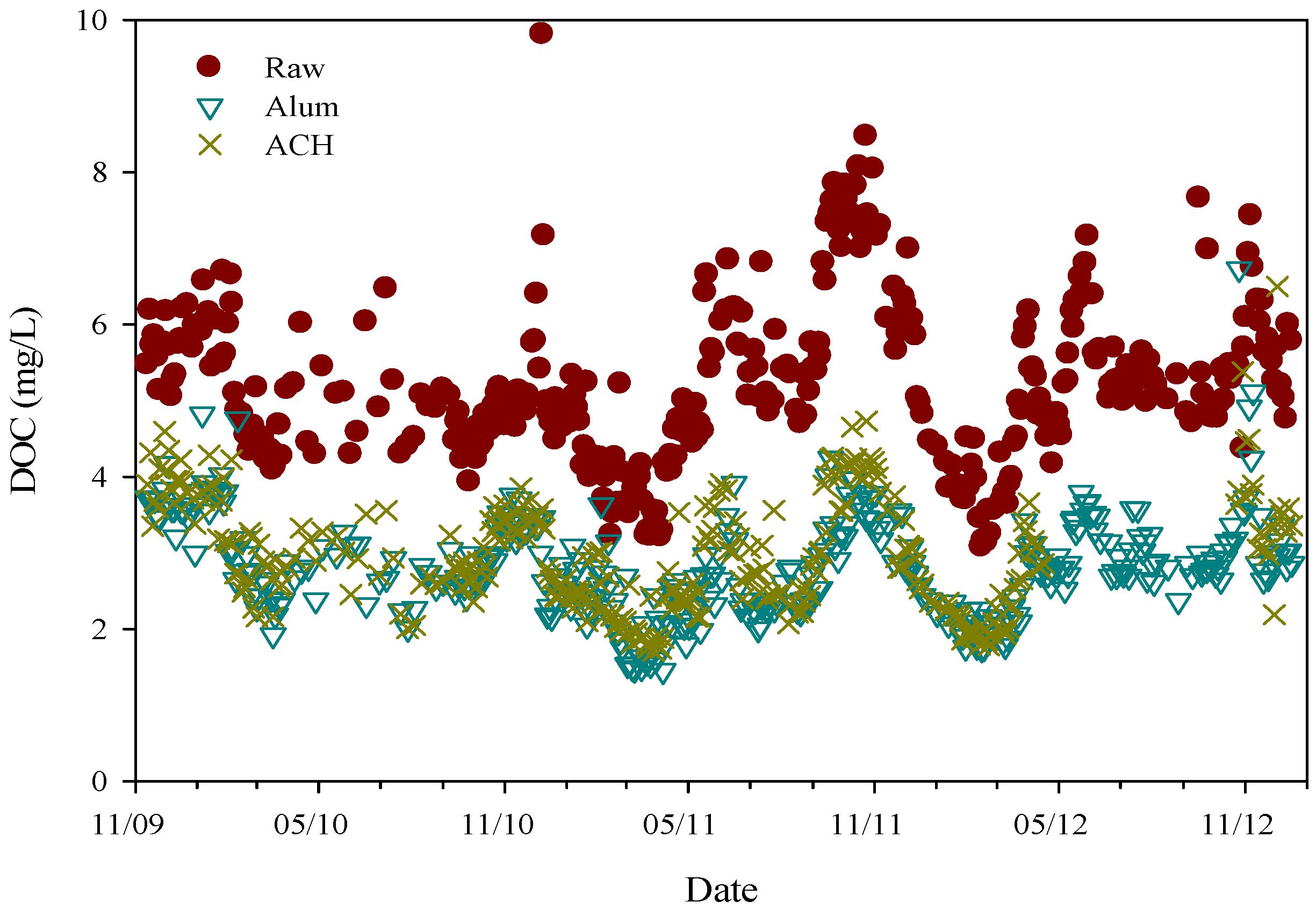

| Parameter | DOC (mg/L) | SUVA (L/mg-m) | pH | Alkalinity (mg/L as CaCO3) | Turbidity (NTU) |
|---|---|---|---|---|---|
| Mean | 5.38 ± 1.28 | 3.05 ± 0.54 | 7.65 ± 0.35 | 110 ± 24 | 7.92 ± 3.97 |
| Median | 5.03 | 3.02 | 7.68 | 110 | 7.43 |
| Minimum | 3.14 | 1.67 | 6.64 | 64 | 1.90 |
| Maximum | 9.81 | 4.13 | 8.32 | 160 | 29.4 |
| Water | n | DOC (mg/L) | pH | UV254 (cm−1) |
|---|---|---|---|---|
| Raw | 361 | 5.38 ± 1.28 | 7.65 ± 0.35 | 0.149 ± 0.037 |
| Alum | 361 | 2.83 ± 0.66 | 6.99 ± 0.30 | 0.044 ± 0.009 |
| ACH | 299 | 2.97 ± 0.74 | 7.50 ± 0.29 | 0.051 ± 0.014 |
| Water | THM(SD) (ug/L) | THM/DOC(SD) (ug/L) | HAA(SD) (ug/L) | HAA/DOC(SD) (ug/L) |
|---|---|---|---|---|
| Raw | 260(92) | 48(13) | 219(63) | 41(12) |
| Alum | 69(30) | 24(9) | 76(39) | 27(11) |
| ACH | 107(43) | 36(12) | 89(38) | 30(13) |
| Metric | Raw Mean | Alum Mean | ACH Mean | % > Alum | p-Value (t-Test) |
|---|---|---|---|---|---|
| PARAFAC C1 Fmax (R.U.) | 0.501 | 0.277 | 0.335 | 21% | 0.000 |
| PARAFAC C2 Fmax (R.U.) | 0.495 | 0.166 | 0.191 | 15% | 0.000 |
| PARAFAC C3 Fmax (R.U.) | 0.290 | 0.170 | 0.210 | 24% | 0.000 |
| OFI (R.U.) | 969 | 466 | 546 | 17% | 0.000 |
| DOC (mg/L) | 5.24 | 2.83 | 2.97 | 5% | 0.010 |
| UV254 (cm−1) | 0.150 | 0.044 | 0.051 | 17% | 0.000 |
| DBP | Test | All | ||
|---|---|---|---|---|
| – | R | MSE | R | MSE |
| THM | 0.88 | 2289 | 0.91 | 1747 |
| HAA | 0.92 | 1043 | 0.91 | 1105 |
| Parameter | THM (ug/L) | HAA (ug/L) | ||
|---|---|---|---|---|
| – | Alum | ACH | Alum | ACH |
| Minimum | 0 | 0 | 34 | 11 |
| Maximum | 231 | 320 | 527 | 653 |
| Median | 77 | 87 | 81 | 97 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skeriotis, A.T.; Sanchez, N.P.; Kennedy, M.; Johnstone, D.W.; Miller, C.M. Long-Term Comparison of Disinfection By-Product Formation Potential in a Full Scale Treatment Plant Utilizing a Multi-Coagulant Drinking Water Treatment Scheme. Water 2016, 8, 318. https://doi.org/10.3390/w8080318
Skeriotis AT, Sanchez NP, Kennedy M, Johnstone DW, Miller CM. Long-Term Comparison of Disinfection By-Product Formation Potential in a Full Scale Treatment Plant Utilizing a Multi-Coagulant Drinking Water Treatment Scheme. Water. 2016; 8(8):318. https://doi.org/10.3390/w8080318
Chicago/Turabian StyleSkeriotis, Andrew T., Nancy P. Sanchez, Marla Kennedy, David W. Johnstone, and Christopher M. Miller. 2016. "Long-Term Comparison of Disinfection By-Product Formation Potential in a Full Scale Treatment Plant Utilizing a Multi-Coagulant Drinking Water Treatment Scheme" Water 8, no. 8: 318. https://doi.org/10.3390/w8080318
APA StyleSkeriotis, A. T., Sanchez, N. P., Kennedy, M., Johnstone, D. W., & Miller, C. M. (2016). Long-Term Comparison of Disinfection By-Product Formation Potential in a Full Scale Treatment Plant Utilizing a Multi-Coagulant Drinking Water Treatment Scheme. Water, 8(8), 318. https://doi.org/10.3390/w8080318





