Genetic Basis for Geosmin Production by the Water Bloom-Forming Cyanobacterium, Anabaena ucrainica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Cyanobacterial Strains
2.2. Analysis of Geosmin
2.3. General Molecular Techniques and Genome Walking
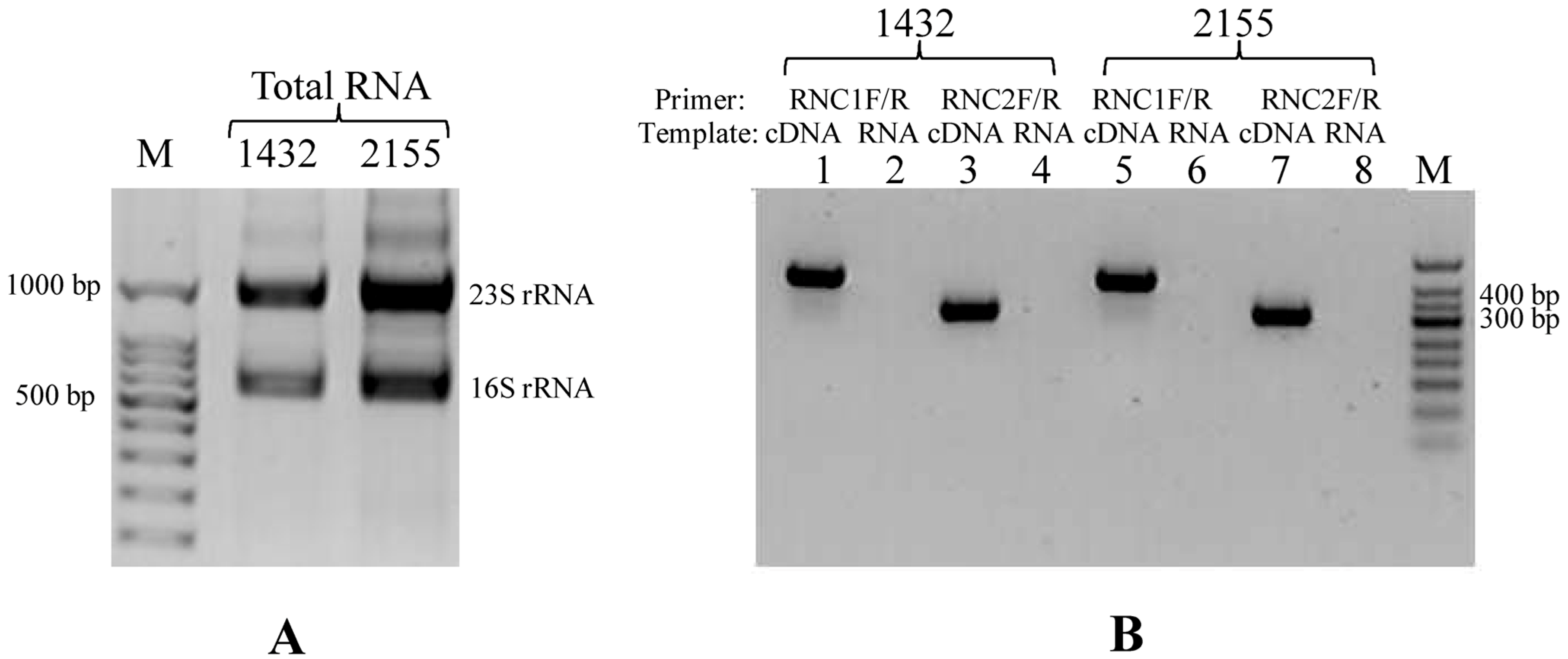
2.4. RNA Extraction and Reverse Transcriptional PCR
2.5. Bioinformatics Analysis
3. Results and Discussion
3.1. Isolation of a Bloom-Forming Cyanobacterium Responsible for Geosmin Production
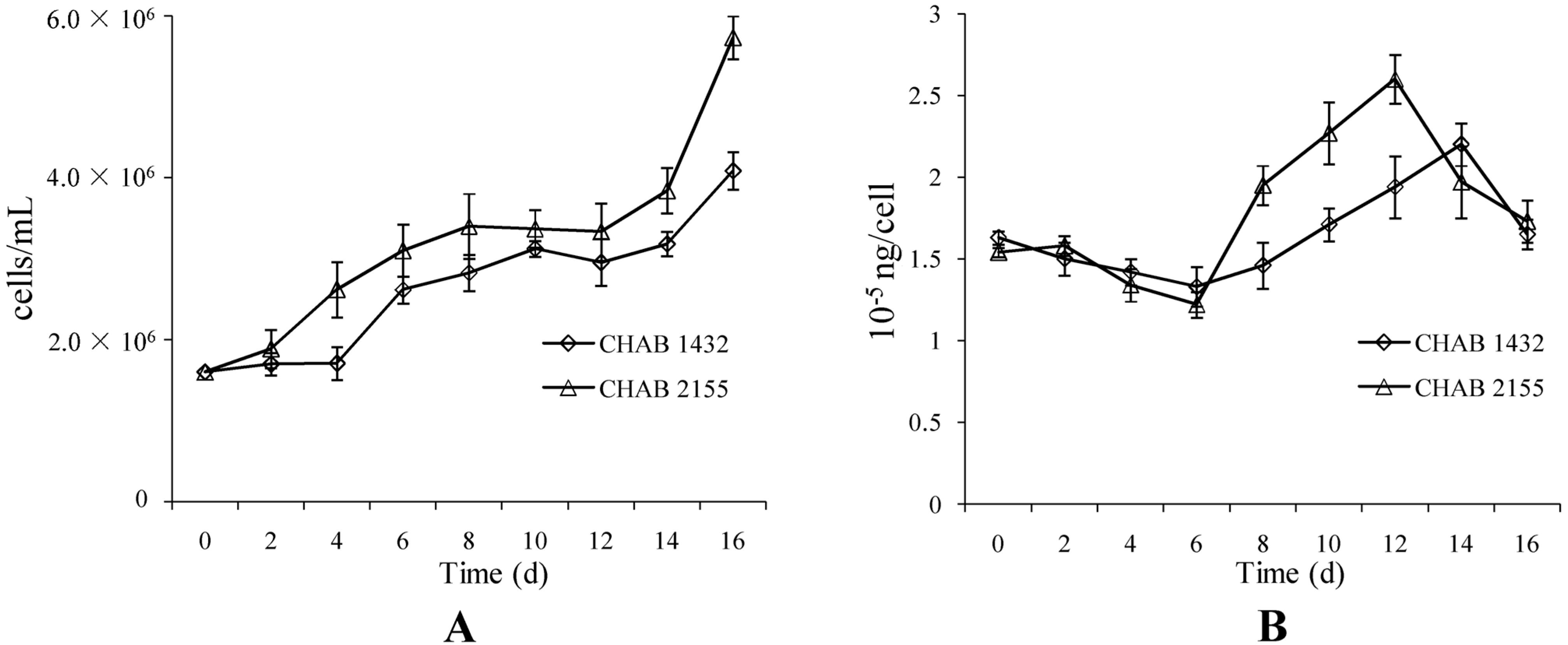
3.2. Identification and Characterization of the Geosmin Synthase Genes of A. ucrainica
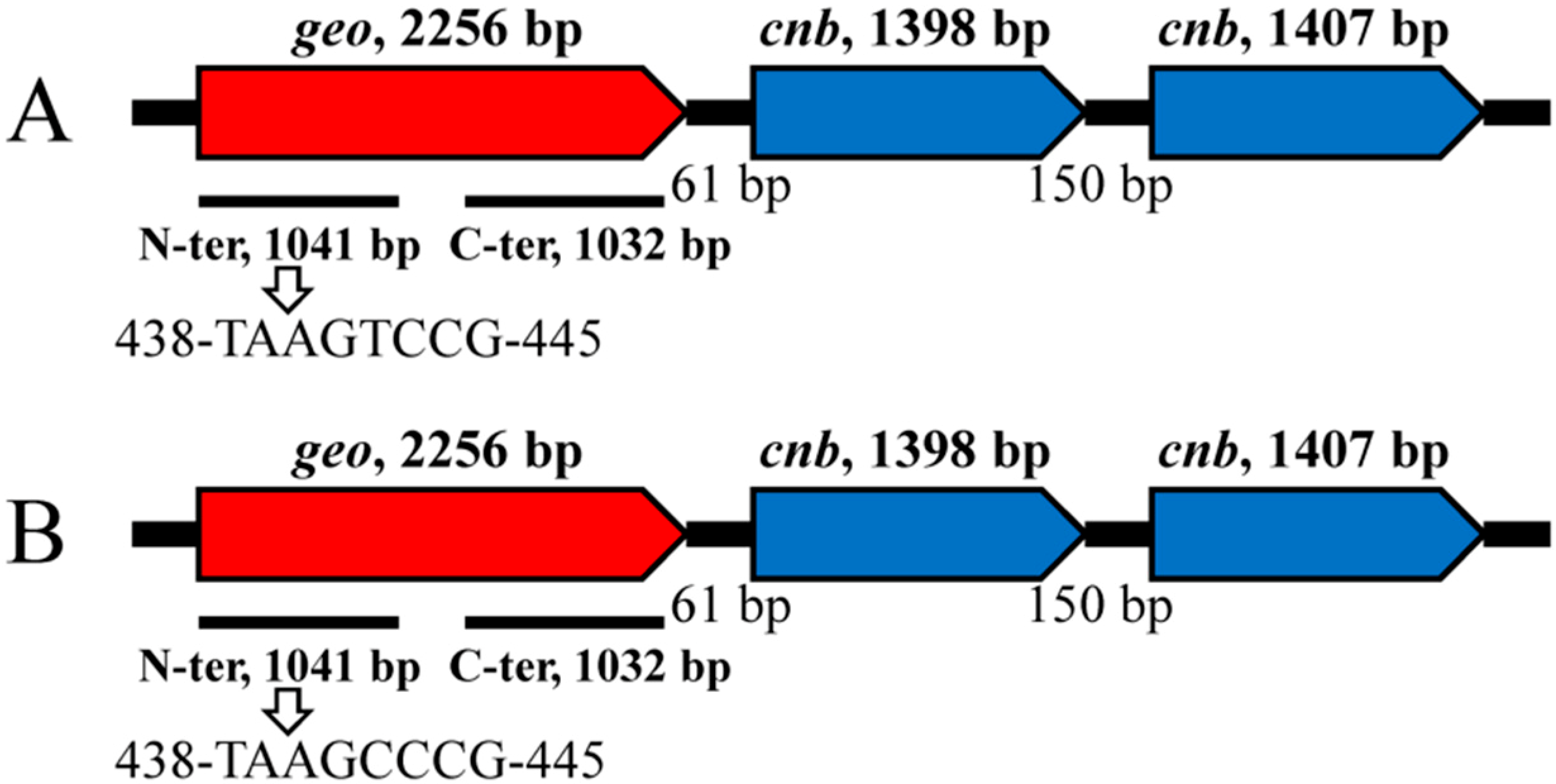
3.3. Structure and Features of the Geosmin Operon

| Species | Taxonomy | Primers GTC1F/R | Primers GTC2F/R | Homology | Organization of genes |
|---|---|---|---|---|---|
| Anabaena sp. 3585 | Nostocales | + a/− b | +/− | + c | geo/cnb/cnb |
| Calothrix sp. 2384 | Nostocales | +/− | +/− | + | geo/cnb/cnb |
| Nostoc sp. 261 | Nostocales | +/− | +/− | + | geo/cnb/cnb |
| Aphanizomenon gracile 2417 | Nostocales | +/− | +/− | + | geo/cnb/cnb |
| Phormidium sp. D6 | Oscillatoriales | +/− | +/− | + | geo/cnb/cnb |
| Lyngbya kuetzingii 388 | Oscillatoriales | +/− | +/− | + | geo/cnb/cnb |
| Tychonema bourrellyi 663 | Oscillatoriales | +/− | +/− | + | geo/cnb/cnb |
3.4. Analysis of Geosmin-Associated Genes: Origin and Evolution
3.4.1. Inconsistent Topologies between Geosmin Gene and 16S rDNA Phylogenetic Trees
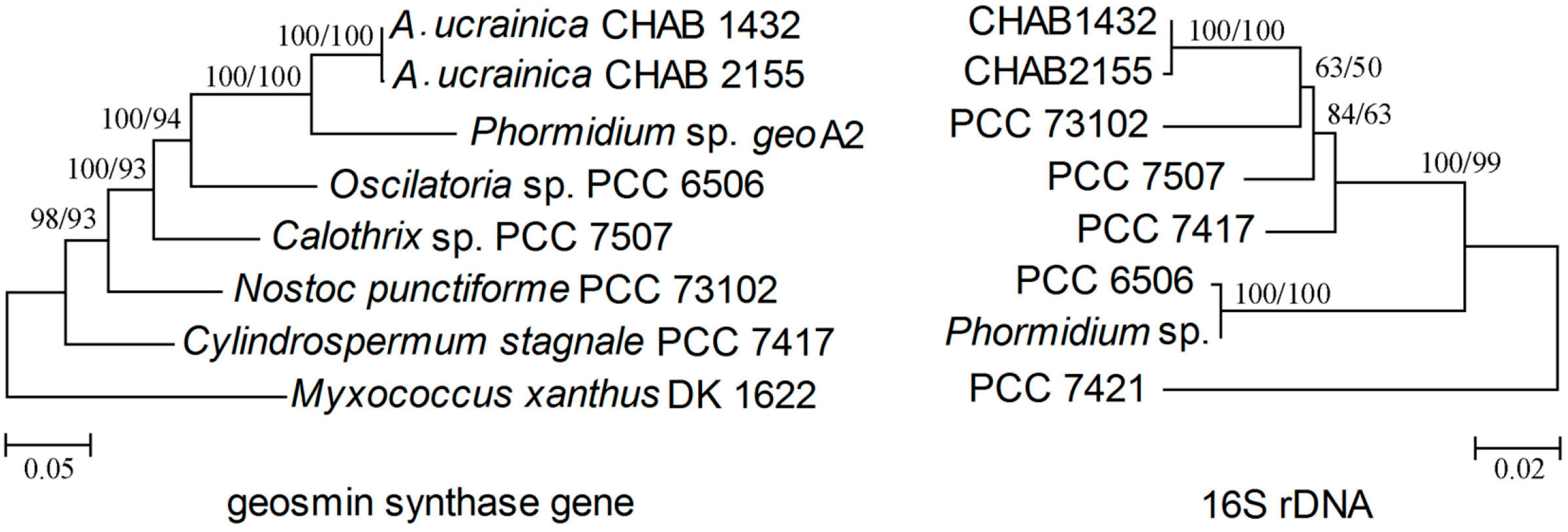
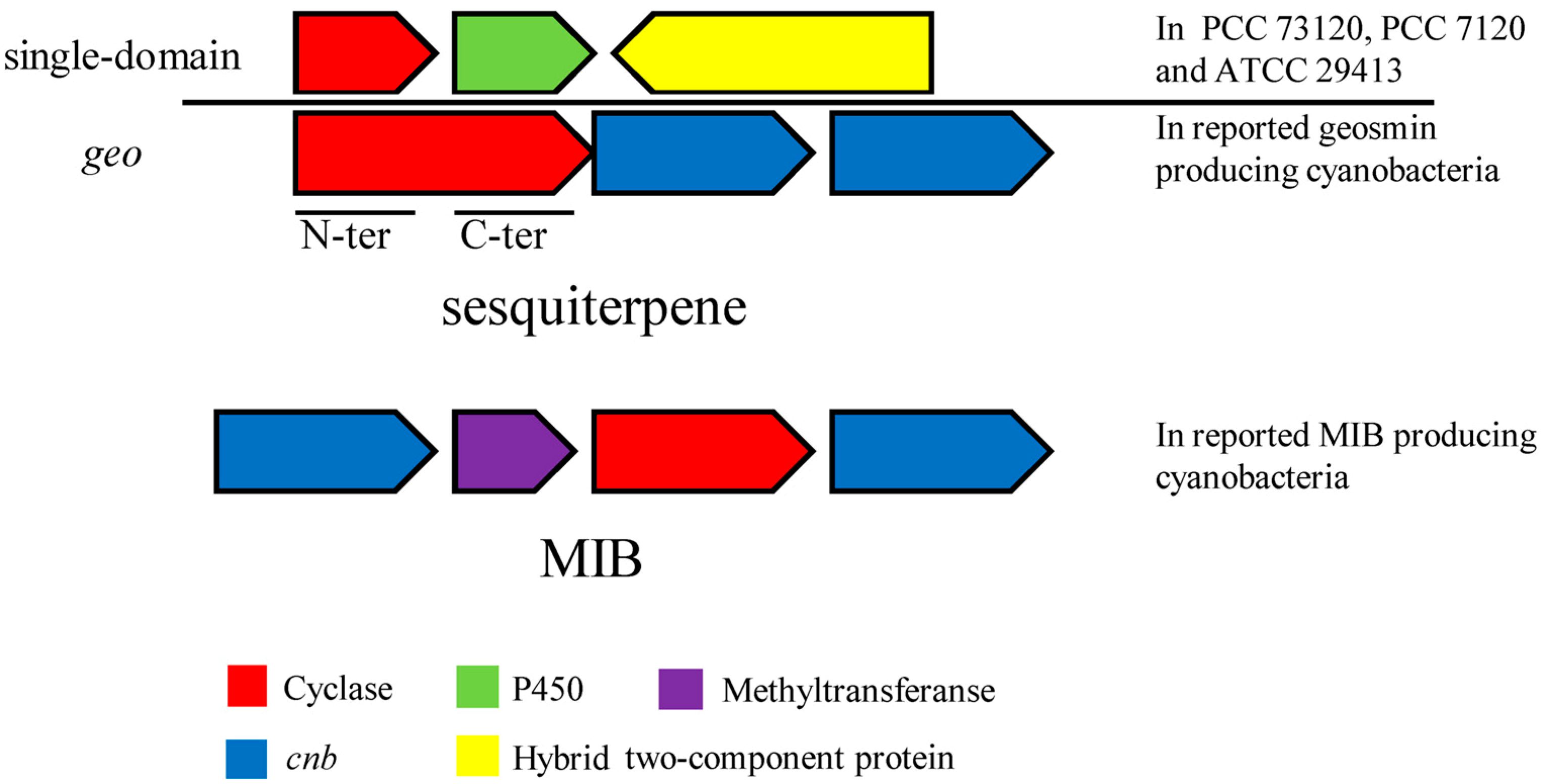
3.4.2. Comparison of geo, Single-Domain Sesquiterpene Gene, MIB Synthesis Gene and Their Related Gene Clusters in Cyanobacteria
| Sequences | A | B | C | D | E |
|---|---|---|---|---|---|
| A | - | - | - | - | - |
| B | 0.773 | - | - | - | - |
| C | 0.874 | 0.791 | - | - | - |
| D | 0.771 | 0.885 | 0.803 | - | - |
| E | 0.615 | 0.6F | 0.604 | 0.606 | - |
| F | 0.628 | 0.612 | 0.605 | 0.601 | 0.73 |
4. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Graham, J.L.; Loetin, K.A.; Meyer, M.T.; Ziegler, A.A. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacteria blooms from the midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G.; Taylor, W.D. A guide to geosmin- and MIB-producing cyanobacteria in the United States. Water Sci. Technol. 2004, 49, 19–24. [Google Scholar] [PubMed]
- Li, L.; Wan, N.; Gan, N.; Xiao, B.D.; Song, L.R. Annual dynamics and origins of the odorous compounds in the pilot experimental area of Lake Dianchi, China. Water Sci. Technol. 2007, 55, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Gerber, N.N.; Lecheval, H.A. Geosmin an earthy-smelling substance isolated from actinomycetes. Appl. Microbiol. 1965, 13, 935–938. [Google Scholar] [PubMed]
- Watson, S.B.; Brownlee, B.; Satchwill, T.; Hargesheimer, E.E. Quantitative analysis of trace levels of geosmin and MIB in source and drinking water using headspace SPME. Water Res. 2000, 34, 2818–2828. [Google Scholar] [CrossRef]
- Jüttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Trowitzsch, W.; Witte, L.; Reichenbach, H. . Geosmin from earthy smelling cultures of Nannocystis exedens (Myxobacterales). FEMS Microbiol. Lett 1981, 12, 257–260. [Google Scholar]
- Cane, D.E.; Watt, R.M. Expression and mechanistic analysis of a germacradienol synthase from Streptomyces coelicolor implicated in geosmin biosynthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, X.; Cane, D.E. Biosynthesis of the earthy odorant geosmin by a bifunctional Streptomyces coelicolor enzyme. Nat. Chem. Biol. 2007, 3, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, F.; Medger, A.; Börnick, H.; Opitz, M.; Lang, K.; Göttfert, M.; Röskel, I. Identification and expression analyses of putative sesquiterpene synthase genes in Phormidium sp. and prevalence of geoA-like genes in a drinking water reservoir. Appl. Environ. Microbiol. 2007, 73, 6988–6993. [Google Scholar] [CrossRef] [PubMed]
- Agger, S.A.; Lopez-Gallego, F.; Hoye, T.R.; Schmidt-Dannert, C. Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC 7120. J. Bacteriol. 2008, 190, 6084–6096. [Google Scholar] [CrossRef] [PubMed]
- Giglio, S.; Jiang, J.Y.; Saint, C.P.; Cane, D.E.; Monis, P.T. Isolation and characterization of the gene associated with geosmin production in cyanobacteria. Environ. Sci. Technol. 2008, 42, 8027–8032. [Google Scholar] [CrossRef]
- Giglio, S.; Saint, C.P.; Monis, P.T. Expression of the geosmin synthase gene in the cyanobacterium Anabaena circinalis AWQC318. J. Phycol. 2011, 47, 1338–1343. [Google Scholar] [CrossRef]
- Guo, L. Ecology doing battle with the green monster of Taihu Lake. Science 2007, 317, 1166. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.M.; Hiroki, M. NIES-Collection List of Strains: Algae and Protozoa; National Institute for Environmental Studies, Environment Agency: Tsukuba, Japan, 1997; p. 140. [Google Scholar]
- National Library of Medicine. Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 7 May 2014).
- Marchler-Bauer, A.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; Deweese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; et al. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009, 37, 205–210. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; . Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Su, M.; Gaget, V.; Giglio, S.; Burch, M.; An, W.; Yang, M. Establishment of quantitative PCR methods for the quantification of geosmin-producing potential and Anabaena sp. in freshwater systems. Water Res. 2013, 47, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Okubo, T. Development of Anabaena blooms in a small reservoir with dense sediment akinete population, with special reference to temperature and irradiance. J. Plankton Res. 2003, 25, 1059–1067. [Google Scholar] [CrossRef]
- Watson, S.B. Aquatic taste and odor: A primary signal of drinking-water integrity. J. Toxicol. Environ. Health A 2004, 67, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.W.; Michinaka, A.; Yen, H.K.; Giglio, S.; Hobson, P.; Monis, P.; Lin, T.F. Monitoring of geosmin producing Anabaena circinalis using quantitative PCR. Water Res. 2014, 49, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Giglio, S.; Chou, W.K.W.; Ikeda, H.; Cane, D.E.; Monis, P.T. Biosynthesis of 2-methylisoborneol in cyanobacteria. Environ. Sci. Technol. 2011, 45, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xu, Y.; Shao, J.H.; Wang, J.; Li, R.H. Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: Isolation, characterization, and expression in response to light. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Körner, H.; Sofia, H.J.; Zumft, W.G. Phylogeny of the bacterial superfamily of Crp–Fnr transcription regulators: Exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 2003, 27, 559–592. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, R.; Michali, T.K.; Neilan, B.A. Identification of a saxitoxin biosynthesis gene with a history of frequent horizontal gene transfers. J. Mol. Evol. 2008, 67, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Leikoski, N.; Fewer, D.P.; Sivonen, K. Widespread occurrence and lateral transfer of the cyanobactin biosynthesis gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2009, 75, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, D.; Wang, G.; Song, L.; Li, L. Identification and expression analysis of the gene associated with geosmin production in Lyngbya kuetzingii UTEX 1547 (cyanobacteria). Harmful Algae 2014, 39, 127–133. [Google Scholar] [CrossRef]
- Agger, S.; Lopez-Gallego, F.; Schmidt-Dannert, C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbio. 2009, 72, 1181–1195. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shao, J.; Xu, Y.; Yan, B.; Li, R. Genetic Basis for Geosmin Production by the Water Bloom-Forming Cyanobacterium, Anabaena ucrainica. Water 2015, 7, 175-187. https://doi.org/10.3390/w7010175
Wang Z, Shao J, Xu Y, Yan B, Li R. Genetic Basis for Geosmin Production by the Water Bloom-Forming Cyanobacterium, Anabaena ucrainica. Water. 2015; 7(1):175-187. https://doi.org/10.3390/w7010175
Chicago/Turabian StyleWang, Zhongjie, Jihai Shao, Yao Xu, Biao Yan, and Renhui Li. 2015. "Genetic Basis for Geosmin Production by the Water Bloom-Forming Cyanobacterium, Anabaena ucrainica" Water 7, no. 1: 175-187. https://doi.org/10.3390/w7010175
APA StyleWang, Z., Shao, J., Xu, Y., Yan, B., & Li, R. (2015). Genetic Basis for Geosmin Production by the Water Bloom-Forming Cyanobacterium, Anabaena ucrainica. Water, 7(1), 175-187. https://doi.org/10.3390/w7010175






