Effect of Selected Plant Extracts and D- and L-Lysine on the Cyanobacterium Microcystis aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Test Compounds
2.3. Preparation Extracts
2.4. Dose-Range Finding Experiment
2.5. Bioassay
| F. mume (mg L−1) | S. miltiorrhiza (mg L−1) | M. oleifera (mg L−1) | D- and L-lysine (mg L−1) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 30 | 40 | 4 | 0.5 |
| 60 | 80 | 8 | 1.4 |
| 120 | 160 | 16 | 4.3 |
| 240 | 320 | 32 | 13 |
| 480 | 640 | 64 | 38.9 |
2.6. Data Analysis
 . In addition, growth rates were also calculated similarly based on changes in biovolume concentrations. For each compound the estimated growth rates (µ) at different concentrations were compared by a one-way ANOVA or when prerequisites for parametric testing were not met by Kruskal-Wallis One Way Analysis of Variance on Ranks. As some products caused a decline in population growth yielding negative growth rates, finite rates of increase of the population (λ) were calculated (λ = eµ) based on which EC50 values were determined by four parameter logistic regression in the tool pack SigmaPlot version 12.5. The effect of each extract/compound on ΦPSII was tested by running repeated measure ANOVAs in the statistical program IBM SPSS version 19 (IBM Corporation, Armonk, NY, USA).
. In addition, growth rates were also calculated similarly based on changes in biovolume concentrations. For each compound the estimated growth rates (µ) at different concentrations were compared by a one-way ANOVA or when prerequisites for parametric testing were not met by Kruskal-Wallis One Way Analysis of Variance on Ranks. As some products caused a decline in population growth yielding negative growth rates, finite rates of increase of the population (λ) were calculated (λ = eµ) based on which EC50 values were determined by four parameter logistic regression in the tool pack SigmaPlot version 12.5. The effect of each extract/compound on ΦPSII was tested by running repeated measure ANOVAs in the statistical program IBM SPSS version 19 (IBM Corporation, Armonk, NY, USA).3. Results
3.1. Range Finding Assay
3.2. Bioassay
| Growth Rate (d−1) | ||||
|---|---|---|---|---|
| Concentration | l-lysine | Concentration | F. mume | S. miltiorrhiza |
| 0 | 0.67 (0.03) a | 0 | 0.46 (0.05) α | 0.46 (0.05) α |
| 1 | 0.52 (0.02) b | 3 | 0.45 (0.02) α | 0.40 (0.05) α |
| 5 | 0.21 (0.02) c | 300 | −0.35 (0.04) β | 0.05 (0.04) γ |
| 10 | 0.17 (0.01) c | Extract | F1,17 = 37.3 | p < 0.001 |
| 40 | 0.16 (0.04) c | Concentration | F2,17 = 428.9 | p < 0.001 |
| F4,14 = 282.3 p < 0.001 | Interaction | F2,17 = 56.7 | p < 0.001 | |
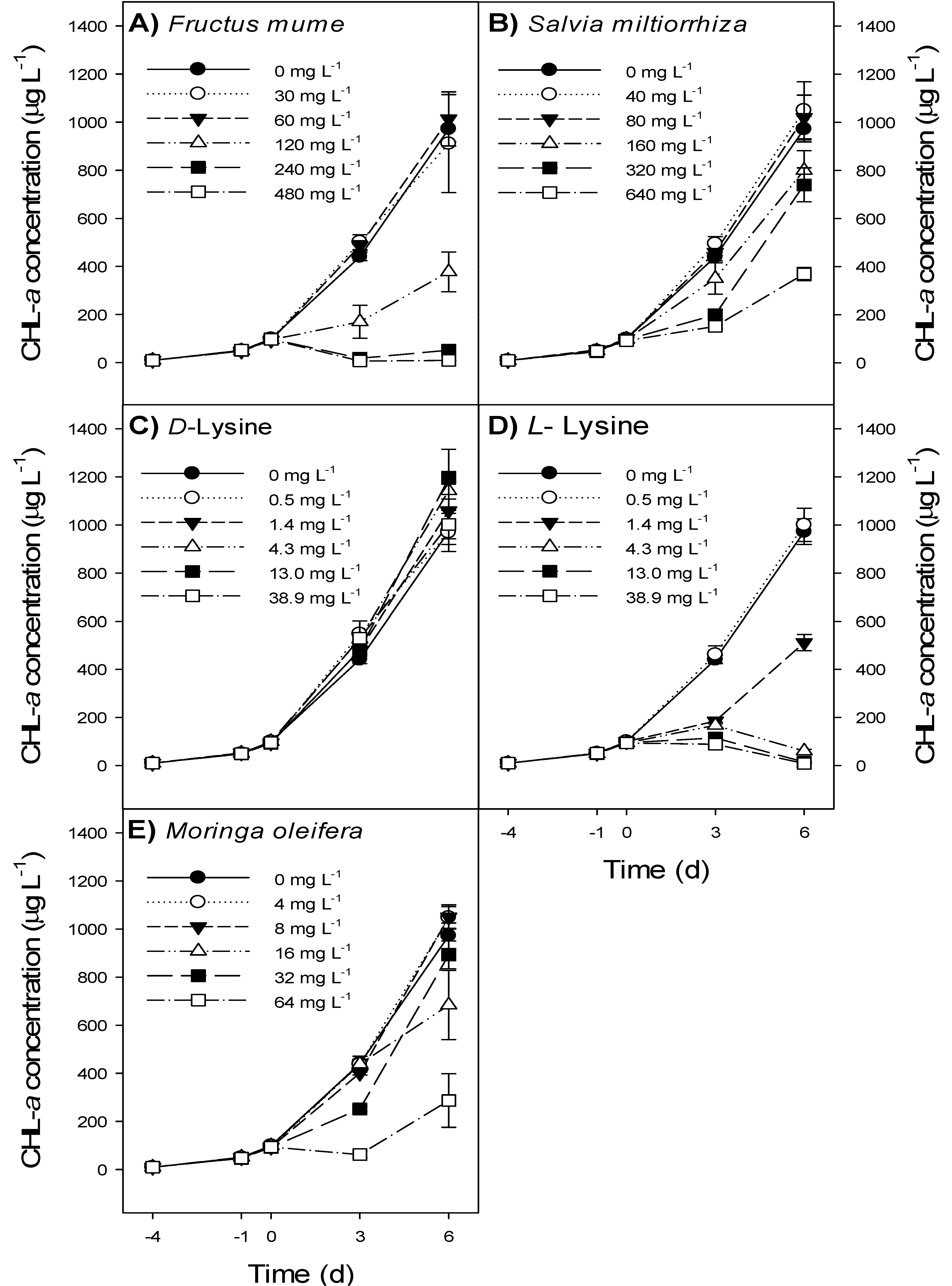
| F. mume | S. miltiorrhiza | d-Lysine | l-Lysine | M. oleifera | |||||
|---|---|---|---|---|---|---|---|---|---|
| [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) |
| 0 | 0.47 (0.01) a | 0 | 0.47 (0.01) a | 0 | 0.47 (0.01) a | 0 | 0.47 (0.01) a | 0 | 0.47 (0.01) a |
| 30 | 0.45 (0.08) a | 40 | 0.48 (0.02) a | 0.5 | 0.48 (0.01) a | 0.5 | 0.48 (0.01) a | 4 | 0.48 (0.01) a |
| 60 | 0.48 (0.03) a | 80 | 0.48 (0.02) a | 1.4 | 0.48 (0.01) a | 1.4 | 0.38 (0.01) ab | 8 | 0.48 (0.01) a |
| 120 | 0.33 (0.05) ab | 160 | 0.44 (0.02) b | 4.3 | 0.49 (0.01) a | 4.3 | −0.02 (0.01) b | 16 | 0.43 (0.07) ab |
| 240 | −0.04 (0.03) ab | 320 | 0.42 (0.02) b | 13 | 0.49 (0.02) a | 13 | −0.09 (0.02) b | 32 | 0.45 (0.02) ab |
| 480 | −0.12 (0.01) b | 640 | 0.35 (0.02) c | 38.9 | 0.48 (0.02) a | 38.9 | −0.13 (0.02) b | 64 | 0.26 (0.11) b |
| H5 = 26.6 p < 0.001 | F5,34 = 40.6 p < 0.001 | F5,34 = 1.94 p = 0.118 | H5 = 31.0 p < 0.001 | H5 = 20.7 p < 0.001 | |||||
| EC50 (mg L−1) | |||||||||
| 148 | >640 | >38.9 | 2.2 | >64 | |||||
| r2adj | |||||||||
| 0.994 | --- | --- | 0.998 | --- | |||||
| F. mume | S. miltiorrhiza | d-Lysine | l-Lysine | M. oleifera | |||||
|---|---|---|---|---|---|---|---|---|---|
| [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) | [C] | growth (d-1) |
| 0 | 0.31 (0.05) a | 0 | 0.31 (0.05) a | 0 | 0.31 (0.05) a | 0 | 0.31 (0.05) a | 0 | 0.31 (0.05) a |
| 30 | 0.28 (0.04) a | 40 | 0.30 (0.00) a | 0.5 | 0.30 (0.00) a | 0.5 | 0.30 (0.00) a | 4 | 0.31 (0.02) a |
| 60 | 0.31 (0.07) a | 80 | 0.30 (0.04) a | 1.4 | 0.30 (0.04) a | 1.4 | 0.30 (0.04) a | 8 | 0.31 (0.05) a |
| 120 | 0.25 (0.06) a | 160 | 0.31 (0.04) a | 4.3 | 0.31 (0.04) a | 4.3 | 0.31 (0.04) a | 16 | 0.30 (0.04) a |
| 240 | 0.00 (0.01) b | 320 | 0.31 (0.07) a | 13 | 0.31 (0.07) a | 13 | 0.31 (0.07) a | 32 | 0.29 (0.07) a |
| 480 | 0.00 (0.01) b | 640 | 0.23 (0.10) a | 38.9 | 0.23 (0.10) a | 38.9 | 0.23 (0.10) a | 64 | 0.23 (0.05) a |
| F5,34 = 51.6 p < 0.001 | F5,34 = 1.57 p = 0.201 | F5,34 = 1.57 p = 0.201 | F5,34 = 1.57 p = 0.201 | F5,34 = 2.00 p = 0.109 | |||||
| EC50 (mg L−1) | |||||||||
| 128 | >640 | >38.9 | 1.7 | >64 | |||||
| r2adj | |||||||||
| 0.985 | --- | --- | 0.991 | --- | |||||

| F. mume | S. miltiorrhiza | |||||
|---|---|---|---|---|---|---|
| Tests of Within-Subjects Effects | ||||||
| Source | df | F | P | df | F | P |
| Time | 3.79 | 84.4 | <0.001 | 4 | 134.4 | <0.001 |
| Time × concentration | 18.9 | 8.13 | <0.001 | 20 | 4.52 | <0.001 |
| Error | 53.1 | 60 | ||||
| Tests of between-Subjects Effects | ||||||
| Source | df | F | P | df | F | P |
| Concentration | 5 | 9.91 | <0.001 | 5 | 6.24 | 0.003 |
| Error | 15 | 15 | ||||
| D-lysine | L-lysine | |||||
| Tests of Within-Subjects Effects | ||||||
| Source | df | F | P | df | F | P |
| Time | 3.82 | 193.6 | <0.001 | 4 | 183.1 | <0.001 |
| Time × concentration | 19.1 | 2.29 | 0.008 | 20 | 26.2 | <0.001 |
| Error | 57.3 | 60 | ||||
| Tests of between-Subjects Effects | ||||||
| Source | df | F | P | df | F | P |
| Concentration | 5 | 1.06 | 0.422 | 5 | 21.8 | <0.001 |
| Error | 15 | 15 | ||||
| M. oleifera | ||||||
| Tests of Within-Subjects Effects | ||||||
| Source | df | F | P | |||
| Time | 4 | 123.7 | <0.001 | |||
| Time × concentration | 20 | 3.48 | <0.001 | |||
| Error | 60 | |||||
| Tests of between-Subjects Effects | ||||||
| Source | df | F | P | |||
| Concentration | 5 | 2.66 | 0.065 | |||
| Error | 15 | |||||
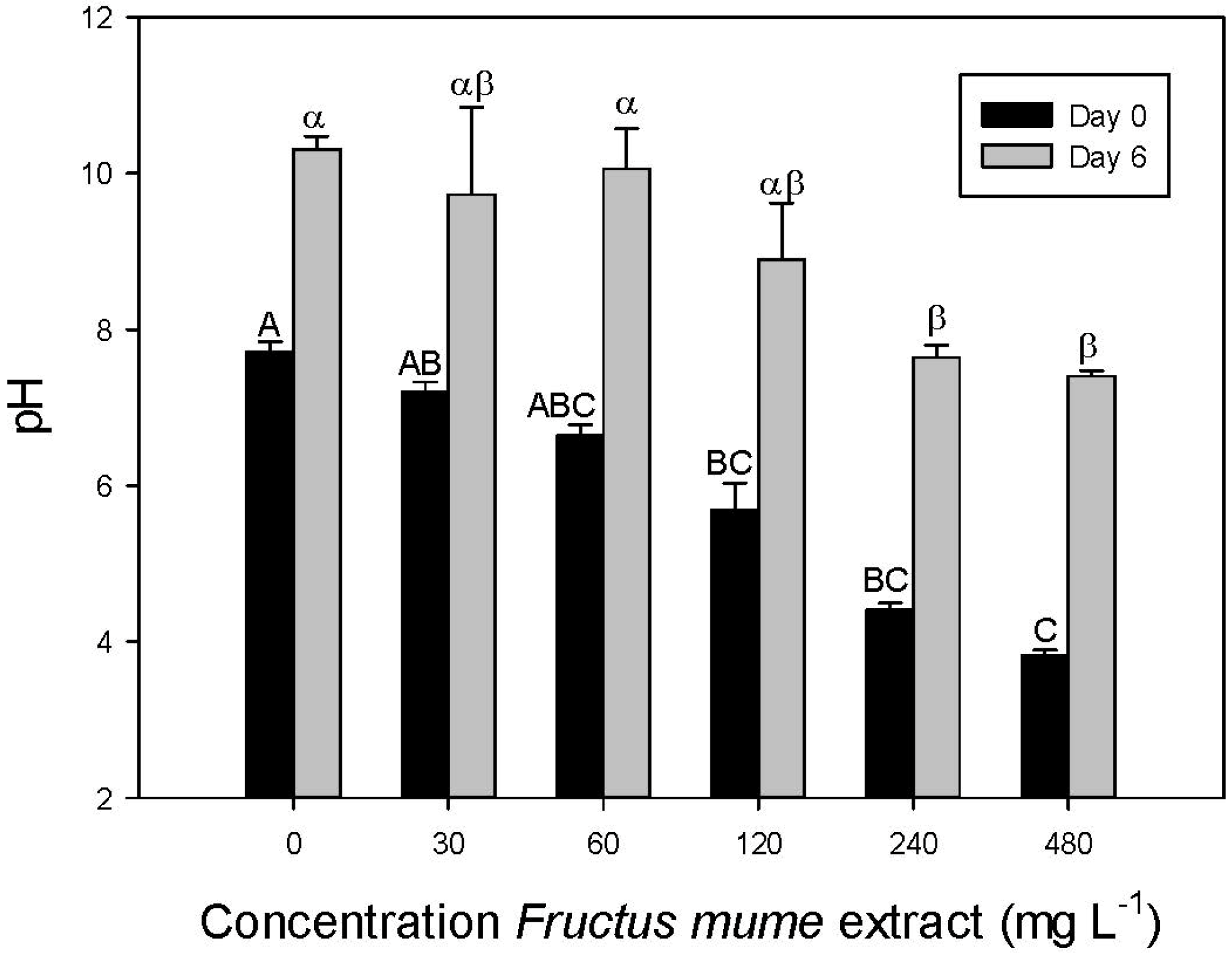
4. Discussion
| Species/Strain | Effect | Concentration L-lysine | Reference |
|---|---|---|---|
| M. aeruginosa N–87, N–98 | complete lysis | 1.2 mg L−1 | [38,39] |
| M. viridis N–102 | “ | “ | “ |
| M. aeruginosa N–88 | lysis | 2.4 mg L−1 | “ |
| M. wesenbergii N–604 | “ | “ | “ |
| M. aeruginosa N-90 | “ | 5 mg L−1 | “ |
| M. viridis | killed | 1 mg L−1 | [22] |
| M. aeruginosa N-44, N-298 | inhibited, no complete lysis | 10 mg L−1 | [39] |
| M. aeruginosa | no effect/inhibition | 0.5/5 mg L−1 | [25] |
| M. aeruginosa 3 strains | decline/stabilization | 5 mg L−1 | [40] |
| M. viridis NIES 102 | inhibited | 3.65 mg L−1 | [23] |
| M. novacekii | “ | 7.3 mg L−1 | “ |
| M. aeruginosa TAC 71-1, M. ichthioblabe | significant growth | 7.3 mg L−1 | “ |
| M. wesenbergii N-104, N–111 | hardly affected/no effect | 10 mg L−1 | [38] |
5. Conclusions
- Fructus mume extract at ≥240 mg L−1 and l-lysine at ≥4.3 mg L−1 caused a decline in Microcystis aeruginosa NIVA-CYA 43 biomass.
- The effect of F. mume extracts is most probably caused by low pH.
- Salvia miltiorrhiza, Moringa oleifera and d-Lysine had no relevant effect on M. aeruginosa biomass.
- All treatments had a short-lived effect only and did not cause a complete wipe-out of M. aeruginosa.
- Our results do not favor the application of the compounds tested as method to mitigate blooms of cyanobacteria in the field.
Acknowledgments
Author Contributions
Appendix
| Cyanobacteria | Medium | Volume | Initial density | Light (µmol m−2 s−1) | Temp | Shaking | Time | Reference |
|---|---|---|---|---|---|---|---|---|
| Mv | MA | 5 mL | 105 cells mL−1 | ?? | ?? | no | 48 h | Kaya and Sano 1996 [22] |
| Ma, Pa, Pp | BG11 | 550 mL | ?? | 53−12:12 L:D | 22 °C | yes | 96 h | Zimba et al., 2001 [25] |
| Ma, Mv, Mw | CB | 10 mL | 106 cells mL−1 | 50−12:12 L:D | 22 °C | no | 48 h | Hehmann et al., 2002 [38] |
| Ma, Mi, Mn, Mv | CT | 100 mL | ?? | 2000 lx | 30 °C | no | 35 d | Takamura et al., 2004 [23] |
| Ma | BG11 | 0.2 mL | 106 cells mL−1 | 3000 lx−16:8 L:D | 25 °C | no | 7 d | Yang et al., 2009 [19] |
| Ma | WC | 50 mL | 13.4, 269 µg CHLa L−1 | ~57−18:6 L:D | 22 °C | 40 rpm | 11,14 d | Lürling and Beekman 2010 [21] |
| Ma | BG11 | 20 mL | 106 cells mL−1 | 90−14:10 L:D (?) | 25 °C (?) | 3 × d−1 | 7 d | Yi et al., 2012 [20] |
| Ma | BG11 | ?? | 4.6 × 106 cells mL−1 (?) | 20−12:12 | 30 °C | 40 rpm | ?? | Liu et al., 2013 [24] |
Conflicts of Interest
References
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication by reducing both nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Health B 2000, 3, 323–347. [Google Scholar] [CrossRef]
- Codd, G.A. Cyanobacterial toxins, the perception of water quality, and the prioritization of eutrophication control. Ecol. Eng. 2000, 16, 51–60. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F. Case study on the efficacy of a lanthanum-enriched clay (Phoslock®) in controlling eutrophication in Lake Het Groene Eiland (The Netherlands). Hydrobiologia 2013, 710, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Q.; Liu, P.-W. Strategy of water pollution prevention in Taihu Lake and its effects analysis. J. Great Lakes Res. 2010, 36, 150–158. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E&FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- de Figueirdo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Stroom, J.M.; Lürling, M.F.L.L.W.; Kardinaal, W.E.A. Netherlands: Risks of toxic cyanobacterial blooms in recreational waters and guidelines. In Current Approaches to Cyanotoxin Risk Management, Risk Management and Regulations in Different Countries; Chorus, I., Ed.; Federal Environment Agency: Dessau Roβlau, Germany, 2012; pp. 82–96. [Google Scholar]
- Faassen, E.J.; Lürling, M. Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar. Drugs 2013, 11, 2643–2654. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EG of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy of 23 October. Off. J. Eur. Union 2000, L327, 1–72. [Google Scholar]
- European Union. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, L64, 37–51. [Google Scholar]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Nat. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Hu, H.; Hong, Y. Algal-bloom control by allelopathy of aquatic macrophytes—A review. Front Environ. Sci. Engin China 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Shao, J.; Li, R.; Lepo, J.E.; Gu, J.-D. Potential for control of harmful cyanobacterial blooms using biologically derived substances: Problems and prospects. J. Environ. Manag. 2013, 125, 149–155. [Google Scholar] [CrossRef]
- Yang, J.-D.; Hu, L.-B.; Zhou, W.; Yin, Y.-F.; Chen, J.; Shi, Z.-Q. Lysis of Microcystis aeruginosa with extracts from Chinese medicinal herbs. Int. J. Mol. Sci. 2009, 10, 4157–4167. [Google Scholar] [CrossRef]
- Yi, Y.-L.; Lei, Y.; Yin, Y.-B.; Zhang, H.-Y.; Wang, G.-X. The antialgal activity of 40 medicinal plants against Microcystis aeruginosa. J. Appl. Phycol. 2012, 24, 847–856. [Google Scholar] [CrossRef]
- Lürling, M.; Beekman, W. Anti-cyanobacterial activity of Moringa oleifera seeds. J. Appl. Phycol. 2010, 22, 503–510. [Google Scholar] [CrossRef]
- Kaya, K.; Sano, T. Algicidal compounds in yeast extract as a component of microbial culture media. Phycologia 1996, 35, 117–119. [Google Scholar] [CrossRef]
- Takamura, Y.; Yamada, T.; Kimoto, A.; Kanehama, N.; Tanaka, T.; Nakadaira, S.; Yagi, O. Growth inhibition of Microcystis cyanobacteria by L-Lysine and disappearance of natural Microcystis blooms with spraying. Microb. Environ. 2004, 19, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Chen, M.-J.; Wang, M.-H.; Jia, R.-B.; Li, L. Inhibition of Microcystis aeruginosa by the extracellular substances from an Aeromonas sp. J. Microb. Biotechnol. 2013, 23, 1304–1307. [Google Scholar] [CrossRef]
- Zimba, P.V.; Dionigi, C.P.; Brashear, S.S. Selective toxicity of exogenous l-lysine to cyanobacteria, relative to a chlorophyte and a diatom. Phycologia 2001, 40, 483–486. [Google Scholar] [CrossRef]
- Qiang, H.; Richmond, A. Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. J. Appl. Phycol. 1996, 8, 139–145. [Google Scholar] [CrossRef]
- Shao, J.; Peng, L.; Luo, S.; Yu, G.; Gu, J.-D.; Lin, S.; Li, R. First report on the allelopathic effect of Tychonema bourrellyi (Cyanobacteria) against Microcystis aeruginosa (Cyanobacteria). J. Appl. Phycol. 2013, 25, 1567–1573. [Google Scholar] [CrossRef]
- Lürling, M.; Beekman, W. Palmelloids formation in Chlamydomonas reinhardtii: Defence against rotifer predators? Ann. Limnol. 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Lürling, M.; van der Grinten, E. Life-history characteristics of Daphnia exposed to dissolved microcystin-LR and to the cyanobacterium Microcystis aeruginosa with and without microcystins. Environ. Toxicol. Chem. 2003, 22, 1281–1287. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, H.; Matsunaga, R. Rapid determination of the main organic acid composition of raw Japanese apricot fruit juices using near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 9652–9657. [Google Scholar] [CrossRef]
- Lee, L.; Lustigman, B.; Chu, I.-Y.; Jou, H.-L. Effect of aluminum and pH on the growth of Anacystis nidulans. Bull. Environ. Contam Toxicol. 1991, 46, 720–726. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y.; Euwe, M. Mitigating cyanobacterial blooms: How effective are “effective microorganisms”? Lakes Reserv. Res. Manag. 2009, 14, 353–363. [Google Scholar] [CrossRef]
- Van Egeraat, A.W.S.M. EM1 and the growth of microorganisms. In Microorganisms in EM; Wageningen University: Wageningen, The Netherlands, 1998. [Google Scholar]
- Na, J.-R.; Oh, K.-N.; Park, S.-U.; Bae, D.; Choi, E.J.; Jung, M.A.; Choi, C.Y.; Lee, D.-W.; Jun, W.; Lee, K.Y.; et al. The laxative effects of Maesil (Prunus mume Siebold & Zucc.) on constipation induced by a low-fibre diet in a rat model. Int. J. Food Sci. Nutr. 2013, 64, 333–345. [Google Scholar] [CrossRef]
- Evers, N. Getalswaarden bij de goede ecologische toestand voor oppervlaktewater voor de algemene fysisch-chemische kwaliteitselementen temperatuur, zuurgraad, doorzicht, zoutgehalte en zuurstof; STOWA: Utrecht, The Netherlands, 2007. (In Dutch) [Google Scholar]
- Geng, L.; Qin, B.; Yang, Z. Unicellular Microcystis aeruginosa cannot revert back to colonial form after short-term exposure to natural conditions. Biochem. Syst. Ecol. 2013, 51, 104–108. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.-L.; Hao, K.; Liu, G.-L.; Wang, G.-X. Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa—Towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere 2013, 93, 997–1004. [Google Scholar] [CrossRef]
- Hehmann, A.; Kaya, K.; Watanabe, M.M. Selective control of Microcystis using an amino acid—A laboratory assay. J. Appl. Phycol. 2002, 14, 85–89. [Google Scholar] [CrossRef]
- Hehmann, A.; Watanabe, M.M.; Kaya, K. Killing of Microcystis by an amino acid: Results of laboratory and enclosure experiments. Verh. Int. Verein Limnol. 2002, 28, 1147–1150. [Google Scholar]
- Lin, B.-G.; Yang, L.-Y.; Xiao, L.; Ji, J.; Yuan, Y.; Yao, Y. Mechanism of lysine inhibiting Microcystis aeruginosa cells. J. Ecol. Rural Environ. 2008, 24, 68–72. [Google Scholar]
- Moreno-Garrido, I.; Lubian, L.M.; Soares, A.M.V.M. Influence of cellular density on determination of EC50 in microalgal growth inhibition tests. Ecotoxicol. Environ. Saf. 2000, 47, 112–116. [Google Scholar] [CrossRef]
- Katayon, S.; Megat Mohd Noor, M.J.; Asma, M.; Abdul Ghani, L.A.; Thamer, A.M.; Azni, I.; Ahmad, J.; Khor, B.C.; Suleyman, A.M. Effects of storage conditions of Moringa oleifera seeds on its performance in coagulation. Biores Technol. 2006, 97, 1455–1460. [Google Scholar] [CrossRef]
- Shehata, S.; Badr, S.; Wahba, S. Drinking water treatment options for eliminating freshwater algae. Int. J. Environ. Stud. 2002, 59, 679–688. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Subba Narasiah, K.; Talbot, B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Nishi, L.; Madrona, G.S.; Guilherme, A.L.F.; Vieira, A.M.S.; Araújo, A.A.; Ugri, M.C.B.A.; Bergamasco, R. Cyanobacteria removal by coagulation/flocculation with seeds of the natural coagulant Moringa oleifera Lam. Chem. Engin Trans. 2011, 24, 1129–1134. [Google Scholar]
- Camacho, F.P.; Bongiovani, M.C.; Arakawa, F.S.; Shimabuku, Q.L.; Vieira, A.M.S.; Bergamasco, R. Advanced processes of cyanobacteria and cyanotoxins removal in supply water treatment. Chem. Eng. Trans. 2013, 32, 421–426. [Google Scholar]
- Madsen, M.; Schlundt, J.; Omer, E.F.E. Effect of water coagulation by seeds of Moringa oleifera on bacterial concentrations. J. Trop. Med. Hyg. 1987, 90, 101–109. [Google Scholar]
- Ndabigengesere, A.; Narasiah, K.D. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Made-in-China.com. Available online: http://www.made-in-china.com/products-search/hot-china-products/L-Lysine.html (accessed on 25 April 2014).
- Kaya, K.; Liu, Y.-D.; Shen, Y.-W.; Xiao, B.-D.; Sano, T. Selective control of toxic Microcystis water blooms using lysine and malonic acid: An enclosure experiment. Environ. Toxicol. 2005, 20, 170–178. [Google Scholar] [CrossRef]
- Zhou, S.; Shao, Y.; Gao, N.; Deng, Y.; Qiao, J.; Ou, H.; Deng, J. Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci. Total Environ. 2013, 463–464, 111–119. [Google Scholar] [CrossRef]
- Li, L.; Pan, G. A universal method for flocculating harmful algal blooms in marine and fresh waters using modified sand. Environ. Sci. Technol. 2013, 47, 4555–4562. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lürling, M.; Van Oosterhout, F. Effect of Selected Plant Extracts and D- and L-Lysine on the Cyanobacterium Microcystis aeruginosa. Water 2014, 6, 1807-1825. https://doi.org/10.3390/w6061807
Lürling M, Van Oosterhout F. Effect of Selected Plant Extracts and D- and L-Lysine on the Cyanobacterium Microcystis aeruginosa. Water. 2014; 6(6):1807-1825. https://doi.org/10.3390/w6061807
Chicago/Turabian StyleLürling, Miquel, and Frank Van Oosterhout. 2014. "Effect of Selected Plant Extracts and D- and L-Lysine on the Cyanobacterium Microcystis aeruginosa" Water 6, no. 6: 1807-1825. https://doi.org/10.3390/w6061807
APA StyleLürling, M., & Van Oosterhout, F. (2014). Effect of Selected Plant Extracts and D- and L-Lysine on the Cyanobacterium Microcystis aeruginosa. Water, 6(6), 1807-1825. https://doi.org/10.3390/w6061807






