Fecal Coliform and E. coli Concentrations in Effluent-Dominated Streams of the Upper Santa Cruz Watershed
Abstract
:1. Introduction
2. Study Location: Santa Cruz Watershed
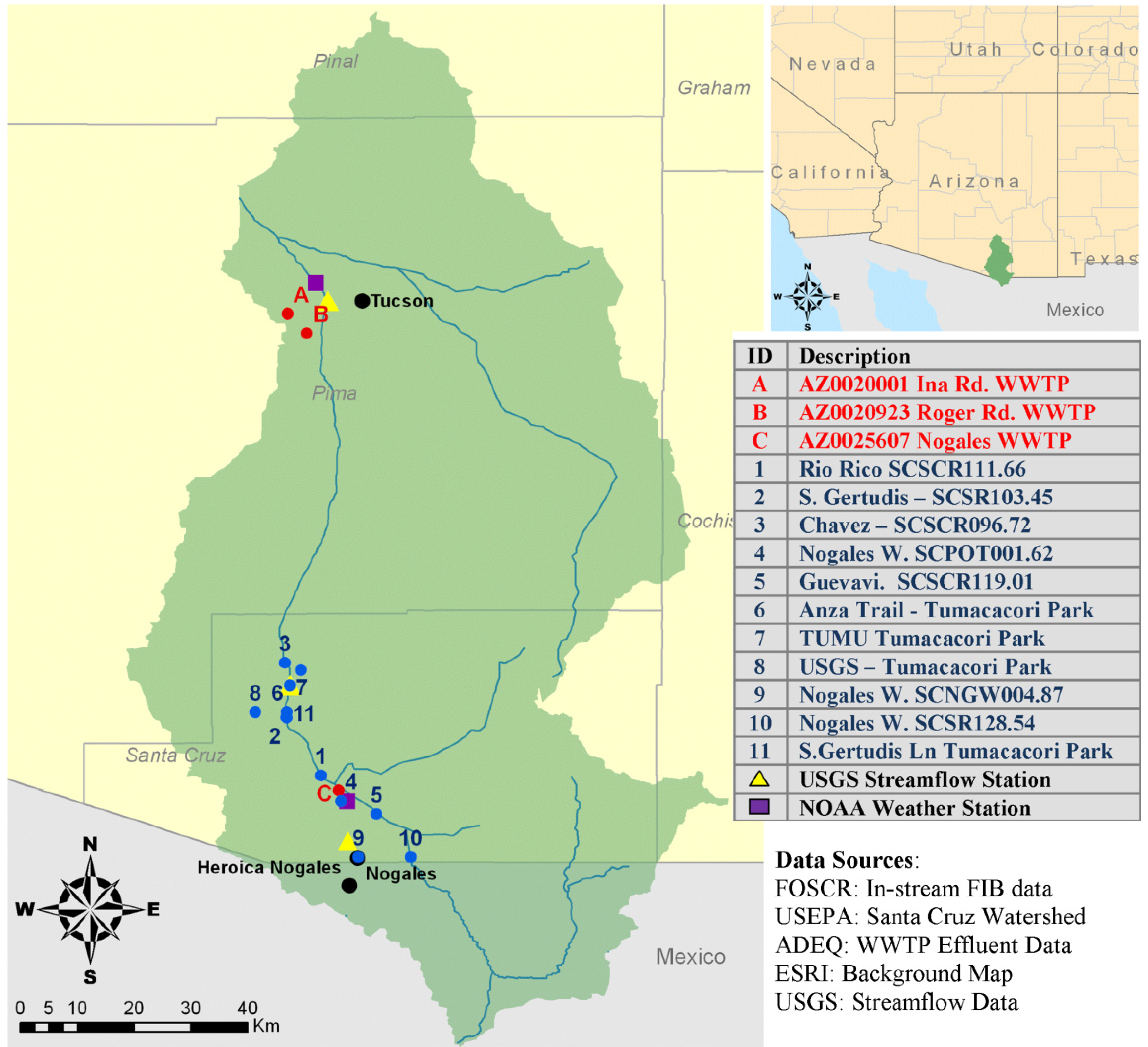
3. Data Collection and Analysis
| E. coli a | ||
| Water Quality Criteria | FBC d | PBC e |
| Geometric Mean c | 126 | 126 |
| Single sample maximum | 235 | 575 |
| Fecal Coliform b | ||
| Water Quality Criteria | FBC d | Other Designated Uses f |
| Geometric Mean c | 200 | 1000 |
| 10% of samples over 30 days | 400 | 2000 |
| Single Sample Maximum | 800 | 4000 |
4. Results
4.1. Fecal Coliform and E. coli Concentrations from Point Source WWTP Effluent
| Facility Name Permit ID | # of Reporting Periods a | The highest value of Maximum concentrations reported by the facility during DMRs period | Mean of the Maximum Concentrations reported during DMRs period | Reporting Periods >800 cfu/100 mL (Fecal) | Reporting Periods >235 cfu/100 mL (FBC E. coli) | Reporting Periods >575 cfu/100 mL (PBC E. coli) |
|---|---|---|---|---|---|---|
| Pima County Ina Road WWTP AZ0020001 | 94 | 1600 | 231 | 13% | ---- | ---- |
| Roger Road WWTP AZ0020923 | 98 | 1600 | 269 | 15% | ---- | ---- |
| Nogales International WWTP AZ0025607 | 27 | 2400 | 330 | ---- | 29% | 18% |
| Facility Name Permit ID | # of Reporting Periods a | The highest value of Average Concentrations b reported by the facility during DMRs period | Mean of the Average Concentrations b reported by the facility during DMRs period | Reporting Periods >200 cfu/100 mL (Fecal) | Reporting Periods >126 cfu/100 mL (E. coli) |
|---|---|---|---|---|---|
| Pima County Ina Road WWTP AZ0020001 | 94 | 79 | 16.2 | 0 | --- |
| Roger Road WWTP AZ0020923 | 98 | 104 | 17.4 | 0 | --- |
| Nogales International WWTP AZ0025607 | 27 | 229 | 41.6 | ---- | 11% |
4.2. In-Stream Fecal Coliform and E. coli Data Analysis
4.2.1. Fecal Coliform and E. coli Concentrations from Nonpoint In-Stream Sources
| Reach ID ADEQ ID | # of samples | Start Date | End Date | Single Sample Max | Geometric Mean | % > 800 (Fecal) |
|---|---|---|---|---|---|---|
| Rio Rico SCSCR111.66 ADEQ 100238 | 112 | 3/1988 | 12/2008 | 139,000 | 161 | 19% |
| S. Gertudis SCSCR103.45 ADEQ 100247 | 98 | 2/1993 | 12/2008 | 27,100 | 149 | 21% |
| Chavez SCSCR096.72 ADEQ 100244 | 89 | 11/1992 | 12/2008 | 49,200 | 99 | 15% |
| Nogales W. (Portero Creek) SCPOT001.62ADEQ 100571 | 70 | 3/1996 | 12/2008 | 24,000 | 146 | 24% |
| Nogales Guevavi SCSCR119.01 ADEQ 100246 | 32 | 11/1992 | 7/2001 | 79,000 | 39 | 13% |
| Reaches ID ADEQ ID | # of samples | Start Date | End Date | MAX | Geometric Mean | % > 235 (FBC E. coli) | % > 575 (PBC E. coli) |
|---|---|---|---|---|---|---|---|
| Santa Gertudis Lane Tubac Basin Tumacacori Park (NPS) | 159 | 6/2007 | 9/2010 | 547,500 | 668 | 61% | 45% |
| Anza Trail River Crossing Tubac Basin Tumacacori Park (NPS) | 64 | 6/2007 | 9/2010 | 173,290 | 316 | 53% | 33% |
| TUMA Educational Site Tubac Basin Tumacacori Park (NPS) | 88 | 7/2007 | 9/2010 | 241,960 | 609 | 57% | 42% |
| Rio Rico SCSCR111.66 ADEQ 100238 | 29 | 2/2008 | 5/2011 | 241,920 a | 306 | 34% | 24% |
| S. Gertudis SCSCR103.45 ADEQ 100247 | 22 | 2/2008 | 5/2011 | 241,920 a | 367 | 41% | 18% |
| Chavez SCSCR096.72 ADEQ 100244 | 19 | 2/2008 | 4/2011 | 141,300 | 491 | 52% | 26% |
| Nogales W. (Portero Creek) SCPOT001.62 ADEQ 100571 | 21 | 2/2008 | 5/2011 | 241,920 a | 792 | 61% | 38% |
| USGS Tumacacori Tubac | 16 | 6/2/2010 | 9/8/2010 | 210,000 | 2265 | 75% | 56% |
4.2.2. Correlation of In-Stream Fecal Coliform and E. coli Concentrations to Streamflow and Precipitation

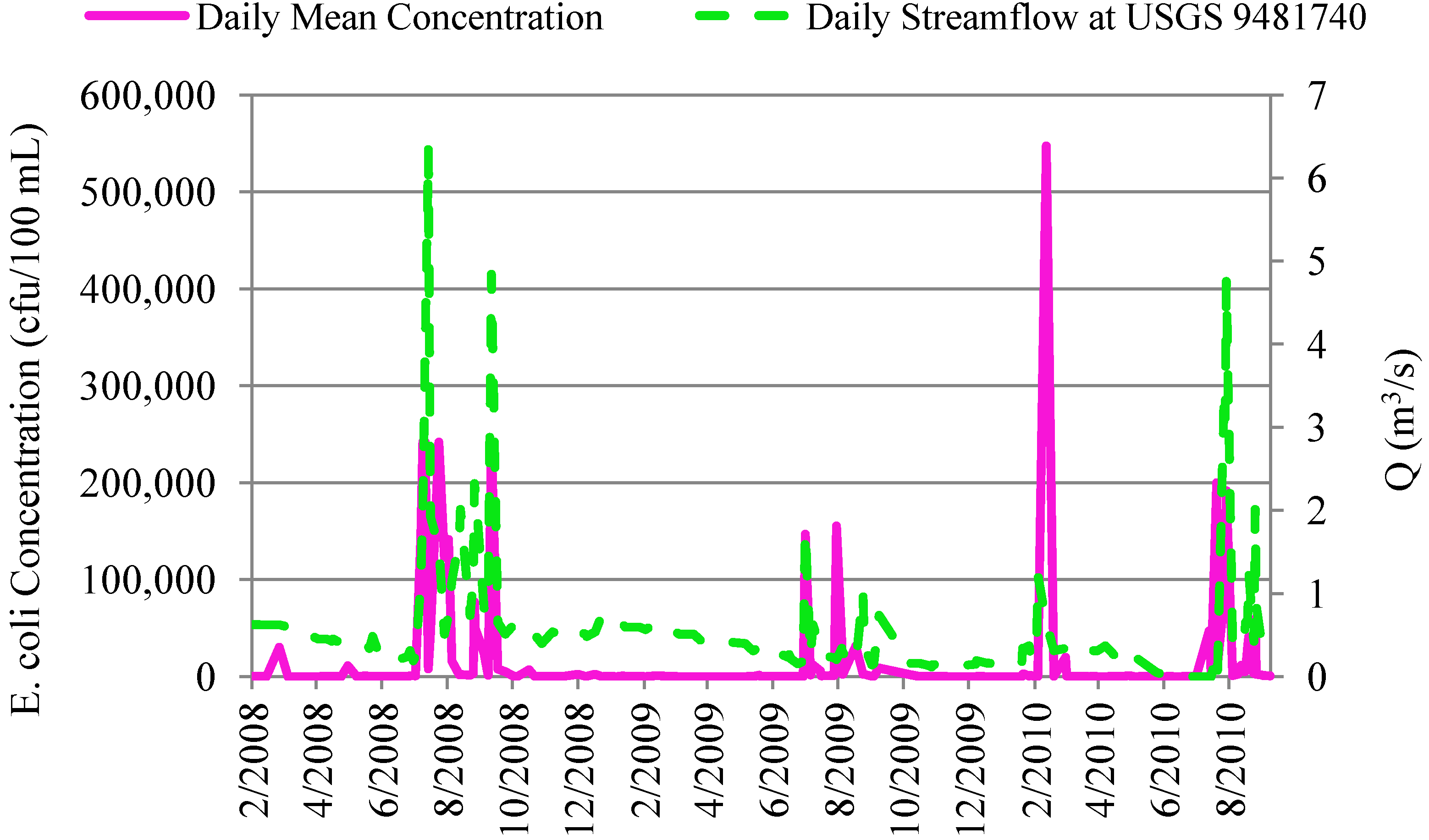
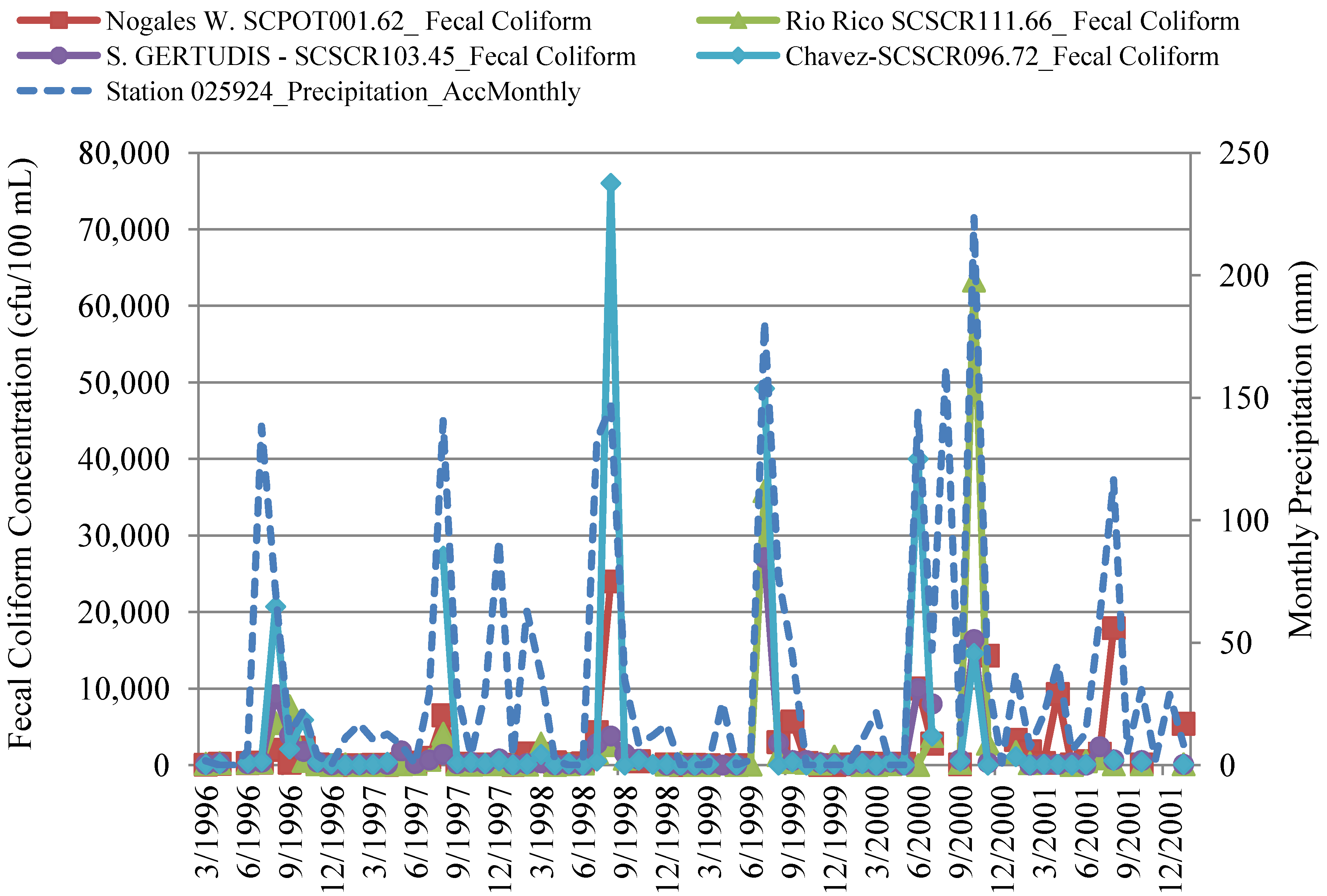
4.3. In-Stream Concentrations versus WWTP Effluent Concentrations
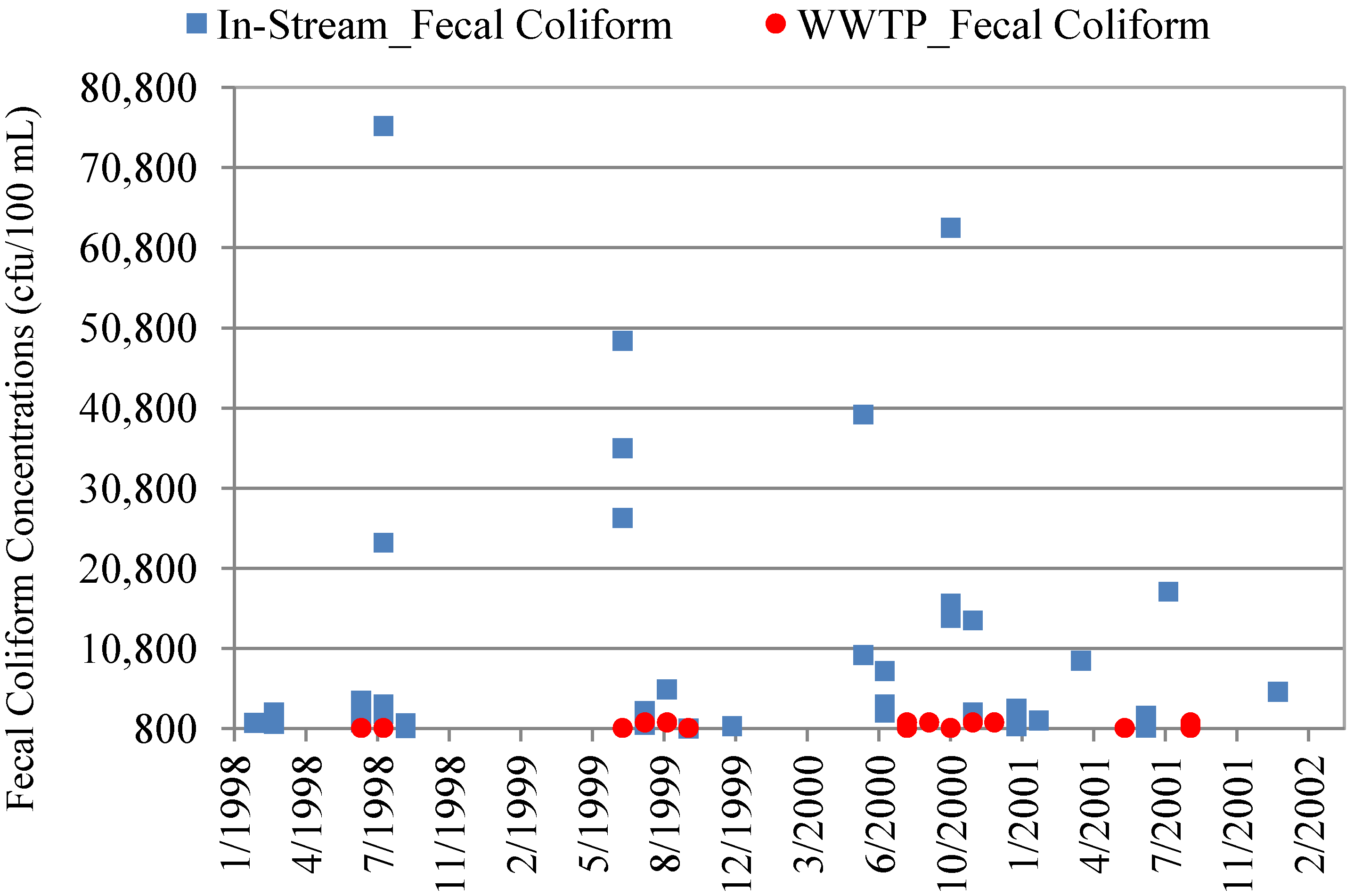
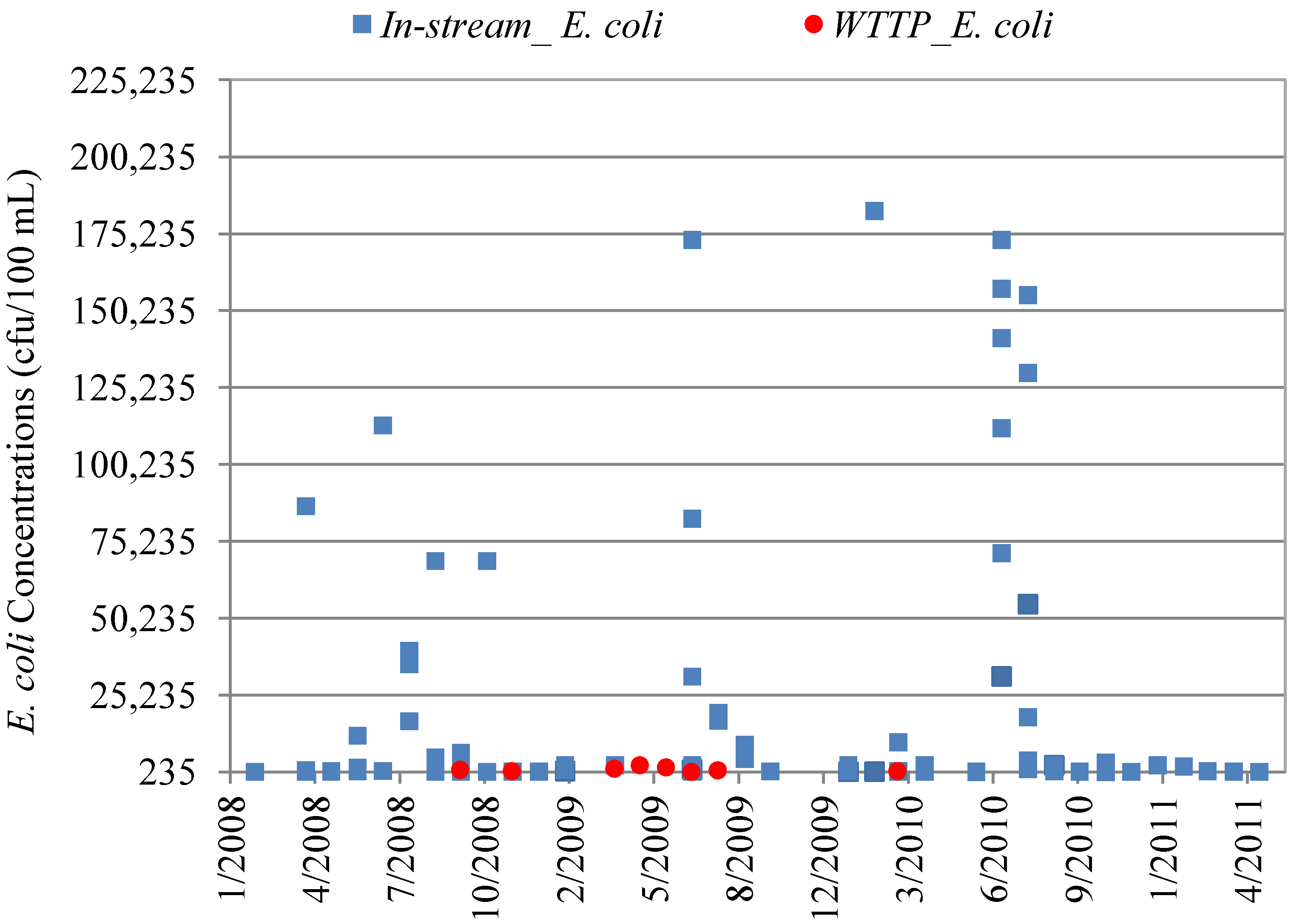
| Data Set | Mean Concentration (cfu/100 mL) | Minimum Concentration | Maximum Concentration | p-value * |
|---|---|---|---|---|
| WWTP Effluent E. coli | 330 | <1.0 | 2400 | 0.000002 |
| In-stream E. coli | 1,745 | <1.0 | 139,000 | |
| WWTP Effluent Fecal Coliform (DMR Maximums **) | 285 | 3 | 1,600 | 0.0002 |
| In-stream Fecal Coliform | 2,519 | <1.0 | 139,000 |
5. Discussion
6. Conclusions and Recommendations
Acknowledgments
References
- Fayer, R.; Speer, C.A.; Dubey, J.P. Cryptosporidium and Cryptosporidiosis, 2nd ed; CRC Press: Boca Raton, FL, USA, 1997; pp. 1–42. [Google Scholar]
- Gaffield, S.J.; Goo, R.L.; Richards, L.A.; Jackson, R.J. Public health effects of inadequately managed stormwater runoff. Am. J. Public Health 2003, 93, 1527–1533. [Google Scholar] [CrossRef]
- Asano, T.; Levine, A.D. Wastewater reclamation, recycling and reuse: Past, present, and future. Water Sci. Technol. 1996, 33, 1–14. [Google Scholar]
- Asano, T.; Cotruvo, J.A. Groundwater recharge with reclaimed municipal wastewater: Health and regulatory considerations. Water Res. 2004, 38, 1941–1951. [Google Scholar] [CrossRef]
- Arizona Department of Water Resources (ADWR) Web Page. Recharge. Available online: http://www.azwater.gov/AzDWR/WaterManagement/Recharge/default.htm (accessed on 6 July 2011).
- Schladweiler, J. Tracking Down the Roots of Our Sanitary Sewers. Available online: https://www.sewerhistory.org (accessed on 8 August 2011).
- Simon, T. Reuse of effluent water—Benefits and risks. Agric. Water Manag. 2006, 80, 147–159. [Google Scholar] [CrossRef]
- Brooks, B.; Riley, T.; Taylor, R. Water quality of effluent-dominated ecosystems: Ecotoxicological, hydrological, and management consideration. Hydrobiologia 2006, 556, 365–379. [Google Scholar] [CrossRef]
- Gannon, J.; Busse, M. E. coli and enterococci levels in urban stormwater, river water and chlorinated treatment plant effluent. Water Res. 1989, 23, 1167–1176. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA) Web Page. 5.11 Fecal Bacteria. Available online: http://water.epa.gov/type/rsl/monitoring/vms511.cfm (accessed on 26 July 2011).
- U.S. Environmental Protection Agency (USEPA), Review of Published Studies to Characterize Relative Risks From Different Sources of Fecal Contamination in Recreational Water; EPA 822-R-09-001; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- U.S. Environmental Protection Agency (USEPA), Ambient Water Quality Criteria for Bacteria—1986; EPA 440/5-84-002; U.S. Environmental Protection Agency: Washington, DC, USA, 1986.
- U.S. Environmental Protection Agency (USEPA), Test Methods for Escherichia coli and enterococci in Water by the Membrane Filter Procedure (Method #1103.1); EPA 600/4-85-076; U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory: Cincinatti, OH, USA, 1985.
- U.S. Environmental Protection Agency (USEPA), Bacterial Water Quality Standards for Recreational Waters (Freshwater and Marine Waters); EPA-823-R-03-008; U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- American Public Health Association (APHA), Standard Methods for the Examination of Water and Wastewater, 18th edAmerican Public Health Association: Washington, DC, USA, 1992.
- Gronewold, A.D.; Borsuk, M.E.; Wolpert, R.L.; Reckhow, K.H. An assessment of fecal indicator bacteria-based water quality standards. Environ. Sci. Technol. 2008, 42, 4676–4682. [Google Scholar] [CrossRef]
- Harwood, V.J.; Levine, A.D.; Scott, T.M.; Chivukula, V.; Lukasik, J.; Farrah, S.R.; Rose, J.B. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 2005, 71, 3163–3170. [Google Scholar]
- Leclerc, H.; Mossel, D.A.A.; Edberg, S.C.; Struijk, C.B. Advances in the bacteriology of the Coliform Group: Their suitability as markers of microbial water safety. Annu. Rev. Microbiol. 2001, 55, 201–234. [Google Scholar] [CrossRef]
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water qualit. Water Res. 2007, 41, 3517–3538. [Google Scholar]
- Arizona Department of Environmental Quality (ADEQ). 2006/2008 Status of Ambient Surface Water Quality in Arizona: Arizona’s Integrated 305(b) Assessment and 303(d) Listing Report. Available online: http://www.azdeq.gov/environ/water/assessment/assess.html (accessed on 26 July 2011).
- U.S. Environmental Protection Agency (USEPA), Bacteriological criteria for those states not complying with Clean Water Act section 303(i)(1)(A)Title 40: Part 131.41 Arizona; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Marino, R.; Gannon, J. Survival of fecal coliforms and fecal streptococci in storm drain sediment. Water Res. 1991, 25, 1089–1098. [Google Scholar] [CrossRef]
- National Research Council, Issues in Potable Reuse—The Viabilty of Augmenting Drinking Water Supplies with Reclaimed Water; National Academy Press: Washington, DC, USA, 1998.
- U.S. Environmental Protection Agency (USEPA) Web Page. Watershed Priorities: Santa Cruz River Watershed, AZ. Available online: http://www.epa.gov/region9/water/watershed/santacruz.html (accessed on 15 September 2011).
- Scott, C.; Pasqualetti, M.; Hoover, J.; Garfin, G.; Varady, R.; Guhathakurta, S. Water and Energy Sustainability with Rapid Growth and Climate Change in the Airzona-Sonora Border Region; A Report to the Arizona Water Institute: Temple, AZ, USA, 2009. [Google Scholar]
- U.S. Census Bureau. Table A1: Interim Projections of the Total Population for the United States and States: April 1, 2000 to July 1, 2030. Available online: http://wonder.cdc.gov/wonder/help/populations/population-projections/SummaryTabA1.pdf (accessed on 14 September 2011).
- Sprouse, T.W. Water Issues on the Arizona–Mexico Border: The Santa Cruz, San Pedro, and Colorado Rivers; Water Resources Research Center, The University of Arizona: Tucson, AZ, USA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Web Page. US-Mexico Border 2012. Available online: http://www.epa.gov/usmexicoborder/index.html (accessed on 30 September 2011).
- U.S. Environmental Protection Agency (USEPA) Web Page. Envirofacts Database. Available online: http://www.epa.gov/enviro/ (accessed on 7 September 2011).
- Arizona Department of Water Resources (ADWR) Web Page. Active Management Area Water Supply—Central Arizona Project Water. Available online: http://www.azwater.gov/azdwr/StatewidePlanning/WaterAtlas/ActiveManagementAreas/PlanningAreaOverview/WaterSupply.htm (accessed on 30 September 2011).
- Pima County Regional Wastewater Reclamation Department (RWRD), RWRD’s 2010 Effluent Generation and Utilization Report; Pima County Regional Wastewater Reclamation Department: Tucson, AZ, USA, 2011.
- CH2MHILL, Nogales International Wastewater Treatment Plant Maximum Allowable Headworks Loading Development; CH2MHILL: El Paso, TX, USA, 2009.
- Lim, K.J.; Engel, B.A.; Tang, Z.; Choi, J.; Kim, K.-S.; Muthukrishnan, S.; Tripathy, D. Automated web GIS based hydrograph analysis tool (WHAT). JAWRA J. Am. Water Resour. Assoc. 2005, 41, 1407–1416. [Google Scholar]
- Arizona Department of Environmental Quality (ADEQ), Total Maximum Daily Load For: Oak Creek- Slide Rock State Park Parameters: Escherichia coliform; Open File Report 09-08; Arizona Department of Environmental Quality: Phoenix, AZ, USA, 1999.
- U.S. Environmental Protection Agency (USEPA), Water Quality Standards Handbook: Second Edition; EPA-823-B-12-002; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Lipp, E.; Kurz, R.; Vincent, R.; Rodriguez-Palacios, C.; Farrah, S.; Rose, J. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries Coasts 2001, 24, 266–276. [Google Scholar] [CrossRef]
- Kinnaman, A.; Surbeck, C.Q.; Usner, D. Coliform bacteria: The effect of sediments on decay rates and on required detention times in stormwater BMPs. J. Environ. Prot. 2012, 3, 787–797. [Google Scholar] [CrossRef]
- Easton, J.H.; Gauthier, J.J.; Lalor, M.M.; Pitt, R.E. Die-off of pathogenic E. coli O157:H7 in sewage contaminated waters. JAWRA J. Am. Water Resour. Assoc. 2005, 41, 1187–1193. [Google Scholar] [CrossRef]
- Litton, R.M.; Ahn, J.H.; Sercu, B.; Holden, P.A.; Sedlak, D.L.; Grant, S.B. Evaluation of chemical, molecular, and traditional markers of fecal contamination in an effluent dominated urban stream. Environ. Sci. Technol. 2010, 44, 7369–7375. [Google Scholar]
- Hunt, W.; Smith, J.; Jadlocki, S.; Hathaway, J.; Eubanks, P. Pollutant removal and peak flow mitigation by a bioretention cell in urban Charlotte, N.C. J. Environ. Eng. 2008, 134, 403–408. [Google Scholar] [CrossRef]
- Van der Valk, A.G.; Jolly, R.W. Recommendations for research to develop guidelines for the use of wetlands to control rural nonpoint source pollution. Ecolog. Eng. 1992, 1, 115–134. [Google Scholar] [CrossRef]
- Baffaut, C.; Sadeghi, A. Bacteria modeling with SWAT for assessment and remediation studies: A review. 2010, 53, 1585–1594. [Google Scholar]
- Benham, B.L.; Baffaut, C.; Zeckoski, R.W.; Mankin, K.R.; Pachepsky, Y.A.; Sadeghi, A.M.; Brannan, K.M.; Soupir, M.L.; Habersack, M.J. Modeling bacteria fate and transport in watersheds to support TMDLs. Transact. ASABE 2006, 49, 987–1002. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sanders, E.C.; Yuan, Y.; Pitchford, A. Fecal Coliform and E. coli Concentrations in Effluent-Dominated Streams of the Upper Santa Cruz Watershed. Water 2013, 5, 243-261. https://doi.org/10.3390/w5010243
Sanders EC, Yuan Y, Pitchford A. Fecal Coliform and E. coli Concentrations in Effluent-Dominated Streams of the Upper Santa Cruz Watershed. Water. 2013; 5(1):243-261. https://doi.org/10.3390/w5010243
Chicago/Turabian StyleSanders, Emily C., Yongping Yuan, and Ann Pitchford. 2013. "Fecal Coliform and E. coli Concentrations in Effluent-Dominated Streams of the Upper Santa Cruz Watershed" Water 5, no. 1: 243-261. https://doi.org/10.3390/w5010243
APA StyleSanders, E. C., Yuan, Y., & Pitchford, A. (2013). Fecal Coliform and E. coli Concentrations in Effluent-Dominated Streams of the Upper Santa Cruz Watershed. Water, 5(1), 243-261. https://doi.org/10.3390/w5010243




