Insight on the Ultrafast Water Treatment over NiFe-Layered Double Hydroxides via Electroactivation of Ferrate(VI): The Role of Spin State Regulation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalysts Preparation
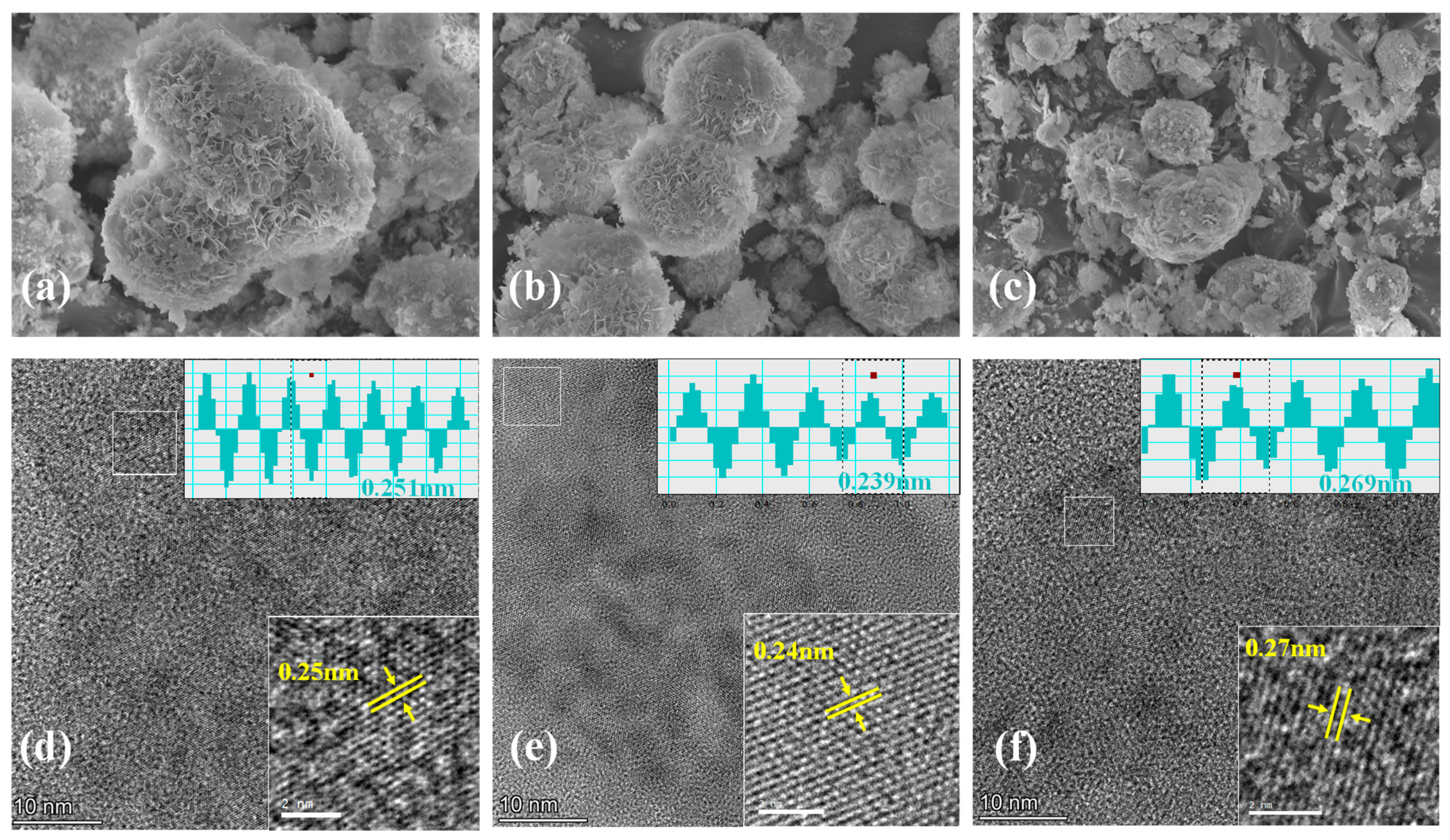
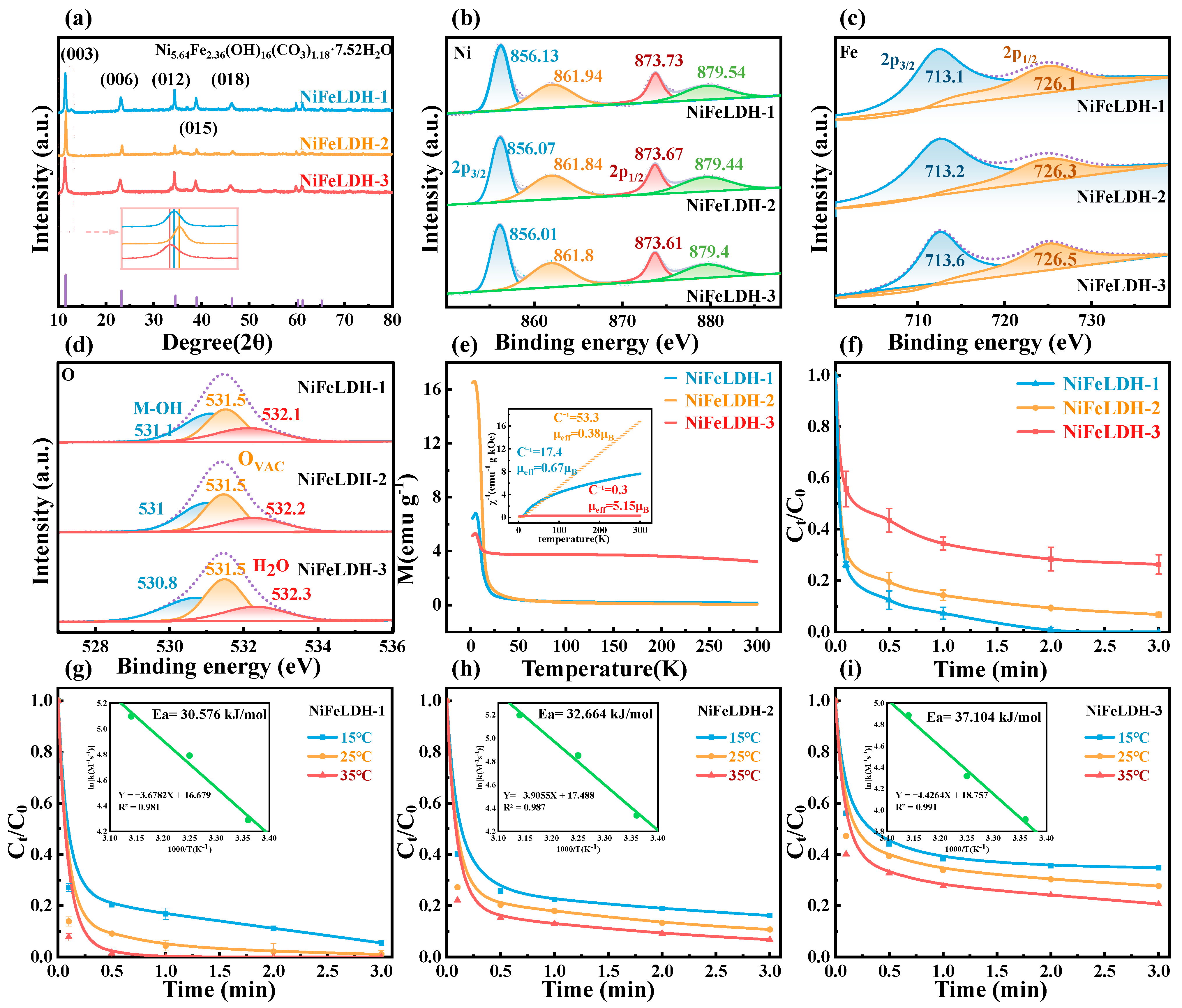
2.3. Structural Characterization
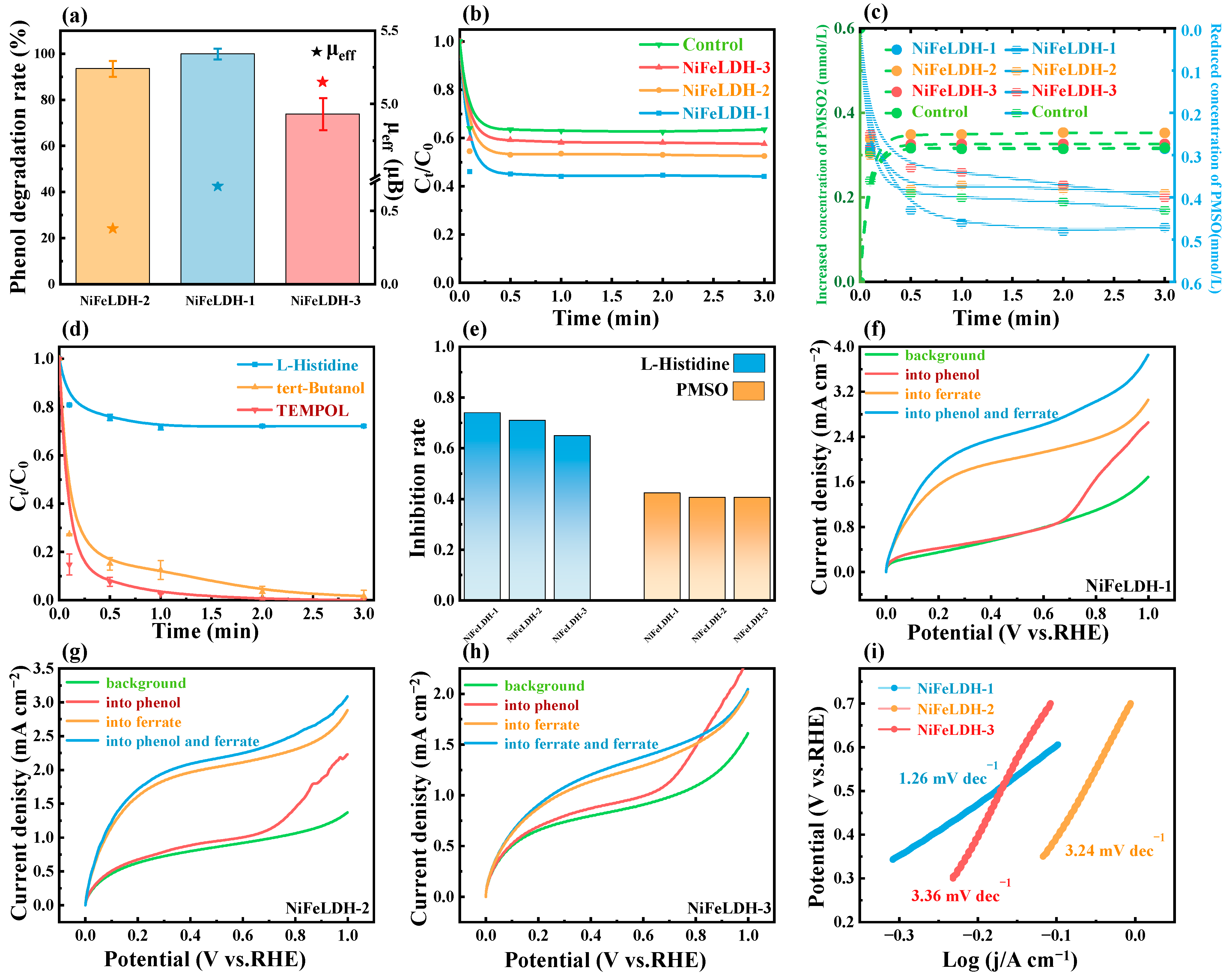
2.4. Electrochemical Measurements
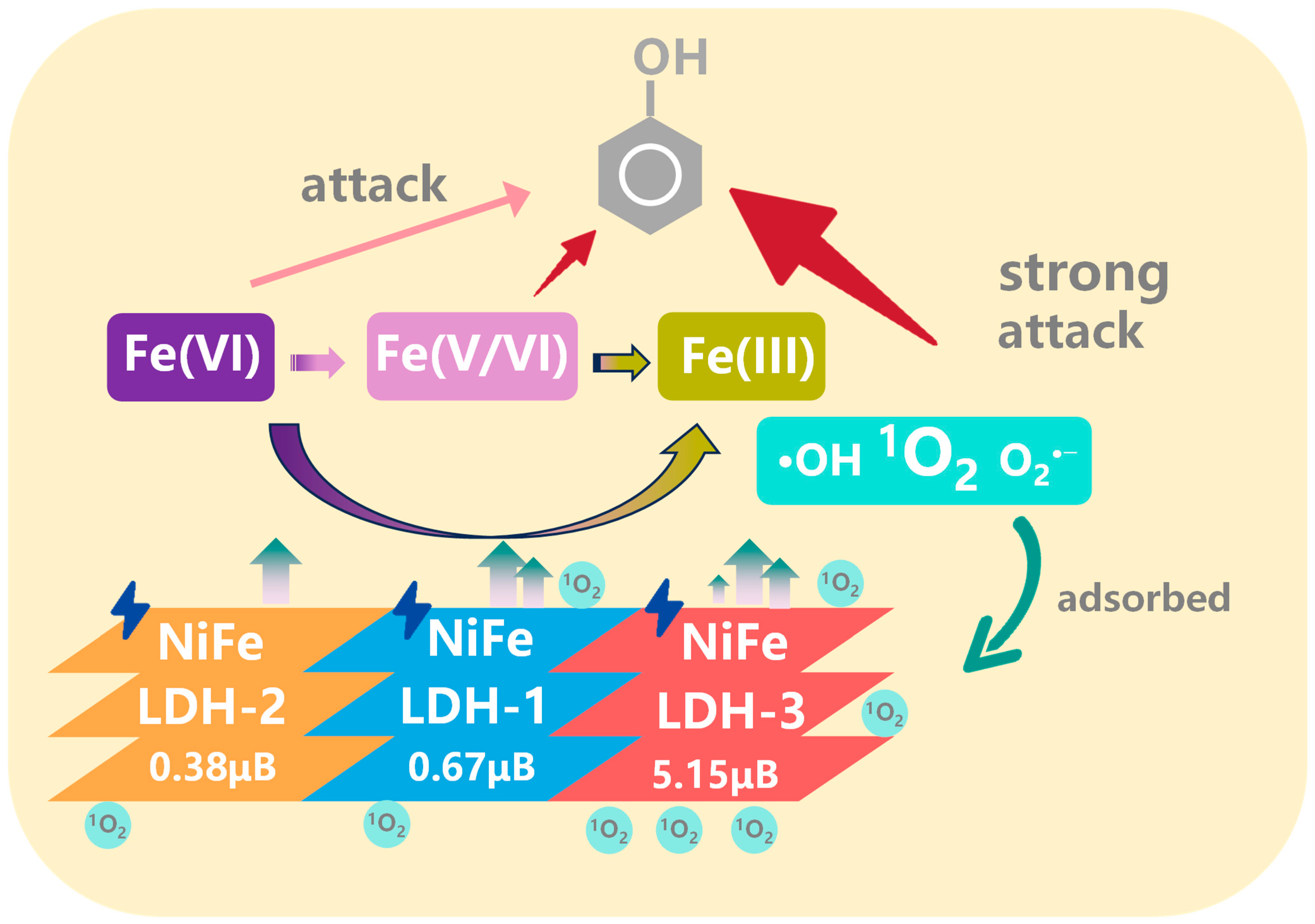
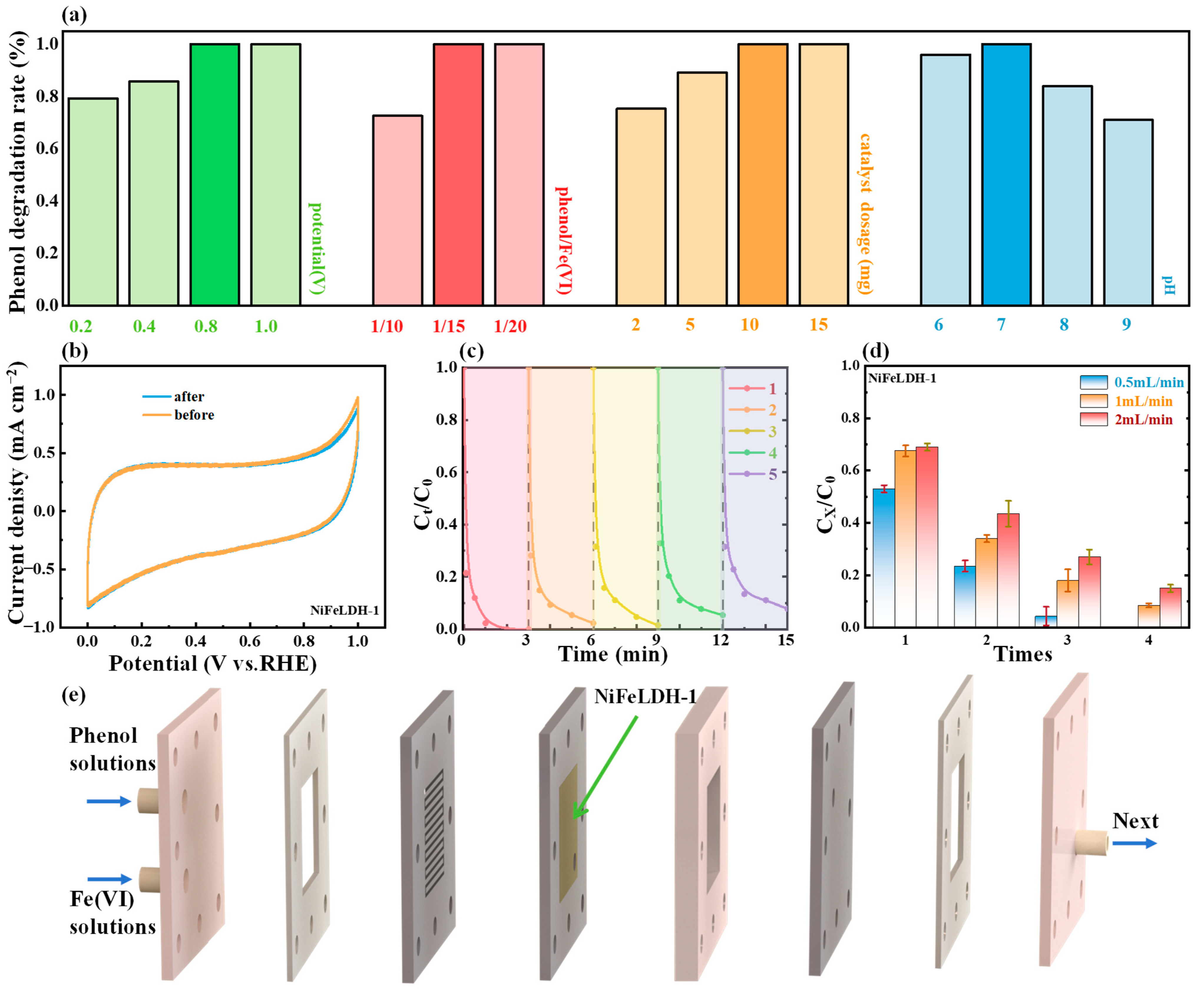
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Abdel-Shafy, H.I. Barriers to ferrate(VI) application in water and wastewater treatment. Environ. Sci. Technol. 2024, 58, 3057–3060. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q.; Stanford, C.; Petri, M. Practical application of ferrate(VI) for water and wastewater treatment–Site study’s approach. Water-Energy Nexus 2018, 1, 42–46. [Google Scholar] [CrossRef]
- Shu, J.; Xu, X.; Zhang, Y.; Wang, K.; Zhu, Y.; Lian, X.; Wang, H. Insight into the mechanism of ferrate(VI) activation by mineral zincite for carbamazepine degradation: Role of Fe(V) species and free radical induction. Chem. Eng. J. 2023, 473, 145360. [Google Scholar] [CrossRef]
- Yu, J.; Sumita; Zhang, K.; Zhu, Q.; Wu, C.; Huang, S.; Zhang, Y.; Yao, S.; Pang, W. A review of research progress in the preparation and application of ferrate(VI). Water 2023, 15, 699. [Google Scholar] [CrossRef]
- Cao, J.Y.; Du, Y.; Dai, X.; Liu, T.; Wang, Z.J.; Li, J.; Zhang, H.; Zhou, P.; Lai, B. Ferrate(VI)-based synergistic oxidation processes (Fe(VI)-SOPs): Promoted reactive species production, micropollutant/microorganism elimination, and toxicity reduction. Chem. Eng. J. 2024, 489, 151180. [Google Scholar] [CrossRef]
- Li, J.; Fu, C.; Lin, Q.; Zeng, T.; Wang, D.; Huang, X.; Song, S.; Li, C.; Dong, F. Fe(VI) activation system mediated by a solar-driven TiO2 nanotubes electrode for CLQ degradation: Performances, mechanisms and pathways. J. Hazard. Mater. 2023, 452, 131274. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Qiao, J.; Guan, X. Application of Fe(VI) in abating contaminants in water: State of art and knowledge gaps. Front. Environ. Sci. Eng. 2021, 15, 80. [Google Scholar] [CrossRef]
- Dai, X.; Liu, T.; Du, Y.; Cao, J.Y.; Wang, Z.J.; Li, J.; Zhou, P.; Zhang, H.; Lai, B. Synergistic effect in enhancing treatment of micro-pollutants by ferrate and carbon materials: A review. Chin. Chem. Lett. 2024, 81, 110548. [Google Scholar] [CrossRef]
- He, S.; Xue, N.; Lin, G.; Chen, Y.; Gai, X.; Yu, Y.; Liu, F.; Guo, Z.; Qiu, P. The efficient Ferrate activation by Co modified N-doped carbon nanosheets for the ultrafast water treatment. J. Environ. Chem. Eng. 2024, 12, 114982. [Google Scholar] [CrossRef]
- Deng, Y.; Guan, X. Unlocking the potential of ferrate(VI) in water treatment: Toward one-step multifunctional solutions. J. Hazard. Mater. 2024, 464, 132920. [Google Scholar] [CrossRef]
- Petkova, A.P.; Nekrasov, R.E.; Dyachenko, V.A.; Brunman, M.V. Comparison of ferrate production methods and assessment of its possible applications for water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 539, 012029. [Google Scholar] [CrossRef]
- Jang, E.; Cho, J.; Kim, J. WC nanoparticles and NiFe alloy co-encapsulated in N-doped carbon nanocage for exceptional OER and ORR bifunctional electrocatalysis. Appl. Surf. Sci. 2024, 663, 160201. [Google Scholar] [CrossRef]
- Gao, B.; Tan, J.; Wang, R.; Zeng, Q.; Wen, Y.; Zhang, Q.; Wang, J.; Zeng, Q. Intensive investigation of the synergistic effects between electrocatalysis and peroxymonosulfate activation for efficient organic elimination. J. Hazard. Mater. 2024, 479, 135719. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Zhao, C.; Mousset, E.; Khan, J.A.; Dionysiou, D.D. Decomposition of refractory organics in wastewater by electrocatalytic reduction: Mechanism, challenges, and future perspectives. Curr. Opin. Chem. Eng. 2024, 44, 101016. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Z.; Zhang, X.; Sun, A.; Liu, J. Interface engineering of double-layered nanosheets via cosynergistic modification by LDH interlayer carbonate anion and molybdate for accelerated industrial water splitting at high current density. Appl. Surf. Sci. 2022, 598, 153690. [Google Scholar] [CrossRef]
- Hu, X.; Guo, X.; Meng, T.; Guo, Q.; Cheng, J.; Wang, Y.; Huang, W. NiAlFe catalysts based on hydrotalcite-like precursors for low temperature CO2 methanation: Electronic effects among components and intrinsic activity of Ni site. Appl. Surf. Sci. 2024, 670, 160705. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.D.; Liu, J.; Yao, Y.; Yan, X.; Gu, Z.G. Intercalation-induced partial exfoliation of NiFe LDHs with abundant active edge sites for highly enhanced oxygen evolution reaction. J. Colloid Interface Sci. 2022, 607, 1353–1361. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Xie, R.; Chen, L.; Li, M.; Li, J.; Ji, B.; Hu, Z.; Li, J.; Song, L.; et al. Controlled self-assembled NiFe layered double hydroxides/reduced graphene oxide nanohybrids based on the solid-phase exfoliation strategy as an excellent electrocatalyst for the oxygen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 13545–13556. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, Q.; Wang, Z.; Li, Z.; Zhou, Q.; Li, X.; Zhao, D.; Lu, J.; Tian, S.; Li, Y.; et al. Two-Dimensional SnS Mediates NiFe-LDH-Layered Electrocatalyst toward Boosting OER Activity for Water Splitting. ACS Appl. Mater. Interfaces 2024, 16, 23054–23060. [Google Scholar] [CrossRef]
- Lu, X.; Xue, H.; Gong, H.; Bai, M.; Tang, D.; Ma, R.; Sasaki, T. 2D layered double hydroxide nanosheets and their derivatives toward efficient oxygen evolution reaction. Nano-Micro Lett. 2020, 12, 86. [Google Scholar] [CrossRef]
- He, L.; Zhou, Y.; Wang, M.; Li, S.; Lai, Y. Recent Progress on Stability of Layered Double Hydroxide-Based Catalysts for Oxygen Evolution Reaction. Nanomaterials 2024, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Gu, Q.; Zhu, S.; Wang, L.; Peng, H.; Zhao, Y. Implanting cation vacancies in Ni-Fe LDHs for efficient oxygen evolution reactions of lithium-oxygen batteries. Appl. Catal. B Environ. 2021, 285, 119792. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Sun, S.; Sun, H.; Yang, C.; Cai, Z.; Zhang, H.; Yue, M.; Zhang, M.; Wang, H.; et al. Oxalate anions-intercalated NiFe layered double hydroxide as a highly active and stable electrocatalyst for alkaline seawater oxidation. J. Colloid Interface Sci. 2024, 662, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Deng, X.; Li, W.; Ma, Z.; Wang, X. Electrochemical-induced surface reconstruction to NiFe-LDHs-based heterostructure as novel positive electrode for supercapacitors with enhanced performance in neutral electrolyte. Chem. Eng. J. 2022, 449, 137886. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, K.; Xie, J.; Wang, Y.; Wang, J. Construction of core-shell NiFe LDH/Co(OH) F amorphous/crystalline heterostructure for synergistically enhanced electrocatalytic water oxidation. J. Alloys Compd. 2024, 1010, 177889. [Google Scholar] [CrossRef]
- Pastor, E.; Sachs, M.; Selim, S.; Durrant, J.R.; Bakulin, A.A.; Walsh, A. Electronic defects in metal oxide photocatalysts. J. Nat. Rev. Mater. 2024, 7, 503–521. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Yan, Z.; Chen, Y.; Liu, H.; Du, X. Tailoring the oxygen evolution reaction activity of lanthanide-doped NiFe-LDHs through lanthanide contraction. Chem. Eng. J. 2024, 496, 154059. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Huang, J.; Yao, S.; Jiang, R.; Hou, Y.; Tang, W.; Sun, P.; Huang, H.; Wang, M.; et al. Redox-active ligands enhance oxygen evolution reaction activity: Regulating the spin state of ferric ions and accelerating electron transfer. J. Colloid Interface Sci. 2023, 650, 1182–1192. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Q.; Yao, Z.; Liang, T.; Zhang, S.; Zhu, T.; Xing, C.; Pan, J.; Yu, Z.; Liang, K. Inducing spin polarization via Co doping in the BiVO4 cell to enhance the built-in electric field for promotion of photocatalytic CO2 reduction. J. Colloid Interface Sci. 2024, 664, 500–510. [Google Scholar] [CrossRef]
- Getzner, L.; Paliwoda, D.; Vendier, L.; Lawson-Daku, L.M.; Rotaru, A.; Molnár, G.; Cobo, S.; Bousseksou, A. Combining electron transfer, spin crossover, and redox properties in metal-organic frameworks. Nat. Commun. 2024, 15, 7192. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Z.; Liu, Y.; Tian, W.; Huang, Z.; Zhao, X.; Wang, L.; Wang, S.; Ma, J. Enhanced ferrate(VI) oxidation of organic pollutants through direct electron transfer. Water Res. 2023, 244, 120506. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, S.; Yang, H.; Xi, S.; Gracia, J.; Xu, Z.J. Spin-related electron transfer and orbital interactions in oxygen electrocatalysis. Adv. Mater. 2020, 32, 2003297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Borca, C.N.; Huthwelker, T.; Yüzbasi, N.S.; Baster, D.; El Kazzi, M.; Schneider, C.W.; Schmidt, T.J.; Fabbri, E. Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment. Nat. Commun. 2024, 15, 3067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Gong, Z.; Wang, R.; Guo, M.; Zeng, R.; Li, Y.; Xiao, Z.; Qie, L.; Liu, J. Band Structure and Spin-State-Induced Electronic Configuration Regulation for Efficient Sulfur Redox Reaction. Adv. Funct. Mater. 2024, 35, 2417730. [Google Scholar] [CrossRef]
- He, Z.D.; Tesch, R.; Eslamibidgoli, M.J.; Eikerling, M.H.; Kowalski, P.M. Low-spin state of Fe in Fe-doped NiOOH electrocatalysts. Nat. Commun. 2023, 14, 3498. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Wang, Z.-L.; Zhu, H.-B. Regulating Fe-spin state by doping P heteroatom in Fe-NC catalyst derived from colloidal nanoparticles encapsulated ZIF-8 to boost oxygen reduction activity. J. Energy Storage 2024, 98, 113197. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Cai, W.; Song, W.; Guo, S.; Zhu, C. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 2023, 9, 181–197. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.S.; Wang, H.F.; Zhang, Q.; Tian, G.L.; Nie, J.Q.; Wei, F. Spatially confined hybridization of nanometer-sized NiFe hydroxides into nitrogen-doped graphene frameworks leading to superior oxygen evolution reactivity. Adv. Mater. 2015, 27, 4516–4522. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning electronic structure of NiFe layered double hydroxides with vanadium doping toward high efficient electrocatalytic water oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, F.; Zhang, S.J.; Fisher, A.; Zhou, Y.; Wang, Z.; Li, Y.; Xu, B.B.; Li, J.T.; Sun, S.G. Interfacial interaction between FeOOH and Ni-Fe LDH to modulate the local electronic structure for enhanced OER electrocatalysis. ACS Catal. 2018, 8, 11342–11351. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, R.; Pan, L.; Hou, F.; Zhao, Y.; Shen, Z.; Mi, W.; Shi, C.; Wang, Q.; Zhang, X.; et al. Regulating the spin state of FeIII by atomically anchoring on ultrathin titanium dioxide for efficient oxygen evolution electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Widhiastuti, F.; Fan, L.; Paz-Ferreiro, J.; Chiang, K. Oxidative treatment of bisphenol A by Fe(VI) and Fe(VI)/H2O2 and identification of the degradation products. Environ. Technol. Innov. 2022, 28, 102643. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, Y.; Niu, L.; Huang, X.; Yu, X.; Feng, M. Fe(IV)/Fe(V)-mediated polyferric sulfate/periodate system: A novel coagulant/oxidant strategy in promoting micropollutant abatement. J. Hazard. Mater. 2024, 466, 133614. [Google Scholar] [CrossRef]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, Q.; Liu, Y.; Deng, Y.; Fan, X.; Xu, K.; Ma, Y.; He, J. Efficient activation of peroxymonosulfate by Fe single-atom: The key role of Fe-pyrrolic nitrogen coordination in generating singlet oxygen and high-valent Fe species. J. Hazard. Mater. 2024, 462, 132753. [Google Scholar] [CrossRef]
- Mehdi, M.; An, B.-S.; Kim, H.; Lee, S.; Lee, C.; Seo, M.; Noh, M.W.; Cho, W.-C.; Kim, C.-H.; Choi, C.H.; et al. Rational design of a stable Fe-rich Ni-Fe layered double hydroxide for the industrially relevant dynamic operation of alkaline water electrolyzers. Adv. Energy Mater. 2023, 13, 2204403. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, X.; Xue, N.; Qiu, P.; Chen, Y.; Teng, D.; Zhang, Z.; Liu, F.; Liu, Z.; Guo, Z. Insight on the Ultrafast Water Treatment over NiFe-Layered Double Hydroxides via Electroactivation of Ferrate(VI): The Role of Spin State Regulation. Water 2025, 17, 1369. https://doi.org/10.3390/w17091369
Gai X, Xue N, Qiu P, Chen Y, Teng D, Zhang Z, Liu F, Liu Z, Guo Z. Insight on the Ultrafast Water Treatment over NiFe-Layered Double Hydroxides via Electroactivation of Ferrate(VI): The Role of Spin State Regulation. Water. 2025; 17(9):1369. https://doi.org/10.3390/w17091369
Chicago/Turabian StyleGai, Xinyu, Ningxuan Xue, Pengxiang Qiu, Yiyang Chen, Da Teng, Zhihui Zhang, Fengling Liu, Zhongyi Liu, and Zhaobing Guo. 2025. "Insight on the Ultrafast Water Treatment over NiFe-Layered Double Hydroxides via Electroactivation of Ferrate(VI): The Role of Spin State Regulation" Water 17, no. 9: 1369. https://doi.org/10.3390/w17091369
APA StyleGai, X., Xue, N., Qiu, P., Chen, Y., Teng, D., Zhang, Z., Liu, F., Liu, Z., & Guo, Z. (2025). Insight on the Ultrafast Water Treatment over NiFe-Layered Double Hydroxides via Electroactivation of Ferrate(VI): The Role of Spin State Regulation. Water, 17(9), 1369. https://doi.org/10.3390/w17091369





