Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater
Abstract
1. Introduction
2. Leading Treatment Techniques of PFASs
2.1. Adsorption
2.1.1. Activated Carbon
2.1.2. Resins
2.1.3. Minerals
2.1.4. Molecularly Imprinted Polymer
2.1.5. New-Generation Adsorbents
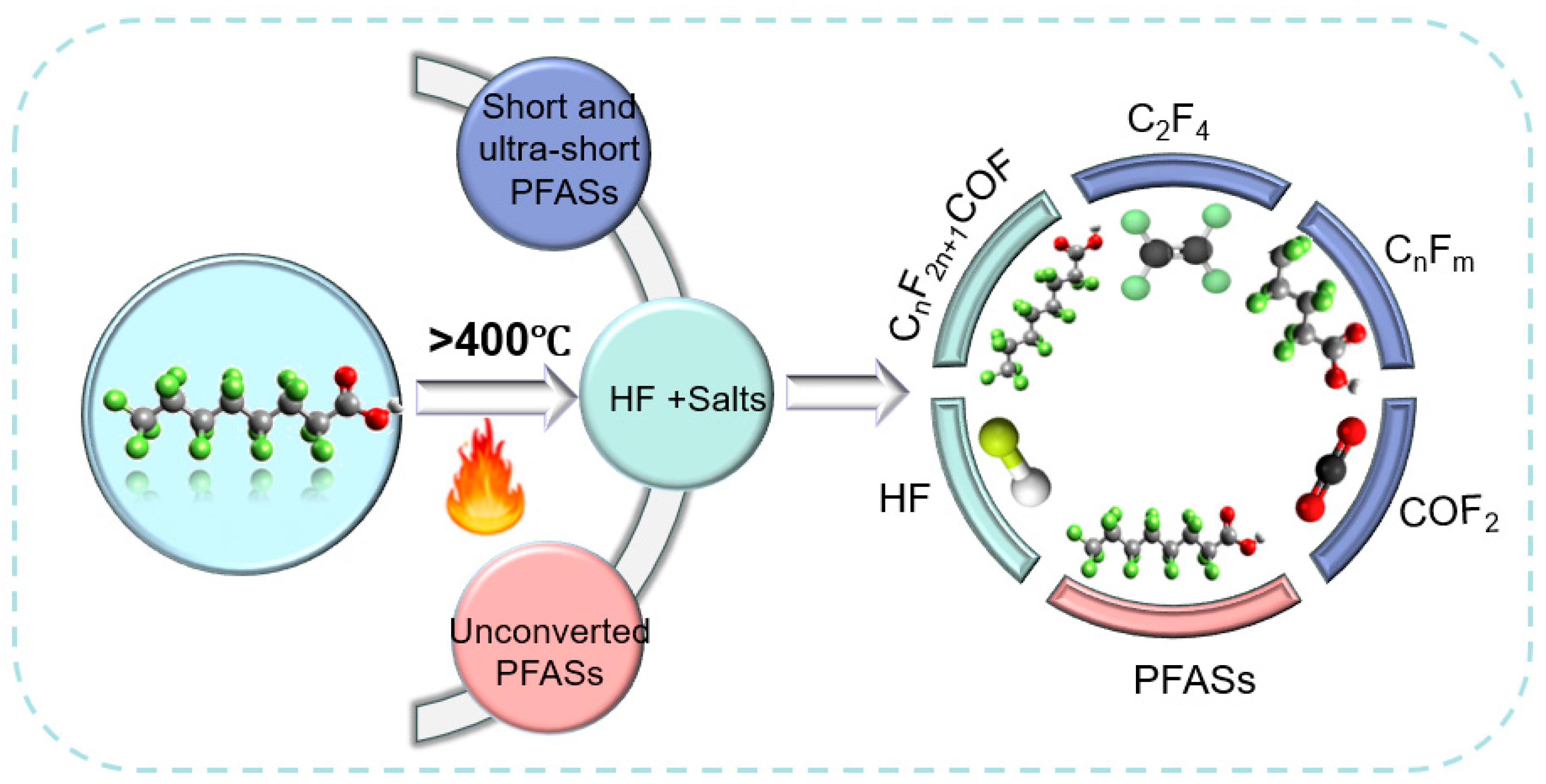
2.2. Thermal Treatment/Thermal Degradation
2.3. Biodegradation Processes
2.4. Oxidation Technologies
2.4.1. Photodegradation
2.4.2. Fenton Oxidation
2.4.3. Sonochemical Degradation
2.4.4. Electrochemical Oxidation
3. Alternative Treatment Techniques
3.1. Foam Fractionation
3.2. Constructed Wetland
3.3. Piezoelectric Ball Milling
4. Future Research Directions
4.1. Removal of Short-Chain PFASs
4.2. Remediation of PFASs Contamination
4.3. Exploring the Degradation Mechanism of Multi-Structured PFAS
4.4. Increased Exploration of Low-Cost Environmental Remediation Technologies for PFASs
4.5. Development of PFASs Alternatives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghanbari, R.; Wu, D.; Heynderickx, P.M. Fabrication of MXene-based membranes and their application in per- and polyfluorinated substances removal: Comparison with commercial membranes, challenges, and future improvements. Coord. Chem. Rev. 2025, 523, 216253. [Google Scholar] [CrossRef]
- Kang, P.; Zhao, Y.; Wei, T.; Cai, Y.; Ji, B.; Addo-Bankas, O. Interactions between MPs and PFASs in aquatic environments: A dual-character situation. J. Environ. Manag. 2024, 351, 119907. [Google Scholar] [CrossRef]
- Yan, P.-F.; Dong, S.; Pennell, K.D.; Cápiro, N.L. A review of the occurrence and microbial transformation of per- and polyfluoroalkyl substances (PFAS) in aqueous film-forming foam (AFFF)-impacted environments. Sci. Total Environ. 2024, 927, 171883. [Google Scholar] [CrossRef] [PubMed]
- Restivo, J.; Orge, C.A.; Soares, O.S.G.P.; Pereira, M.F.R. A review of current and prospective catalytic routes for the management of PFAs contamination in water. J. Environ. Chem. Eng. 2024, 12, 112859. [Google Scholar] [CrossRef]
- Eichler, C.M.A.; Little, J.C. A framework to model exposure to per- and polyfluoroalkyl substances in indoor environments. Environ. Sci. Process. Impacts 2020, 22, 500–511. [Google Scholar] [CrossRef]
- Hartmann, H.; Weiss, V.; Loberg, M.; Lee, E. PFAS-mediated disruptions on thyroid histology. Am. J. Clin. Pathol. 2024, 162, S94. [Google Scholar] [CrossRef]
- Wallace, M.A.G.; Smeltz, M.G.; Mattila, J.M.; Liberatore, H.K.; Jackson, S.R.; Shields, E.P.; Xhani, X.; Li, E.Y.; Johansson, J.H. A review of sample collection and analytical methods for detecting per- and polyfluoroalkyl substances in indoor and outdoor air. Chemosphere 2024, 358, 142129. [Google Scholar] [CrossRef]
- Alcorn, T. Extensive contamination with potential carcinogen prompts regulation across the USA. Lancet Oncol. 2020, 21, 340. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X. Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review. Foods 2023, 12, 2624. [Google Scholar] [CrossRef]
- Tulcan, R.X.S.; Yarleque, C.M.H.; Lu, X.; Yeerkenbieke, G.; Herrera, V.O.; Gunarathne, V.; Yánez-Jácome, G.S. Characterization of per- and polyfluoroalkyl substances (PFASs) in Chinese river and lake sediments. J. Hazard. Mater. 2025, 489, 137680. [Google Scholar] [CrossRef]
- Guo, P.; Furnary, T.; Vasiliou, V.; Yan, Q.; Nyhan, K.; Jones, D.P.; Johnson, C.H.; Liew, Z. Non-targeted metabolomics and associations with per- and polyfluoroalkyl substances (PFAS) exposure in humans: A scoping review. Environ. Int. 2022, 162, 107159. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Y.; Li, Y.; Li, R.; Pei, Y.; Shi, Y.; Wang, H. Constructing multiple sites porous organic polymers for highly efficient and reversible adsorption of triiodide ion from water. Green Energy Environ. 2025; in press. [Google Scholar] [CrossRef]
- Xu, D.; Wu, S.; Li, Z.; Wu, S.; Xu, J.; Gu, C. Aerobic granular sludge treating tannery wastewater and particle size control. AIP Adv. 2025, 15, 025128. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.-L.; Min, Y.-T.; Chen, S.; Yang, W.; Gu, J.-T.; Feng, W.-J.; Li, Y.; Hong, C.; Du, J.; et al. Fukushima Contaminated Water Risk Factor: Global Implications. Environ. Sci. Technol. 2025, 59, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, A.-H.; Seo, Y.E.; Kho, Y.; Choi, K. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Potable Groundwater near Military Bases in South Korea. Environ. Sci. Technol. Lett. 2025, 12, 440–446. [Google Scholar] [CrossRef]
- Davidsen, N.; Lauvås, A.J.; Myhre, O.; Ropstad, E.; Carpi, D.; Gyves, E.M.-d.; Berntsen, H.F.; Dirven, H.; Paulsen, R.E.; Bal-Price, A.; et al. Exposure to human relevant mixtures of halogenated persistent organic pollutants (POPs) alters neurodevelopmental processes in human neural stem cells undergoing differentiation. Reprod. Toxicol. 2021, 100, 17–34. [Google Scholar] [CrossRef]
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J.; Kanno, J.; Abdallah, M.; Blystone, C.R.; Calkins, M.M.; Dong, G.-H.; Dorman, D.C.; et al. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. Lancet Oncol. 2023, 25, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. A review of the occurrence and distribution of Per- and polyfluoroalkyl substances (PFAS) in human organs and fetal tissues. Environ. Res. 2025, 272, 121181. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Li, W.; Shen, J.; Zhang, B.; Li, P.; Han, R.; Cao, C.; Wang, R. Association between exposure to per- and polyfluoroalkyl substances (PFAS) and chronic cough in American adults: Results from NHANES 2003–2012. Ecotoxicol. Environ. Saf. 2025, 291, 117901. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Ji, Q.; Yang, S.; Yang, F. Association of per- and polyfluoroalkylated substances/heavy metals and bone health in children and adolescents. Front. Public Health 2024, 12, 1431001. [Google Scholar] [CrossRef]
- Zhu, H.; Xia, Y.; Zhang, Y.; Kang, Y.; Ding, Y.; Chen, R.; Feng, H. Distribution characteristics and transformation mechanism of per- and polyfluoroalkyl substances in drinking water sources: A review. Sci. Total Environ. 2024, 916, 169566. [Google Scholar] [CrossRef]
- Tewfik, E.-L.; Noisel, N.; Verner, M.-A. Biomonitoring equivalents for perfluorooctanoic acid (PFOA) for the interpretation of biomonitoring data. Environ. Int. 2023, 179, 108170. [Google Scholar] [CrossRef] [PubMed]
- UNEP. PFASs Listed Under the Stockholm Convention [WWW Document]. Stockholm Convention on Persistent Organic Pollutants (POPs). 2024. Available online: https://www.pops.int/Implementation/IndustrialPOPs/PFAS/Overview/tabid/5221/Default.aspx (accessed on 15 March 2025).
- MEEPRC. Amendment to Annex a for the Inclusion of Perfluorooctanoic Acid (PFOA), Its Salts and Its Related Compounds; MEEPRC: Beijing, China, 2020. (In Chinese) [Google Scholar]
- MEEPRC. Key Controlled List of New Pollutants (2023 Edition); MEEPRC: Beijing, China, 2023. [Google Scholar]
- Liu, J.; Xie, Y.; Zhou, L.; Lu, G.; Li, Y.; Gao, P.; Hou, J. Co-accumulation characteristics and interaction mechanism of microplastics and PFASs in a large shallow lake. J. Hazard. Mater. 2024, 480, 135780. [Google Scholar] [CrossRef]
- Zeeshan, M.; Tabraiz, S.; Hashmi, S.I.; Iqbal, A.; Dittmann, D.; Abbas, Z.; MacLeod, C.L.; Ruhl, A.S. A comprehensive overview on the occurrence and removal of per- and polyfluoroalkyl substances through adsorption and biodegradation. Bioresour. Technol. Rep. 2025, 29, 102077. [Google Scholar] [CrossRef]
- Hussain, H.N.; Jilani, M.I.; Imtiaz, F.; Ahmed, T.; Arshad, M.B.; Mudassar, M.; Sharif, M.N. Advances in the removal of Polyfluoroalkyl Substances (PFAS) from water using destructive and non-destructive methods. Green Anal. Chem. 2025, 12, 100225. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Bandosz, T.J. Activated carbon versus metal-organic frameworks: A review of their PFAS adsorption performance. J. Hazard. Mater. 2022, 425, 127810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Sun, M. Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res. 2021, 207, 117781. [Google Scholar] [CrossRef] [PubMed]
- Sasi, P.C.; Alinezhad, A.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. Effect of granular activated carbon and other porous materials on thermal decomposition of per- and polyfluoroalkyl substances: Mechanisms and implications for water purification. Water Res. 2021, 200, 117271. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344, 126223. [Google Scholar] [CrossRef]
- Liu, F.; Guan, X.; Xiao, F. Photodegradation of per- and polyfluoroalkyl substances in water: A review of fundamentals and applications. J. Hazard. Mater. 2022, 439, 129580. [Google Scholar] [CrossRef]
- Schlesinger, D.R.; McDermott, C.; Le, N.Q.; Ko, J.S.; Johnson, J.K.; Demirev, P.A.; Xia, Z. Destruction of per/poly-fluorinated alkyl substances by magnetite nanoparticle-catalyzed UV-Fenton reaction. Environ. Sci. Water Res. Technol. 2022, 8, 2732–2743. [Google Scholar] [CrossRef]
- Fuller, M.E.; Zhao, Y.; Hedman, P.C.; Koster van Groos, P.G.; Soto, A.; Boodoo, F.; Yniguez, J.; McKenzie, E.R. Sonochemical degradation of PFAS in ion exchange regeneration wastes. J. Hazard. Mater. 2024, 471, 134291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.; Vo, P.H.N.; Shukla, P.; Ge, L.; Zhao, C.-X. Electrochemical advanced oxidation of per- and polyfluoroalkyl substances (PFASs): Development, challenges and perspectives. Chem. Eng. J. 2024, 500, 157222. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Zhou, B.; Yuan, R.; Chen, Z.; Chen, H. Removal of PFASs from water by carbon-based composite photocatalysis with adsorption and catalytic properties: A review. Sci. Total Environ. 2022, 836, 155652. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Gao, B.; Ji, R.; Li, C.; Wang, S. Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2379–2414. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef] [PubMed]
- Karbassiyazdi, E.; Kasula, M.; Modak, S.; Pala, J.; Kalantari, M.; Altaee, A.; Esfahani, M.R.; Razmjou, A. A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents. Chemosphere 2023, 311, 136933. [Google Scholar] [CrossRef]
- Becskereki, G.; Horvai, G.; Tóth, B. The Selectivity of Molecularly Imprinted Polymers. Polymers 2021, 13, 1781. [Google Scholar] [CrossRef]
- Tasfaout, A.; Ibrahim, F.; Morrin, A.; Brisset, H.; Sorrentino, I.; Nanteuil, C.; Laffite, G.; Nicholls, I.A.; Regan, F.; Branger, C. Molecularly imprinted polymers for per- and polyfluoroalkyl substances enrichment and detection. Talanta 2023, 258, 124434. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of Per- and Polyfluoroalkyl Substances: Mechanisms and Implications for Thermal Treatment Processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef]
- Li, H.; Junker, A.L.; Wen, J.; Ahrens, L.; Sillanpää, M.; Tian, J.; Cui, F.; Vergeynst, L.; Wei, Z. A recent overview of per- and polyfluoroalkyl substances (PFAS) removal by functional framework materials. Chem. Eng. J. 2023, 452, 139202. [Google Scholar] [CrossRef]
- Bi, Y.; Meng, X.; Tan, Z.; Geng, Q.; Peng, J.; Yong, Q.; Sun, X.; Guo, M.; Wang, X. A novel ZIF-L/PEI thin film nanocomposite membrane for removing perfluoroalkyl substances (PFASs) from water: Enhanced retention and high flux. Sci. Total Environ. 2024, 925, 171727. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; He, L.; Wang, J.; Wang, S.; Shi, X.; Zhang, X.; Wang, H.; He, F. Environmental behavior of per- and polyfluoroalkyl substances (PFASs) and the potential role of biochar for its remediation: A review. Biochar 2025, 7, 14. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Gong, T.; Wu, Y.; Chen, G. Cutting-edge technologies and relevant reaction mechanism difference in treatment of long- and short-chain per- and polyfluoroalkyl substances: A review. Chemosphere 2024, 354, 141692. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Lin, T.; Zhang, X.; Jiang, F.; Chen, H. Impact of biological activated carbon filtration and backwashing on the behaviour of PFASs in drinking water treatment plants. J. Hazard. Mater. 2023, 446, 130641. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Boyer, T.H.; Fang, Y.; Liu, C.J.; Strathmann, T.J. Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per- and polyfluoroalkyl substances (PFASs). Water Res. 2023, 243, 120324. [Google Scholar] [CrossRef]
- Fang, Y.; Ellis, A.; Choi, Y.J.; Boyer, T.H.; Higgins, C.P.; Schaefer, C.E.; Strathmann, T.J. Removal of Per- and Polyfluoroalkyl Substances (PFASs) in Aqueous Film-Forming Foam (AFFF) Using Ion-Exchange and Nonionic Resins. Environ. Sci. Technol. 2021, 55, 5001–5011. [Google Scholar] [CrossRef]
- Huang, J.; Fu, K.; Fang, Z.; Luo, J. Enhanced selective removal of PFAS at trace level using quaternized cellulose-functionalized polymer resin: Performance and mechanism. Water Res. 2025, 272, 122937. [Google Scholar] [CrossRef]
- Ahrens, L.; Lundgren, S.; McCleaf, P.; Köhler, S. Removal of perfluoroalkyl substances (PFAS) from different water types by techniques based on anion exchange (AIX), powdered activated carbon (PAC), iron(III) chloride and nanofiltration (NF) membrane—A systematic comparison. Sci. Total Environ. 2025, 970, 179004. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Zhang, W.L.; Liang, Y.N. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—A review. Sci. Total Environ. 2019, 694, 133606. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, J.A.R.; Bourg, I.C. Molecular dynamics simulation of the adsorption of per- and polyfluoroalkyl substances (PFASs) on smectite clay. J. Colloid Interface Sci. 2021, 585, 337–346. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, J.; Liu, Y.; Wang, M.; Deng, S.; Xia, Z. A SPE Method with Two MIPs in Two Steps for Improving the Selectivity of MIPs. Anal. Chem. 2019, 91, 8436–8442. [Google Scholar] [CrossRef] [PubMed]
- Gomri, C.; Benkhaled, B.T.; Cretin, M.; Semsarilar, M. Adsorbent Material Used for the Treatment of Per- and Poly-fluoroalkyl Substances (PFAS): A Short Review. Macromol. Chem. Phys. 2024, 225, 2400012. [Google Scholar] [CrossRef]
- Liu, F.; Pignatello, J.J.; Sun, R.; Guan, X.; Xiao, F. A Comprehensive Review of Novel Adsorbents for Per- and Polyfluoroalkyl Substances in Water. ACS ES&T Water 2024, 4, 1191–1205. [Google Scholar] [CrossRef]
- Ou, X.; He, M.; Chen, B.; Hu, B. Covalent organic frameworks based hierarchical porous hybrid monolithic capillary: Synthesis, characterization, and applications in trace metals analysis. J. Hazard. Mater. 2024, 462, 132680. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Shao, H.; Zhou, S.; Yu, G.; Deng, S. Cationic covalent organic framework for efficient removal of PFOA substitutes from aqueous solution. Chem. Eng. J. 2021, 412, 127509. [Google Scholar] [CrossRef]
- Song, X.; Wang, R.; Wang, X.; Han, H.; Qiao, Z.; Sun, X.; Ji, W. An amine-functionalized olefin-linked covalent organic framework used for the solid-phase microextraction of legacy and emerging per- and polyfluoroalkyl substances in fish. J. Hazard. Mater. 2022, 423, 127226. [Google Scholar] [CrossRef]
- Mohd Azmi, L.H.; Williams, D.R.; Ladewig, B.P. Polymer-assisted modification of metal-organic framework MIL-96 (Al): Influence of HPAM concentration on particle size, crystal morphology and removal of harmful environmental pollutant PFOA. Chemosphere 2021, 262, 128072. [Google Scholar] [CrossRef]
- Ilango, A.K.; Mekkat, R.; Jeyalakshmi, V.; Pervez, M.N.; Jiang, T.; Chand, P.; Kumaran, Y.; Efstathiadis, H.; Sukalingum, D.; Soos, M.; et al. Enhanced removal of PFAS in water using activated ZIF-8 carbons: High adsorption efficiency, repeatable regenerability and reusability. Chem. Eng. J. 2025, 507, 160192. [Google Scholar] [CrossRef]
- Chen, G.; Kobashi, K.; Futaba, D.N. Unexpected structural scaling and predictability in carbon nanotubes. J. Mater. Sci. Technol. 2025, 231, 30–35. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Deng, S.; Wang, W.; Gholami, Z.; Gholami, F.; Dastyar, W.; Mojiri, A.; Blaney, L. Regeneration of exhausted adsorbents after PFAS adsorption: A critical review. J. Hazard. Mater. 2024, 471, 134429. [Google Scholar] [CrossRef] [PubMed]
- Oyetade, O.A.; Varadwaj, G.B.B.; Nyamori, V.O.; Jonnalagadda, S.B.; Martincigh, B.S. A critical review of the occurrence of perfluoroalkyl acids in aqueous environments and their removal by adsorption onto carbon nanotubes. Rev. Environ. Sci. Bio/Technol. 2018, 17, 603–635. [Google Scholar] [CrossRef]
- Yin, S.; Villagrán, D. Design of nanomaterials for the removal of per- and poly-fluoroalkyl substances (PFAS) in water: Strategies, mechanisms, challenges, and opportunities. Sci. Total Environ. 2022, 831, 154939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Wang, B.; Lu, Z.; Wu, C.; He, Z.; Jiang, L.; Wei, P.; Yi, T. Constructing Activatable Photosensitizers Using Covalently Modified Mesoporous Silica. Adv. Sci. 2025, 12, e2406887. [Google Scholar] [CrossRef]
- Barisci, S.; Suri, R. Occurrence and removal of poly/perfluoroalkyl substances (PFAS) in municipal and industrial wastewater treatment plants. Water Sci. Technol. 2021, 84, 3442–3468. [Google Scholar] [CrossRef]
- Lassen, S.; Niemeyer, B. New silica-based adsorbents for water purification: Removal of short- and long-chain perfluoroalkyl sulfonic acids (PFSA) at sub-nanomolar concentrations. Appl. Water Sci. 2024, 14, 183. [Google Scholar] [CrossRef]
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: A comprehensive review. Environ. Sci. Process. Impacts 2022, 24, 196–208. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Garg, A.; Shetti, N.P.; Basu, S.; Nadagouda, M.N.; Aminabhavi, T.M. Treatment technologies for removal of per- and polyfluoroalkyl substances (PFAS) in biosolids. Chem. Eng. J. 2023, 453, 139964. [Google Scholar] [CrossRef]
- DiStefano, R.; Feliciano, T.; Mimna, R.A.; Redding, A.M.; Matthis, J. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediat. J. 2022, 32, 231–238. [Google Scholar] [CrossRef]
- Kumar, R.; Dada, T.K.; Whelan, A.; Cannon, P.; Sheehan, M.; Reeves, L.; Antunes, E. Microbial and thermal treatment techniques for degradation of PFAS in biosolids: A focus on degradation mechanisms and pathways. J. Hazard. Mater. 2023, 452, 131212. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Hedayati Marzbali, M.; Pramanik, B.K.; Paz-Ferreiro, J.; de Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 2020, 7, 638–649. [Google Scholar] [CrossRef]
- Trang, B.; Li, Y.; Xue, X.-S.; Ateia, M.; Houk, K.N.; Dichtel, W.R. Low-temperature mineralization of perfluorocarboxylic acids. Science 2022, 377, 839–845. [Google Scholar] [CrossRef]
- Amin, A.M.; Luo, Y.; Nolan, A.; Mallavarapu, M.; Naidu, R.; Fang, C. Thermal kinetics of PFAS and precursors in soil: Experiment and surface simulation in temperature-time plane. Chemosphere 2023, 318, 138012. [Google Scholar] [CrossRef]
- Berhanu, A.; Mutanda, I.; Taolin, J.; Qaria, M.A.; Yang, B.; Zhu, D. A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): Biotransformation routes and enzymes. Sci. Total Environ. 2022, 859, 160010. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P. Why Is the Biodegradation of Polyfluorinated Compounds So Rare? mSphere 2021, 6, e0072121. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Ribeiro, I.; Pinto, L.M.; Cambra, R.; Oliveira, R.S.; Pereira, F.; Carvalho, M.F. Biodegradation of mono-, di- and trifluoroacetate by microbial cultures with different origins. New Biotechnol. 2018, 43, 23–29. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, V.; Sugumar, V.; Umesh, M.; Sondhi, S.; Chakraborty, P.; Kaur, K.; Thomas, J.; Kamaraj, C.; Maitra, S.S. A comprehensive review on the need for integrated strategies and process modifications for per- and polyfluoroalkyl substances (PFAS) removal: Current insights and future prospects. Case Stud. Chem. Environ. Eng. 2024, 9, 100623. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Gu, L.; Hua, Z.-L.; Wang, D.-W.; Xu, R.-Y.; Ge, X.-Y.; Chu, K.-J. Removal of Per-, Poly-fluoroalkyl substances (PFASs) and multi-biosphere community dynamics in a bacteria-algae symbiotic aquatic ecosystem. Environ. Pollut. 2022, 314, 120266. [Google Scholar] [CrossRef]
- You, J.; Ye, L.; Zhang, S.; Zhao, J.; Zhao, Y.; He, Y.; Chen, J.; Kennes, C.; Chen, D. Electrode functional microorganisms in bioelectrochemical systems and its regulation: A review. Biotechnol. Adv. 2025, 79, 108521. [Google Scholar] [CrossRef]
- Lorah, M.M.; He, K.; Blaney, L.; Akob, D.M.; Harris, C.; Tokranov, A.; Hopkins, Z.; Shedd, B.P. Anaerobic biodegradation of perfluorooctane sulfonate (PFOS) and microbial community composition in soil amended with a dechlorinating culture and chlorinated solvents. Sci. Total Environ. 2024, 932, 172996. [Google Scholar] [CrossRef]
- Che, S.; Jin, B.; Liu, Z.; Yu, Y.; Liu, J.; Men, Y. Structure-Specific Aerobic Defluorination of Short-Chain Fluorinated Carboxylic Acids by Activated Sludge Communities. Environ. Sci. Technol. Lett. 2021, 8, 668–674. [Google Scholar] [CrossRef]
- Grgas, D.; Petrina, A.; Štefanac, T.; Bešlo, D.; Dragičević, T.L. A Review: Per- and Polyfluoroalkyl Substances—Biological Degradation. Toxics 2023, 11, 446. [Google Scholar] [CrossRef]
- Huang, S.; Jaffe, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhu, G.; Pei, T.; Guo, G.; Wang, X.; Zhao, Y. Methods for sample pretreatment and detection of per- and polyfluoroalkyl substances in biological samples: A review. TrAC Trends Anal. Chem. 2025, 187, 118206. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.-X.; Qu, J.-P.; Kang, Y.-B. Photocatalytic low-temperature defluorination of PFASs. Nature 2024, 635, 610–617. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Y.; Yu, X.; Wu, S.; Wang, W.; Yu, Q.; Wang, C.; Liang, Y.; Sun, H. Unveiling the Contribution of Hydrogen Radicals to Per- and Polyfluoroalkyl Substances (PFASs) Defluorination: Applicability and Degradation Mechanisms. Environ. Sci. Technol. 2025, 59, 1875–1886. [Google Scholar] [CrossRef]
- Kim, T.; Eom, S.; Kim, M.-K.; Zoh, K.-D. Degradation and defluorination of C6F13 PFASs with different functional groups by VUV/UV-based reduction and oxidation processes. J. Hazard. Mater. 2025, 488, 137216. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Awoyemi, O.S.; Naidu, R.; Fang, C. Advancements on Ultrasonic Degradation of Per- and Polyfluoroalkyl Substances (PFAS): Toward Hybrid Approaches. Environments 2024, 11, 187. [Google Scholar] [CrossRef]
- Brillas, E. A review on the application of single and combined Fenton, photo-Fenton, and electrochemical advanced oxidation processes to remove diclofenac from aqueous media. J. Environ. Chem. Eng. 2025, 13, 115443. [Google Scholar] [CrossRef]
- Brillas, E. Fenton, photo-Fenton, electro-Fenton, and their combined treatments for the removal of insecticides from waters and soils. A review. Sep. Purif. Technol. 2022, 284, 120290. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochemistry 2020, 69, 105245. [Google Scholar] [CrossRef]
- Jun, B.; Choi, J.; Son, Y. Ultrasonic Activation of Persulfate for the Removal of BPA in 20, 28, and 300 kHz Systems. Ultrason. Sonochemistry 2025, 114, 107281. [Google Scholar] [CrossRef]
- Lei, Y.-J.; Tian, Y.; Sobhani, Z.; Naidu, R.; Fang, C. Synergistic degradation of PFAS in water and soil by dual-frequency ultrasonic activated persulfate. Chem. Eng. J. 2020, 388, 124215. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, C.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Li, L.; Wang, Y.; Huang, Q. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes. Water Res. 2020, 170, 115254. [Google Scholar] [CrossRef]
- Liu, X.; Shu, Y.; Pan, Y.; Zeng, G.; Zhang, M.; Zhu, C.; Xu, Y.; Wan, A.; Wang, M.; Han, Q.; et al. Electrochemical destruction of PFAS at low oxidation potential enabled by CeO2 electrodes utilizing adsorption and activation strategies. J. Hazard. Mater. 2025, 486, 137043. [Google Scholar] [CrossRef]
- Wang, W.; Mi, X.; Zhou, Z.; Zhou, S.; Li, C.; Hu, X.; Qi, D.; Deng, S. Novel insights into the competitive adsorption behavior and mechanism of per- and polyfluoroalkyl substances on the anion-exchange resin. J. Colloid Interface Sci. 2019, 557, 655–663. [Google Scholar] [CrossRef]
- Vo, P.H.N.; Buckley, T.; Xu, X.; Nguyen, T.M.H.; Rudolph, V.; Shukla, P. Foam fractionation of per- and polyfluoroalkyl substances (PFASs) in landfill leachate using different cosurfactants. Chemosphere 2023, 310, 136869. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Lewis, J.; Wiberg, K.; Wall, E.; Ahrens, L. Foam fractionation for removal of per- and polyfluoroalkyl substances: Towards closing the mass balance. Sci. Total Environ. 2023, 871, 162050. [Google Scholar] [CrossRef]
- Malovanyy, A.; Forsén, E.; Lihammar, R. Removal of per- and polyfluoroalkyl substances (PFAS) from municipal wastewater by foam fractionation. Water Res. 2024, 268, 122660. [Google Scholar] [CrossRef]
- Fang, B.; Chen, H.; Zhao, M.; Qiao, B.; Zhou, Y.; Wang, Y.; Zhang, Y.; Gao, M.; Wang, Y.; Yao, Y.; et al. Biotic and abiotic transformations of aqueous film-forming foam (AFFF)-derived emerging polyfluoroalkyl substances in aerobic soil slurry. Water Res. 2025, 276, 123284. [Google Scholar] [CrossRef]
- Savvidou, P.; Dotro, G.; Campo, P.; Coulon, F.; Lyu, T. Constructed wetlands as nature-based solutions in managing per-and poly-fluoroalkyl substances (PFAS): Evidence, mechanisms, and modelling. Sci. Total Environ. 2024, 934, 173237. [Google Scholar] [CrossRef]
- Ma, H.; Kang, Y.; Li, M.; Dong, J.; Wang, Y.; Xiao, J.; Guo, Z. Enhancement of perfluorooctanoic acid and perfluorooctane sulphonic acid removal in constructed wetland using iron mineral: Performance and mechanisms. J. Hazard. Mater. 2023, 447, 130819. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Wang, Y.; Qian, X. The fate and behavior of perfluorooctanoic acid (PFOA) in constructed wetlands: Insights into potential removal and transformation pathway. Sci. Total Environ. 2022, 861, 160309. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Yan, P.; Xie, H.; Zhang, J.; Wu, S.; Wu, H. A review of per- and polyfluoroalkyl substances (PFASs) removal in constructed wetlands: Mechanisms, enhancing strategies and environmental risks. Environ. Res. 2024, 262, 119967. [Google Scholar] [CrossRef]
- Qiao, W.; Li, R.; Tang, T.; Zuh, A.A. Removal, distribution and plant uptake of perfluorooctane sulfonate (PFOS) in a simulated constructed wetland system. Front. Environ. Sci. Eng. 2021, 15, 20. [Google Scholar] [CrossRef]
- Li, X.-q.; Hua, Z.-l.; Wu, J.-y.; Gu, L. Removal of perfluoroalkyl acids (PFAAs) in constructed wetlands: Considerable contributions of submerged macrophytes and the microbial community. Water Res. 2021, 197, 117080. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, C.; Wu, F.; Xu, H.; Zhao, Z.; Han, Z.; Zhang, H.; Yang, S. Enhanced removal of organic, nutrients, and PFCs in the iron-carbon micro-electrolysis constructed wetlands: Mechanism and iron cycle. Chem. Eng. J. 2022, 457, 141174. [Google Scholar] [CrossRef]
- Qian, X.; Huang, J.; Yan, C.; Xiao, J. Stimulation on microbial nitrogen metabolism by iron under PFAS exposure drives effective nitrogen removal in constructed wetland. J. Water Process Eng. 2023, 53, 103879. [Google Scholar] [CrossRef]
- Goukeh, M.N.; Alamdari, N. Removal of Contaminants in Stormwater via Subsurface-Flow Wetlands: A Review with Focus on Nutrients, Heavy Metals, and PFAS. J. Environ. Eng. 2024, 150, 03124001. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, Y.; Yang, Y.; Li, Q.; Man, Y.; Dai, Y.; Fu, J.; Wei, T.; Tai, Y.; Zhang, X. Curbing per- and polyfluoroalkyl substances (PFASs): First investigation in a constructed wetland-microbial fuel cell system. Water Res. 2023, 230, 119530. [Google Scholar] [CrossRef]
- Qian, X.; Huang, J.; Cao, C.; Yao, J. Innovative application of basalt fibers as biological carrier in constructed wetland-microbial fuel cell for improvement of performance under perfluorooctanoic acid exposure. Bioresour. Technol. 2024, 406, 131019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; You, L.; Hu, S.; Fang, J.; Hu, B.; Chen, Z. Enhancement of PFAS stress tolerance and wastewater treatment efficiency by arbuscular mycorrhizal fungi in constructed wetlands. Environ. Res. 2024, 263, 120148. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, X.; Zhu, M.; Liao, X.; Hou, S.; Yu, Y.; Fan, X. The strong alternating built-in electric field sourced by ball milling on Pb2BO3X (X = Cl, Br, I) piezoelectric materials contributes to high catalytic activity. Nano Energy 2022, 101, 107545. [Google Scholar] [CrossRef]
- Yang, N.; Yang, S.; Ma, Q.; Beltran, C.; Guan, Y.; Morsey, M.; Brown, E.; Fernando, S.; Holsen, T.M.; Zhang, W.; et al. Solvent-Free Nonthermal Destruction of PFAS Chemicals and PFAS in Sediment by Piezoelectric Ball Milling. Environ. Sci. Technol. Lett. 2023, 10, 198–203. [Google Scholar] [CrossRef]
- Yang, N.; Guan, Y.; Yang, S.; Ma, Q.; Olive, C.; Fernando, S.; Zhang, W.; Holsen, T.M.; Yang, Y. PFAS Destruction and Near-Complete Defluorination of Undiluted Aqueous Film-Forming Foams at Ambient Conditions by Piezoelectric Ball Milling. Environ. Sci. Technol. 2025, 59, 1854–1863. [Google Scholar] [CrossRef]





| Remediation Methods | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Adsorption | Different kinds of adsorbents were selected to adsorb the PFASs to achieve the removal effect. | Good removal; low cost; simple operation. | Adsorbent regeneration and disposal issues. | [29,30] |
| Thermal treatment | High-temperature conditions expose PFASs to air or oxygen, breaking the chemical bonds within their molecules. | Suitable for different types of PFASs (both long and short chains). | Requiring high-temperature conditions; generating harmful gases. | [31] |
| Biodegradation | Microorganisms destroy C–F bonds and defluorinate under aerobic or anaerobic conditions. | Good approach for treating unsaturated PFASs. | Long degradation cycle; limited degree of mineralization; the degradation efficiency affected by the degree of fluoridation of PFASs. | [32] |
| Photodegradation | PFAS removal using UV or visible light to generate hydrated electrons. | Reduced secondary pollution. | Low efficiency; high cost; small-scale application. | [33] |
| Fenton oxidation | Production of ·OH by reaction between ferrous ions (Fe2+) and hydrogen peroxide (H2O2). | Relatively low cost; highly maneuverable. | Generating byproducts; requiring long processing time. | [34] |
| Sonochemical degradation | A type of advanced oxidation treatment that uses ultrasonic irradiation to form high-temperature bubbles with highly oxidizing substances to degrade PFASs. | No need to add chemicals; compatible with other degradation technologies. | Small-scale application; high cost; easily affected by external influences (e.g., solution pH, viscosity coefficient, surface tension coefficient, solution temperature, etc.). | [35] |
| Electrochemical oxidation | Adsorption of PFASs onto the electrode by direct/indirect anodic oxidation, degradation by the electrode, or by reaction with other liquids. | High removal efficiency; short reaction time. | Generation of short-chain PFASs and toxic byproducts; high maintenance cost. | [36] |
| Adsorbents | Adsorption Principles | Advantages and Disadvantages | Removal Effects | References |
|---|---|---|---|---|
| Activated carbon (AC) | High specific surface area and pore structure for effective adsorption of PFASs through hydrophobic interaction. | Low cost, simple process; applicable to many existing treatment plants; high quality of treated effluent; ineffective against short-chain PFASs. | Conventional GAC removes 12% of PFOA but does not remove PFOS; powdered AC removes over 90% of PFOS and PFOA within 72 h at 25 °C. | [37,38] |
| Ion exchange resin | It is mainly adsorbed on its surface through ion exchange and electrostatic attraction. | Easy to operate and maintain; regeneration and reuse possibilities; high removal efficiency; not very effective at removing short-chain PFASs; performance is pH-sensitive. | Polystyrene-divinylbenzene (PS-DVB) resins removed >90% of all 35 PFAS compounds in 24 h, while polymethacrylate and polyacrylic resins removed >90% of less than half of the compounds. | [39] |
| Mineral materials | Mineral materials with positively charged surfaces adsorb negatively charged compounds (e.g., PFOA, PFOS, etc.) by electrostatic attraction. | Economical; large reserves; variety and wide range of applications, etc. | At concentrations below 1 ppm, the zeolite removes >80% perfluorocarboxylic acid (PFCA) and >60% perfluorosulfonic acid (PFSA) in less than 30 s. | [40] |
| Molecularly imprinted polymers (MIPs) | Synthesis of MIPs using precipitation polymerization enables highly selective adsorption of PFASs. | High selectivity but high synthesis cost. | The binding capacity of MIPs for PFASs varies significantly, ranging from 1.289 to 1455.5 mg⋅g−1 for PFOS and from 5.45 to 12.4 mg⋅g−1 for PFOA. | [41,42] |
| Carbon nanotubes (CNTs) | The specific surface area of CNTs decreases with increasing outer diameter, and those with small diameters adsorb more PFASs. | With a high specific surface area; strong hydrophobicity; poor dispersion in water; good mechanical properties; chemical and thermal stability; ineffective against short-chain PFASs. | Polyaniline nanotubes (PANTs) were prepared by chemical oxidation self-assembly. PFOS and PFOA were adsorbed by electrostatic interaction, while the adsorption capacities were as high as 1651 mg g−1 and 1100 mg g−1, respectively. | [43] |
| Metal–organic frameworks (MOFs) | Mainly dependent on electrostatic and hydrophobic interactions | High surface area; tunable porosity; higher stability; easy to adapt; higher synthesis cost; ineffective against short-chain PFASs. | Thin-film nanocomposite (TFN) membranes achieved retention rates of 97.75% and 97.85% for PFOA and PFOS, respectively. | [44,45] |
| Bio- adsorbents | Porous structure of biochar, chemical interactions between its surface functional groups and PFASs, and possible π-π stacking | Environmentally friendly and cost-effective; wide raw material availability; good physicochemical properties. | The aminated rice husk adsorbent reached the adsorption equilibrium of PFBA, PFOA, and PFOS within 3 h, 5 h, and 9 h, with the adsorption capacities of 1.70, 2.49 mmol g−1, and 2.65 mmol g−1, respectively. | [46] |
| Chemical Compounds | Pyrolysis Temperature | Pyrolysis Product |
|---|---|---|
| Perfluoro hexane (n-C6F14) | Does not decompose at <400 °C, requires palladium catalysts. | Fluoride and carbide. |
| Perfluoro pentane (n-C5F12) | Does not decompose at temperatures below 840 °C. | Hydrogen fluoride, fluorocarbons, fluorides, and oxides. |
| Octafluorocyclobutane (C4F8) | 360~560 °C. | Perfluoro propane and hexane. |
| Perfluoro propane (n-C3F6) | 550~675 °C. | Perfluoro-2-butene and PFIB. |
| 2H-heptafluoropropane (HFP, C3HF7) | Does not decompose at temperatures below 640 °C. | Hydrogen fluoride, hexafluoro propane, and trifluoroacetic acid. |
| Perfluoro ethane (N-C2F4) | <550 °C; 550~700 °C; 700~750 °C. | Octafluorocyclobutane; perfluoro propane and butane; hexafluoroethane and perfluoro isobutene. |
| Remediation Techniques | Remediation Principles | Methods |
|---|---|---|
| Bioremediation | Utilization of microbial decomposition of PFASs. | Soil biological treatment or biomobilization. |
| Chemical remediation | Utilizes chemicals to break down PFASs. | Advanced oxidation treatment. |
| Physical remediation | Physical mechanics are utilized to separate and remove PFASs. | Adsorbents and redox treatments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhao, Y.; Wei, T.; Shen, C. Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater. Water 2025, 17, 1319. https://doi.org/10.3390/w17091319
Chen Z, Zhao Y, Wei T, Shen C. Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater. Water. 2025; 17(9):1319. https://doi.org/10.3390/w17091319
Chicago/Turabian StyleChen, Zhenzhen, Yaqian Zhao, Ting Wei, and Cheng Shen. 2025. "Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater" Water 17, no. 9: 1319. https://doi.org/10.3390/w17091319
APA StyleChen, Z., Zhao, Y., Wei, T., & Shen, C. (2025). Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater. Water, 17(9), 1319. https://doi.org/10.3390/w17091319







