Novel MIL-53(Fe)@C Magnetic Composite Electrode for Efficient Dechlorination of Disinfection By-Product Trichloroacetic Acid in Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
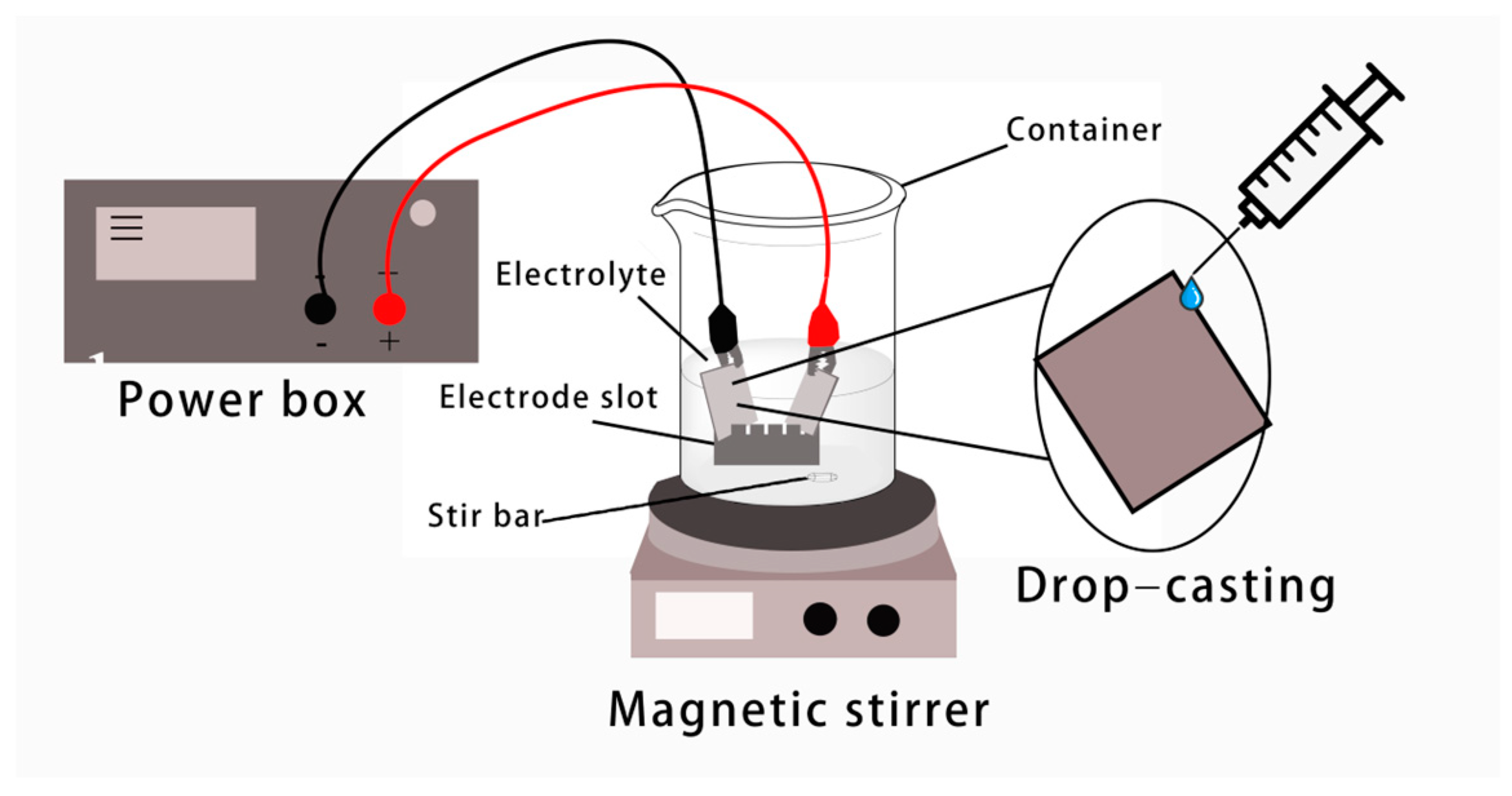
2.2. Preparation of MIL-53(Fe)@C-MAG Electrode
2.3. TCAA Dechlorination Experiments
2.4. Analytical Methods
2.5. Characterization Methods
3. Results and Discussion
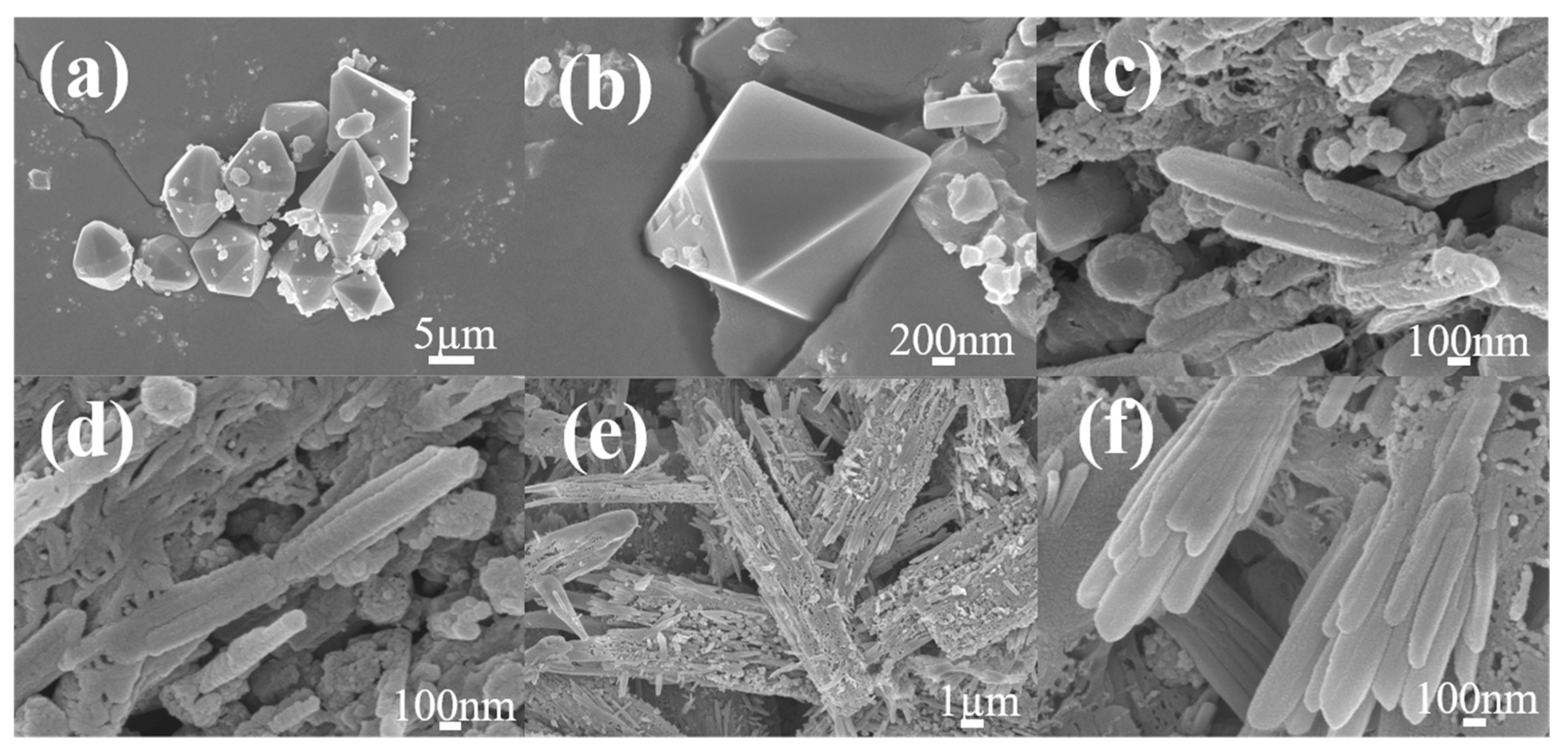
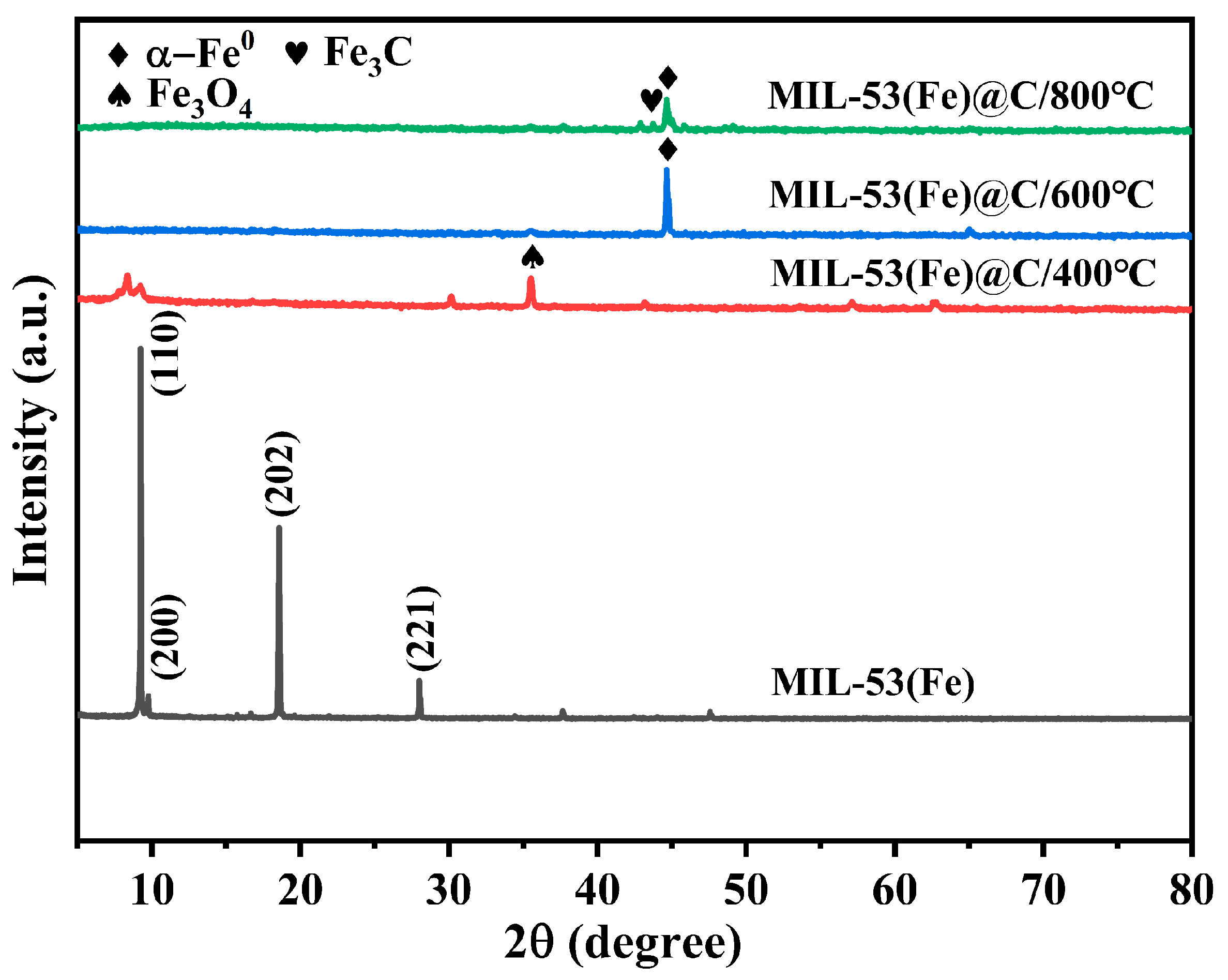
3.1. Preparation and Characterization of MIL-53(Fe) and MIL-53(Fe)@C Series
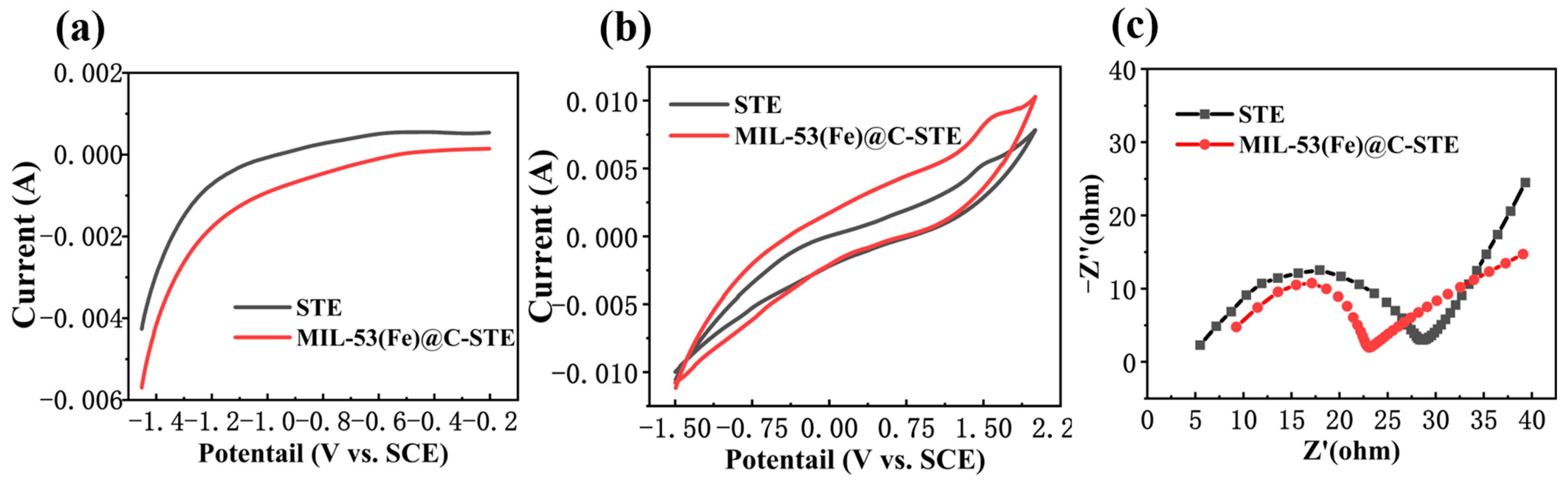
3.2. Electrochemical Performance Analysis
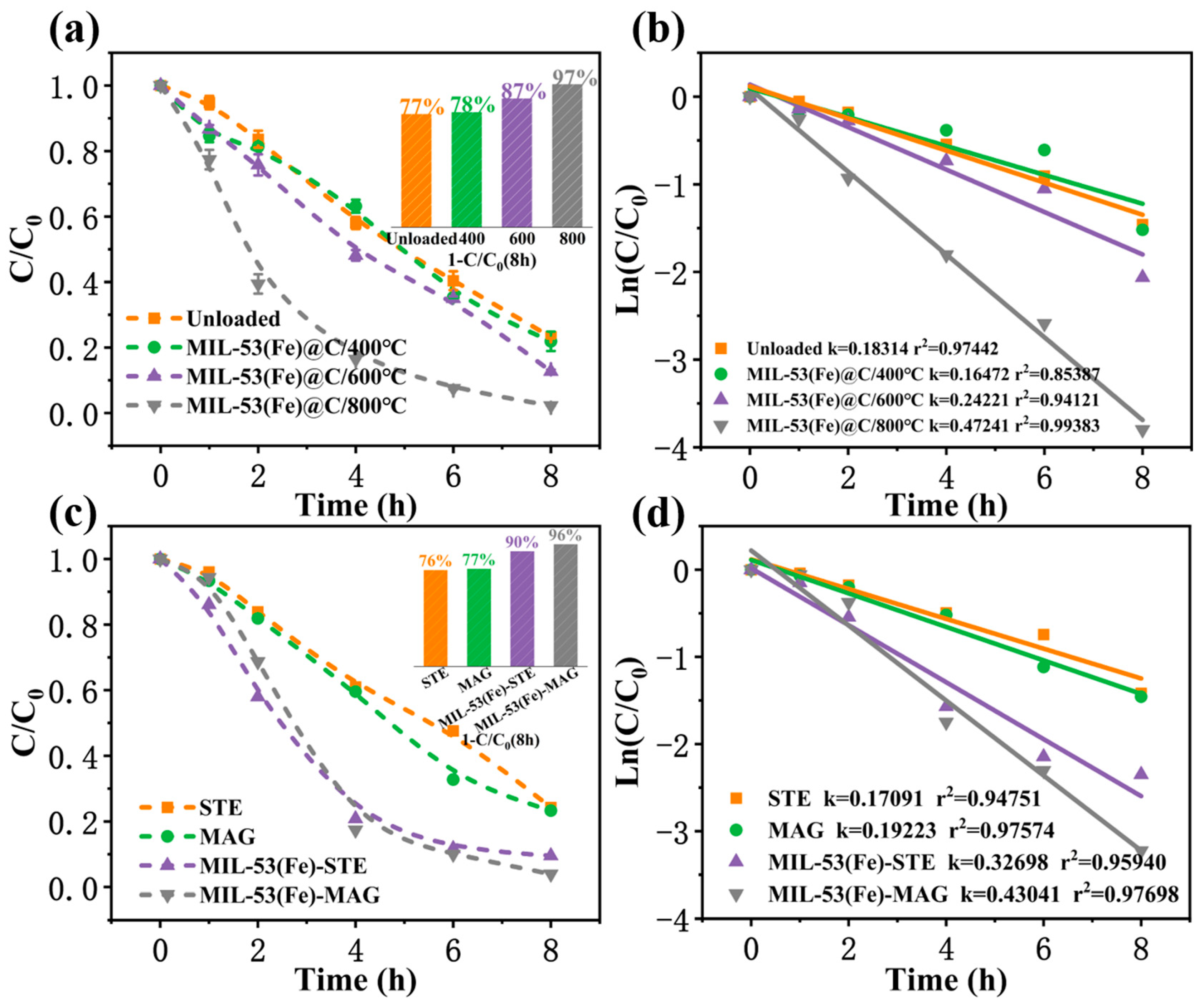
3.3. Performance of TCAA Degradation Under Different Synthesis Conditions
3.4. Effect of Influencing Factors on the Dechlorination of TCAA by MIL-53(Fe)@C-MAG
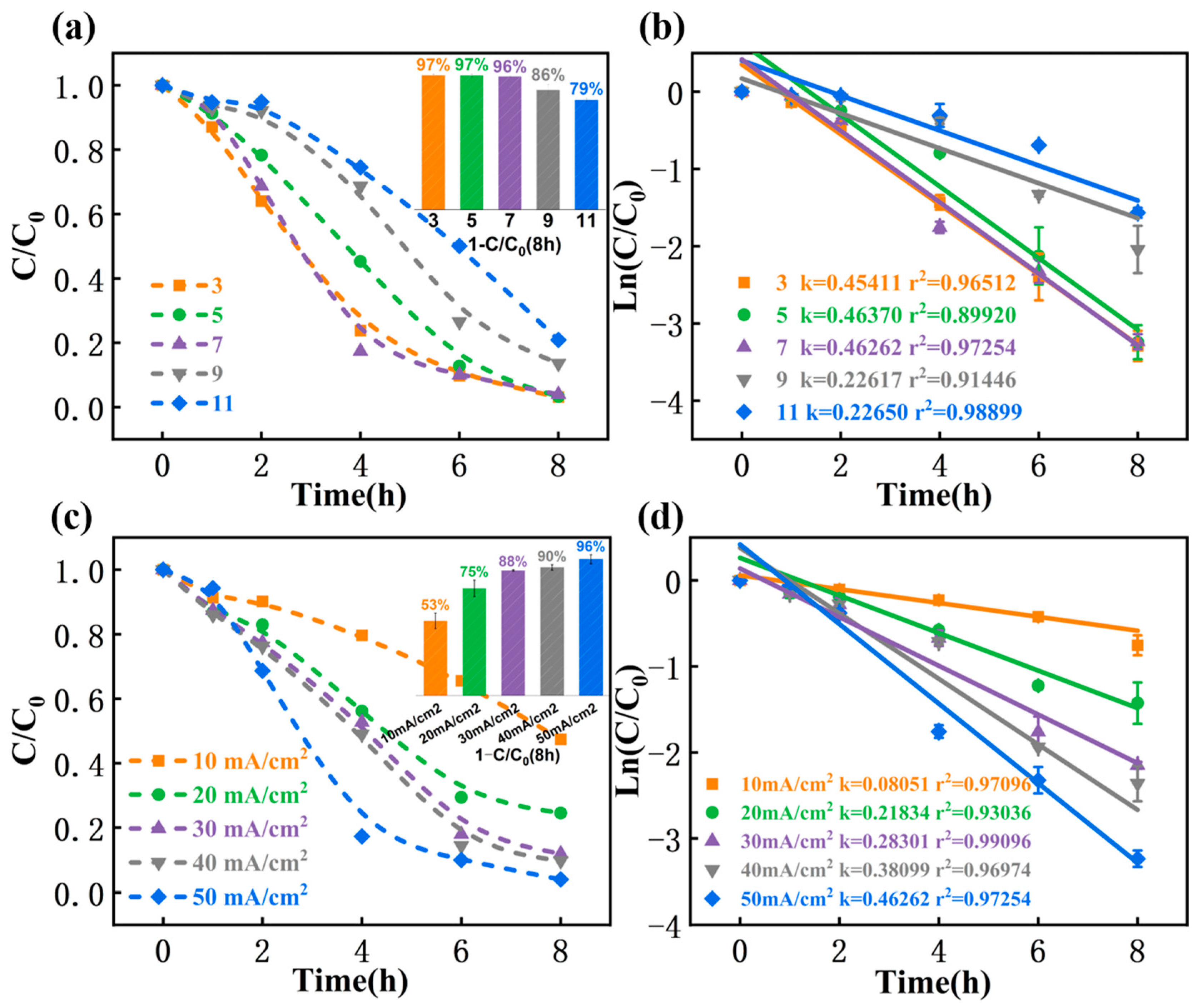
3.4.1. pH
3.4.2. Current Density
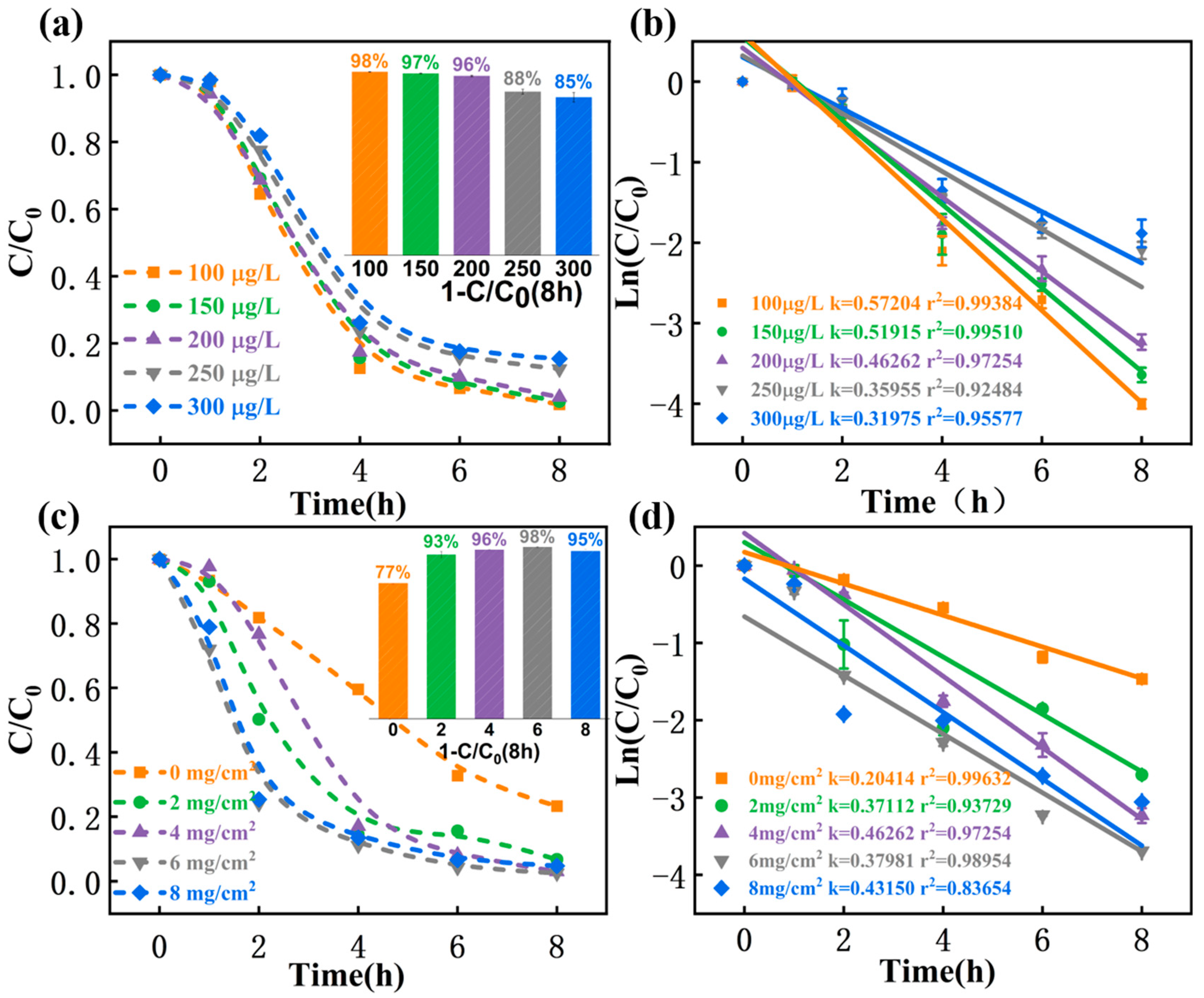
3.4.3. Initial TCAA Concentrations
3.4.4. MIL-53(Fe)@C Loading
3.5. Reaction Mechanism
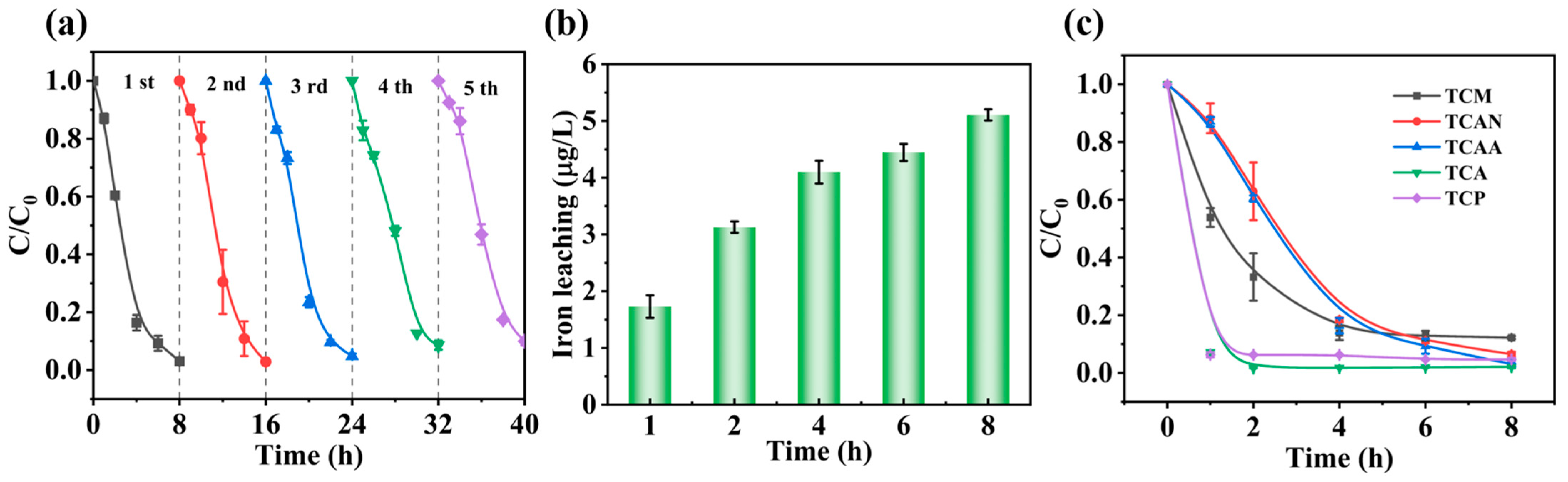
3.6. Stability of MIL-53(Fe)@C-MAG Electrodes
3.7. Dechlorination of Other DBPs by MIL-53(Fe)@C-MAG Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Yang, D.; Li, S.; Yang, L.; Yan, W.; Xu, H. Advances on Electrochemical Disinfection Research: Mechanisms, Influencing Factors and Applications. Sci. Total Environ. 2024, 912, 169043. [Google Scholar] [CrossRef] [PubMed]
- Rolbiecki, D.; Paukszto, Ł.; Krawczyk, K.; Korzeniewska, E.; Sawicki, J.; Harnisz, M. Chlorine Disinfection Modifies the Microbiome, Resistome and Mobilome of Hospital Wastewater—A Nanopore Long-Read Metagenomic Approach. J. Hazard. Mater. 2023, 459, 132298. [Google Scholar] [CrossRef]
- McGuire, M.J. Eight Revolutions in the History of US Drinking Water Disinfection. J. AWWA 2006, 98, 123–149. [Google Scholar] [CrossRef]
- Du, J.; Shi, X.; Wang, Y.; Tang, A.; Zhang, Z.; Fu, M.-L.; Sun, W.; Yuan, B. Effects of Chlorination on the Nitrosamines Formation from Two Algae Species in Drinking Water Source-M. Aeruginosa and C. Meneghiniana. Chemosphere 2022, 287, 132093. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wang, J.; Chen, C. Characterization of Dissolved Organic Matter as N-Nitrosamine Precursors Based on Hydrophobicity, Molecular Weight and Fluorescence. J. Environ. Sci. 2013, 25, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Song, S.; Graham, N.J.D.; Yu, W. Direct Generation of DBPs from City Dust During Chlorine-Based Disinfection. Water Res. 2024, 248, 120839. [Google Scholar] [CrossRef]
- Benítez, J.S.; Rodríguez, C.M.; Casas, A.F. Disinfection Byproducts (DBPs) in Drinking Water Supply Systems: A Systematic Review. Phys. Chem. Earth Parts ABC 2021, 123, 102987. [Google Scholar] [CrossRef]
- Li, J.; Aziz, M.T.; Granger, C.O.; Richardson, S.D. Halocyclopentadienes: An Emerging Class of Toxic DBPs in Chlor(Am)Inated Drinking Water. Environ. Sci. Technol. 2022, 56, 11387–11397. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, J.; Wei, W.; Song, M.; Shi, W.; Li, A.; Zhou, Q.; Pan, Y. Comparative Cytotoxicity Analyses of Disinfection Byproducts in Drinking Water Using Dimensionless Parameter Scaling Method: Effect of Halogen Substitution Type and Number. Water Res. 2023, 240, 120087. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, S.N.; Wang, G.S. Determination of Ten Haloacetic Acids in Drinking Water Using High-Performance and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. Sci. 2009, 47, 67–74. [Google Scholar] [CrossRef]
- Wagner, E.D.; Plewa, M.J. CHO Cell Cytotoxicity and Genotoxicity Analyses of Disinfection By-Products: An Updated Review. J. Environ. Sci. 2017, 58, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Xiang, Y.; Jiang, J.; Yin, R. Role of UV-Based Advanced Oxidation Processes on NOM Alteration and DBP Formation in Drinking Water Treatment: A State-of-the-Art Review. Chemosphere 2023, 311, 136870. [Google Scholar] [CrossRef]
- Park, K.Y.; Yu, Y.J.; Yun, S.J.; Kweon, J.H. Natural Organic Matter Removal from Algal-Rich Water and Disinfection by-Products Formation Potential Reduction by Powdered Activated Carbon Adsorption. J. Environ. Manag. 2019, 235, 310–318. [Google Scholar] [CrossRef]
- Zhao, X.; Li, A.; Mao, R.; Liu, H.; Qu, J. Electrochemical Removal of Haloacetic Acids in a Three-Dimensional Electrochemical Reactor with Pd-GAC Particles as Fixed Filler and Pd-Modified Carbon Paper as Cathode. Water Res. 2014, 51, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Korshin, G.V.; Jensen, M.D. Electrochemical Reduction of Haloacetic Acids and Exploration of Their Removal by Electrochemical Treatment. Electrochim. Acta 2001, 47, 747–751. [Google Scholar] [CrossRef]
- Li, Y.P.; Cao, H.B.; Zhang, Y. Reductive Dehalogenation of Haloacetic Acids by Hemoglobin-Loaded Carbon Nanotube Electrode. Water Res. 2007, 41, 197–205. [Google Scholar] [CrossRef]
- Jian, M.; Wang, Y. Construction of MXenes/COFs Heterojunctions for Photo/Electrocatalytic Applications: Opportunities and Challenges. Chem. Eng. J. 2025, 507, 160631. [Google Scholar] [CrossRef]
- Sundar Rajan, A.P.; Senthil, R.A.; Moon, C.J.; Kumar, A.; Min, A.; Ubaidullah, M.; Choi, M.Y. Insights into Dual-Functional Catalytic Activity of Laser-Driven CoGaIr-LDH Nanosheets in Water Electrolysis for Green Hydrogen Production via in-Situ Raman and Theoretical Analyses. Chem. Eng. J. 2024, 502, 157848. [Google Scholar] [CrossRef]
- Han, Y.P.; Wang, Z.R.; Yan, Y.Y.; Li, Q.H.; Shao, P.; Zhang, H.X.; Han, L.L.; Wang, F.; Zhang, J. Coplanar Two-Dimensional Cu-MOF with Dual-Cu Sites for Electrocatalytic CO2 Reduction to C2H4. Chem. Eng. J. 2025, 507, 160493. [Google Scholar] [CrossRef]
- Yang, C.; Tang, Y.; Yang, Q.; Wang, B.; Liu, X.; Li, Y.; Yang, W.; Zhao, K.; Wang, G.; Wang, Z.; et al. Copper–Nickel–MOF/Nickel Foam Catalysts Grown in Situ for Efficient Electrochemical Nitrate Reduction to Ammonia. J. Hazard. Mater. 2024, 480, 136036. [Google Scholar] [CrossRef]
- Yılmaz, E.; Sert, E.; Atalay, F.S. Synthesis, Characterization of a Metal Organic Framework: MIL-53 (Fe) and Adsorption Mechanisms of Methyl Red onto MIL-53 (Fe). J. Taiwan Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Tran, T.V.; Jalil, A.A.; Nguyen, D.T.C.; Alhassan, M.; Nabgan, W.; Cao, A.N.T.; Nguyen, T.M.; Vo, D.-V.N. A Critical Review on the Synthesis of NH2-MIL-53(Al) Based Materials for Detection and Removal of Hazardous Pollutants. Environ. Res. 2023, 216, 114422. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Faghihian, H. Application of Novel Metal Organic Framework, MIL-53(Fe) and Its Magnetic Hybrid: For Removal of Pharmaceutical Pollutant, Doxycycline from Aqueous Solutions. Environ. Toxicol. Pharmacol. 2017, 53, 121–132. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Ahmad, A.; Shinde, A.; Das, I.; Madhao Ghangrekar, M. Anodic Degradation of Salicylic Acid and Simultaneous Bio-Electricity Recovery in Microbial Fuel Cell Using Waste-Banana-Peels Derived Biochar-Supported MIL-53(Fe)-Metal-Organic Framework as Cathode Catalyst. J. Electroanal. Chem. 2024, 967, 118451. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Huang, S.; Feng, J.; Wu, Y.; Deng, J.; Liu, J.; Tu, X.; Ma, X.; Li, X.; et al. Enhanced Deep Dehalogenation of Brominated DBPs on Vitamin B12 Composite Electrodes by the Synergistic Effect of UV Irradiation: Accelerated Formation of Co-Br Bonds and Enhanced Mediation by Atomic H*. Chem. Eng. J. 2024, 485, 149873. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Deng, J.; Liu, J.; Wu, Y.; Huang, S.; Ma, X.; Li, X.; Dietrich, A.M. Enhanced Dehalogenation of Brominated DBPs by Catalyzed Electrolysis Using Vitamin B12 Modified Electrodes: Kinetics, Mechanisms, and Mass Balances. J. Hazard. Mater. 2023, 449, 131052. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.; Jahn, D. Microbial Production of Vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Lee, D.E.; Moru, S.; Jo, W.K.; Tonda, S. Structurally Engineered Vitamin B12 on Graphene as a Bioinspired Metal–N–C-Based Electrocatalyst for Effective Overall Water Splitting in Alkaline Media. Appl. Surf. Sci. 2022, 575, 151729. [Google Scholar] [CrossRef]
- Song, G.; Wang, Z.; Wang, L.; Li, G.; Huang, M.; Yin, F. Preparation of MOF(Fe) and Its Catalytic Activity for Oxygen Reduction Reaction in an Alkaline Electrolyte. Chin. J. Catal. 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Zhang, Q.; Zhou, M. S-Doped MIL-53 as Efficient Heterogeneous Electro-Fenton Catalyst for Degradation of Sulfamethazine at Circumneutral pH. J. Hazard. Mater. 2022, 424, 127674. [Google Scholar] [CrossRef]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Moulin, B.; Heurtaux, D.; Clet, G.; Vimont, A.; Grenèche, J.-M.; et al. Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host–Guest Interactions. J. Am. Chem. Soc. 2010, 132, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like Carbon Nanotube/Fe3C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of Active Hydrogen Species on Palladium Nanoparticles for an Enhanced Electrocatalytic Hydrodechlorination of 2,4-Dichlorophenol in Water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef]
- Zhu, Y.; Nie, X.; Liu, X. Preparation of Fe/Co Bimetallic MOF Photofenton Catalysts and Their Performance in Degrading Tetracycline Hydrochloride Pollutants Efficiently under Visible Light. J. Alloys Compd. 2025, 1010, 176967. [Google Scholar] [CrossRef]
- Dou, J.; Cheng, J.; Lu, Z.; Tian, Z.; Xu, J.; He, Y. Biochar Co-Doped with Nitrogen and Boron Switching the Free Radical Based Peroxydisulfate Activation into the Electron-Transfer Dominated Nonradical Process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Yan, L.; Ma, H.; Wang, B.; Wang, Y.; Chen, Y. Electrochemical Treatment of Petroleum Refinery Wastewater with Three-Dimensional Multi-Phase Electrode. Desalination 2011, 276, 397–402. [Google Scholar] [CrossRef]
- Zhou, M.; Lei, L. The Role of Activated Carbon on the Removal of p-Nitrophenol in an Integrated Three-Phase Electrochemical Reactor. Chemosphere 2006, 65, 1197–1203. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, P.; Qu, G.; Ning, P.; Ren, N.; Wang, J.; Wu, F.; Chen, X.; Wang, Z.; Zhang, T.; et al. Study on the Mechanism of Rapid Degradation of Rhodamine B with Fe/Cu@antimony Tailing Nano Catalytic Particle Electrode in a Three Dimensional Electrochemical Reactor. Water Res. 2023, 244, 120487. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Jonoush, Z.A.; Rezaee, A. Three-Dimensional Electro-Fenton System Supplied with a Nanocomposite of Microbial Cellulose/Fe3O4 for Effective Degradation of Tetracycline. Chemosphere 2023, 317, 137890. [Google Scholar] [CrossRef]
- Mao, R.; Zhao, X.; Lan, H.; Liu, H.; Qu, J. Graphene-Modified Pd/C Cathode and Pd/GAC Particles for Enhanced Electrocatalytic Removal of Bromate in a Continuous Three-Dimensional Electrochemical Reactor. Water Res. 2015, 77, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, R.; Tong, Y.; Lan, H.; Zhang, G.; Liu, H.; Qu, J. Reductive Dechlorination of Trichloroacetic Acid (TCAA) by Electrochemical Process over Pd-In/Al2O3 Catalyst. Electrochim. Acta 2017, 232, 13–21. [Google Scholar] [CrossRef]
- Li, G.; Liu, W.; Gao, S.; Lu, H.; Fu, D.; Wang, M.; Liu, X. MXene-Based Composite Aerogels with Bifunctional Ferrous Ions for the Efficient Degradation of Phenol from Wastewater. Chemosphere 2024, 358, 142151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hou, Y.; Yu, Z.; Liu, Y.; Huang, J.; Qian, L.; Xiong, J. Three-Dimensional Electro-Fenton Degradation of Rhodamine B with Efficient Fe-Cu/Kaolin Particle Electrodes: Electrodes Optimization, Kinetics, Influencing Factors and Mechanism. Sep. Purif. Technol. 2019, 210, 60–68. [Google Scholar] [CrossRef]
- Li, A.; Zhao, X.; Hou, Y.; Liu, H.; Wu, L.; Qu, J. The Electrocatalytic Dechlorination of Chloroacetic Acids at Electrodeposited Pd/Fe-Modified Carbon Paper Electrode. Appl. Catal. B Environ. 2012, 111–112, 628–635. [Google Scholar] [CrossRef]
- Yin, H.; Cao, X.; Lei, C.; Chen, W.; Huang, B. Insights into Electroreductive Dehalogenation Mechanisms of Chlorinated Environmental Pollutants. ChemElectroChem 2020, 7, 1825–1837. [Google Scholar] [CrossRef]
- Yao, Q.; Zhou, X.; Xiao, S.; Chen, J.; Abdelhafeez, I.A.; Yu, Z.; Chu, H.; Zhang, Y. Amorphous Nickel Phosphide as a Noble Metal-Free Cathode for Electrochemical Dechlorination. Water Res. 2019, 165, 114930. [Google Scholar] [CrossRef]
- Lou, Z.; Xu, J.; Zhou, J.; Yang, K.; Cao, Z.; Li, Y.; Liu, Y.; Lou, L.; Xu, X. Insight into Atomic H* Generation, H2 Evolution, and Cathode Potential of MnO2 Induced Pd/Ni Foam Cathode for Electrocatalytic Hydrodechlorination. Chem. Eng. J. 2019, 374, 211–220. [Google Scholar] [CrossRef]
- Palisoc, S.T.; Uy, D.J.S.; Natividad, M.T.; Lopez, T.B.G. Determination of Heavy Metals in Mussel and Oyster Samples with Tris (2,2′-Bipyridyl) Ruthenium (II)/Graphene/Nafion® Modified Glassy Carbon Electrodes. Mater. Res. Express 2017, 4, 116406. [Google Scholar] [CrossRef]
- Steiniger, K.A.; Lambert, T.H. Olefination of Carbonyls with Alkenes Enabled by Electrophotocatalytic Generation of Distonic Radical Cations. Sci. Adv. 2023, 9, eadg3026. [Google Scholar] [CrossRef]
- Fan, C.; Pu, S.; Liu, G.; Yang, T. Substituent Position Effect on the Properties of New Unsymmetrical Isomeric Diarylethenes Having a Chlorine Atom. J. Photochem. Photobiol. Chem. 2008, 197, 415–425. [Google Scholar] [CrossRef]
- Sakurama, K.; Kawai, A.; Tuan Giam Chuang, V.; Kanamori, Y.; Osa, M.; Taguchi, K.; Seo, H.; Maruyama, T.; Imoto, S.; Yamasaki, K.; et al. Analysis of the Binding of Aripiprazole to Human Serum Albumin: The Importance of a Chloro-Group in the Chemical Structure. ACS Omega 2018, 3, 13790–13797. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.A.; Roberts, A.L. Pathways and Kinetics of Chlorinated Ethylene and Chlorinated Acetylene Reaction with Fe(0) Particles. Environ. Sci. Technol. 2000, 34, 1794–1805. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Quan, R.; Cao, W.; Zhang, W.; Jiang, S.; Feng, J.; Wang, J.; Giannakis, S. Novel MIL-53(Fe)@C Magnetic Composite Electrode for Efficient Dechlorination of Disinfection By-Product Trichloroacetic Acid in Water Treatment. Water 2025, 17, 1309. https://doi.org/10.3390/w17091309
Ma X, Quan R, Cao W, Zhang W, Jiang S, Feng J, Wang J, Giannakis S. Novel MIL-53(Fe)@C Magnetic Composite Electrode for Efficient Dechlorination of Disinfection By-Product Trichloroacetic Acid in Water Treatment. Water. 2025; 17(9):1309. https://doi.org/10.3390/w17091309
Chicago/Turabian StyleMa, Xiaoyan, Rongbin Quan, Wenqing Cao, Weijie Zhang, Su Jiang, Jiao Feng, Jiulong Wang, and Stefanos Giannakis. 2025. "Novel MIL-53(Fe)@C Magnetic Composite Electrode for Efficient Dechlorination of Disinfection By-Product Trichloroacetic Acid in Water Treatment" Water 17, no. 9: 1309. https://doi.org/10.3390/w17091309
APA StyleMa, X., Quan, R., Cao, W., Zhang, W., Jiang, S., Feng, J., Wang, J., & Giannakis, S. (2025). Novel MIL-53(Fe)@C Magnetic Composite Electrode for Efficient Dechlorination of Disinfection By-Product Trichloroacetic Acid in Water Treatment. Water, 17(9), 1309. https://doi.org/10.3390/w17091309







