Sentinel-2 Images Discover How Extraordinary Water Inputs Allow the Ephemeral Resurgence of Najas marina in a Shallow Hypertrophic Lagoon (Albufera of Valencia, Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. In Situ Observations
2.3. Remote Sensing Images
2.4. Extraction of Hydrochemical Variables
2.5. Plant Area Extraction
2.6. Data Analysis
2.7. Thematic Maps
3. Results
3.1. Field Observations and Remote Sensing Results
3.2. Multivariate Statistics
3.3. Thematic Maps
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSS | Total Suspended Solids |
| Chl-a | Chlorophyll-a |
| ZSD | Secchi Disk Depth |
References
- Siddiqui, A.J.; Jahan, S.; Adnan, M.; Ashraf, S.A.; Singh, R. Macrophytes and Their Role in Wetland Ecosystems. In Aquatic Macrophytes: Ecology, Functions and Services; Kumar, S., Bauddh, K., Singh, R., Kumar, N., Kumar, R., Eds.; Springer Nature: Singapore, 2023; pp. 119–138. [Google Scholar]
- Preiner, S.; Dai, Y.; Pucher, M.; Reitsema, R.E.; Schoelynck, J.; Meire, P.; Hein, T. Effects of macrophytes on ecosystem metabolism and net nutrient uptake in a groundwater fed lowland river. Sci. Total Environ. 2020, 721, 137620. [Google Scholar] [CrossRef]
- Newman, R.M. Herbivory and detritivory on freshwater macrophytes by invertebrates: A review. J. N. Am. Benthol. Soc. 1991, 10, 89–114. [Google Scholar] [CrossRef]
- Swe, T.; Lombardo, P.; Ballot, A.; Thrane, J.E.; Sample, J.; Eriksen, T.E.; Mjelde, M. The importance of aquatic macrophytes in a eutrophic tropical shallow lake. Limnologica 2021, 90, 125910. [Google Scholar] [CrossRef]
- Germ, M.; Janež, V.; Gaberščik, A.; Zelnik, I. Diversity of macrophytes and environmental assessment of the Ljubljanica river (Slovenia). Diversity 2021, 13, 278. [Google Scholar] [CrossRef]
- Dvořák, J. An example of relationships between macrophytes, macroinvertebrates and their food resources in a shallow euthrophic lake. Hydrobiologia 1996, 339, 27–36. [Google Scholar] [CrossRef]
- Harvey, R.M.; Pickett, J.R.; Bates, R.D. Environmental factors controlling the growth and distribution of submersed aquatic macrophytes in two South Carolina reservoirs. Lake Reserv. Manag. 1987, 3, 243–255. [Google Scholar] [CrossRef][Green Version]
- Chambers, P.A.; Lacoul, P.; Murphy, K.J.; Thomaz, S.M. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 2007, 595, 9–26. [Google Scholar] [CrossRef]
- Lacoul, P.; Freedman, B. Environmental influences on aquatic plants in freshwater ecosystems. Environ. Rev. 2006, 14, 89–136. [Google Scholar] [CrossRef]
- Zhu, G.; Li, W.; Zhang, M.; Ni, L.; Wang, S. Adaptation of submerged macrophytes to both water depth and flood intensity as revealed by their mechanical resistance. Hydrobiologia 2012, 696, 77–93. [Google Scholar] [CrossRef]
- Madsen, J.D.; Adams, M.S. The distribution of submerged aquatic macrophyte biomass in a eutrophic stream, Badfish Creek—The effect of environment. Hydrobiologia 1989, 171, 111–119. [Google Scholar] [CrossRef]
- Engloner, A.; Szalma, E.; Sipos, K.; Dinka, M. Occurrence and habitat preference of aquatic macrophytes in a large river channel. Community Ecol. 2013, 14, 243–248. [Google Scholar] [CrossRef]
- Shields, E.C.; Moore, K.A. Effects of sediment and salinity on the growth and competitive abilities of three submersed macrophytes. Aquat. Bot. 2016, 132, 24–29. [Google Scholar] [CrossRef][Green Version]
- Kading, J.; Xu, L. Documenting macrophytes and their habitat preferences in southeastern South Dakota. Proc. South. Dak. Acad. Sci. 2021, 100, 37–52. [Google Scholar]
- Alexander, T.J.; Vonlanthen, P.; Seehausen, O. Does Eutrophication-Driven Evolution Change Aquatic Ecosystems? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160041. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Ough, K.M.; Scroggie, M.P.; Schreiber, E.S.G.; Kohout, M. Assessing changes in macrophyte assemblages with salinity in non-riverine wetlands: A Bayesian approach. Aquat. Bot. 2009, 90, 137–142. [Google Scholar] [CrossRef]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Iwan Jones, J. How green is my river? A new paradigm of eutrophication in rivers. Sci. Tot. Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Ecology of Freshwaters, Man and Medium, Past to Future, 3rd ed.; Blackwell Science: Oxford, UK, 1998; ISBN 978-1-444-31342-0. [Google Scholar]
- Scheffer, M.; van Nes, E.H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 2007, 584, 455–466. [Google Scholar] [CrossRef]
- Molner, J.V.; Mellinas-Coperias, I.; Canós-López, C.; Pérez-González, R.; Sendra, M.D.; Soria, J.M. Seasonal Dynamics and Environmental Drivers of Phytoplankton in the Albufera Coastal Lagoon (Valencia, Spain). Environments 2025, 12, 23. [Google Scholar] [CrossRef]
- Viinikka, Y. Najas marina L. (Najadaceae). Karyotypes, cultivation and morphological variation. Ann. Bot. Fenn. 1976, 13, 119–131. [Google Scholar]
- Agami, M.; Waisel, Y. The effect of temperature and photoperiod on growth of Najas marina L. In Proceedings of the International Symposium on Aquatic Macrophytes, Nijmegen, The Netherlands, 18–23 September 1983. [Google Scholar]
- Soria, J.M.; Molner, J.V.; Pérez-González, R.; Alvado, B.; Vera-Herrera, L.; Romo, S. Monitoring the Extraordinary Ephemeral Emergence of Myriophyllum spicatum L. in the Coastal Lagoon Albufera of Valencia (Spain) and Assessing the Impact of Environmental Variables Using a Remote Sensing Approach. J. Mar. Sci. Eng. 2024, 12, 260. [Google Scholar] [CrossRef]
- Confederación Hidrográfica del Júcar (Spanish Government). A Delegation of Representatives of the Administrations Responsible for the Management of l’Albufera Made a Technical Visit to the Area Where the Najas Marina Have Proliferated. Valencia, Spain, 2022. (In Spanish). Available online: https://www.chj.es/es-es/ciudadano/salaprensa/Paginas/Visita-Albufera-najas-marinas.aspx (accessed on 16 December 2024).
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Malthus, T.J. Bio-optical Modeling and Remote Sensing of Aquatic Macrophytes. In Bio-Optical Modeling and Remote Sensing of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–308. [Google Scholar] [CrossRef]
- Soria, J.M. Past, present and future of la Albufera of Valencia Natural Park. Limnetica 2006, 25, 135–142. [Google Scholar] [CrossRef]
- Jégou, A.; Sanchis-Ibor, C. The opaque lagoon. Water management and governance in l’Albufera de València wetland (Spain). Limnetica 2019, 38, 503–515. [Google Scholar] [CrossRef]
- Soria, J.; Romo, S.; Vera-Herrera, L.; Calvo, S.; Sòria-Perpinyà, X.; Pérez, J. Evolución de la conductividad en la Albufera de Valencia entre 1985 y 2018. Limnetica 2021, 40, 223–232. [Google Scholar] [CrossRef]
- Mondria, M. Infrastructures and Eutrophication in l’Albufera de València. The Cabhal model (in Spanish). Ph.D. Thesis, Polythecnic University of Valencia, Valencia, Spain, 10 March 2011. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Soria-Perpinya, X.; Urrego, P.; Pereira-Sandoval, M.; Ruíz-Verdú, A.; Peña, R.; Soria, J.; Delegido, J.; Vicente, E.; Moreno, J. Monitoring the ecological state of a hypertrophic lake (Albufera of València, Spain) using multitemporal Sentinel-2 images. Limnetica 2019, 38, 457–469. [Google Scholar] [CrossRef]
- Molner, J.V.; Soria, J.M.; Pérez-González, R.; Sòria-Perpinyà, X. Measurement of Turbidity and Total Suspended Matter in the Albufera of Valencia Lagoon (Spain) Using Sentinel-2 Images. J. Mar. Sci. Eng. 2023, 11, 1894. [Google Scholar] [CrossRef]
- Molner, J.V.; Soria, J.M.; Pérez-González, R.; Sòria-Perpinyà, X. Estimating Water Transparency Using Sentinel-2 Images in a Shallow Hypertrophic Lagoon (The Albufera of Valencia, Spain). Water 2023, 15, 3669. [Google Scholar] [CrossRef]
- Hoffmann, M.; Sacher, M.; Lehner, S.; Raeder, U.; Melzer, A. Influence of sediment on the growth of the invasive macrophyte Najas marina ssp intermedia in lakes. Limnologica 2013, 43, 265–271. [Google Scholar] [CrossRef]
- Proctor, V.I. Storage and germination of Chara oospores. J. Phycol. 1967, 3, 90–92. [Google Scholar] [CrossRef]
- Triest, L. Electrophoretic polymorphism and divergence in Najas marina L.(Najadaceae): Molecular markers for individuals, hybrids, cytodemes, lower taxa, ecodemes and conservation of genetic diversity. Aquat. Bot. 1989, 33, 301–380. [Google Scholar] [CrossRef]
- Handley, R.J.; Davy, A.J. Seedling root establishment may limit Najas marina L. to sediments of low cohesive strength. Aquat. Bot. 2002, 73, 129–136. [Google Scholar] [CrossRef]
- Forsberg, C. Sterile Germination of Oospores of Chara and Seeds of Najas marina. Physiol. Plant 1965, 18, 128–137. [Google Scholar] [CrossRef]
- Mazej, Z.; Epšek, M. The macrophytes of lake Velenjsko Jezero, Slovenia–the succession of macrophytes after restoration of the lake. Acta Biol. Slov. 2005, 48, 21–31. [Google Scholar] [CrossRef]
- Nichols, S.A.; Shaw, B.H. Ecological Life Histories of the Three Aquatic Nuisance Plants, Myriophyllum spicatum, Potamogeton crispus and Elodea canadensis. Hydrobiologia 1986, 131, 3–21. [Google Scholar] [CrossRef]
- Bolduan, B.R.; Van Eeckhout, G.C.; Quade, H.W.; Gannon, J.E. Potamogeton crispus—The other invader. Lake Reserv. Manag. 1994, 10, 113–125. [Google Scholar] [CrossRef]
- Sanderson, B.G.; Asaeda, T.; Rajapakse, L.; Redden, A.M. Mechanisms affecting biomass and distribution of charophytes and Najas marina in Myall Lake, New South Wales, Australia. Hydrobiologia 2008, 608, 99–119. [Google Scholar] [CrossRef]
- Abd Ellah, R.G. Water resources in Egypt and their challenges, Lake Nasser case study. Egypt. J. Aquat. Res. 2020, 46, 1–12. [Google Scholar] [CrossRef]
- Ma, R.; Duan, H.; Gu, X.; Zhang, S. Detecting Aquatic Vegetation Changes in Taihu Lake, China Using Multi-temporal Satellite Imagery. Sensors 2008, 8, 3988–4005. [Google Scholar] [CrossRef]
- Song, S.; Wu, X.; Hou, J.; Peng, S.; Lin, X.; Ge, X.; Yan, D.; Lin, G. Monitoring the Dynamics of Aquatic Vegetation in a Typical Shallow Lake Using the Water Bloom Index Algorithm—A Case Study in Bao’ an Lake in the Middle Reaches of the Yangtze River. Plants 2024, 13, 3090. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Li, W.; Qu, X.; Shen, D.; Liu, J.; Qiao, R.; Zhao, Y.; Xiong, F.; Wang, H. Impacts of eutrophication and fishery production on thesuccession of submerged macrophytes in Bao’ an lake. Acta Hydrobiol. Sin. 2023, 47, 1769–1779. [Google Scholar]
- Bresciani, M.; Sotgia, C.; Fila, G.L.; Musanti, M.; Bolpagni, R. Assessing Common Reed Bed Health and Management Strategies in Lake Garda (Italy) by Means of Leaf Area Index Measurements. Ital. J. Remote Sens. 2011, 43, 9–22. [Google Scholar] [CrossRef]
- Sotgia, C. Censimento e Studio Delle Aree a Canneto del Lago di Garda Meridionale Attraverso Tecniche di Telerilevamento. Ph.D. Thesis, Università di Parma, Parma, Italy, 2010. [Google Scholar]
- Silva, S.D.G.T.M.; Dahanayaka, D.D.G.L.; Wijeyaratne, M.J.S. A remote sensing approach for assessing the invasion of Najas marina in Madu Ganga Estuary, Sri Lanka. Int. J. Aquat. Biol. 2020, 8, 377–382. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, Y.; Zhu, Z.L. Growth response of the submerged macrophyte Myriophyllum spicatum to sediment nutrient levels and water-level fluctuations. Aquat. Biol. 2012, 17, 295–303. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Villa, P.; Bresciani, M.; Bolpagni, R.; Pinardi, M.; Giardino, C. A rule-based approach for mapping macrophyte communities using multi-temporal aquatic vegetation indices. Remote Sens. Environ. 2015, 171, 218–233. [Google Scholar] [CrossRef]
- Powell, S.J.; Jakeman, A.; Croke, B. Can NDVI response indicate the effective flood extent in macrophyte dominated floodplain wetlands? Ecol. Indic. 2014, 45, 486–493. [Google Scholar] [CrossRef]
- Soria, J.M.; Muñoz, R.; Campillo-Tamarit, N.; Molner, J.V. Flash-Flood-Induced Changes in the Hydrochemistry of the Albufera of Valencia Coastal Lagoon. Diversity 2025, 17, 119. [Google Scholar] [CrossRef]


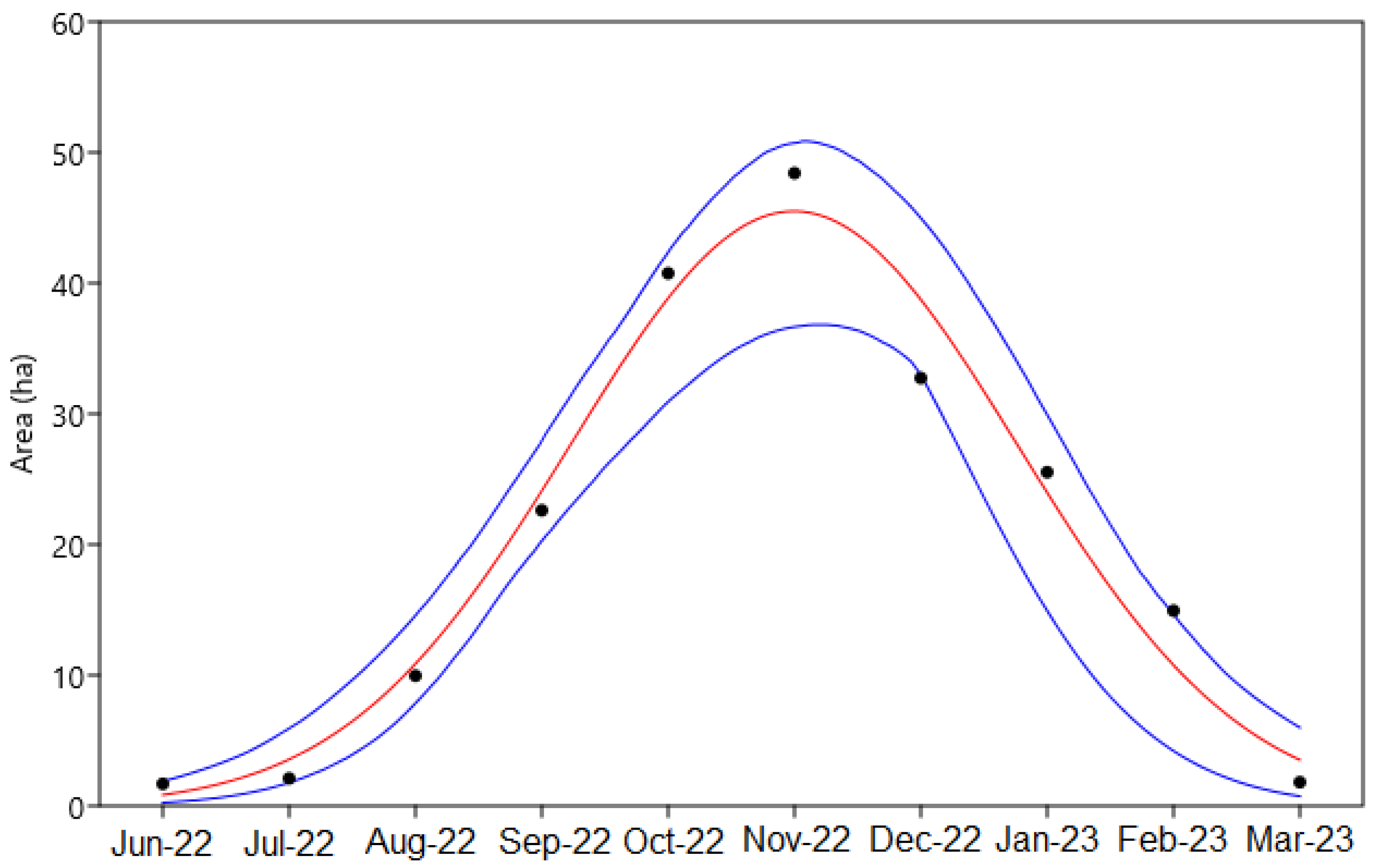
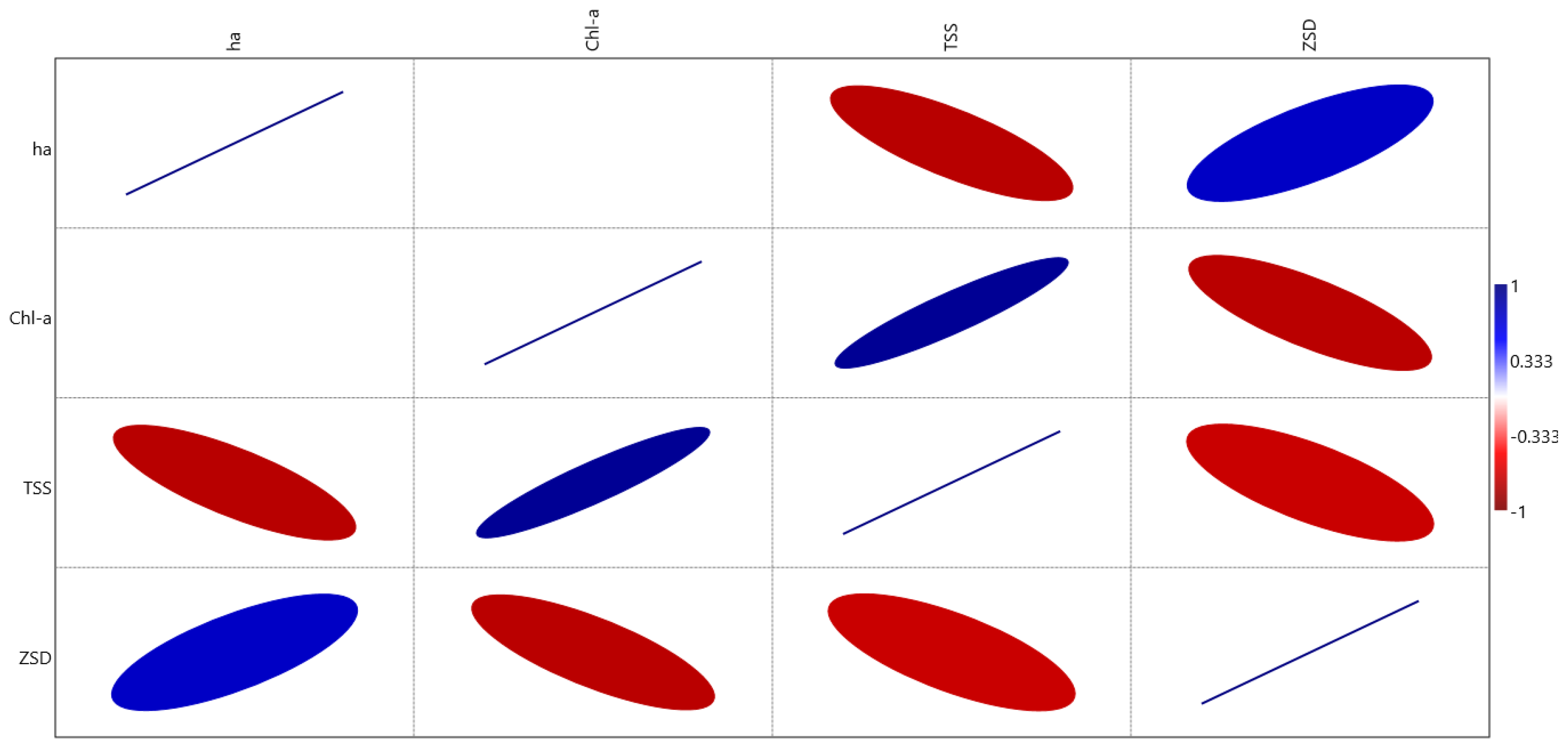
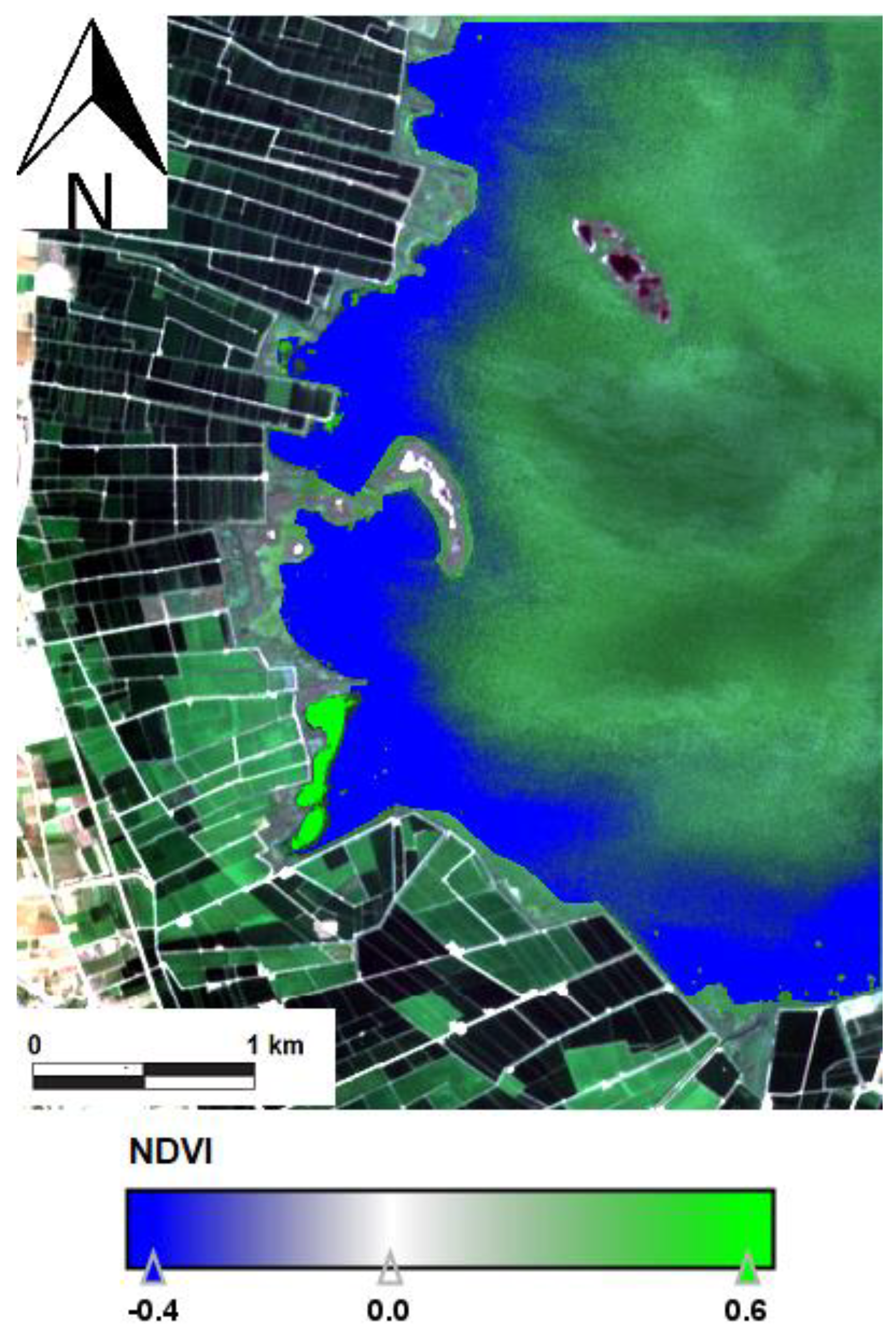
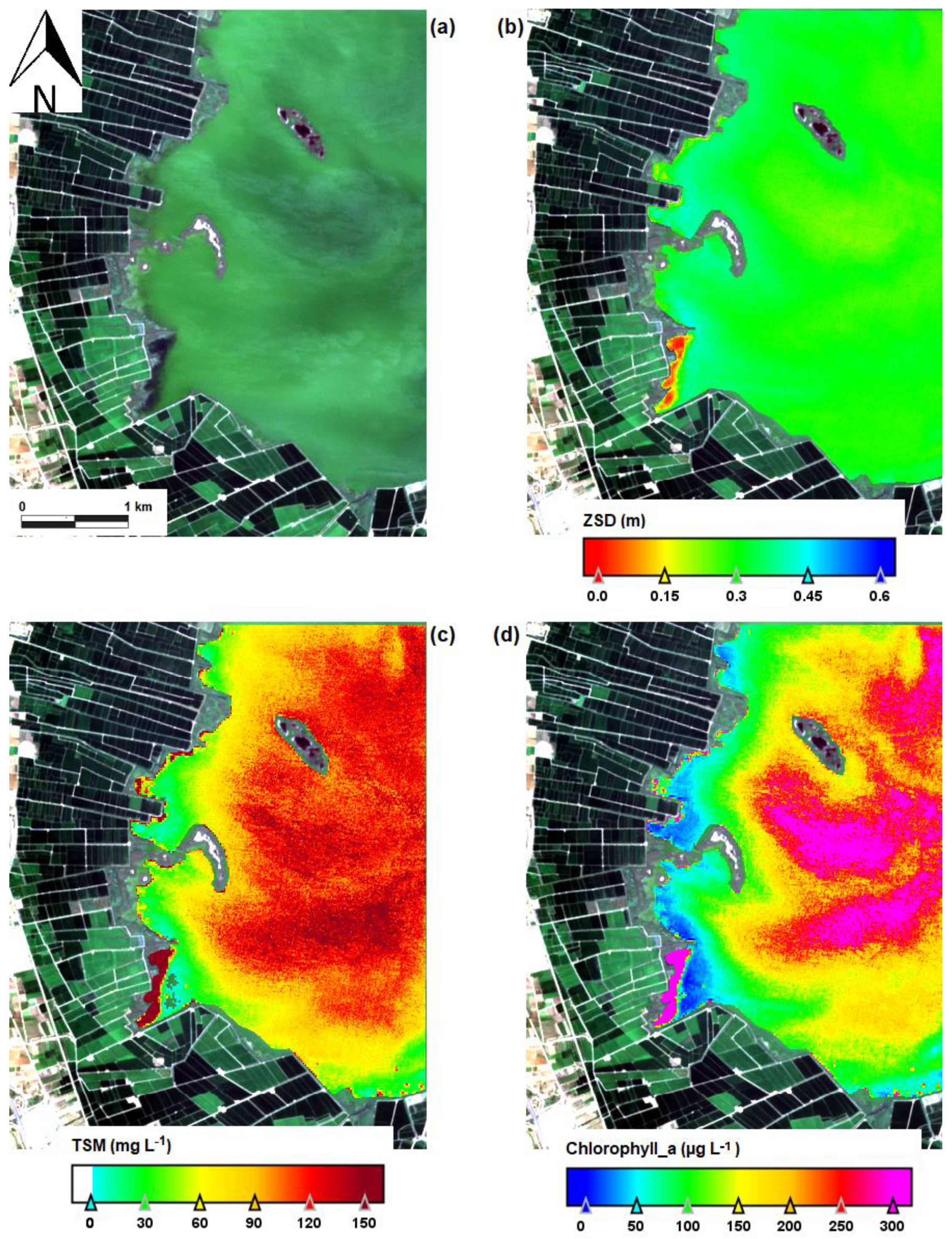
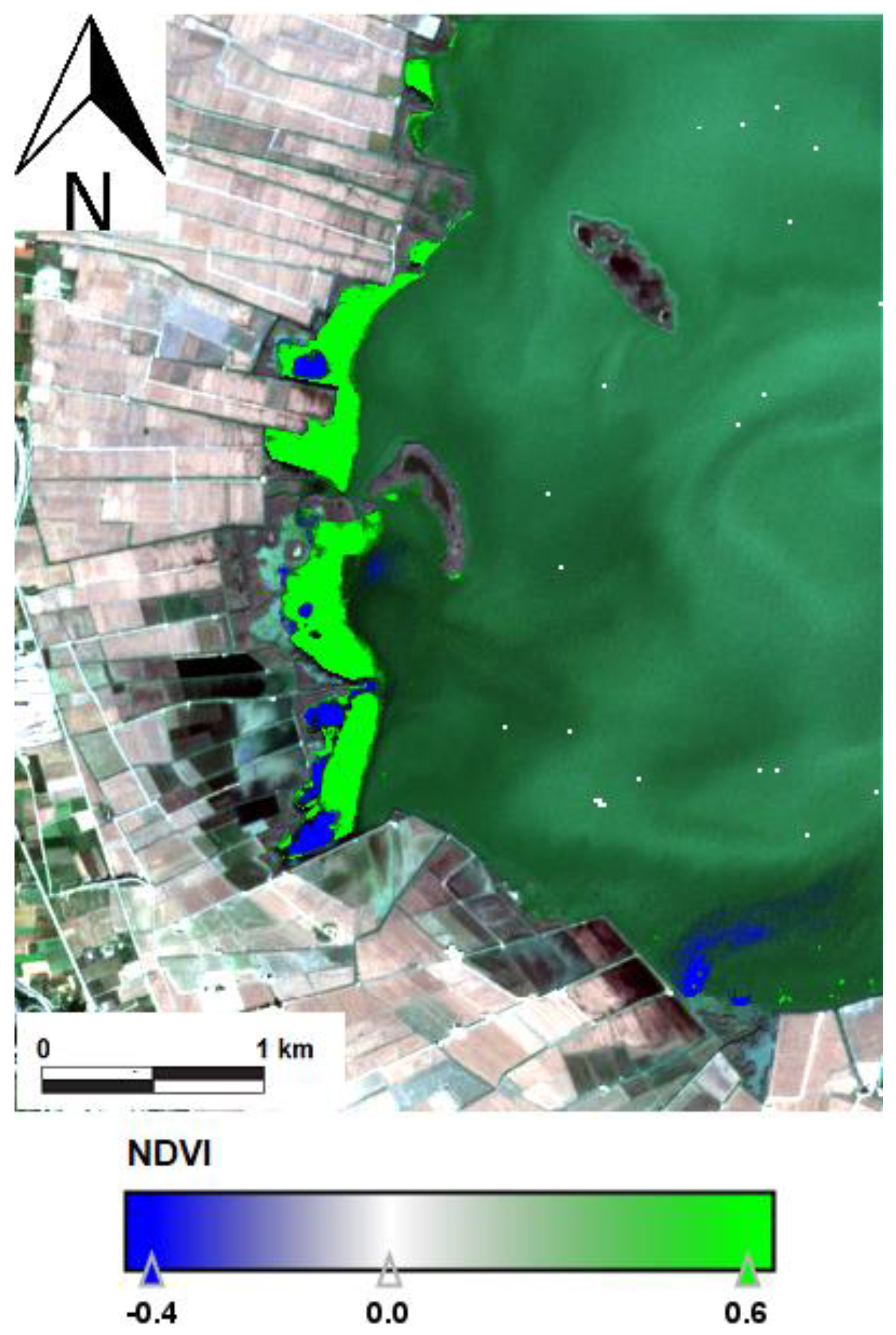
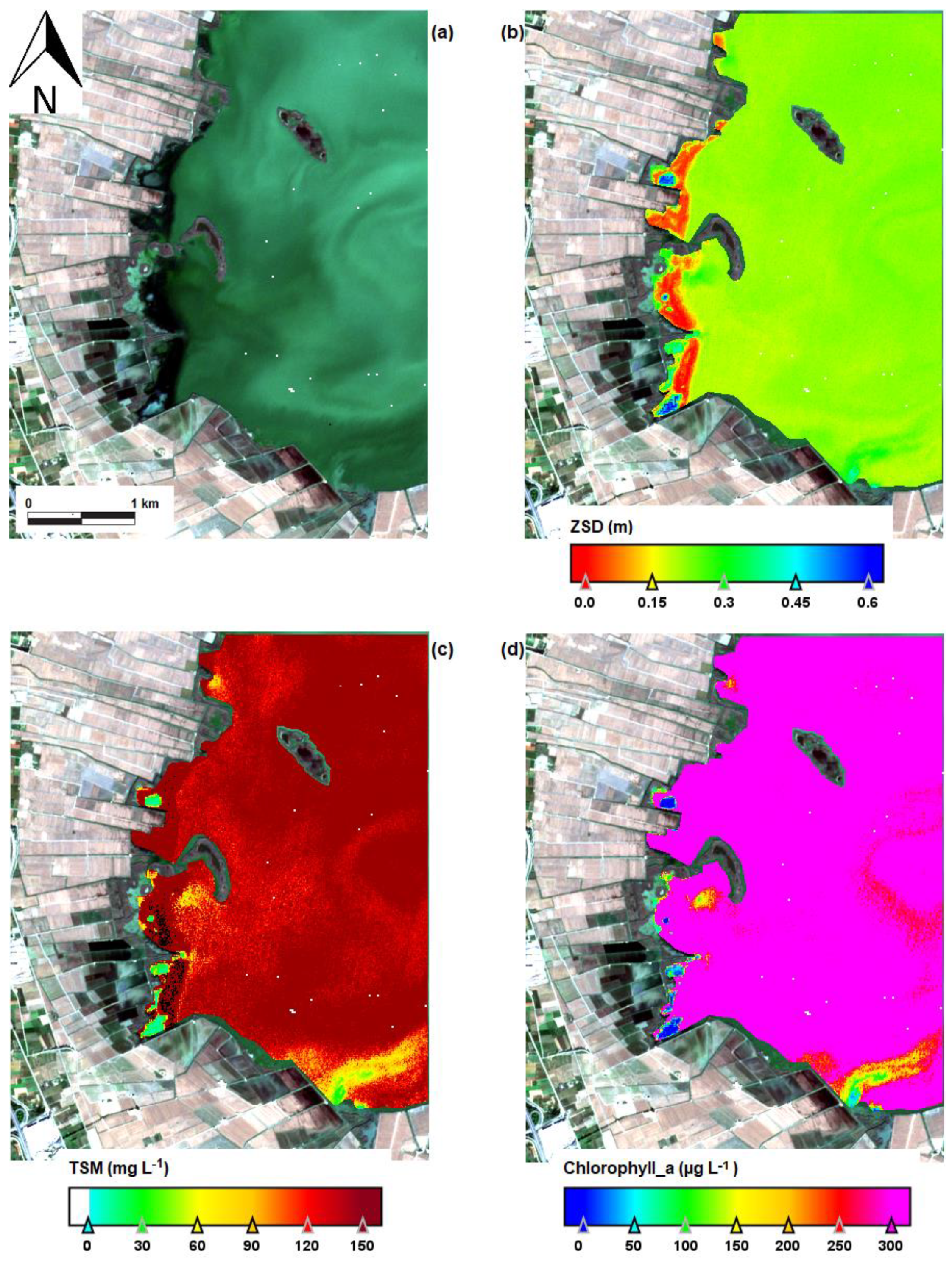

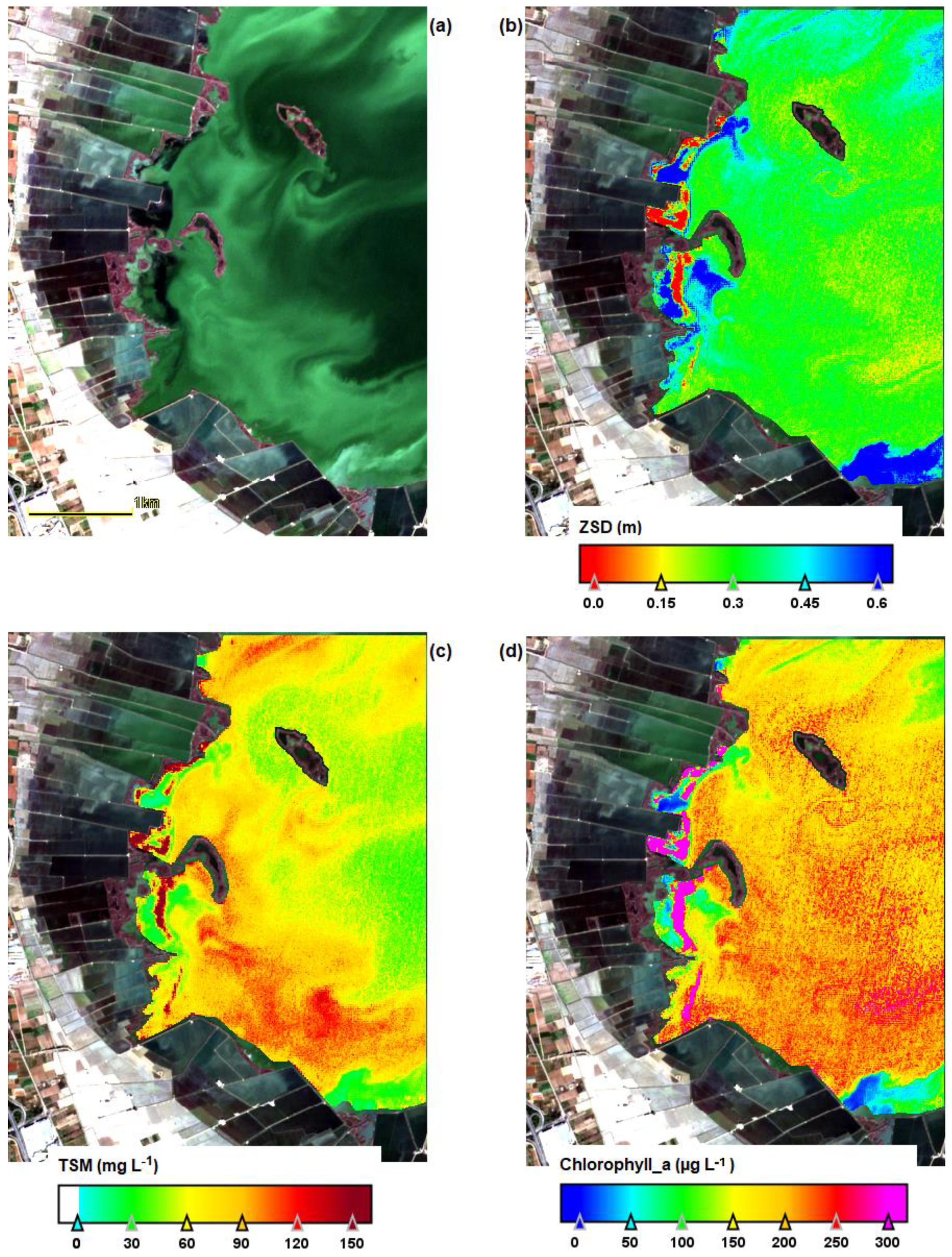
| Date | Area (ha) | Chl-a (mg m−3) | TSS (mg L−1) | ZSD (m) |
|---|---|---|---|---|
| 09 June 2022 | 1.69 | 187.70 | 127.48 | 0.35 |
| 09 July 2022 | 2.11 | 137.67 | 50.54 | 0.29 |
| 08 August 2022 | 9.96 | 58.60 | 30.60 | 0.37 |
| 07 September 2022 | 22.62 | 26.91 | 19.41 | 0.49 |
| 02 October 2022 | 40.76 | 33.82 | 14.41 | 0.49 |
| 06 November 2022 | 48.42 | 363.50 | 145.30 | 0.21 |
| 16 December 2022 | 32.74 | 110.22 | 38.41 | 0.43 |
| 05 January 2023 | 25.54 | 121.98 | 32.72 | 0.32 |
| 04 February 2023 | 14.94 | 145.10 | 53.20 | 0.31 |
| 01 March 2023 | 1.82 | 113.62 | 65.66 | 0.32 |
| Area (ha) | Chl-a (mg m−3) | TSS (mg L−1) | ZSD (m) | |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 |
| Shapiro–Wilk W | 0.918 | 0.843 | 0.827 | 0.935 |
| p (normal) | 0.345 | 0.049 | 0.031 | 0.507 (*) |
| Area (ha) | Chl-a (mg m−3) | TSS (mg L−1) | ZSD (m) | |
|---|---|---|---|---|
| N | 9 | 9 | 9 | 9 |
| Shapiro–Wilk W | 0.912 | 0.938 | 0.833 | 0.862 |
| p (normal) | 0.329 (*) | 0.557 (*) | 0.048 | 0.102 (*) |
| Area (ha) | Chl-a (mg m−3) | TSS (mg L−1) | ZSD (m) | |
|---|---|---|---|---|
| N | 9 | 9 | 9 | 9 |
| Shapiro–Wilk W | 0.912 | 0.938 | 0.983 | 0.862 |
| p (normal) | 0.329 (*) | 0.557 (*) | 0.979 (*) | 0.102 (*) |
| Area (ha) | Chl-a (mg m−3) | TSS (mg L−1) | ZSD (m) | |
|---|---|---|---|---|
| Area (ha) | p = 0.108 | p = 0.014 (*) | p = 0.027 (*) | |
| Chl-a (mg m−3) | r = −0.571 | p = 0.001 (***) | p = 0.016 (*) | |
| TSS (mg L−1) | r = −0.774 | r = 0.914 | p = 0.033 (*) | |
| ZSD (m) | r = 0.723 | r = −0.767 | r = −0.708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria, J.M.; Campillo-Tamarit, N.; Molner, J.V.; Soria-Perpinyà, X. Sentinel-2 Images Discover How Extraordinary Water Inputs Allow the Ephemeral Resurgence of Najas marina in a Shallow Hypertrophic Lagoon (Albufera of Valencia, Spain). Water 2025, 17, 1302. https://doi.org/10.3390/w17091302
Soria JM, Campillo-Tamarit N, Molner JV, Soria-Perpinyà X. Sentinel-2 Images Discover How Extraordinary Water Inputs Allow the Ephemeral Resurgence of Najas marina in a Shallow Hypertrophic Lagoon (Albufera of Valencia, Spain). Water. 2025; 17(9):1302. https://doi.org/10.3390/w17091302
Chicago/Turabian StyleSoria, Juan M., Noelia Campillo-Tamarit, Juan Víctor Molner, and Xavier Soria-Perpinyà. 2025. "Sentinel-2 Images Discover How Extraordinary Water Inputs Allow the Ephemeral Resurgence of Najas marina in a Shallow Hypertrophic Lagoon (Albufera of Valencia, Spain)" Water 17, no. 9: 1302. https://doi.org/10.3390/w17091302
APA StyleSoria, J. M., Campillo-Tamarit, N., Molner, J. V., & Soria-Perpinyà, X. (2025). Sentinel-2 Images Discover How Extraordinary Water Inputs Allow the Ephemeral Resurgence of Najas marina in a Shallow Hypertrophic Lagoon (Albufera of Valencia, Spain). Water, 17(9), 1302. https://doi.org/10.3390/w17091302









