Assessing the Potential of Magnetic Water Treatment of Groundwater for Calcium Carbonate Scale Mitigation in Drinking Water Distribution Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
Water Collection and Treatment
- FF121 AM System (Fluid Force). This system comprised four rectangular magnets (162 mm × 150 mm × 55 mm), encased in plastic covers and arranged around an 8-inch PVC pipe. Their north poles faced the center, creating mutual repulsion. As shown in Figure 1, this system—installed by JMAS in Quinta Versalles—featured direct surface contact between the magnets and the pipe exterior, although the magnets did not interact with the water;
- FF5 System (Fluid Force). This system featured two radially magnetized, arc-shaped Fluid Force FF5 magnets installed around a 1-inch PVC pipe, with their north poles oriented toward the center. Located in the CIMAV magnetism laboratory, it regulated water flow via a ball valve, allowing water to pass through the magnetic field before collection. According to the manufacturer, these magnets are designed for iron pipes requiring a strong magnetic signal and are commonly used in domestic water networks and industrial machinery. Each magnet measures 106 mm × 68 mm × 27 mm;
- IH System (Magnetic Solutions). A 1-Tesla Halbach cylinder from Magnetic Solutions was used for laboratory-scale studies at the CIMAV magnetism laboratory. This system produced a uniform radial magnetic field within a 1-inch central hole, with a field gradient at both the inlet and outlet. A 1-inch PVC pipe was positioned over the cylinder, allowing water to flow through the magnetic field without direct contact. Opening the red valve enabled water to pass through, undergo magnetization, and be collected.
- Physicochemical—Electrical conductivity and chemical composition (Al, Ba, Ca, Cu, Fe, K, Mg, Mn, Mo, Na, Si, Sr, Zn) were assessed via inductively coupled plasma (ICP) spectroscopy, and pH via an Orion Versa Star meter (Thermo Scientific, Waltham, MA, USA);
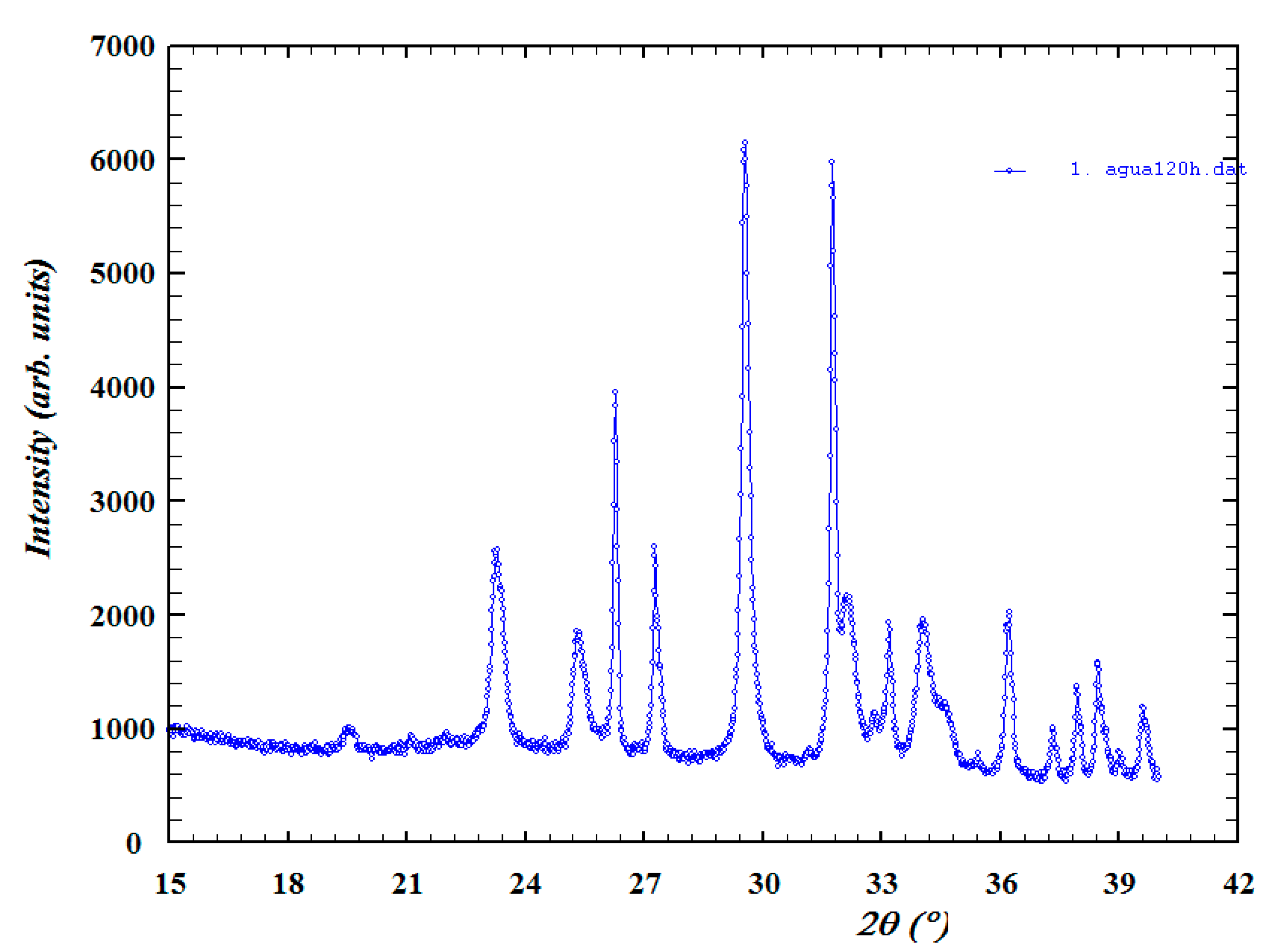
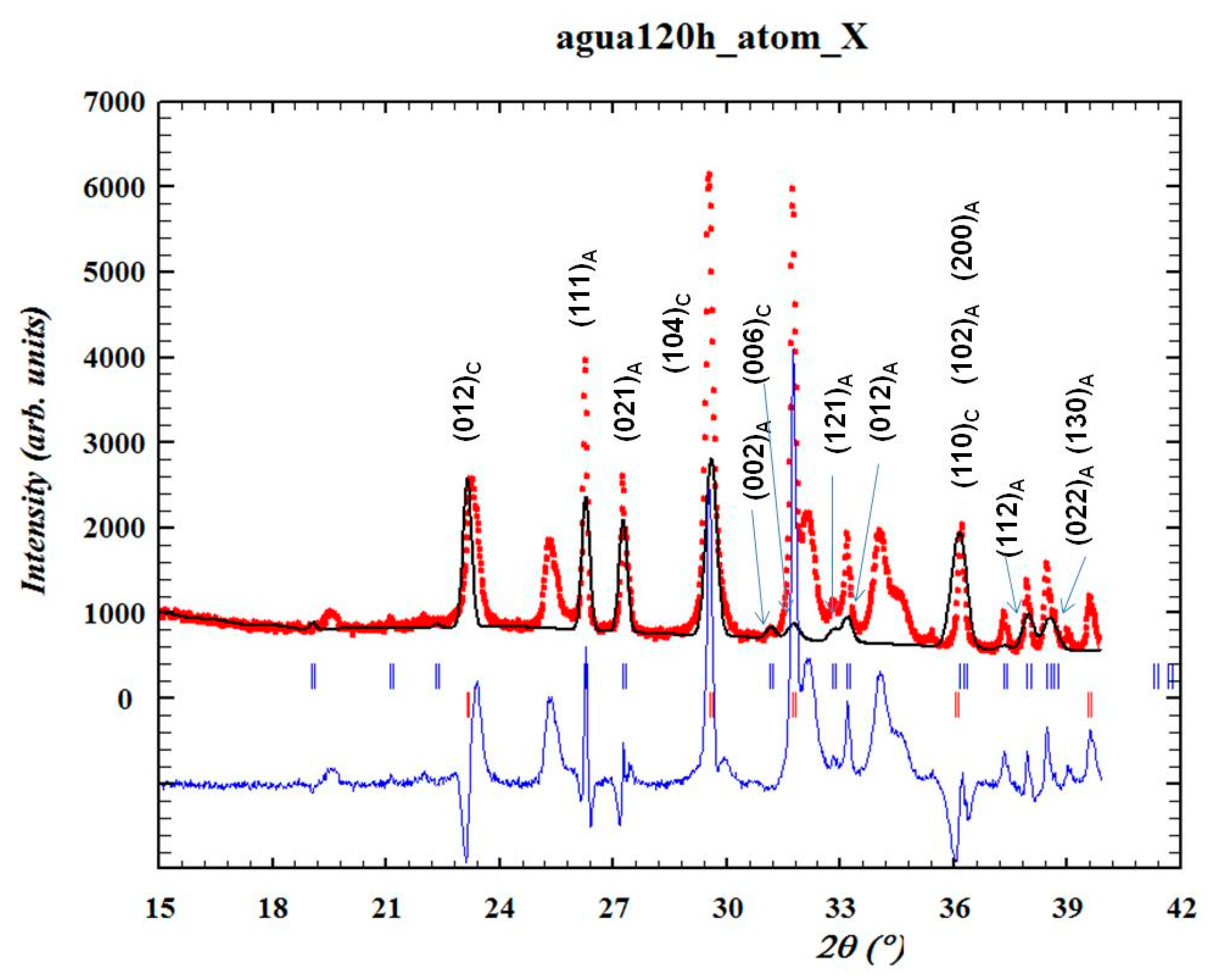
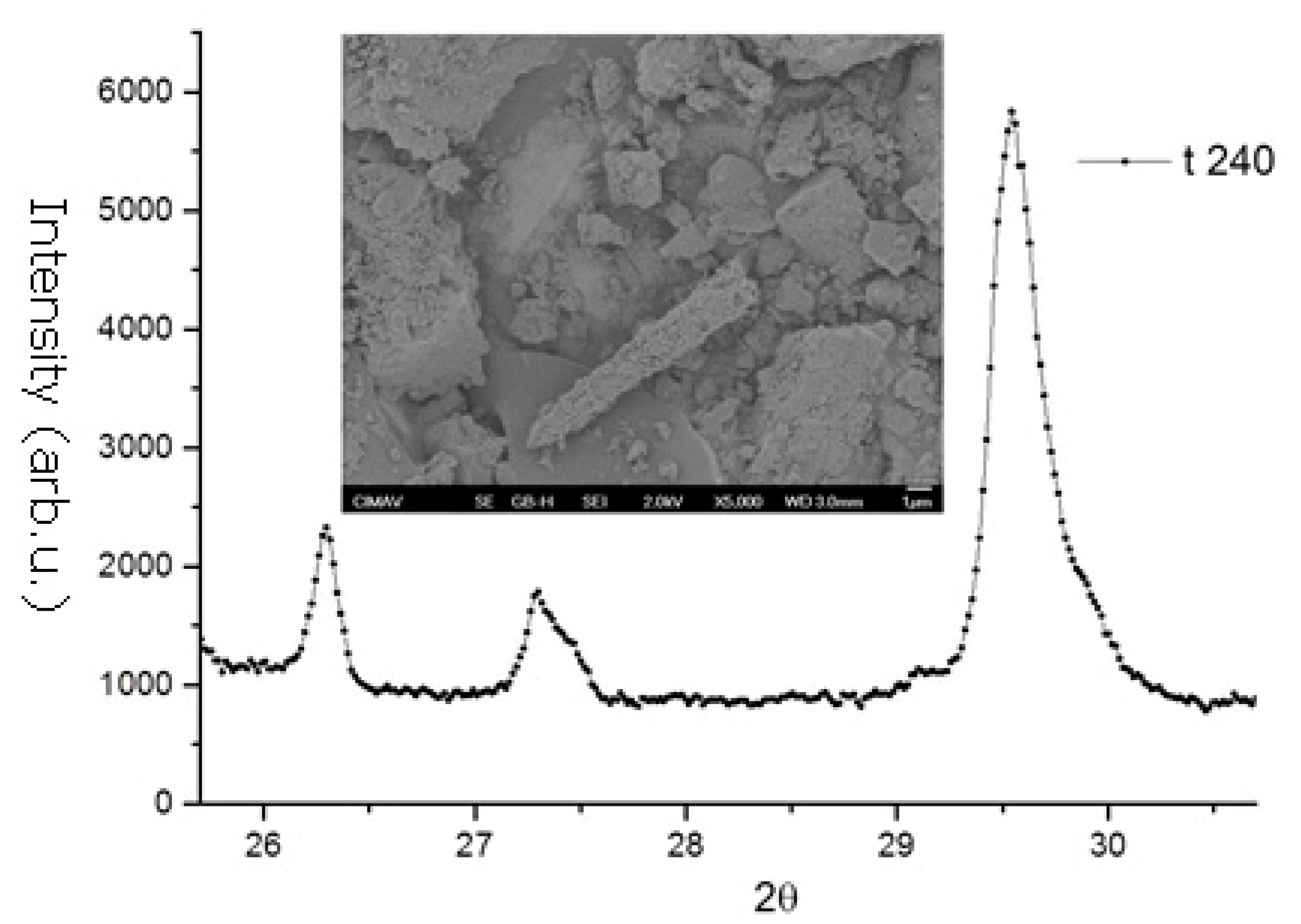
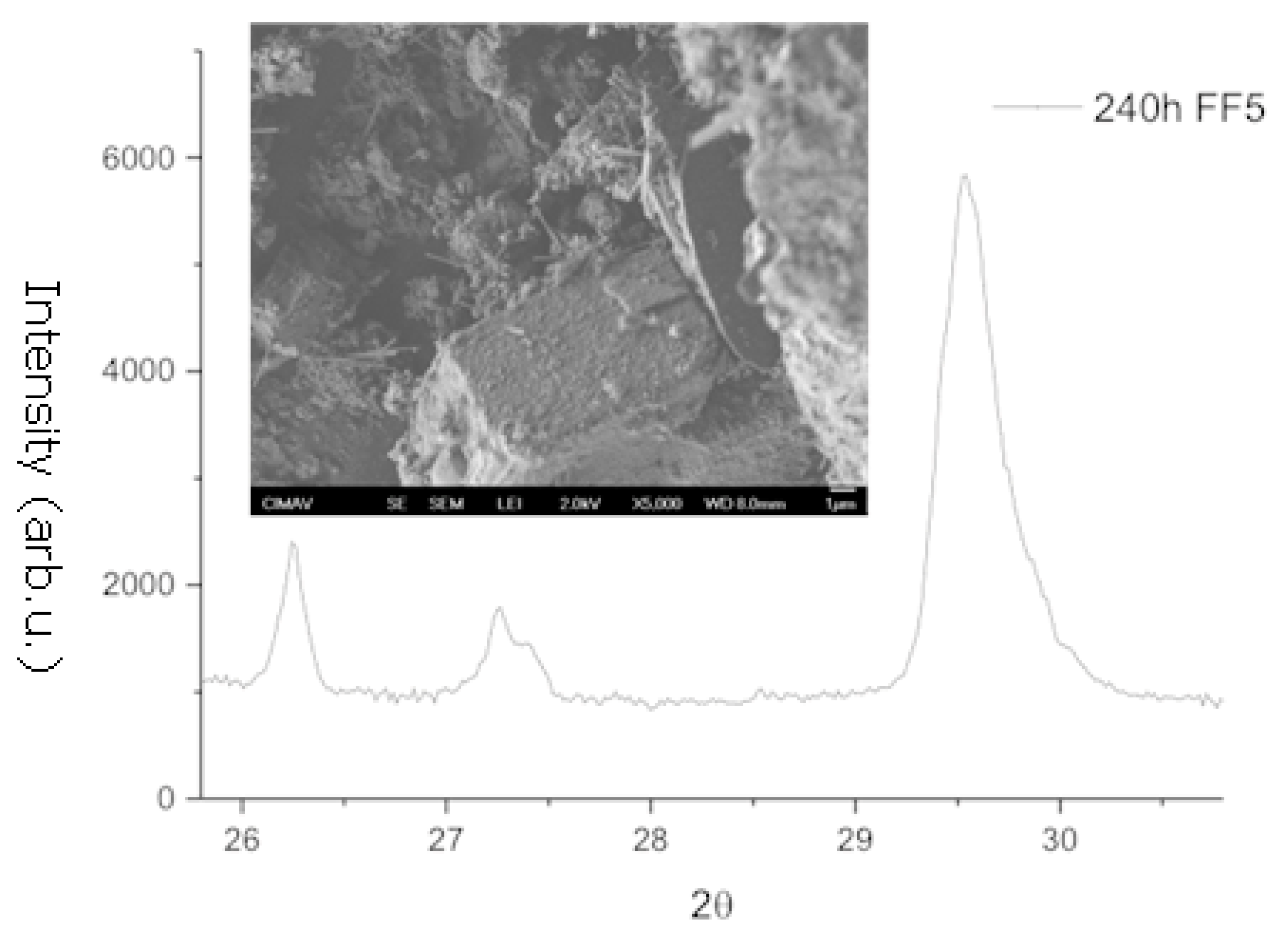
- Structural analysis—X-ray diffraction (XRD) using a Bruker D8 Discover diffractometer using Cu-Kα radiation (Billerica, MA, USA). The crystallographic evolution of calcite and aragonite was assessed using the Rietveld refinement method in FullProf. The aragonite fraction (A) was determined usingwhere I104, I111, and I021 correspond to calcite and aragonite XRD peak intensities.
3. Results
3.1. X-Ray Diffraction and Scanning Electron Microscopy
3.2. Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced Polymer Induced Precipitation of Polymorphous in Calcium Carbonate: Calcite Aragonite Vaterite Phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Antony, A.; How Low, J.; Gray, S.; EChildress, A.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Li, Z. Effect of magnetic field on the physical properties of water. Results Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Su, M.; Han, J.; Li, Y.; Chen, J.; Zhao, Y.; Chadwick, K. Ultrasonic Crystallization of Calcium Carbonate in Presence of Seawater Ions. Desalination 2015, 369, 85–90. [Google Scholar] [CrossRef]
- Zarga, Y.; Ben Boubaker, H.; Ghaffour, N.; Elfil, H. Study of calcium carbonate and sulfate co-precipitation. Chem. Eng. Sci. 2013, 96, 33–41. [Google Scholar] [CrossRef]
- Younes, A.A.; El-Maghrabi, H.H.; Ali, H.R. Novel polyacrylamide-based solid scale inhibitor. J. Hazard. Mater. 2017, 334, 1–9. [Google Scholar] [CrossRef]
- Othman, A.; Sohaili, J.; Sumaiyyah Supian, N. A Review: Methodologies Review of Magnetic Water Treatment as Green Approach of Water Pipeline System. Pertanika J. Sci. Technol. 2019, 27, 218–296. [Google Scholar]
- ElMassalami, M.; Teixeira, M.S.; Elzubair, A. Investigating the Antiscale Magnetic Treatment Controversy: Insights from the Model Calcium Carbonate Scalant. Sci. Rep. 2025, 15, 3441. [Google Scholar] [CrossRef]
- Busch, K.W.; Busch, M.A.; Parker, D.H.; Darling, R.E.; McAtee, J.L. Studies of a water treatment device that uses magnetic fields. Corrosion 1986, 42, 211–221. [Google Scholar] [CrossRef]
- Alimi, F.; Tlili, M.M.; Ben Amor, M.; Maurin, G.; Gabrielli, C. Effect of magnetic water treatment on calcium carbonate precipitation: Influence of the pipe material. Chem. Eng. Process. Process. Intensif. 2009, 48, 1327–1332. [Google Scholar] [CrossRef]
- Baker, J.S.; Judd, S.J. Magnetic amelioration of scale formation. Water Res. 1996, 30, 247–260. [Google Scholar] [CrossRef]
- Ali, A. Effect of Magnetic Treatment on Temporary Hardness of Groundwater. Asian J. Chem. 2019, 31, 1017. [Google Scholar]
- Dalas, E.; Koutsoukos, P.G. The effect of magnetic fields on calcium carbonate scale formation. J. Cryst. Growth 1989, 96, 802–806. [Google Scholar] [CrossRef]
- Alabi, A.; Chiesa, M.; Garlisi, C.; Palmisano, G. Advances in anti-scale magnetic water treatment. Environ. Sci. Water Res. Technol. 2015, 1, 408–425. [Google Scholar] [CrossRef]
- Al Helal, A.; Soames, A.; Gubner, R.; Iglauer, S.; Barifcani, A. Influence of magnetic fields on calcium carbonate scaling in aqueous solutions at 150 °C and 1 bar. J. Colloid Interface Sci. 2018, 509, 472–484. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, X.; Xu, P. A critical review of the application of electromagnetic fields for scaling control in water systems: Mechanisms, characterization, and operation. NPJ Clean Water 2020, 3, 25. [Google Scholar] [CrossRef]
- Chung, A.; Sheng, H.; Ling, J. Magnetic Induction on pH, Oxidation-Reduction Potential, Conductivity and Dissolved Oxygen Level of Water and Its Properties: A State-of-the-Art Review. In Sustainable Groundwater and Environment: Challenges and Solutions; Li, P., He, X., Wu, J., Elumalai, V., Eds.; Springer: Cham, Switzerland, 2025; pp. 279–309. [Google Scholar]
- Bian, Y.; Li, S.; Luo, H.; Lv, L.; Zan, S.; Ren, B.; Zhu, G. Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification. Water 2023, 15, 3391. [Google Scholar] [CrossRef]
- Tai, Y.C.; Wu, C.K.; Chang, M.C. Effects of magnetic field on the crystallization of CaCO3 using permanent magnets. Chem. Eng. Sci. 2008, 63, 5606–5612. [Google Scholar] [CrossRef]
- Liu, C.Z.; Lin, C.H.; Yeh, M.S.; Chao, Y.M.; Shen, P. Surface Modification and Planar Defects of Calcium Carbonates by Magnetic Water Treatment. Nanoscale Res. Lett. 2010, 5, 1982–1991. [Google Scholar] [CrossRef]
- Piyadasa, C.; Yeager, T.R.; Gray, S.R.; Stewart, M.B.; Ridgway, H.F.; Pelekani, C.; Orbell, J.D. The influence of electromagnetic fields from two commercially available water-treatment devices on calcium carbonate precipitation. Environ. Sci. Water Res. Technol. 2017, 3, 566–572. [Google Scholar] [CrossRef]
- Karar, A.; Henni, A.; Namoune, F.; Rosei, F. Inhibition of nucleation and crystal growth of calcium carbonate in hard waters using Paronychia arabica in an arid desert region. Water Environ. J. 2020, 34, 979–987. [Google Scholar] [CrossRef]
- Martínez, S.; Boluda, N. Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Latva, M.; Inkinen, J.; Rämö, J.; Kaunisto, T.; Mäkinen, R.; Ahonen, M.; Matilainen, J.; Pehkonen, S. Studies on the magnetic water treatment in new pilot scale drinking water system and in old existing real-life water system. J. Water Process. Eng. 2016, 9, 215–224. [Google Scholar] [CrossRef]
- Silva, I.B.; Neto, J.C.Q.; Petri, D.F. The effect of magnetic field on ion hydration and sulfate scale. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 175–183. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Parkin, S.S.P. Handbook of Magnetism and Magnetic Materials; Springer Nature Link: Cham, Switzerland, 2020. [Google Scholar]
- Mghaiouini, R.; Benzbiria, N.; Belghiti, M.; Belghiti, H.; Monkade, M.; El Bouari, A. Optical properties of water under the action of the electromagnetic field in the infrared spectrum. Mater. Today Proc. 2020, 30, 1046–1051. [Google Scholar] [CrossRef]
- Alimi, F.; Tlili, M.; Ben Amor, M.; Gabrielli, C.; Maurin, G. Influence of magnetic field on calcium carbonate precipitation. Desalination 2006, 206, 163–168. [Google Scholar] [CrossRef]
- Kozic, V.; Krope, J.; Lipus, L.C.; Ticar, I. Magnetic field analysis on electromagnetic water treatment device. Hung. J. Ind. Chem. Veszprém 2006, 34, 51–54. [Google Scholar]
- Higashitani, K.; Kage, A.; Katamura, S.; Imai, K.; Hatade, S. Effects of a Magnetic Field on the Formation of CaCO3 Particles. J. Colloid Interface Sci. 1993, 156, 90–95. [Google Scholar] [CrossRef]
- Pouget, E.M.; Bomans, P.H.H.; Goos, J.A.C.M.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM. Science 2009, 323, 1455–1458. [Google Scholar] [CrossRef]
- INEGI. INEGI. 2020. Available online: https://www.inegi.org.mx/programas/ccpv/2020/default.html#Datos_abiertos (accessed on 28 December 2023).



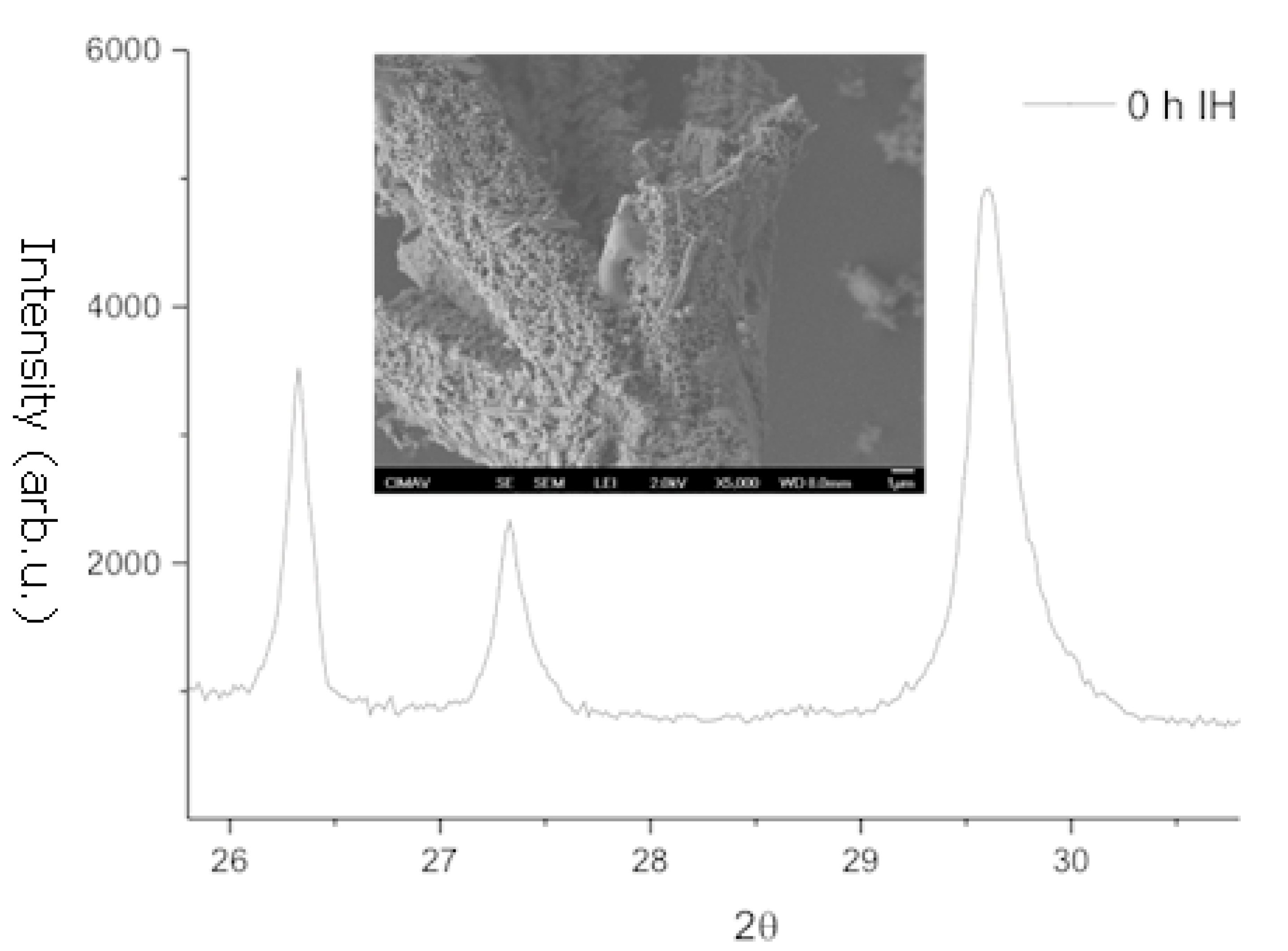
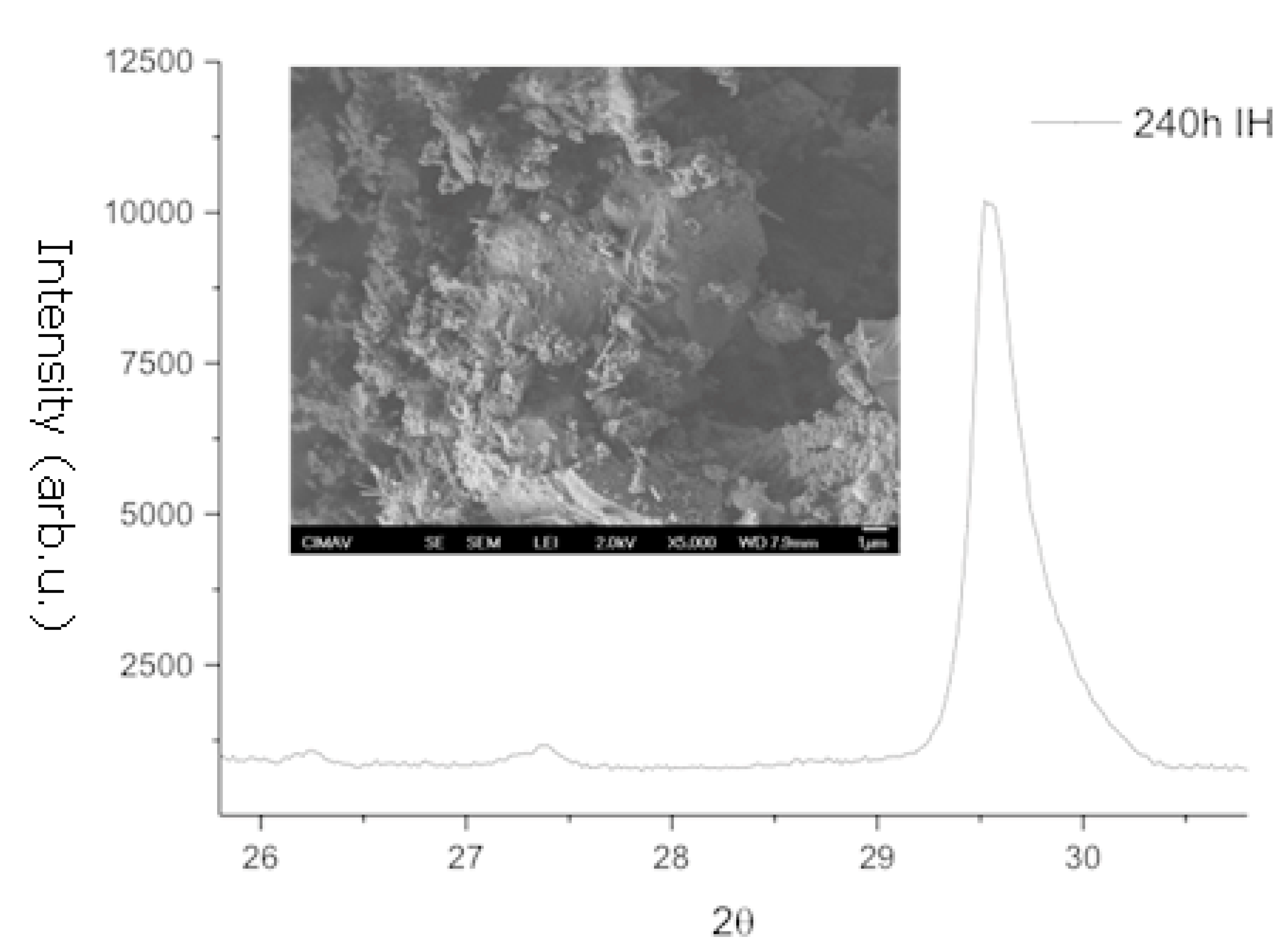



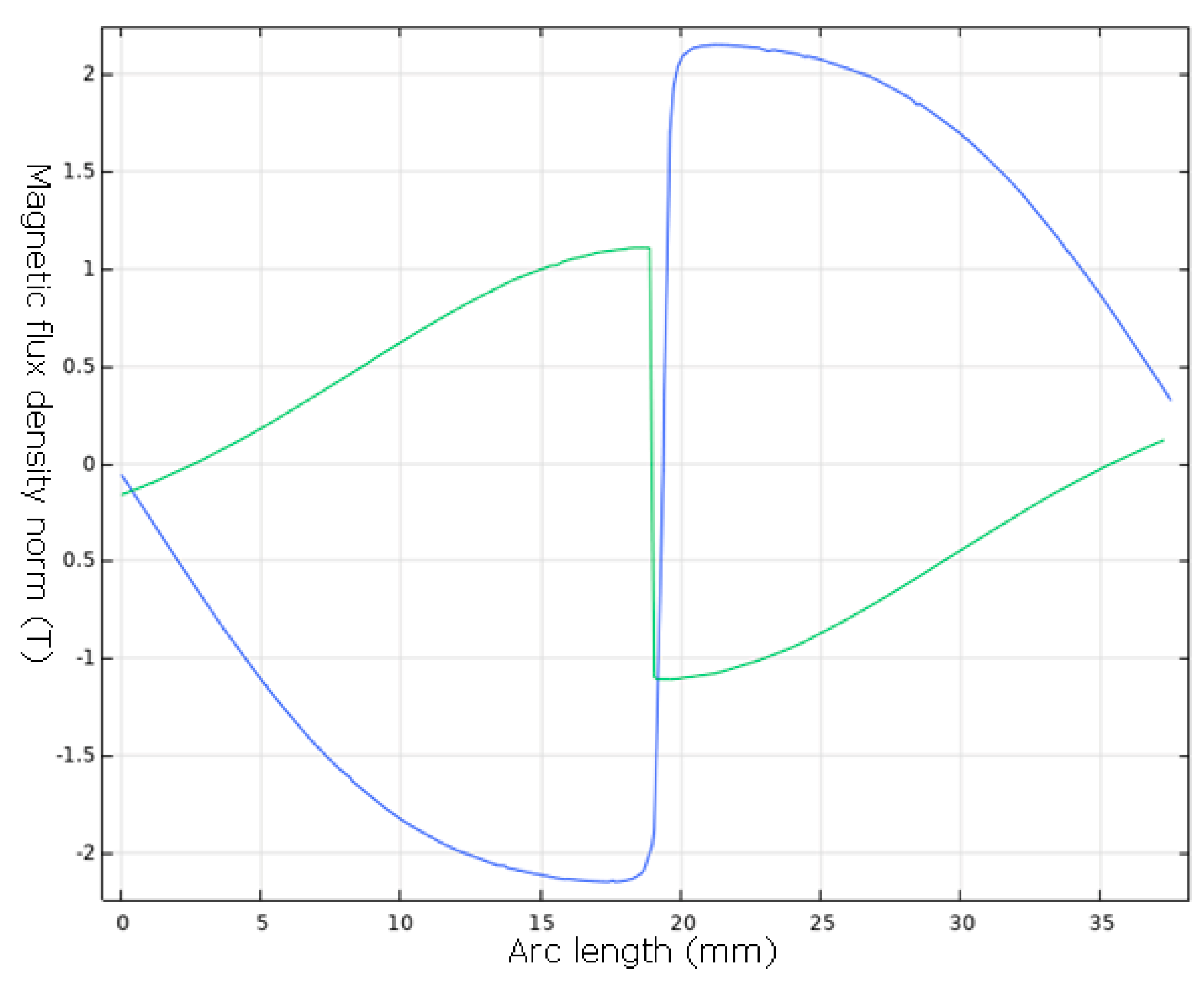
| Element | MW (mg/L) | Element (mg/L) |
|---|---|---|
| Al | 0.02 | 0.014 |
| Ba | 0.03 | 0.031 |
| Ca | 49.187 | 49.031 |
| Cu | N.D. | N.D. |
| Fe | N.D. | N.D. |
| K | 2.237 | 2.277 |
| Mg | 5.68 | 5.721 |
| Mn | N.D. | N.D. |
| Mo | N.D. | N.D. |
| Na | 81.242 | 80.664 |
| Si | 20.651 | 20.477 |
| Sr | 0.447 | 0.477 |
| Zn | 0.008 | 0.034 |
| Measurement | NMW 2 | MW FF121 1 | MW FF5 | MW IH |
|---|---|---|---|---|
| pH | 8.799 | 8.352 | 8.214 | 8.314 |
| Conductivity (µS/cm) | 585 | 583 | 585 | 596 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, D.; Herrera-Peraza, E.; Navarro-Gomez, C.; Sanchez-Navarro, J.R. Assessing the Potential of Magnetic Water Treatment of Groundwater for Calcium Carbonate Scale Mitigation in Drinking Water Distribution Networks. Water 2025, 17, 1265. https://doi.org/10.3390/w17091265
Sanchez D, Herrera-Peraza E, Navarro-Gomez C, Sanchez-Navarro JR. Assessing the Potential of Magnetic Water Treatment of Groundwater for Calcium Carbonate Scale Mitigation in Drinking Water Distribution Networks. Water. 2025; 17(9):1265. https://doi.org/10.3390/w17091265
Chicago/Turabian StyleSanchez, David, Eduardo Herrera-Peraza, Carmen Navarro-Gomez, and Jesus Ruben Sanchez-Navarro. 2025. "Assessing the Potential of Magnetic Water Treatment of Groundwater for Calcium Carbonate Scale Mitigation in Drinking Water Distribution Networks" Water 17, no. 9: 1265. https://doi.org/10.3390/w17091265
APA StyleSanchez, D., Herrera-Peraza, E., Navarro-Gomez, C., & Sanchez-Navarro, J. R. (2025). Assessing the Potential of Magnetic Water Treatment of Groundwater for Calcium Carbonate Scale Mitigation in Drinking Water Distribution Networks. Water, 17(9), 1265. https://doi.org/10.3390/w17091265






