Advanced Analytical Approaches for Phenolic Compounds in Groundwater: A PRISMA Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
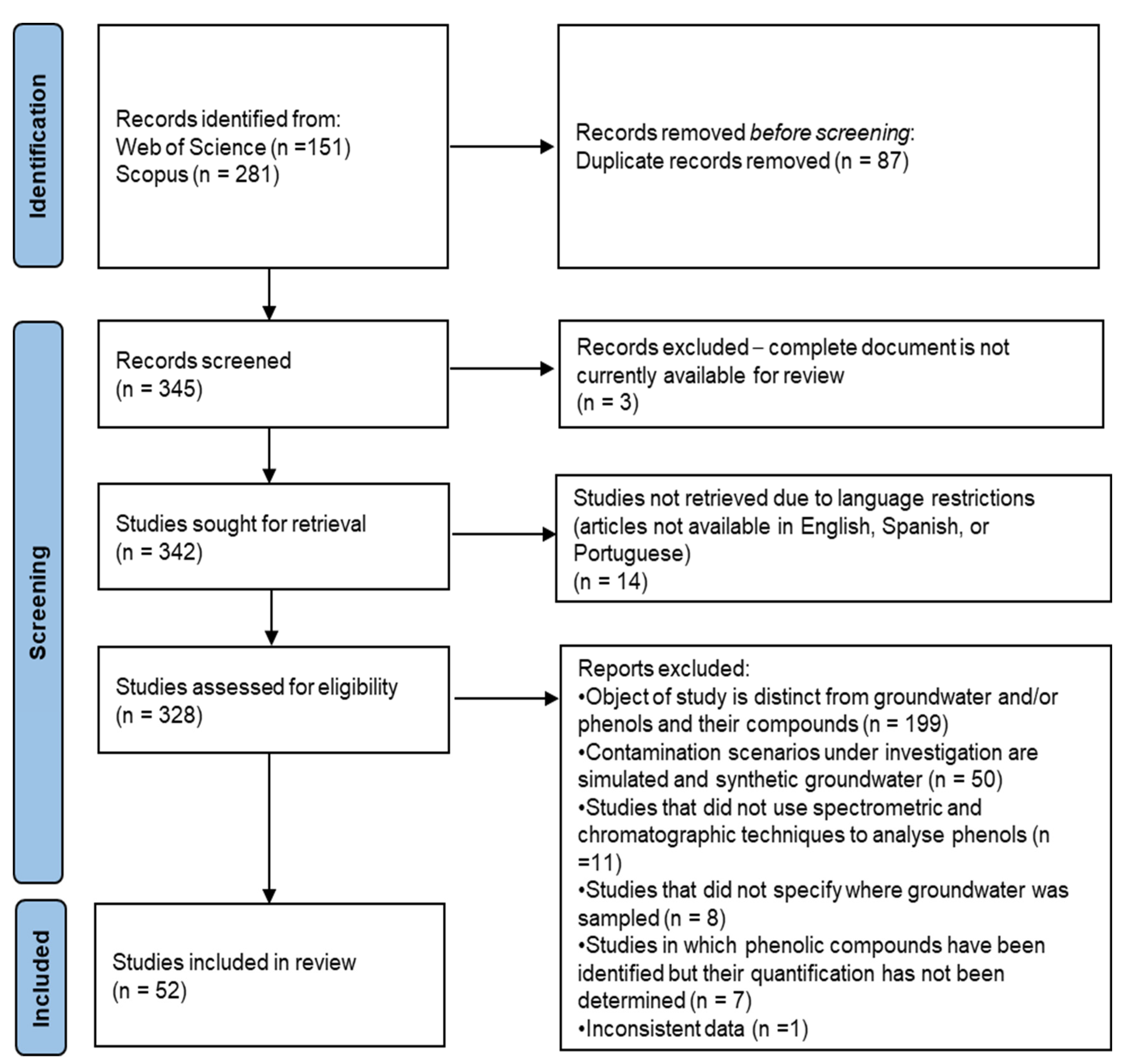
2.3. Search and Selection Strategies
| WoS | (“phenol*”) (Topic) and (“ groundwater*” or “ ground water*” or “subsurface water*” or “Underground water*”) (Topic) and (“spectrometry” OR “chromatography”) (Topic) |
| Scopus | (TITLE-ABS-KEY ((“phenol*”)) AND TITLE-ABS-KEY ((“groundwater*” OR “ground water*” OR “subsurface water*” OR “Underground water*”)) AND TITLE-ABS-KEY ((“spectrometry” OR “chromatography”))) |
2.4. Data Extraction, Evaluation and Synthesis
2.5. Classification of Specific Phenolic Compounds
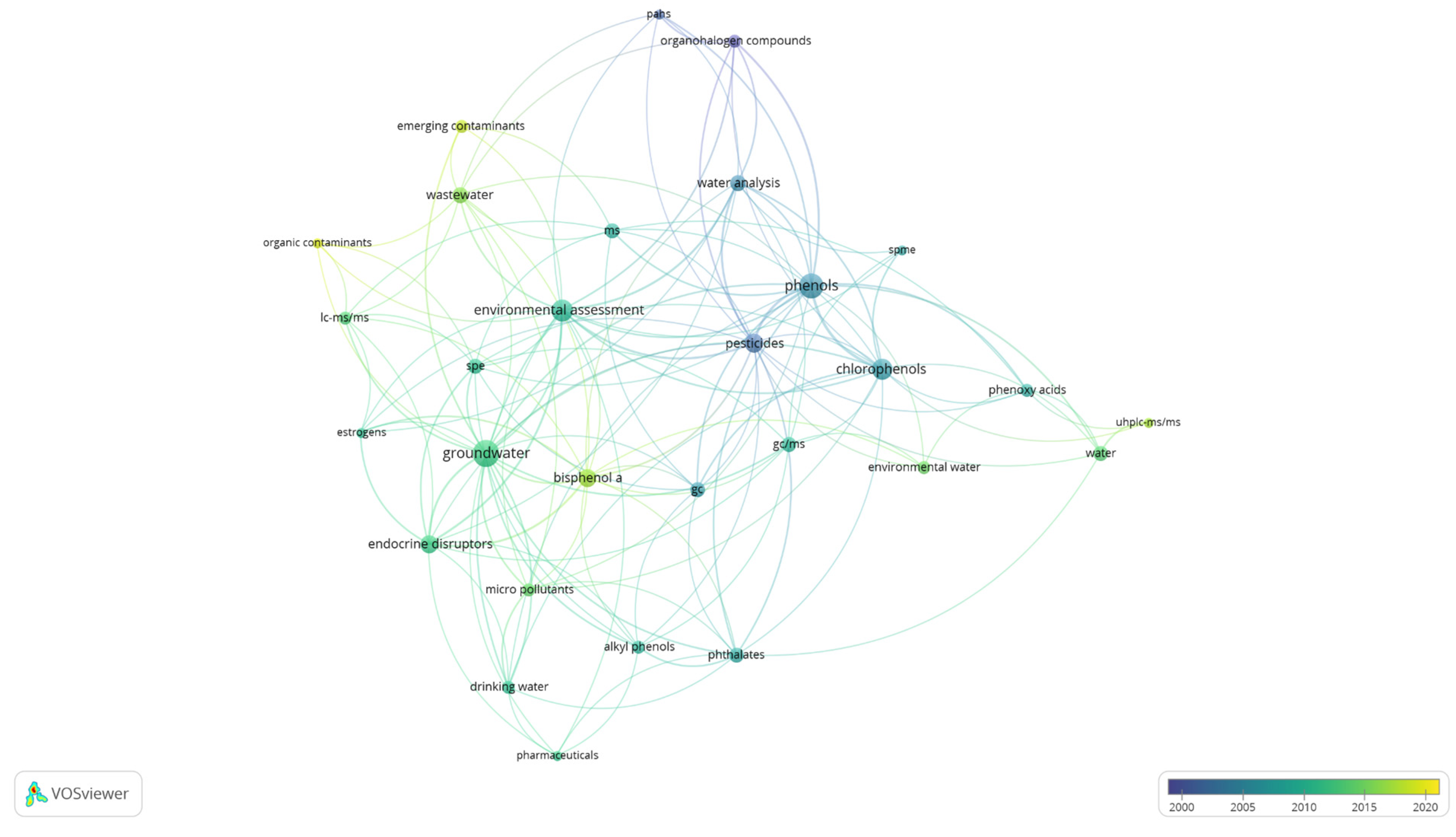
2.6. Data Analysis and Synthesis
3. Results and Discussion
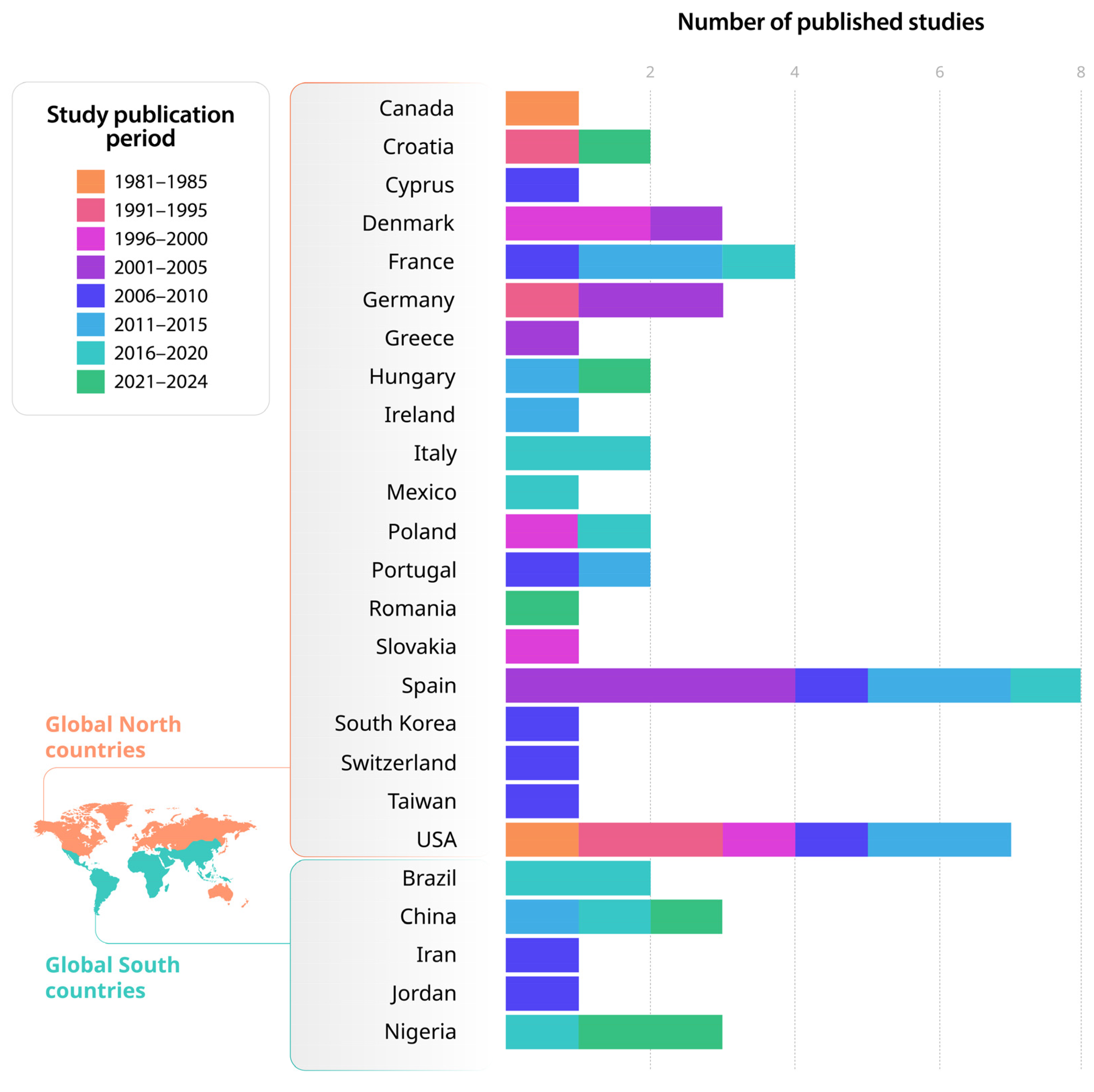
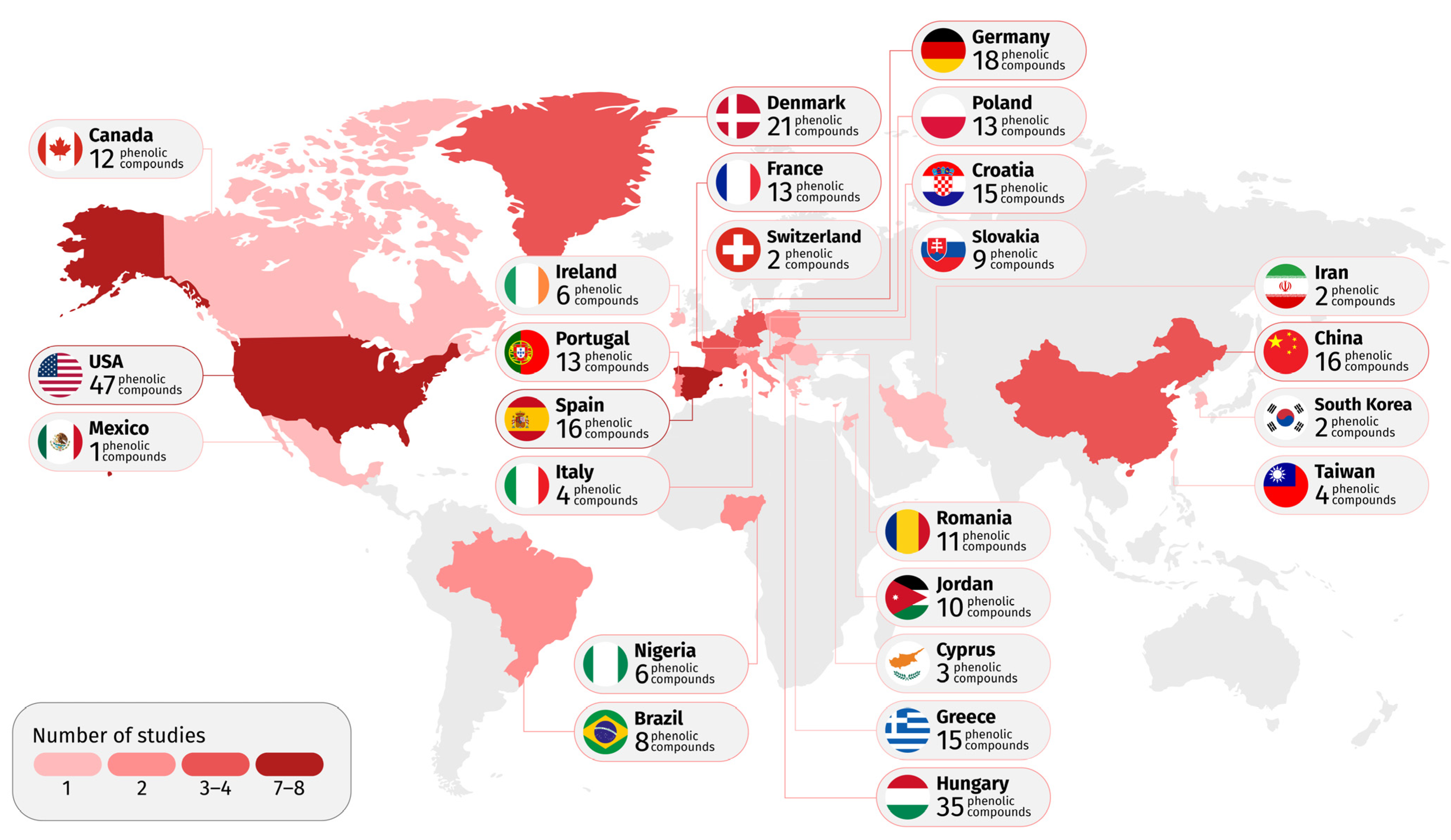
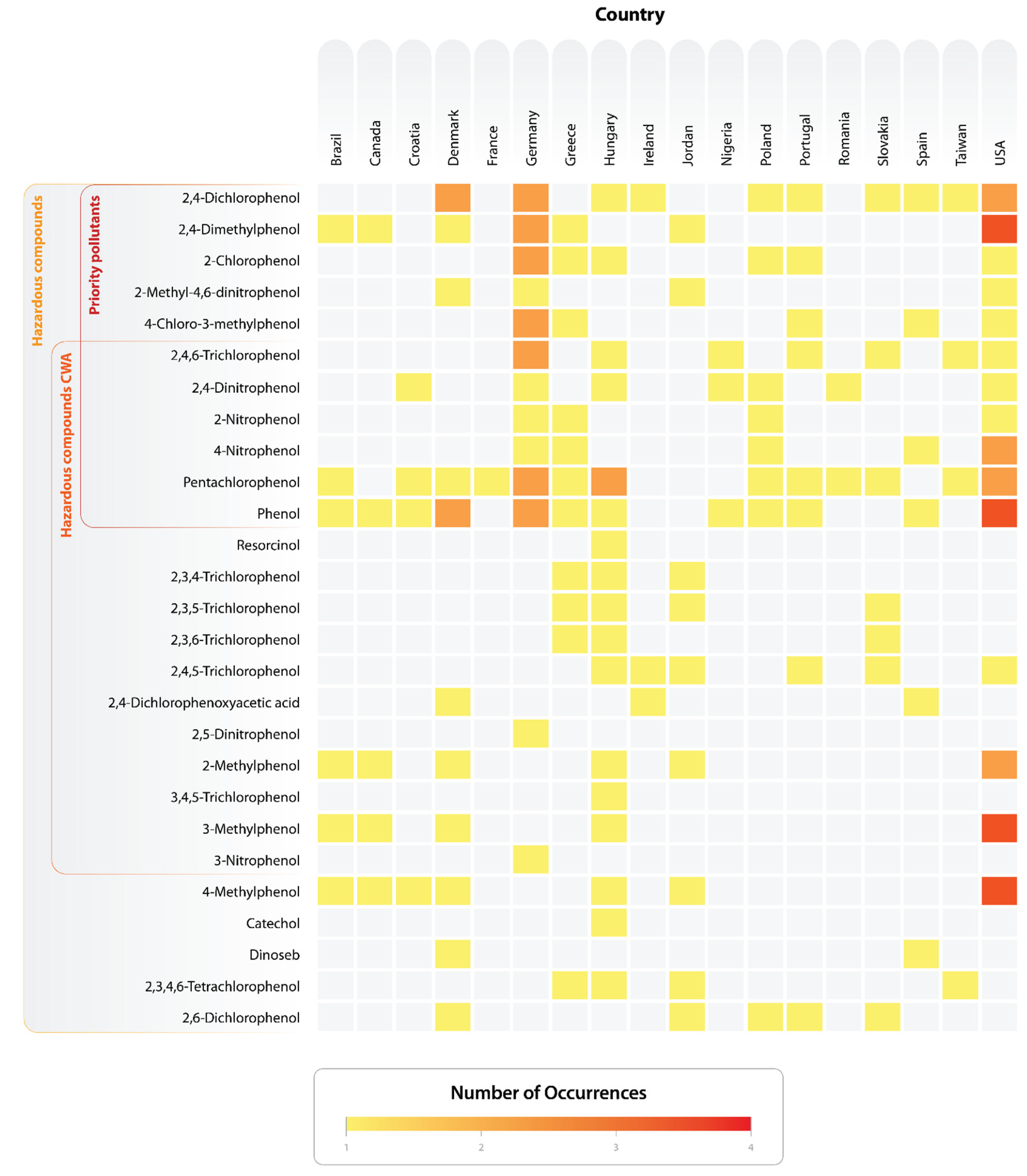
3.1. Temporal Evolution, Sources of Contamination and Global Distribution of Groundwater Contamination by Phenolic Compounds
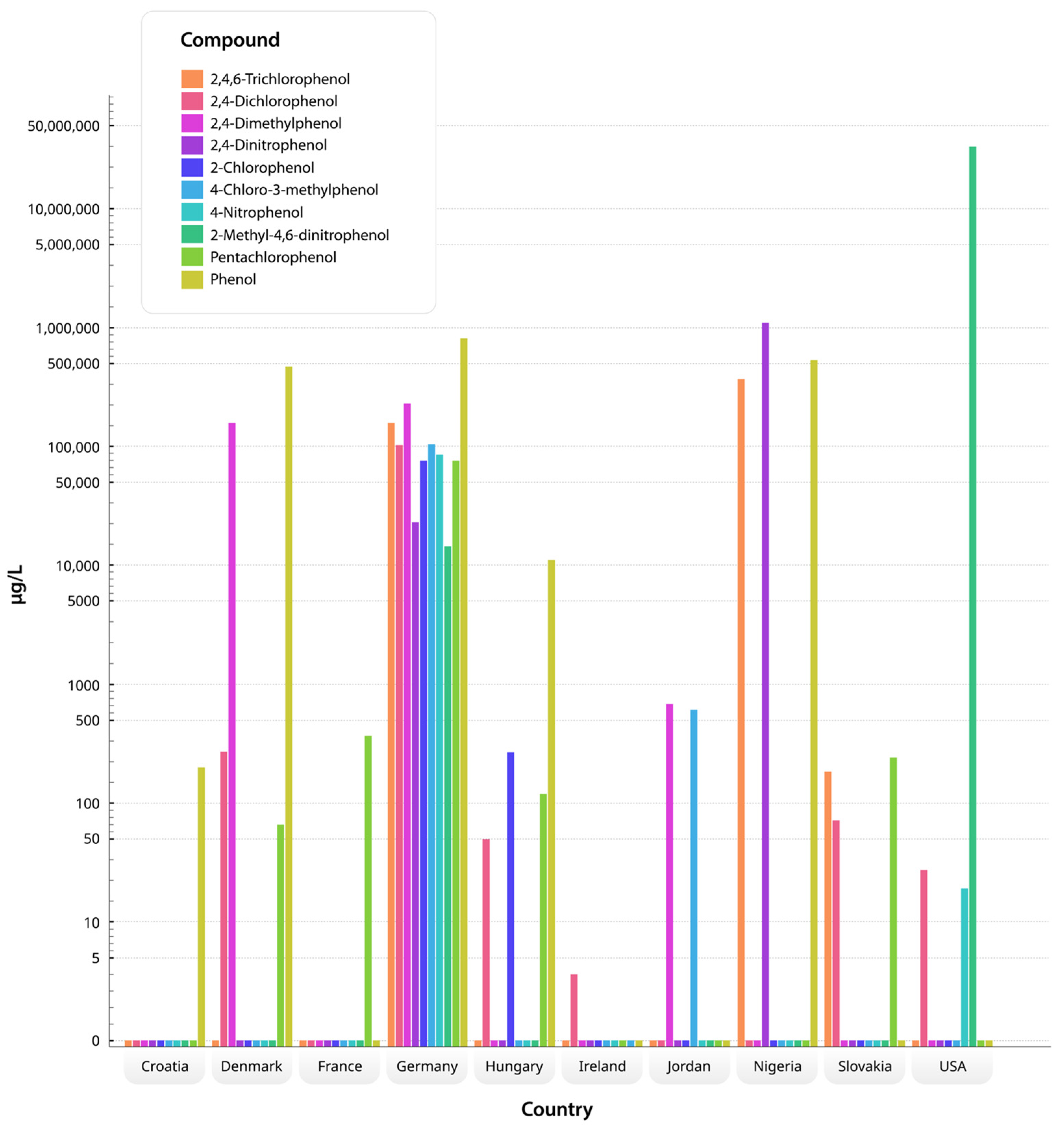
3.2. Phenolic Compounds Evaluated and Detected by Groundwater Studies
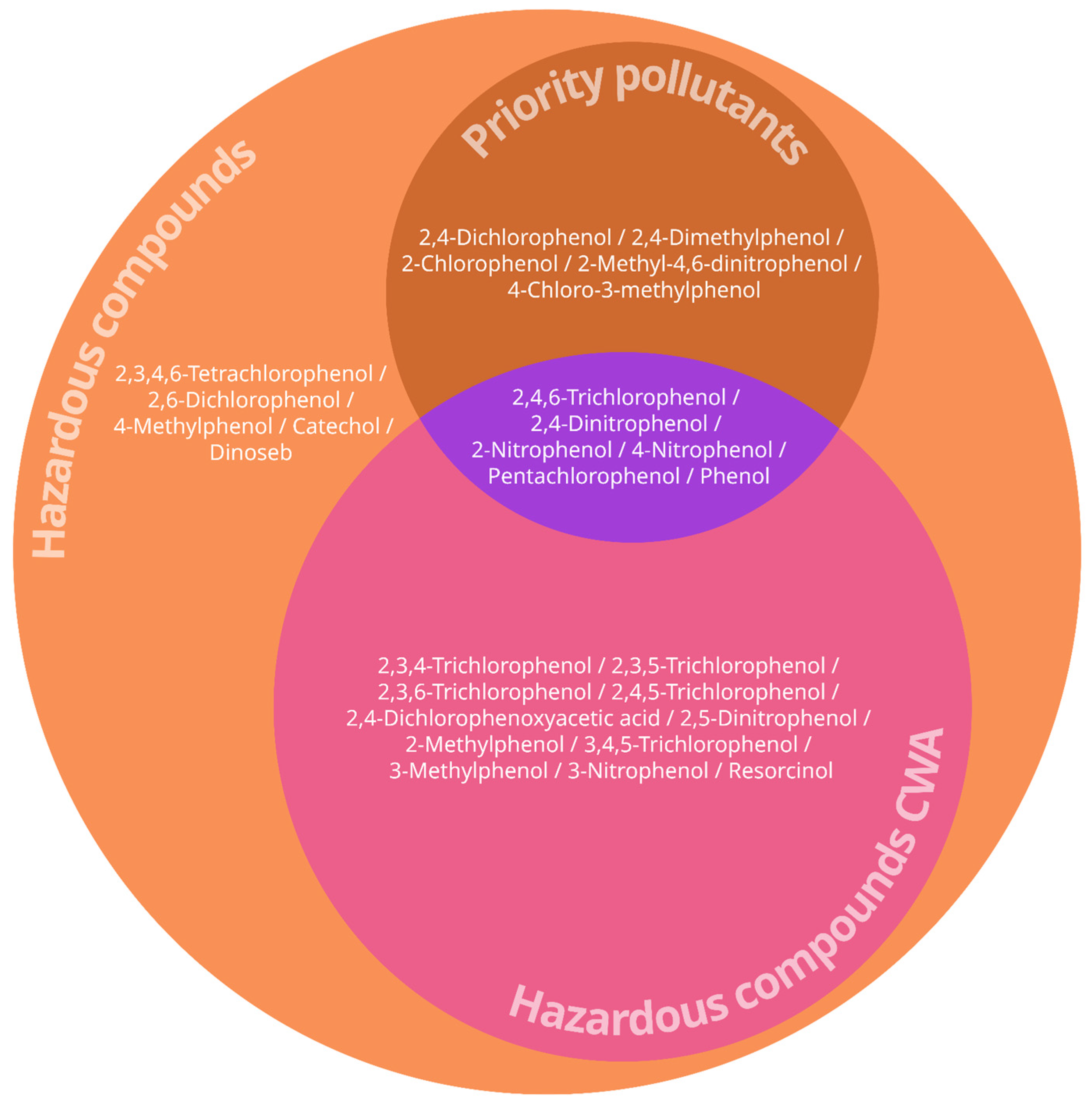
3.3. Priority Pollutants and Hazardous Compounds Identified in Contaminated Groundwater
3.4. Performance of Analytical Techniques for the Detection and Quantification of Priority Phenolic Compounds in Groundwater
4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| µg/L | Microgram per Liter; |
| 1H NMR | Proton Nuclear Magnetic Resonance; |
| 2,4,6-TBP | 2,4,6-Tribromophenol; |
| 2,4-MCP | 4-chloro-2-methylpenol; |
| APs | Alkylphenols; |
| A-SPE | Admicellar Solid-Phase Extraction; |
| BPA | Bisphenol A; |
| BSTFA | Bis(trimethylsilyl)trifluoroacetamide; |
| C18 | Octadecyl; |
| CAS | Chemical Abstracts Service; |
| CERCLA | Comprehensive Environmental Response, Compensation and Liability Act of 1980; |
| CFR | Code of Federal Regulations (United States); |
| CG | Gas Chromatography; |
| GC-MS | Gas Chromatography; |
| CLSA | Closed-Loop Striping Analysis; |
| CWA | Clean Water Act; |
| DBP | Dibromophenol; |
| DCM | Dichlorometano; |
| DOM | Overseas Departments; |
| DSDG | Division for Sustainable Development Goals; |
| EC | Europe Commission; |
| ECs | Emerging Contaminants; |
| ECD | Electron Capture Detector; |
| EDs | Endocrine Disruptors; |
| EDCs | Endocrine-Disrupting Chemicals; |
| FID | Flame Ionization Detector; |
| GC | Gas Chromatography; |
| GC-ECD | Gas Chromatography with Electron Capture Detector; |
| GC-FID | Gas Chromatography with a Flame Ionization Detector; |
| GC-GC-TOFMS | Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry; |
| GC-NPD | Gas Chromatography–Nitrogen–Phosphorus Detector; |
| GC-MS | Gas Chromatography–Mass Spectrometry; |
| GC-MS/MS | Gas Chromatography–Tandem Mass Spectrometry; |
| GRUMO | The National Groundwater-Monitoring Program; |
| GWD | The EU Groundwater Directive; |
| HPLC | High-Performance Liquid Chromatography; |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry; |
| HPLC-SPE-NMR-TOF-MS | High-Performance Liquid Chromatography coupled to Solid-Phase Extraction and Nuclear Magnetic Resonance and Time-of-Flight Mass Spectrometry; |
| IAG | Generative Artificial Intelligence; |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry; |
| IPCC | Intergovernmental Panel on Climate Change; |
| JP8 | Jet Propulsion; |
| LC | Liquid Chromatography; |
| LC-MS | Liquid chromatography–Mass Spectrometry; |
| LC-MS/GC-MS | Liquid chromatography–Mass Spectrometry-Gas Chromatography–Mass Spectrometry; |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry; |
| LC-UV | Liquid Chromatography-Ultraviolet; |
| LD | Detection Limit; |
| LOD | Limit of Detection; |
| LOQ | Limit of Quantification; |
| LQ | Quantification Limit; |
| LLE | Liquid–Liquid Extraction; |
| LME | Liquid-Phase Micro Extraction; |
| LPME | Liquid-Phase Micro Extraction; |
| LPME-GC-MS | Liquid-Phase Micro Extraction–Tandem Mass Spectrometry; |
| LPME-LC-UV | Liquid-Phase Micro Extraction-Liquid Chromatography-Ultraviolet; |
| MASE | Microwave-Assisted Solvent; |
| MIP | Molecularly Imprinted Polymer; |
| MQL | Method Quantification Limit; |
| MS | Mass Spectrometry; |
| MTBE | Methyl Tertiary Butyl Ether; |
| ng/L | Nanogram per Liter; |
| NIH | National Institutes of Health; |
| NLM | National Library of Medicine; |
| NMR | Nuclear Magnetic Resonance; |
| NMW | Natural Mineral Water; |
| ONU | Organização das Nações Unidas; |
| OPFRs | Organophosphate Flame Retardants; |
| PFASs | Perfluoroalkyl Substances; |
| PICo | Population, Interest and Context; |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analyses; |
| R | Recovery (%); |
| RQ | Reportable Quantity; |
| SBSE | Stir Bar Sorptive Extraction; |
| SDG | Sustainable Development Goal; |
| SERS | Surface Enhanced Raman Spectroscopy; |
| SLR | Systematic Literature Review; |
| SPE | Solid-Phase Extraction; |
| SPE-LC-MS-MS | Solid-Phase Extraction–Liquid Chromatography–Tandem Mass Spectrometry; |
| SPE-LC-APCI-MS | Solid-Phase Extraction–Liquid Chromatography Atmospheric Pressure Chemical Ionization–Mass Spectrometry; |
| SPE (on line)-LC-MS-MS | Solid-Phase Extraction (on line)–Liquid Chromatography–Tandem Mass Spectrometry; |
| SPME | Solid-Phase Microextraction; |
| SPME-GC-MS | Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry; |
| SPW | Spring Water |
| TBP | Tribromophenol; |
| TCLP | Toxicity Characteristic Leaching Procedure; |
| TD-SBSE | Thermodesorption System–Stir Bar Sorptive Extraction; |
| TMCS | Trimethylchlorosilane; |
| TMSDMC | Trimethylsilyl-N,N-dimethylcarbamate; |
| TOF-MS | Time-of-Flight Mass Spectrometry; |
| TRI | Toxics Release Inventory; |
| UN | United Nations; |
| UPLC | Ultra-Performance Liquid Chromatography; |
| UPLC-MS/MS | Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry; |
| UHPLC | Ultra-High-Performance Liquid Chromatography; |
| USA | United States of America; |
| US CFR | United States Code of Federal Regulations; |
| USEPA | United States Environmental Protection Agency; |
| WHO | World Health Organization; |
| WoS | Web of Science |
References
- UN. General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed on 23 February 2025).
- UN-DSDG. Division for Sustainable Development Goals. 2025. Available online: https://sdgs.un.org/about (accessed on 23 February 2025).
- Huang, Z.; Yuan, X.; Liu, X. The key drivers for the changes in global water scarcity: Water withdrawal versus water availability. J. Hydrol. 2021, 601, 126658. [Google Scholar] [CrossRef]
- Rodrigues, E.A.; Ferreira, M.L.; de Carvalho, A.R.; Bustillos, J.O.W.V.; Victor, R.A.B.M.; Sodré, M.G.; de Andrade, D.A. Land, water, and climate issues in large and megacities under the lens of nuclear science: An approach for achieving sustainable development goal (SDG11). Sustainability 2022, 14, 13646. [Google Scholar] [CrossRef]
- Rodrigues, E.A.; Carvalho, A.R.D.; Ferreira, M.L.; Victor, R.A.B.M.; Luca, E.F.D.; Rocha, G.C.D.; Andrade, D.A.D. Beyond the Rising Tide: Towards Effective Climate Policy in Coastal Urban Centers. Land 2024, 13, 2071. [Google Scholar] [CrossRef]
- UN. The United Nations World Water Development Report 2024: Water for Prosperity and Peace; UNESCO: Paris, France, 2024; 153p, Available online: https://unesdoc.unesco.org/ark:/48223/pf0000388948 (accessed on 23 February 2025).
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report; Lee, H., Romero, J., Eds.; Contribution of Working Groups I II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 1–34. Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_SPM.pdf (accessed on 23 February 2025).
- Gleick, P.H.; Cooley, H. Freshwater scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- UN. The United Nations World Water Development Report 2022: Groundwater: Making the Invisible Visible; UNESCO: Paris, France, 2022; 225p, Available online: https://unesdoc.unesco.org/ark:/48223/pf0000380721 (accessed on 23 February 2025).
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- UN-Habitat. World Cities Report 2022: Envisaging the Future of Cities; UN-Habitat: Nairobi, Kenya, 2022; 422p, Available online: https://unhabitat.org/sites/default/files/2022/06/wcr_2022.pdf (accessed on 23 February 2025).
- Ravindiran, G.; Rajamanickam, S.; Sivarethinamohan, S.; Karupaiya Sathaiah, B.; Ravindran, G.; Muniasamy, S.K.; Hayder, G. A Review of the Status, Effects, Prevention, and Remediation of Groundwater Contamination for Sustainable Environment. Water 2023, 15, 3662. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Ramos, R.L.; Moreira, V.R.; Amaral, M.C.S. Phenolic compounds in water: Review of occurrence, risk, and retention by membrane technology. J. Environ. Manag. 2024, 351, 119772. [Google Scholar] [CrossRef] [PubMed]
- Ayad, R.; Akkal, S. Phytochemistry and biological activities of algerian Centaura an related genera. Stud. Nat. Prod. Chem. 2019, 63, 357–414. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Elsevier Saunders: Amsterdan, The Netherlands, 2015; Volume 2, pp. 3294–3309. [Google Scholar] [CrossRef]
- Silah, H.; Erkmen, C.; Unal, D.N.; Uslu, B. Sensing of phenol and chlorophenols using carbon nanotubes modified glassy carbon electrode. In Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simuants, and Their Toxicologial Aspects; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 13; pp. 297–329. [Google Scholar] [CrossRef]
- Abdollahi, M.; Hassani, S.; Derakhshani, M. Phenol. In Philip Wexler, Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 871–873. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R.; Gadore, V.; Yadav, G.; Roy, S.; Bhattacharjee, B.; Kumari, K. Phenolic Compounds in Water: From Toxicity and Source to Sustainable Solutions-An Integrated Review of Removal Methods, Advanced Technologies, Cost Analysis, and Future Prospects. J. Environ. Chem. Eng. 2024, 12, 112964. [Google Scholar] [CrossRef]
- Sarkar, S.; Gill, S.S.; Das Gupta, G.; Kumar Verma, S. Water toxicants: A comprehension on their health concerns, detection, and remediation. Environ. Sci. Pollut. Res. 2022, 29, 53934–53953. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shi, J.; Pi, K.; Deng, Y.; Yan, B.; Tong, L.; Jiang, G. Groundwater quality and public health. Annu. Rev. Environ. Resour. 2023, 48, 395–418. [Google Scholar] [CrossRef]
- Deng, Z.H.; Li, N.; Jiang, H.L.; Lin, J.M.; Zhao, R.S. Pretreatment techniques and analytical methods for phenolic endocrine disrupting chemicals in food and environmental samples. TrAC Trends Anal. Chem. 2019, 119, 115592. [Google Scholar] [CrossRef]
- Vaye, O.; Ngumbu, R.S.; Xia, D. A review of the application of comprehensive two-dimensional gas chromatography MS-based techniques for the analysis of persistent organic pollutants and ultra-trace level of organic pollutants in environmental samples. Rev. Anal. Chem. 2022, 41, 63–73. [Google Scholar] [CrossRef]
- Vega-Bustillos, O.; Redígolo, M. Princípios e Aplicações da Espectrometria de Massas, 1st ed.; Scortecci: São Paulo, Brazil, 2024. [Google Scholar]
- PRISMA. Welcome to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Website. 2025. Available online: https://www.prisma-statement.org/ (accessed on 23 February 2025).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. JBI Evid. Implement. 2015, 13, 179–187. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, P.; Karmakar, M.; Leta, J.; Mayr, P. The journal coverage of Web of Science, Scopus and Dimensions: A comparative analysis. Scientometrics 2021, 126, 5113–5142. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Martín-Martín, A.; Orduna-Malea, E.; Thelwall, M.; López-Cózar, E.D. Google Scholar, Web of Science, and Scopus: A systematic comparison of citations in 252 subject categories. J. Informetr. 2018, 12, 1160–1177. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Martín-Martín, A.; Thelwall, M.; Orduna-Malea, E.; Delgado López-Cózar, E. Google Scholar, Microsoft Academic, Scopus, Dimensions, Web of Science, and OpenCitations’ COCI: A multidisciplinary comparison of coverage via citations. Scientometrics 2021, 126, 871–906. [Google Scholar] [CrossRef] [PubMed]
- Asubiaro, T.; Onaolapo, S.; Mills, D. Regional disparities in Web of Science and Scopus journal coverage. Scientometrics 2024, 129, 1469–1491. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The titans of bibliographic information in today’s academic world. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Gioia, D. A systematic methodology for doing qualitative research. J. Appl. Behav. Sci. 2021, 57, 20–29. [Google Scholar] [CrossRef]
- Corbin, J.; Strauss, A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 4th ed.; Sage Publications: Thousand Oaks, CA, USA, 2014; 434p. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. Atualização do PubChem 2025. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- USEPA. U.S. Environmental Protection Agency. 2025. Available online: https://www.epa.gov/ (accessed on 23 February 2025).
- US-eCFR. Title 40. Title 40 Was Last Amended 2/07/2025. 2025. Available online: https://www.ecfr.gov/current/title-40 (accessed on 23 February 2025).
- Guo, L.C.; Zeng, E.Y. Broadening the global reach of the United States environmental protection agency (USEPA) is vital to combating globalized environmental problems. Environ. Sci. Technol. 2010, 44, 6911–6913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, H.Z.; Ho, Y.S. Characteristics and trends on global environmental monitoring research: A bibliometric analysis based on Science Citation Index Expanded. Environ. Sci. Pollut. Res. 2017, 24, 26079–26091. [Google Scholar] [CrossRef]
- USEPA. Summary of the Clean Water Act. 2025. Available online: https://www.epa.gov/laws-regulations/summary-clean-water-act (accessed on 23 February 2025).
- US-eCFR. Title 40, Part 116.4: Designation of Hazardous Substances. 2025. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-116#116.4 (accessed on 27 February 2025).
- USEPA. Hazardous Substance Designations and Release Notifications. 2025. Available online: https://www.epa.gov/epcra/hazardous-substance-designations-and-release-notifications (accessed on 23 February 2025).
- US-eCFR. Title 40, Part 302.—Designation, Reportable Quantities, and Notification. 2025. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-J/part-302 (accessed on 23 February 2025).
- US-eCFR. Title 40 Part 423—Steam electric power generating point source category: Appendix A to Part 423–126 Priority Pollutants. 2025. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-N/part-423 (accessed on 23 February 2025).
- Bhuyan, A.; Sanguri, K.; Sharma, H. Improving the keyword co-occurrence analysis: An integrated semantic similarity approach. In Proceedings of the 2021 IEEE International Conference on Industrial Engineering and Engineering Management (IEEM), Singapore, 13–16 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 482–487. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact: Methods and Practice; Springer International Publishing: Cham, The Netherland, 2014; pp. 285–320. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual: Manual for VOSviewer Version 1.6.18. Universiteit Leiden/CWTS Meaningful metrics. 2022. Available online: https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.18.pdf (accessed on 17 February 2025).
- Lees, N. The Brandt Line after forty years: The more North–South relations change, the more they stay the same? Rev. Int. Stud. 2021, 47, 85–106. [Google Scholar] [CrossRef]
- Solarz, M.W. North–South, Commemorating the First Brandt Report: Searching for the contemporary spatial picture of the global rift. Third World Q. 2012, 33, 559–569. [Google Scholar] [CrossRef]
- FCSSC—Finance Center for South-South Cooperation. Organization in Special Consultative Status with ECOSOC of the United Nations. Global South Countries (Group of 77 and China). 2025. Available online: http://www.fc-ssc.org/en/partnership_program/south_south_countries (accessed on 23 February 2025).
- Hargesheimer, E.E.; Coutts, R.T.; MacKinnon, M.D. Characterization of simple phenols in oil sands extraction-process water. Environ. Technol. 1984, 5, 433–440. [Google Scholar] [CrossRef]
- Ahel, M. Infiltration of organic pollutants into groundwater: Field studies in the alluvial aquifer of the Sava River. In Organic Micropollutants in the Aquatic Environment: Proceedings of the Sixth European Symposium, Lisbon, Portugal, 22–24 May 1990; Springer: Dordrecht, The Netherlands, 1990; pp. 423–427. [Google Scholar] [CrossRef]
- Veningerová, M.; Prachar, V.; Kovacicová, J.; Uhnák, J. Analytical methods for the determination of organochlorine compounds: Application to environmental samples in the Slovak Republic. J. Chromatogr. A 1997, 774, 333–347. [Google Scholar] [CrossRef]
- Biziuk, M.; Namieśnik, J.; Czerwiński, J.; Gorlo, D.; Makuch, B.; Janicki, W.; Wolska, L. Occurrence and determination of organic pollutants in tap and surface waters of the Gdańsk district. J. Chromatogr. A 1996, 733, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Wennrich, L.; Efer, J.; Engewald, W. Gas Chromatographic trace analysis of underivatized nitrophenols. Chromatographia 1995, 41, 361–366. [Google Scholar] [CrossRef]
- Køppen, B.; Spliid, N.H. Determination of acidic herbicides using liquid chromatography with pneumatically assisted electrospray ionization mass spectrometric and tandem mass spectrometric detection. J. Chromatogr. A 1998, 803, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.S.; Hansen, A.B.; Mosboek, H.; Arvin, E. Identification of heteroaromatic and other organic compounds in ground water at Creosote-contaminated sites in Denmark. Groundw. Monit. Remediat. 2009, 17, 106–115. [Google Scholar] [CrossRef]
- Sawhney, B.L.; Kozloski, R.P. Organic Pollutants in Leachates from Landfill Sites. J. Environ. Qual. 1984, 13, 349–352. [Google Scholar] [CrossRef]
- Ham, R.K.; Boyle, W.C.; Engroff, E.C.; Fero, R.L. Organic compounds in ferrous foundry process waste leachates. J. Environ. Eng. 1993, 119, 34–55. [Google Scholar] [CrossRef]
- Beihoffer, J.; Ferguson, C. Determination of selected carboxylic acids and alcohols in groundwater by GC—MS. J. Chromatogr. Sci. 1994, 32, 102–106. [Google Scholar] [CrossRef]
- Rudel, R.A.; Melly, S.J.; Geno, P.W.; Sun, G.; Brody, J.G. Identification of alkylphenols and other estrogenic phenolic compounds in wastewater, septage, and groundwater on Cape Cod, Massachusetts. Environ. Sci. Technol. 1998, 32, 861–869. [Google Scholar] [CrossRef]
- Lacorte, S.; Latorre, A.; Guillamon, M.; Barceló, D. Nonylphenol, octyphenol, and bisphenol a in groundwaters as a result of agronomic practices. Sci. World J. 2002, 2, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Lacorte, S.; Barceló, D. Presence of nonylphenol, octyphenol and bisphenol a in two aquifers close to agricultural, industrial and urban areas. Chromatographia 2003, 57, 111–116. [Google Scholar] [CrossRef]
- López-Roldán, P.; López de Alda, M.J.; Barceló, D. Simultaneous determination of selected endocrine disrupters (pesticides, phenols and phthalates) in water by in-field solid-phase extraction (SPE) using the prototype PROFEXS followed by on-line SPE (PROSPEKT) and analysis by liquid chromatography-atmospheric pressure chemical ionisation-mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 599–609. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Barceló, D. Monitoring of estrogens, pesticides and bisphenol A in natural waters and drinking water treatment plants by solid-phase extraction–liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1045, 85–92. [Google Scholar] [CrossRef]
- Moral, A.; Sicilia, M.D.; Rubio, S.; Pérez-Bendito, D. Multifunctional sorbents for the extraction of pesticide multiresidues from natural waters. Anal. Chim. Acta 2008, 608, 61–72. [Google Scholar] [CrossRef]
- Bono-Blay, F.; Guart, A.; de la Fuente, B.; Pedemonte, M.; Pastor, M.C.; Borrell, A.; Lacorte, S. Survey of phthalates, alkylphenols, bisphenol A and herbicides in Spanish source waters intended for bottling. Environ. Sci. Pollut. Res. 2012, 19, 3339–3349. [Google Scholar] [CrossRef]
- Quintana, J.; Hernández, A.; Ventura, F.; Devesa, R.; Boleda, M.R. Identification of 3-(trifluoromethyl) phenol as the malodorous compound in a pollution incident in the water supply in Catalonia (NE Spain). Environ. Sci. Pollut. Res. 2019, 26, 16076–16084. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Rodrígues-Gonzala, E.; Andrade, M.S.; Sánchez-González, S.; Carabias-Martinéz, R. Occurrence of phenols and phenoxyacid herbicides in environmental waters using an imprinted polymer as a selective sorbent. Sci. Total Environ. 2013, 454–455, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zemo, D.A.; O’Reilly, K.T.; Mohler, R.E.; Tiwary, A.K.; Magaw, R.I.; Synowiec, K.A. Nature and estimated human toxicity of polar metabolite mixtures in groundwater quantified as TPHd/DRO at biodegrading fuel release sites. Groundw. Monit. Remediat. 2013, 33, 44–56. [Google Scholar] [CrossRef]
- Mohler, R.E.; O’Reilly, K.T.; Zemo, D.A.; Tiwary, A.K.; Magaw, R.I.; Synowiec, K.A. Non-targeted analysis of petroleum metabolites in groundwater using GC× GC–TOFMS. Environ. Sci. Technol. 2013, 47, 10471–10476. [Google Scholar] [CrossRef]
- Swartz, C.H.; Reddy, S.; Benotti, M.J.; Yin, H.; Barber, L.B.; Brownawell, B.J.; Rudel, R.A. Steroid estrogens, nonylphenol ethoxylate metabolites, and other wastewater contaminants in groundwater affected by a residential septic system on Cape Cod, MA. Environ. Sci. Technol. 2006, 40, 4894–4902. [Google Scholar] [CrossRef]
- Baugros, J.B.; Giroud, B.; Dessalces, G.; Grenier-Loustalot, M.F.; Cren-Olivé, C. Multiresidue analytical methods for the ultra-trace quantification of 33 priority substances present in the list of REACH in real water samples. Anal. Chim. Acta 2008, 607, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Dévier, M.H.; Le Menach, K.; Viglino, L.; Di Gioia, L.; Lachassagne, P.; Budzinski, H. Ultra-trace analysis of hormones, pharmaceutical substances, alkylphenols and phthalates in two French natural mineral waters. Sci. Total Environ. 2013, 443, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Le Coadou, L.; Le Ménach, K.; Labadie, P.; Dévier, M.H.; Pardon, P.; Augagneur, S.; Budzinski, H. Quality survey of natural mineral water and spring water sold in France: Monitoring of hormones, pharmaceuticals, pesticides, perfluoroalkyl substances, phthalates, and alkylphenols at the ultra-trace level. Sci. Total Environ. 2017, 603, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Tournier, M.; Vauchez, A.; Wiest, L.; Baudot, R.; Lafay, F.; Cren-Olivé, C. Survey regarding the occurrence of selected organic micropollutants in the groundwaters of overseas departments. Environ. Sci. Pollut. Res. 2014, 21, 7512–7521. [Google Scholar] [CrossRef]
- Montero, L.; Conradi, S.; Weiss, H.; Popp, P. Determination of phenols in lake and ground water samples by stir bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2005, 1071, 163–169. [Google Scholar] [CrossRef]
- Schellin, M.; Popp, P. Membrane-assisted solvent extraction of seven phenols combined with large volume injection–gas chromatography–mass spectrometric detection. J. Chromatogr. A 2005, 1072, 37–43. [Google Scholar] [CrossRef]
- Pignotti, E.; Farré, M.; Barceló, D.; Dinelli, E. Occurrence and distribution of six selected endocrine disrupting compounds in surface and groundwaters of the Romagna area (North Italy). Environ. Sci. Pollut. Res. 2017, 24, 21153–21167. [Google Scholar] [CrossRef]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Mass balance of emerging contaminants in the water cycle of a highly urbanized and industrialized area of Italy. Water Res. 2018, 131, 287–298. [Google Scholar] [CrossRef]
- Simões, N.G.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.J.; Almeida, C.M. Experimental and statistical validation of SPME-GC–MS analysis of phenol and chlorophenols in raw and treated water. Chemosphere 2007, 68, 501–510. [Google Scholar] [CrossRef]
- Carvalho, A.R.M.; Cardoso, V.V.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Duarte, E.A. Occurrence and analysis of endocrine-disrupting compounds in a water supply system. Environ. Monit. Assess. 2015, 187, 1–8. [Google Scholar] [CrossRef]
- Makris, K.C.; Snyder, S.A. Screening of pharmaceuticals and endocrine-disrupting compounds in water supplies of Cyprus. Water Sci. Technol. 2010, 62, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Juhler, R.K.; Felding, G. Monitoring methyl tertiary butyl ether (MTBE) and other organic micropollutants in groundwater: Results from the Danish National Monitoring Program. Water Air Soil Pollut. 2003, 149, 145–161. [Google Scholar] [CrossRef]
- Papadopoulou-Mourkidou, E.; Patsias, J.; Papadakis, E.; Koukourikou, A. Use of an automated on-line SPE–HPLC method to monitor caffeine and selected aniline and phenol compounds in aquatic systems of Macedonia–Thrace, Greece. Fresenius’ J. Anal. Chem. 2001, 371, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.; Mörtl, M.; Kende, A. Development and optimization of a method for the analysis of phenols and chlorophenols from aqueous samples by gas chromatography–mass spectrometry, after solid-phase extraction and trimethylsilylation. Microchem. J. 2011, 99, 125–131. [Google Scholar] [CrossRef]
- McManus, S.L.; Moloney, M.; Richards, K.G.; Coxon, C.E.; Danaher, M. Determination and occurrence of phenoxyacetic acid herbicides and their transformation products in groundwater using ultra high performance liquid chromatography coupled to tandem mass spectrometry. Molecules 2014, 19, 20627–20649. [Google Scholar] [CrossRef]
- Godejohann, M.; Heintz, L.; Daolio, C.; Berset, J.D.; Muff, D. Comprehensive non-targeted analysis of contaminated groundwater of a former ammunition destruction site using 1H-NMR and HPLC-SPE-NMR/TOF-MS. Environ. Sci. Technol. 2009, 43, 7055–7061. [Google Scholar] [CrossRef]
- Chung, L.W.; Lee, M.R. Evaluation of liquid-phase microextraction conditions for determination of chlorophenols in environmental samples using gas chromatography–mass spectrometry without derivatization. Talanta 2008, 76, 154–160. [Google Scholar] [CrossRef]
- Shin, H.S. GC–MS determination of antioxidants in ground water contaminated with JP-8. Chromatographia 2007, 66, 893–897. [Google Scholar] [CrossRef]
- Kapelewska, J.; Kotowska, U.; Karpinska, J.; Kowalczuk, D.; Arciszewska ASwirydo, A. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 2018, 137, 292–301. [Google Scholar] [CrossRef]
- Cruz-López, A.; Dávila-Pórcel, R.A.; de León-Gómez, H.; Rodríguez-Martínez, J.M.; Suárez-Vázquez, S.I.; Cardona-Benavides, A.; Castro-Larragoitia, G.J.; Boreselli, L.; Villalba, M.L.; Pinales-Munguía, A.; et al. Exploratory study on the presence of bisphenol A and bis(2-ethylhexyl)phthalate in the Santa Catarina river in Monterrey, N.L., Mexico. Environ. Monit. Assess. 2020, 192, 488. [Google Scholar] [CrossRef]
- Dolatto, R.G.; Messerschmidt, I.; Pereira, B.F.; Martinazzo, R.; Abate, G. Preconcentration of polar phenolic compounds from water samples and soil extract by liquid-phase microextraction and determination via liquid chromatography with ultraviolet detection. Talanta 2016, 148, 292–300. [Google Scholar] [CrossRef]
- Al Nasir, F.; Batarseh, M.I. Agricultural reuse of reclaimed water and uptake of organic compounds: Pilot study at Mutah University wastewater treatment plant, Jordan. Chemosphere 2008, 72, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Gao, Y.; Hu, F.; Yu, X.; Xie, H.; Li, H.; Zheng, W. Solid-phase extraction of seventeen alternative flame retardants in water as determined by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1602, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.J.; Abasabad, P.; Salehzadeh, A. Endocrine disrupting contaminants ins water resources and sewage in Hamadan city of Iran. Iran. J. Environ. Health. Sci. Eng. 2009, 6, 89–96. [Google Scholar]
- Hai-Liang, Y.; Tong-Na, Z. Simultaneous determination of nine C4–C9 alkylphenols and bisphenol A in environmental water using gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2022, 50, 100112. [Google Scholar] [CrossRef]
- Ng, K.; Alygizakis, N.; Nika, M.C.; Galani, A.; Oswald, P.; Oswaldova, M.; Slobodnik, J. Wide-scope target screening characterization of legacy and emerging contaminants in the Danube River Basin by liquid and gas chromatography coupled with high-resolution mass spectrometry. Water Res. 2023, 230, 119539. [Google Scholar] [CrossRef]
- Adebesin, T.N.; Lateef, S.A.; Oloruntoba, E.O.; Adejumo, M. Occurrence of bisphenol A, nonylphenol, octylphenol and heavy metals in groundwater from selected communities in Ibadan, Nigeria. J. Water Health 2023, 21, 740–750. [Google Scholar] [CrossRef]
- Otitoju, O.B.; Alfred, M.O.; Ogunlaja, O.O.; Olorunnisola, C.G.; Olukanni, O.D.; Ogunlaja, A.; Omorogie, M.O.; Unuabonah, E.I. Pollution and risk assessment of phenolic compounds in drinking water sources from southwestern Nigeria. Environ. Sci. Pollut. Res. 2023, 30, 76798–76817. [Google Scholar] [CrossRef]
- Maynard, I.F.N.; Cavalcanti, E.B.; da Silva, L.L.; Martins, E.A.J.; Pires, M.A.F.; de Barros, M.L.; Marques, M.N. Assessing the presence of endocrine disruptors and markers of anthropogenic activity in a water supply system in northeastern Brazil. J. Environ. Sci. Health Part A 2019, 54, 891–898. [Google Scholar] [CrossRef]
- Tao, X.; Tang, C.; Wu, P.; Han, Z.; Zhang, C.; Zhang, Y. Occurrence and behaviour of nonylphenol and octylphenol in Nanming River, Guiyang City, China. J. Environ. Monit. 2011, 13, 3269–3276. [Google Scholar] [CrossRef]
- USEPA. National Recommended Water Quality Criteria—Human Health Criteria Table. Environmental Protection Agency of United States. 2019. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-human-health-criteria-table (accessed on 17 February 2025).
- US-eCFR. Title 40 Part 304—Arbitration Procedures for Small Superfund Cost Recovery. 2025. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-J/part-304 (accessed on 23 February 2025).
- WHO—World Healty Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579461/pdf/Bookshelf_NBK579461.pdf (accessed on 17 February 2025).
- EC—European Commission. Phenol—Summary Risk Assessment Report. European Chemicals Bureau, Italy. 2006. Available online: https://echa.europa.eu/documents/10162/3e04f30d-9953-4824-ba04-defa32a130fa (accessed on 17 February 2025).
- Huang, X. Transboundary watershed pollution control analysis for pollution abatement and ecological compensation. Environ. Sci. Pollut. Res. 2023, 30, 44025–44042. [Google Scholar] [CrossRef]
- GOV.UK. Guidance Phenol: General information (Updated 1 October 2024): UK Health Security Agency. 2025. Available online: https://www.gov.uk/government/publications/phenol-properties-incident-management-and-toxicology/phenol-general-information (accessed on 17 February 2025).
- Ye, L.; Guo, J.; Ge, R. Chapter Thirteen—Environmental Pollutants and Hydroxysteroid Dehydrogenases. Vitam. Horm. 2014, 94, 349–390. [Google Scholar] [CrossRef]
- Bdanthadka, M.; Mehendale, H.M. Chlorophenols. In Encyclopedia of Toxicology, 3rd ed.; Elsiever: Amsterdam, The Netherlands, 2014; pp. 896–899. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Manamsa, K.; Talbot, J. Persistent and emerging micro-organic contaminants in Chalk groundwater of England and France. Environ. Pollut. 2015, 203, 214–225. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Information on Chemicals. 2025. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.002.405 (accessed on 17 February 2025).
- Bonacci, O.; Žaknić-Ćatović, A.; Roje-Bonacci, T. Significant Rise in Sava River Water Temperature in the City of Zagreb Identified across Various Time Scales. Water 2024, 16, 2337. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.; Chung, D.S. Sample Preparation for capillary electrophoretic Applications. Compr. Sampl. Sample Prep. 2012, 3, 701–721. [Google Scholar] [CrossRef]
- Janz, D.M. Dinitrophenols. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 177–178. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Duncianu, M.; Olariu, R.; Arsene, C.; Sarakha, M.; Mailhot, G. Assessing the transformation kinetics of 2- and 4-nitrphenol in the atmospheric aqueous phase. Implications for the distribution of both nitroisomers in the atmosphere. Atmos. Environ. 2009, 43, 2321–2327. [Google Scholar] [CrossRef]
- NLM National Library of Medicine; National Institutes of Health; U.S. Department of Health and Human Services. Toxicological Profile for Nitrophenols: Chapter 1 Relevance to Public Health. 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK601147/ (accessed on 17 February 2025).
- Orata, F. Derivatization reactions and reagents for gas chromatography analysis. In Advanced Gas Chromatography—Progress in Agricultural, Biomedical and Industrial Applications; InTech: Rijeka, Croatia, 2012; pp. 83–156. ISBN 978-953-51-0298-4. [Google Scholar] [CrossRef]
- Bilalova, S.; Newig, J.; Tremblay-Lévesque, L.C.; Roux, J.; Herron, C.; Crane, S. Pathways to water sustainability? A global study assessing the benefits of integrated water resources management. J. Environ. Manag. 2023, 343, 118179. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 1148p. [Google Scholar] [CrossRef]
- Brauman, K.A.; Garibaldi, L.A.; Polasky, S.; Aumeeruddy-Thomas, Y.; Brancalion, P.H.; DeClerck, F.; Verma, M. Global trends in nature’s contributions to people. Proc. Natl. Acad. Sci. USA 2020, 117, 32799–32805. [Google Scholar] [CrossRef]
- Hole, D.G.; Collins, P.; Tesfaw, A.; Barrera, L.; Mascia, M.B.; Turner, W.R. Make nature’s role visible to achieve the SDGs. Glob. Sustain. 2022, 5, e8. [Google Scholar] [CrossRef]
- Khiyat, Z. Groundwater in the Arab region: Making the invisible visible. Desalination Water Treat. 2022, 263, 204–206. [Google Scholar] [CrossRef]

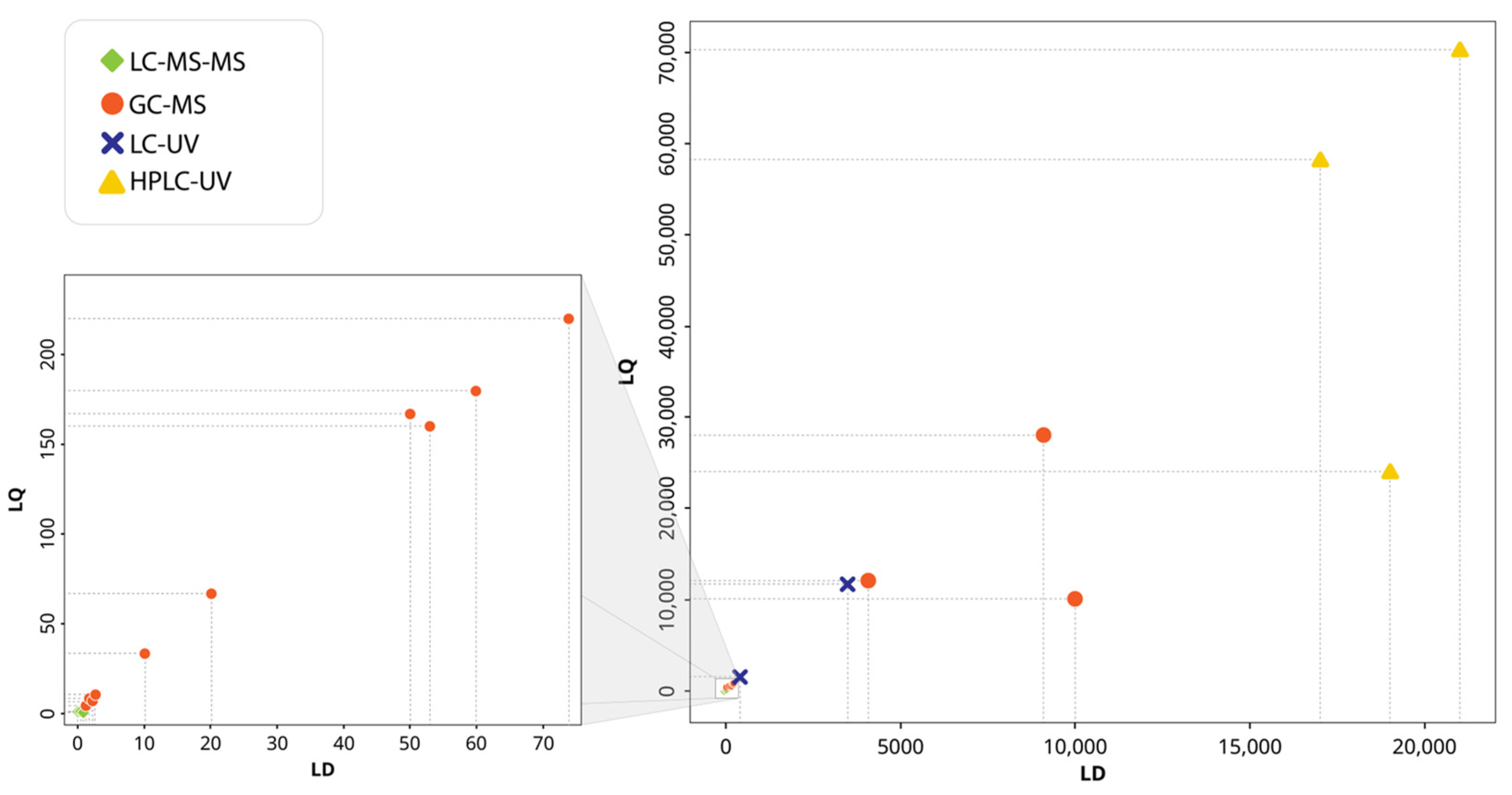
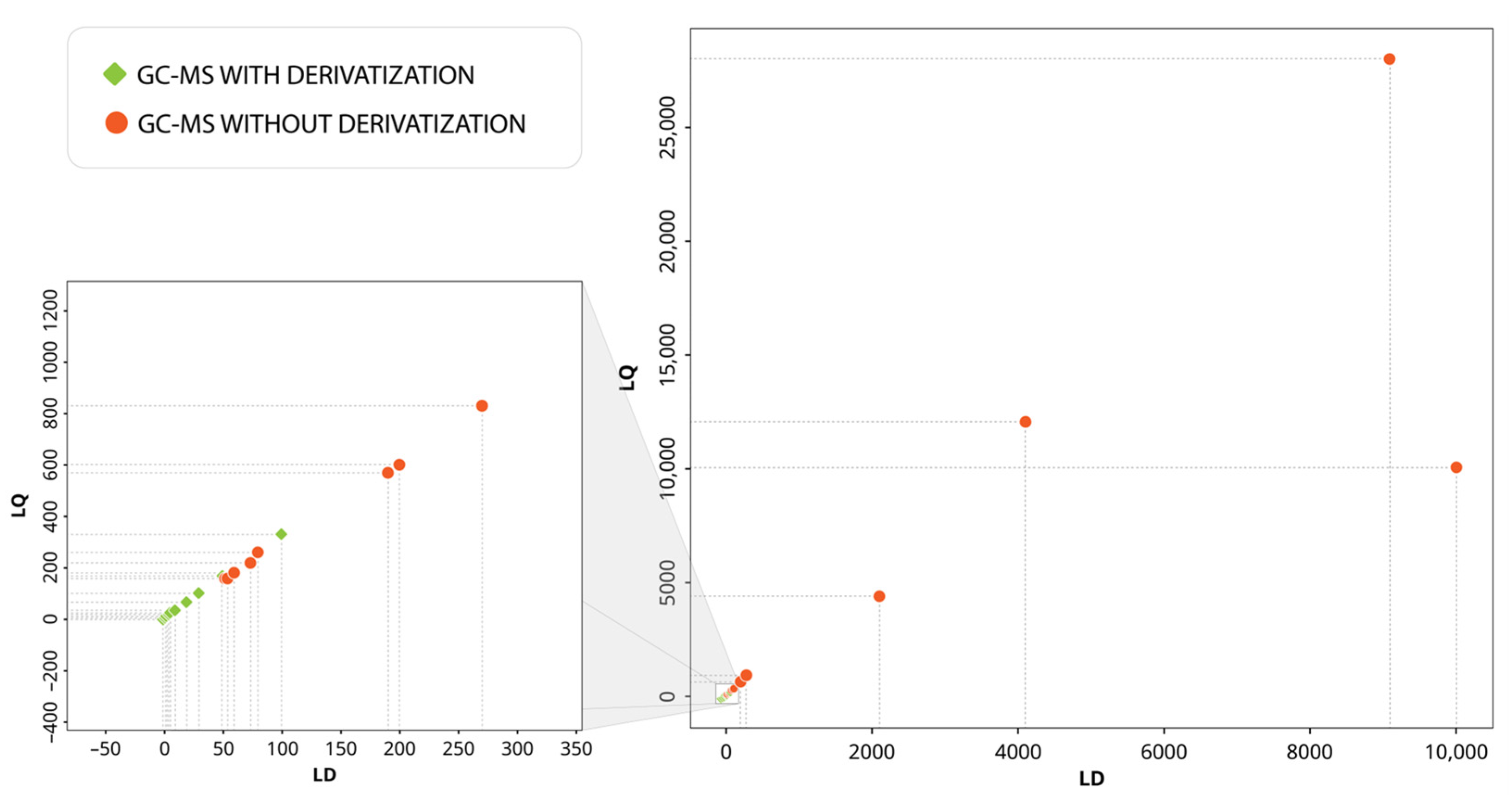


| Analytical Technique | Extraction Technique | Phenolic Group | LD Range (ng/L) | LQ Range (ng/L) | Detected Concentration Range (ng/L) | Pollution Source | Country (Authors) |
|---|---|---|---|---|---|---|---|
| GC-ECD; GC-FID; GC-MS | - | Chlorophenols | - | - | 20 to 340 | Natural substance degradation, Fuel, Pesticides | Denmark [87] |
| Alkylphenols | 600 to 4200 | ||||||
| Alkylphenols ethoxylates | - | ||||||
| Phenols | 70 to 5100 | ||||||
| GC-FID | TCLP, Purge and trap, LLE, SPE and Derivatization | Alkylphenols | 1000 to 10,000 | 2000 to 3000 | 180 to 2.09 × 106 | Creosote Ferrous foundry Landfill leaching, Municipal wastewater, Industrial wastewater, Pesticides Treated wastewater | Canada [54], Jordan [97], USA [61,62], Denmark [60] |
| Chlorocresols | - | 40,000 | <LQ | ||||
| Chlorophenols | - | 40,000 to 180,000 | 710 to 1740 | ||||
| Nitrocresols | - | 80,000 | 640 | ||||
| Nitrophenol | - | 60,000 to 140,000 | <LQ | ||||
| Phenols | 1000 to 10,000 | 30,000 | 11,000 to 1.2 × 106 | ||||
| GC-GC-TOFMS | - | Alkylphenols | - | 1 × 106 to 11 × 106 | <LOQ | Fuel | USA [74] |
| GC-MS | CLSA, direct injection, extraction and derivatization, LLE, LPME Clean up and derivatization, MASE, LPME, OASIS-SPE (offline), SPE, SPE (online), SPME, SPME (online), TD-SBSE, SPE LLE, SPME | Alkylphenols | 1 to 100,000 | 2 to 10,000 | 2.2 to 50 × 106 | Alcohols, Carboxylic acids, Chlorination, Endocrine disrupters (EDCs), Fuel, Industrial wastewater, Jet fuel (JP8), Mining, Municipal wastewater, Natural substance degradation, Pesticides, Pharmaceuticals, Phenolic compounds, Phenoxy alkanoic acids | Brazil [104], China [100,105], France [78], Germany [80,81], Hungary [89], Nigeria [102], Portugal [84], South Korea [93], Spain [65,66,70,71], Taiwan [92], USA [63,64,73], Poland [94] |
| Alkylphenols ethoxylates | 3.3 to 86.7 | 15.6 to 260 | - | ||||
| Biphenols | 1.1 to 2.9 | 4.2 to 10,000 | 14 to 33 | ||||
| Bisphenols | 3 to 1370 | 1 to 16.2 | 6 to 6880 | ||||
| Bromophenols | 190 | 570 | <LQ | ||||
| Chlorocresols | 74 to 200 | 220 | 7000 to 113,910 | ||||
| Chlorophenols | 1.4 to 4100 | 2 to 12,000 | 2.08 to 216,200 | ||||
| Halogenated Phenols | 10 | - | 17,000 | ||||
| Nitrophenols | 2.2 | 6.6 | <LQ | ||||
| Phenols | 0.05 to 100,000 | 0.167 to 28,000 | 720 to 40 × 106 | ||||
| GC-NPD/GC-ECD | SPE-LLE | Nitrophenols | 10,000 to 30,000 | - | 15,000 to 88,000 | Explosive and Heavy metals | Germany [58] |
| Nitrocresols | 10,000 to 30,000 | - | 15,000 to 31,000 | ||||
| HPLC-SPE-NMR-TOF-MS | SPE by 1H NMR | Bisphenols | - | - | 12,000 to 13,000 | Explosives, Heavy metals | Switzerland [91] |
| Nitrophenols | - | - | - | ||||
| LC-MS | SPE, Profexs and Prospekt, SPE Oasis | Alkylphenols | 52.9 to 264.7 | - | 374.2 to 20,000 | Endocrine disrupters (EDCs), Industrial wastewater, Pesticides, Municipal wastewater | Denmark [59], Italy [82], Spain [67,68], USA [75], Mexico [95] |
| Alkylphenols ethoxylates | - | - | 2500 | ||||
| Bisphenols | 0.61 to 6.3 | 2,02 | 5 to 25,000 | ||||
| Chlorocresols | 34.9 to 38.5 | - | - | ||||
| Chlorophenols | 3 to 87.7 | - | - | ||||
| Nitrocresols | 3 to 7 | - | - | ||||
| Phenols | 1216.2 | - | - | ||||
| LC-MS/GC-MS | LLE | Alkylphenols | - | - | 31 to 78 | Municipal wastewater, Industrial wastewater, | Croatia [55] |
| Alkylphenols ethoxylates | - | - | 10 | ||||
| Phenols | - | - | 120 to 280 | ||||
| LC-MS-MS | SPE, SPE (negative and positive mode) SPE (on line) SPE (on line), SPME (on line) SPE Oasis (on line and off line) SPME | Alkylphenols | 3.32 to 220 | 1 to 10 | 4.9 to 106 | Bromophenols, Emerging contaminants (ECs), Endocrine disrupters (EDCs), Industrial wastewater, Laboratory contamination, Municipal wastewater, Organophosphate flame retardants (OPFRs), Pesticides, Pharmaceuticals, Phenoxyacetic acid herbicides | China [98], Cyprus [86], France [76,77,79], Ireland [90], Italy [83], Portugal [85], Romania—Croatia—Hungary [101] |
| Alkylphenols ethoxylates | 0.7 to 38.8 | 2 to 10 | 2.2 to 211.9 | ||||
| Bisphenols | 0.93 to 6.29 | 1 to 10 | 6.29 to 7400 | ||||
| Bromocresols | 0.1 | 0.25 | - | ||||
| Bromophenols | 0.1 to 2.5 | 0.25 to 5 | 1.29 to 15.57 | ||||
| Chlorocresols | 0.1 | 0.2 | 5 to 1364 | ||||
| Chlorophenols | 0.08 to 1.2 | 0.2 to 4.9 | 1 to 1461 | ||||
| Nitrophenols | 0.06 | 0.17 | - | ||||
| Phenols | 1.17 | 3.5 | - | ||||
| Phthalene | 1.33 | 4 | - | ||||
| LC-UV | A-SPE; Extraction and derivatization; Molecularly imprinted polymers (MIP); LC-Sorption C18; LPME SPE (online) | Alkylphenols | 10 to 4190 | 1200 to 11,600 | 1 to 1230 | Municipal wastewater, Industrial wastewater, Acid herbicides, Pesticides, Chemical waste, Phenolic compounds | Brazil [96], Spain [69,72], USA [64], Greece [88], Poland [57], Iran [99], Nigeria [103] |
| Alkylphenols ethoxylates | 1950 to 7900 | - | - | ||||
| Bisphenols | - | - | 22 to 9000 | ||||
| Chlorocresols | 100 | - | - | ||||
| Chlorophenols | 50 to 21,000 | 70,000 | 75,000 to 407,000 | ||||
| Nitrocresols | 13,000 | - | - | ||||
| Nitrophenols | 50 to 17,000 | 58,000 | 210,000 to 1,313,000 | ||||
| Phenols | 400 to 19,000 | 1400 to 24,000 | 119,000 to 552,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa-Cruz-Mérida, G.V.; Otomo, J.I.; Araoz-Prado, D.R.; Rodrigues, E.A.; de Andrade, D.A.; Bustillos, O.V. Advanced Analytical Approaches for Phenolic Compounds in Groundwater: A PRISMA Systematic Review. Water 2025, 17, 1173. https://doi.org/10.3390/w17081173
Santa-Cruz-Mérida GV, Otomo JI, Araoz-Prado DR, Rodrigues EA, de Andrade DA, Bustillos OV. Advanced Analytical Approaches for Phenolic Compounds in Groundwater: A PRISMA Systematic Review. Water. 2025; 17(8):1173. https://doi.org/10.3390/w17081173
Chicago/Turabian StyleSanta-Cruz-Mérida, Giancarla Viviana, Juliana Ikebe Otomo, Diego Ricardo Araoz-Prado, Elaine Aparecida Rodrigues, Delvonei Alves de Andrade, and Oscar Vega Bustillos. 2025. "Advanced Analytical Approaches for Phenolic Compounds in Groundwater: A PRISMA Systematic Review" Water 17, no. 8: 1173. https://doi.org/10.3390/w17081173
APA StyleSanta-Cruz-Mérida, G. V., Otomo, J. I., Araoz-Prado, D. R., Rodrigues, E. A., de Andrade, D. A., & Bustillos, O. V. (2025). Advanced Analytical Approaches for Phenolic Compounds in Groundwater: A PRISMA Systematic Review. Water, 17(8), 1173. https://doi.org/10.3390/w17081173






