The Migration and Pollution Risk of Microplastics in Water, Soil, Sediments, and Aquatic Organisms in the Caohai Watershed, Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Sampling
2.3. Analytical Procedures
2.3.1. Soil and Sediment Samples
2.3.2. Water Samples
2.3.3. Organs of Grass Carp
2.4. Statistical Analysis
3. Results
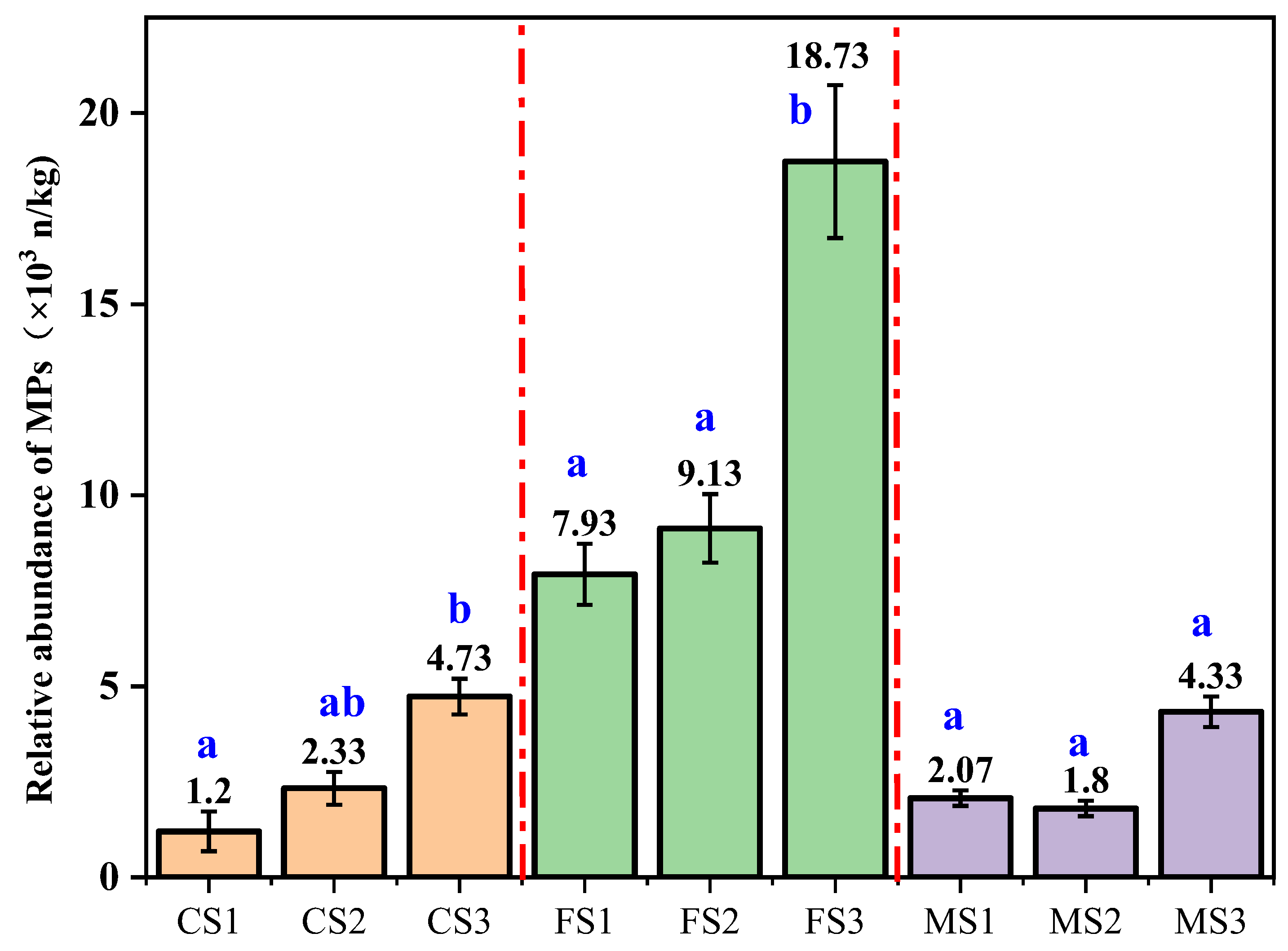
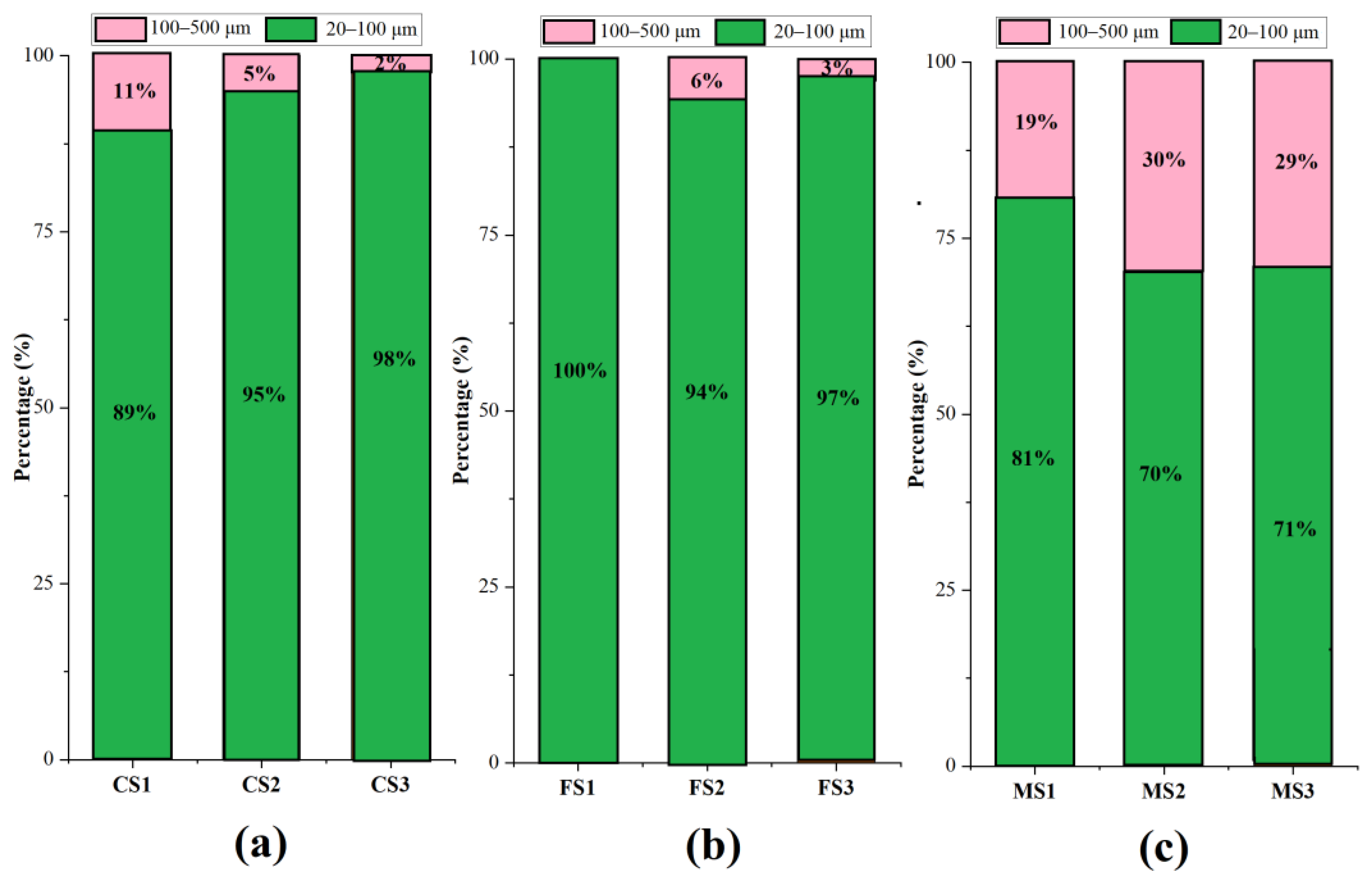
3.1. Relative Abundance of MPs in Soils from Different Land Uses in the Caohai Watershed
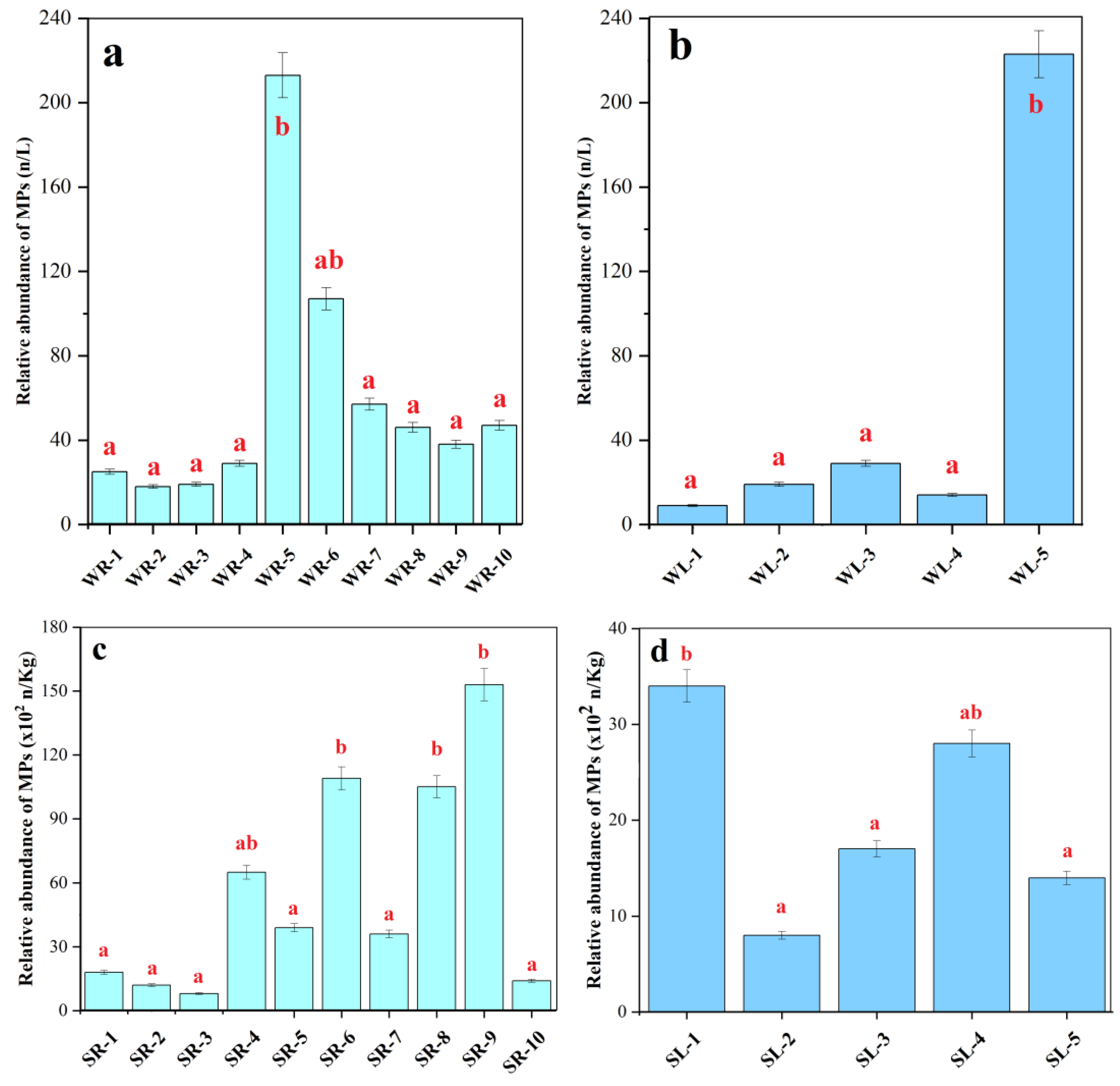
3.2. Relative Abundance and Particle Size of MPs in Water and Sediment from Rivers and Caohai Lake
3.3. MP Components in the Caohai Watershed
3.4. MPs in the Organs of Grass Carp
4. Discussion
4.1. MPs in the Caohai Watershed
4.2. Pollution Sources and Migration of MPs in the Caohai Watershed
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, C.; Wen, M.Y.; Cheng, T. Accessible active sites activated by nano cobalt antimony oxide @ carbon nanotube composite electrocatalyst for highly en-hanced hydrogen evolution reaction. Int. J. Hydrogen Energ. 2023, 48, 7719–7736. [Google Scholar]
- Zhang, Z.M.; Wu, X.L.; Zhang, J.C.; Huang, X.F. Distribution and migration characteristics of microplastics in farmland soils, surface water and sediments in Caohai Lake, southwestern plateau of China. J. Clean. Prod. 2022, 366, 132912. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Montano, S.; Garavaglia, M.G.; Lasagni, M.; Sevesoa, D.; Galli, P. Microplastic and charred microplastic in the Faafu Atoll, Maldives. Mar. Pollut. Bull. 2018, 136, 464–471. [Google Scholar] [CrossRef]
- Hale, R.; Seeley, M.; La Guardia, M.; Mai, L.; Zeng, E. A global perspective on microplastics. J. Geophys. Res. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Liu, H.T.; Sun, K.X.; Liu, X.Y.; Yao, R.; Cao, W.Z.; Zhang, L.; Wang, X.H. Spatial and temporal distributions of microplastics and their macroscopic relationship with algal blooms in Chaohu Lake, China. J. Contam. Hydrol. 2022, 248, 104028. [Google Scholar] [CrossRef]
- Priya, K.L.; Renjith, K.R.; Joseph, C.J.; Indu, M.S.; Srinivas, R.; Haddout, S. Fate, transport and degradation pathway of microplastics in aquatic environment—A critical review. Reg. Stud. Mar. Sci. 2022, 56, 102647. [Google Scholar]
- Wu, X.L.; Liu, H.J.; Guo, X.T.; Zhang, Z.M.; Zhang, J.C.; Huang, X.F. Microplastic distribution and migration in soil, water and sediments in Caohai Lake under the different hydrological periods, Southwest China. Sci. Total Environ. 2023, 865, 161292. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, Z.L.; Zhang, Y.; Kou, T.L.; Yang, H.; Wu, X.L.; Ma, X.W.; Jia, H.T. Distribution characteristics of microplastics in typical facility agriculture soil in North Xinjiang. Environ. Chem. 2024, 44, 1–12. (In Chinese) [Google Scholar]
- Zuo, C.C.; Li, Y.; Chen, Y.Y.; Jiang, J.; Qiu, W.H.; Chen, Q.Q. Leaching of heavy metals from polyester microplastic fibers and the potential risks in simulated real-world scenarios. J. Hazard. Mater. 2024, 461, 132639. [Google Scholar] [CrossRef]
- Kovochich, M.; Liong, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Xi, L.; Kreider, M.L.; Unice, K.M. Chemical Mapping of Tire and Road Wear Particles for Single Particle Analysis. Sci. Total Environ. 2021, 757, 144085. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.J.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, A.S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Sahu, S.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. A review on cutinases enzyme in degradation of microplastics. J. Environ. Manag. 2023, 347, 119193. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics the aquatic environment from marine to food systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Mallik, A.; Xavier, K.A.M.; Naidu, B.C.; Nayak, B.B. Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Sci. Total Environ. 2021, 779, 146433. [Google Scholar] [CrossRef]
- Magadini, D.L.; Goes, J.I.; Ortiz, S.; Lipscomb, J.; Pitiranggon, M.; Yan, B. Assessing the sorption of pharmaceuticals to microplastics through in-situ experiments in New York City waterways. Sci. Total Environ. 2020, 729, 138766. [Google Scholar] [CrossRef]
- Tan, Y.P.; Dai, J.Y.; Xiao, S.W.; Tang, Z.Q.; Zhang, J.M.; Wu, S.Q.; Wu, X.F.; Deng, Y. Occurrence of microplastic pollution in rivers globally: Driving factors of distribution and ecological risk assessment. Sci. Total Environ. 2023, 904, 165979. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–994. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garavena, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.A.; Liu, F.; Klemmensen, N.D.R.; Lykkemark, J.; Vollertsen, J. Retention of microplastics and tyre wear particles in stormwater ponds. Water Res. 2024, 248, 120835. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Li, R.X.; Li, F.; Xu, L.J.; Gan, L.; Li, J.X.; Huang, H.S.; Yan, M.T.; Wang, J. Microplastic pollution in water environment of typical nature reserves and scenery districts in southern China. Sci. Total Environ. 2023, 903, 166628. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.; Sundh, H.; Almroth, B.C. Microplastic exposure in aquatic invertebrates can cause significant negative effects compared to natural particles—A meta-analysis. Environ. Pollut. 2022, 315, 120434. [Google Scholar] [CrossRef]
- Wu, X.L.; Zhang, Z.M.; Guo, X.T. A critical review of the mechanisms underlying the interactions between microplastics and microorganisms in the environment. TrAC-Trend. Anal. Chem. 2024, 172, 117543. [Google Scholar]
- Xia, P.H.; Ma, L.; Yi, Y.; Lin, T. Assessment of heavy metal pollution and exposure risk for migratory birds-a case study of Caohai wetland in Guizhou Plateau (China). Environ. Pollut. 2021, 275, 116564. [Google Scholar] [CrossRef]
- Zhou, C.W.; Yang, R.; Yu, L.F.; Zhang, Y.; Yan, L.B. Hydrological and ecological effects of climate change in caohai watershed based on swat model. Appl. Ecol. Environ. Res. 2019, 17, 161. [Google Scholar] [CrossRef]
- Lo, H.S.; Xu, X.Y.; Wong, C.Y.; Cheung, S.G. Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environ. Pollut. 2018, 236, 208–217. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.N.; Xu, J. Influence of microplastic pollution on the toxicity of potamodromous fish grass carp (Ctenopharyngodon idellus) and its swimming capacity. J. Environ. Chem. Eng. 2024, 12, 113520. [Google Scholar] [CrossRef]
- Gialamas, T.; Gravalos, I.; Kateris, D. Vibration analysis on driver’s seat of agricultural tractors during tillage tests. Span. J. Agric. Res. 2016, 14, e0210. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Guo, J.M.; Sun, S.W. Spatial distribution and risk assessments of mercury in topsoils of Central Asia. Geosci. Front. 2023, 14, 270–279. [Google Scholar] [CrossRef]
- Ali, M.H.; Huang, Y.P.; Johnson, D. Effects of polystyrene microspheres on the swimming behavior and metabolism of grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 2024, 273, 107009. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.M.; Hou, L.L.; Zhang, Y. Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environ. Toxicol. 2024, 39, 1889–2476. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Xie, Y.J. Physiological response and oxidative stress of grass carp (Ctenopharyngodon idellus) under single and combined toxicity of polystyrene microplastics and cadmium. Ecotox. Environ. Safe 2022, 245, 114080. [Google Scholar] [CrossRef]
- Hu, S.Q. Pollution characteristics and ecological risk assessment of microplastics and six phthalate esters in farmland of Shanghai suburb. J. Ecol. Rural. Environ. 2024, 40, 975–984. (In Chinese) [Google Scholar]
- Yang, L.; Kang, S.C.; Wang, Z.Q.; Luo, X.; Guo, J.M.; Guo, T.G.; Chen, P.F.; Yang, C.D.; Zhang, Y.L. Microplastic characteristic in the soil across the Tibetan Plateau. Sci. Total Environ. 2022, 828, 154518. [Google Scholar] [CrossRef]
- Palazot, M.; Soccalingame, L.; Froger, C.; Jolivet, C.; Bispo, A.; Kedzierski, M.; Bruzaud, S. First national reference of microplastic contamination of French soils. Sci. Total Environ. 2024, 918, 170564. [Google Scholar] [CrossRef]
- Yuan, W.K.; Liu, X.N.; Wang, W.F.; Di, M.X.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotox. Environ. Safe 2019, 170, 180–187. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.G.; Li, L.Y.; Yang, D.Q.; Kolandhasamy, P.; Li, D.J.; Shi, H.H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Jiang, C.B.; Yin, L.S.; Wen, X.F.; Du, C.Y.; Wu, L.X.; Long, Y.N.; Liu, Y.Z.; Ma, Y.; Yin, Q.D.; Zhou, Z.Y.; et al. Microplastics in Sediment and Surface Water of West Dongting Lake and South Dongting Lake: Abundance, Source and Composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef]
- Jain, Y.; Govindasamy, H.; Kaur, G.; Ajith, N.; Ramasamy, K.; Robin, R.S.; Ramachandran, P. Microplastic pollution in high-altitude Nainital lake, Uttarakhand, India. Environ. Pollut. 2024, 346, 123598. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Goncalves, F.J.M.; Nogueira, H.; Marques, J.C.; Goncalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antua River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Suni, K.; Kumar, A.; Ambili, A. Spatial distribution and characteristics of microplastics and associated contaminants from mid-altitude lake in NW Himalaya. Chemosphere 2023, 326, 138415. [Google Scholar]
- Hu, D.F.; Zhang, Y.X.; Shen, M.C. Investigation on microplastic pollution of Dongting Lake and its affiliated rivers. Mar. Pollut. Bull. 2020, 160, 111555. [Google Scholar] [CrossRef]
- Munno, K.; Helm, P.A.; Rochman, C.; George, T.; Jackson, D.A. Microplastic contamination in Great Lakes fish. Conserv. Biol. 2021, 36, e13794. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Nagane, S.S.; Maher, D.M.; Verma, S.; Talanikar, A.A.; Wadgaonkar, P.P. Pendant propargyloxy-functionalized aromatic (co)polycarbonates: Synthesis, thermal crosslinking and chemical modification. J. Macromol. Sci. A 2022, 59, 752–763. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Ouyang, S.Y. The native submerged plant, Hydrilla verticillata outperforms its exotic confamilial with exposure to polyamide microplastic pollution: Implication for wetland revegetation and potential driving mechanism. Aquat. Toxicol. 2024, 273, 197029. [Google Scholar] [CrossRef]
- Chen, C.; Wen, M.; Cheng, T. Photocatalytic degradation of tetracycline wastewater through heterojunction based on 2D rhombic ZrMo2O8 nanosheet and nano-TiO2. J. Nanopart. Res. 2024, 24, 172. [Google Scholar] [CrossRef] [PubMed]
- Kanyathare, B.; Asamoah, B.O.; Ishaq, U. Optical transmission spectra study in visible and near-infrared spectral range for identification of rough transparent plastics in aquatic environments. Chemosphere 2020, 248, 126071. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Kerpen, J. Underestimating microplastics? Quantification of the recovery rate of microplastic particles including sampling, sample preparation, subsampling, and detection using μ-Ramanspectroscopy. Anal. Bioanal. Chem. 2023, 415, 2963–2973. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Knight, L.J.; Parker-Jurd, F.N.F.; Al-Sid-Cheikh, M.; Thompson, R.C. Tyre wear particles: An abundant yet widely unreported microplastic? Environ. Sci. Pollut. Res. 2020, 27, 18345–18354. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Järlskog, I.; Strömvall, A.M.; Magnusson, K.; Galfi, H.; Björklund, K.; Polukarova, M.; Garção, R.; Markiewicz, A.; Aronsson, M.; Gustafsson, M.; et al. Traffic-related microplastic particles, metals, and organic pollutants in an urban area under reconstruction. Sci. Total Environ. 2021, 774, 145503. [Google Scholar] [CrossRef]




| Items | Conditions |
|---|---|
| Pyrolyzer | PY-3030D Frontier (Frontier Lab., Kyoto, Japan) |
| Pyrolysis temperature | 550 °C |
| Split ratio | 5:1 |
| Chromatographic column | Rtx-5MS (30 m × 0.25 mm × 0.25 μm) |
| Chromatogram temperature program | Maintain 40 °C for 2 min, heat to 320 °C at a rate of 20 °C/min, maintain for 14 min; total time—30 min. |
| Ionization temperature | 230 °C |
| m/z scanning range | 19–600 |
| PE (mg/kg) | PVC (mg/kg) | PA6 (mg/kg) | PA66 (mg/kg) | |
|---|---|---|---|---|
| Stomach | 54.94 | ND * | ND | ND |
| Intestines | 51.69 | 126.90 | ND | ND |
| Tissue | 27.53 | 78.42 | ND | ND |
| Skin | 34.16 | 98.07 | 27.27 | ND |
| Gills | 20.45 | 33.96 | ND | 34.61 |
| Location (Region) | Date | Abundance | Main Particle Size | Reference | ||
|---|---|---|---|---|---|---|
| Min. | Max. | Mean | ||||
| Soils | ||||||
| Caohai (China) (n/kg) | 2023 | 1200 | 18,733 | 5807 ± 1806 | 20–100 μm | Present study |
| Caohai (China) (n/kg) | 2021 | 3000 * — | 8640 * — | 4783 ± 1892 * 4410 ± 1635 # | 0–0.5 mm | [2] [8] |
| Shanghai (China) (n/kg) | 2023 | 63,400 | 328,000 | — | 20–30 μm | [35] |
| Beijing (China) (n/kg) | 2023 | 14,420 | 38,820 | 24,763 | <0.5 mm | [9] |
| Tibetan Plateau (China) (n/kg) | 2018 | 5 | 340 | 47.12 | 50–500 μm | [36] |
| French territory (France) (n/kg) | 2020 | 258 | 3096 | 597 ± 895 | 1–315 μm | [37] |
| Water | ||||||
| Caohai (China) (n/L) | 2023 | 9 | 223 | 59 ± 17 | 20–100 μm | Present study |
| Caohai (China) (n/L) | 2021 | 4.6 * 2.7 # | 10.1 * 10.5 # | 6.5 ± 3.3 * 5.6 ± 2.0 # | 0–0.5 mm | [2] [8] |
| Poyang Lake (China) (n/L) | 2018 | 5 | 34 | — | <0.5 mm | [38] |
| Taihu Lake (China) (n/L) | 2015 | 3.4 | 25.8 | — | 100–1000 μm | [39] |
| Dongting Lake (China) (n/L) | 2018 | 0.32 | 0.48 | 0.97 ± 0.42 | <0.5 mm | [40] |
| Chao Lake (China) (n/L) | 2019 2020 | 0.33 * 0.24 # | 0.62 * 0.49 # | 2.13 ± 1.53 * 1.68 ± 1.58 # | <1 mm | [6] |
| Nainital Lake (India) (n/L) | 2023 | 8.6 | 56.0 | — | 0.02–1 mm | [41] |
| Antuã River (Portugal) (n/L) | 2016 | 0.058 | 0.1265 | — | - | [42] |
| Lake in NW Himalayas (India) (n/L) | 2022 | 13 | 238 | 130 | 0.9–0.333 mm | [43] |
| Siberian lakes (Russia) (n/L) | 2020 | 4 n/L | 26 n/L | 11 ± 7 n/L | <1.0 mm | [17] |
| Sediment | ||||||
| Caohai (China) (n/kg) | 2023 | 533 | 10,200 | 2933 ± 763 | 20–100 μm | Present study |
| Caohai (China) (n/kg) | 2021 | 1320 * — | 4260 * — | 2094 ± 923 * 1872 ± 1107 # | 0–0.5 mm | [2] [8] |
| Poyang Lake (China) (n/kg) | 2018 | 54 | 506 | — | <0.5 mm | [38] |
| Taihu Lake (China) (n/kg) | 2015 | 11 | 234.6 | — | 100–1000 μm | [39] |
| Dongting Lake (China) (n/kg) | 2018 | 210 | 520 | 385 ± 696 | <0.1 mm | [44] |
| Chao Lake (China) (n/kg) | 2019 | 60 | 1064 | 308 ± 231 | <1 mm | [6] |
| Nainital Lake (India) (n/kg) | 2023 | 400 | 10,600 | — | 0.02–1 mm | [41] |
| Antuã River (Portugal) (n/kg) | 2016 | 18 | 629 | — | - | [42] |
| Fish | ||||||
| Caohai (China) (mg/kg) | 2023 | ND | 126.90 | — | 20–100 μm | Present study |
| Lake Ontario (Canada) (n/fish) | 2015 | 3 | 915 | 59 ± 104 | - | [45] |
| Northeast Atlantic (n/fish) | 2013 | 0 | 2 | — | 1.0–2.0 mm | [46] |
| Poyang Lake (China) (n/fish) | 2018 | 0 | 18 | — | <0.5 mm | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wu, X.; Wang, X.; Xia, P.; Zhang, L.; Huang, X.; Zhang, Z. The Migration and Pollution Risk of Microplastics in Water, Soil, Sediments, and Aquatic Organisms in the Caohai Watershed, Southwest China. Water 2025, 17, 1168. https://doi.org/10.3390/w17081168
Wang X, Wu X, Wang X, Xia P, Zhang L, Huang X, Zhang Z. The Migration and Pollution Risk of Microplastics in Water, Soil, Sediments, and Aquatic Organisms in the Caohai Watershed, Southwest China. Water. 2025; 17(8):1168. https://doi.org/10.3390/w17081168
Chicago/Turabian StyleWang, Xu, Xianliang Wu, Xingfu Wang, Pinhua Xia, Lan Zhang, Xianfei Huang, and Zhenming Zhang. 2025. "The Migration and Pollution Risk of Microplastics in Water, Soil, Sediments, and Aquatic Organisms in the Caohai Watershed, Southwest China" Water 17, no. 8: 1168. https://doi.org/10.3390/w17081168
APA StyleWang, X., Wu, X., Wang, X., Xia, P., Zhang, L., Huang, X., & Zhang, Z. (2025). The Migration and Pollution Risk of Microplastics in Water, Soil, Sediments, and Aquatic Organisms in the Caohai Watershed, Southwest China. Water, 17(8), 1168. https://doi.org/10.3390/w17081168







