Comparative Assessment of Chemical and Isotopic Composition of Geothermal Fluids in the Eastern Part of the Büyük Menderes Graben (Western Türkiye)
Abstract
1. Introduction
2. Geological and Hydrogeological Setting
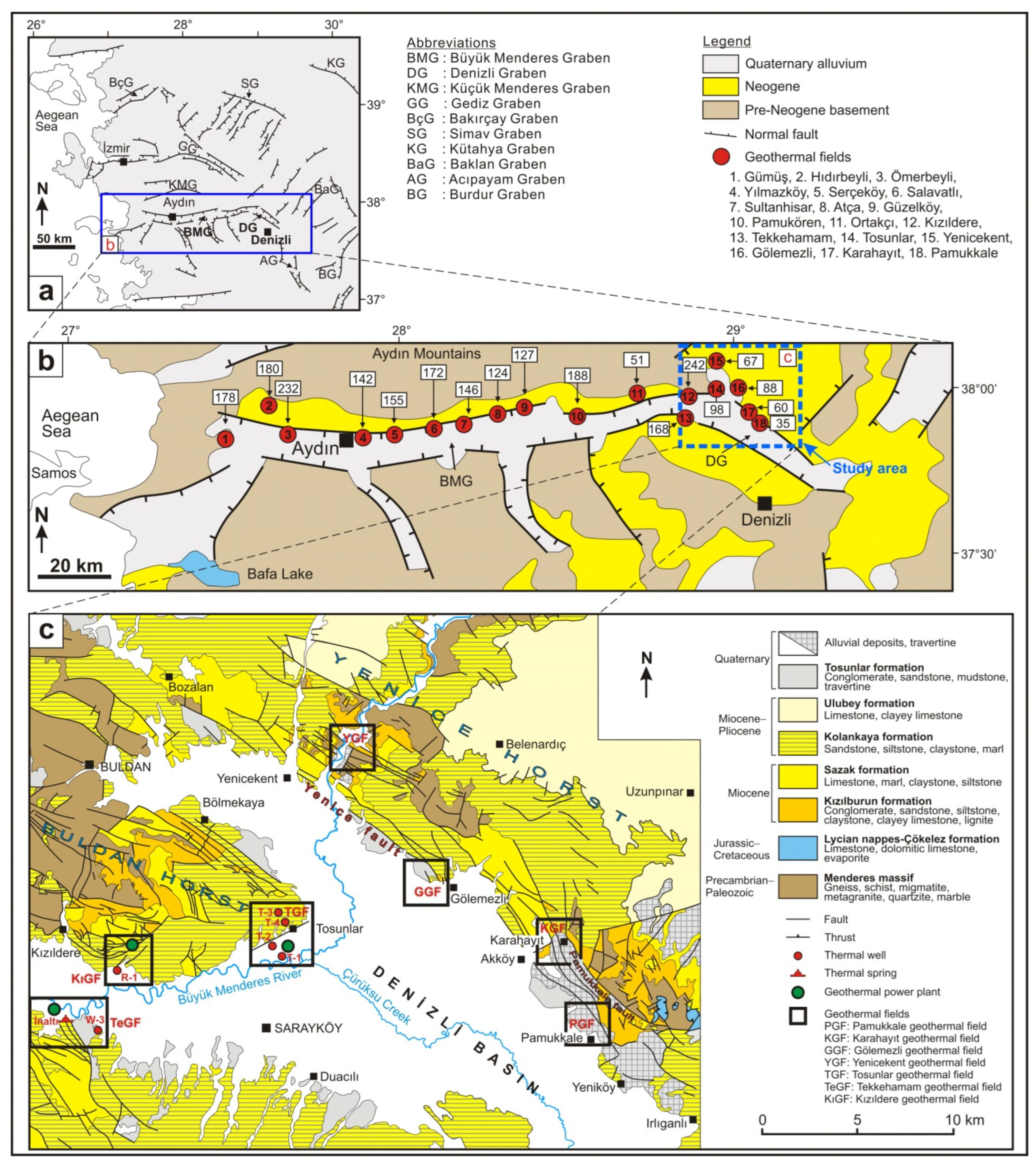
3. Site Description
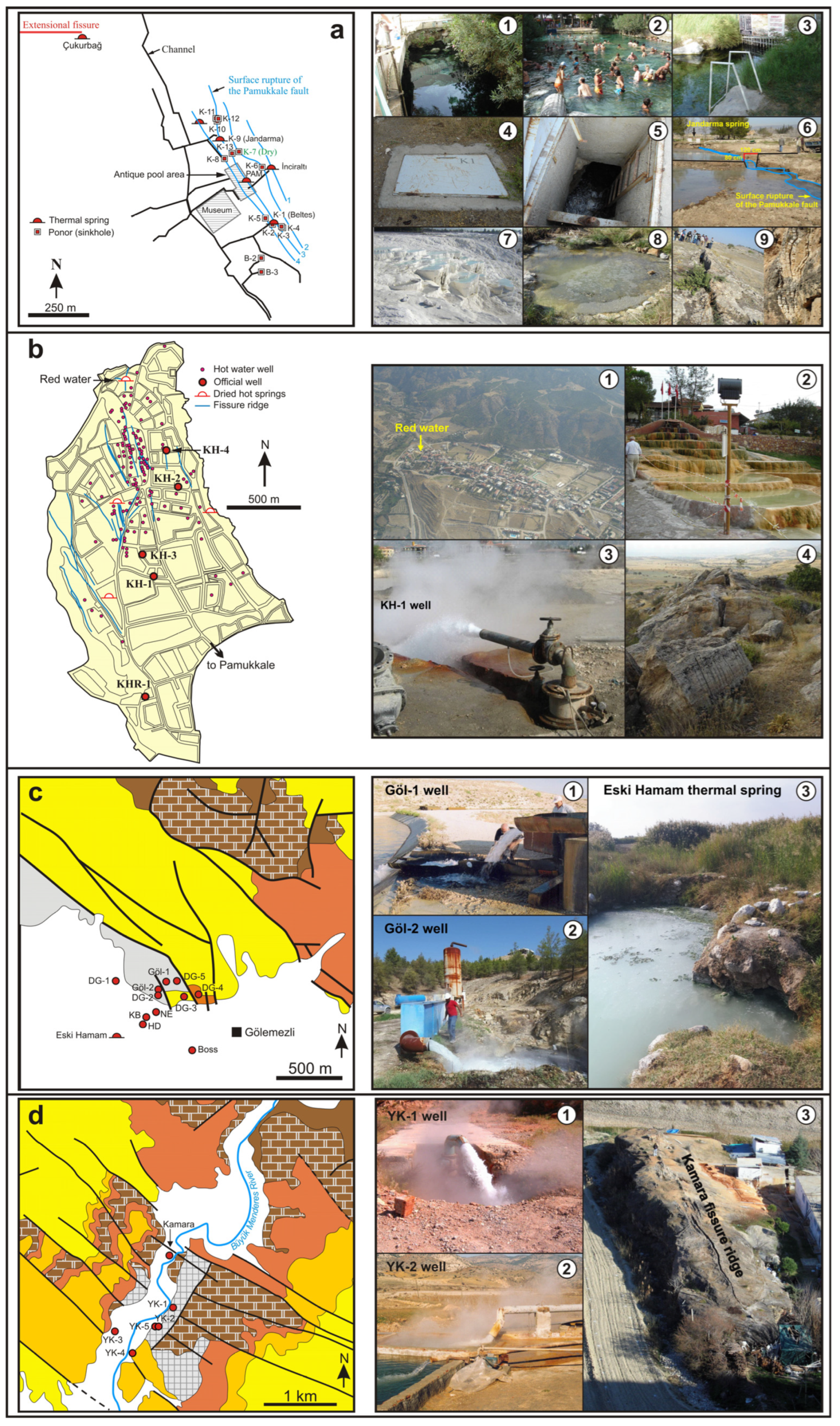
3.1. General
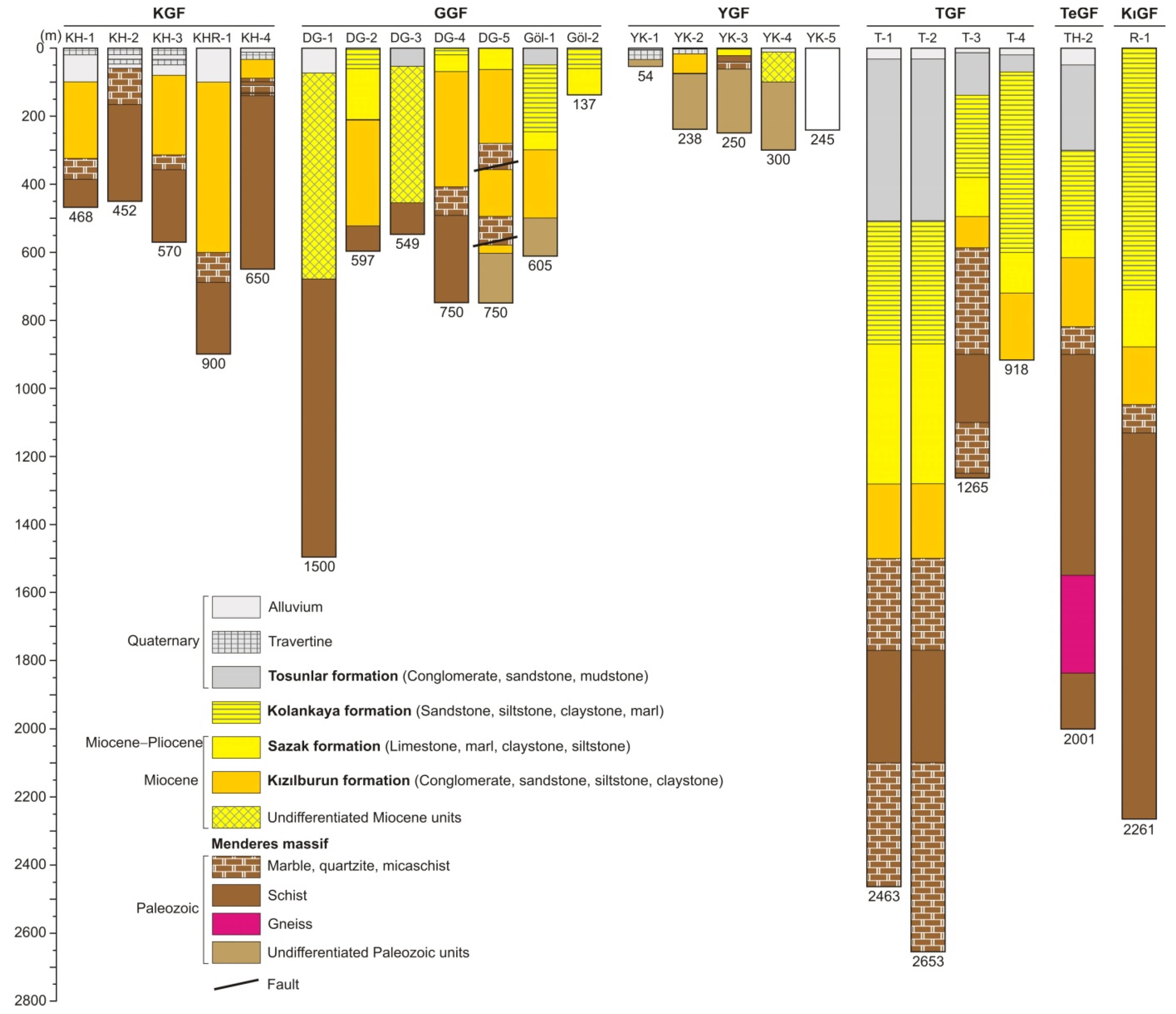
3.2. Geothermal Fields
| Geothermal Field | Well No | Date | Depth (m) | T (°C) | Q (L/s) | Utilization |
|---|---|---|---|---|---|---|
| Karahayıt | KH-1 a | 2007 | 468 | 61.0 | 15 | Thermal bath and spa, hotel and pension heating. |
| KH-2 a | 2007 | 452 | 61.0 | 40 | ||
| KH-3 a | 2007 | 570 | 61.0 | 80 | ||
| KHR-1 b | 2011 | 900 | 54.0 | 27 | ||
| KH-4 c | 2019 | 650 | 54.5 | 30 | ||
| Gölemezli | DG-1 d | 2001 | 1500 | 88.0 | 15 | Greenhouse heating (192,000 m2) and bathing. |
| DG-2 d | 2002 | 597 | 73.0 | 140 | ||
| DG-3 e | 2002 | 549 | 66.0 | 110 | ||
| DG-4 e | 2003 | 750 | 70.0 | 45 | ||
| DG-5 e | 2003 | 750 | 62.0 | 30 | ||
| Göl-1 e | 2008 | 605 | 65.0 | 120 | ||
| Göl-2 e | 2011 | 137 | 68.0 | 136 | ||
| Yenicekent | Kamara f | 1998 | 146 | 55.1 | - | Kamara well water is used for thermal bath.Greenhouse heating (55,000 m2). |
| YK-1 g | 2002 | 54 | 57.0 | 20 | ||
| YK-2 g | 2002 | 238 | 67.0 | 140 | ||
| YK-3 g | 2002 | 250 | 36.0 | 4 | ||
| YK-4 g | 2013 | 300 | 53.0 | 34 | ||
| YK-5 h | 2015 | 245 | 61.0 | 35 | ||
| Tosunlar | T-1 i | 2013 | 2463 | 132.0 | 144 | Electricity production (3.8 MWe), CO2 production andgreenhouse heating (197,000 m2). |
| T-2 i | 2013 | 2653 | 148.8 | 39 | ||
| T-3 i | 2007 | 1265 | 106.6 | 91 | ||
| T-4 i | 2008 | 918 | 85.4 | 73 | ||
| Tekkehamam | TH-2 d | 1997 | 2001 | 168.0 | 12 | Electricity production (106 MWe), thermal bath and spa, greenhouse heating. |
| Kızıldere | R-1 d | 1998 | 2261 | 240.6 | 81 | Electricity production (260 MWe), CO2 production, thermal bath, district heating, greenhouse heating. |
4. Materials and Methods
5. Results and Discussion
5.1. Physicochemical Characteristics
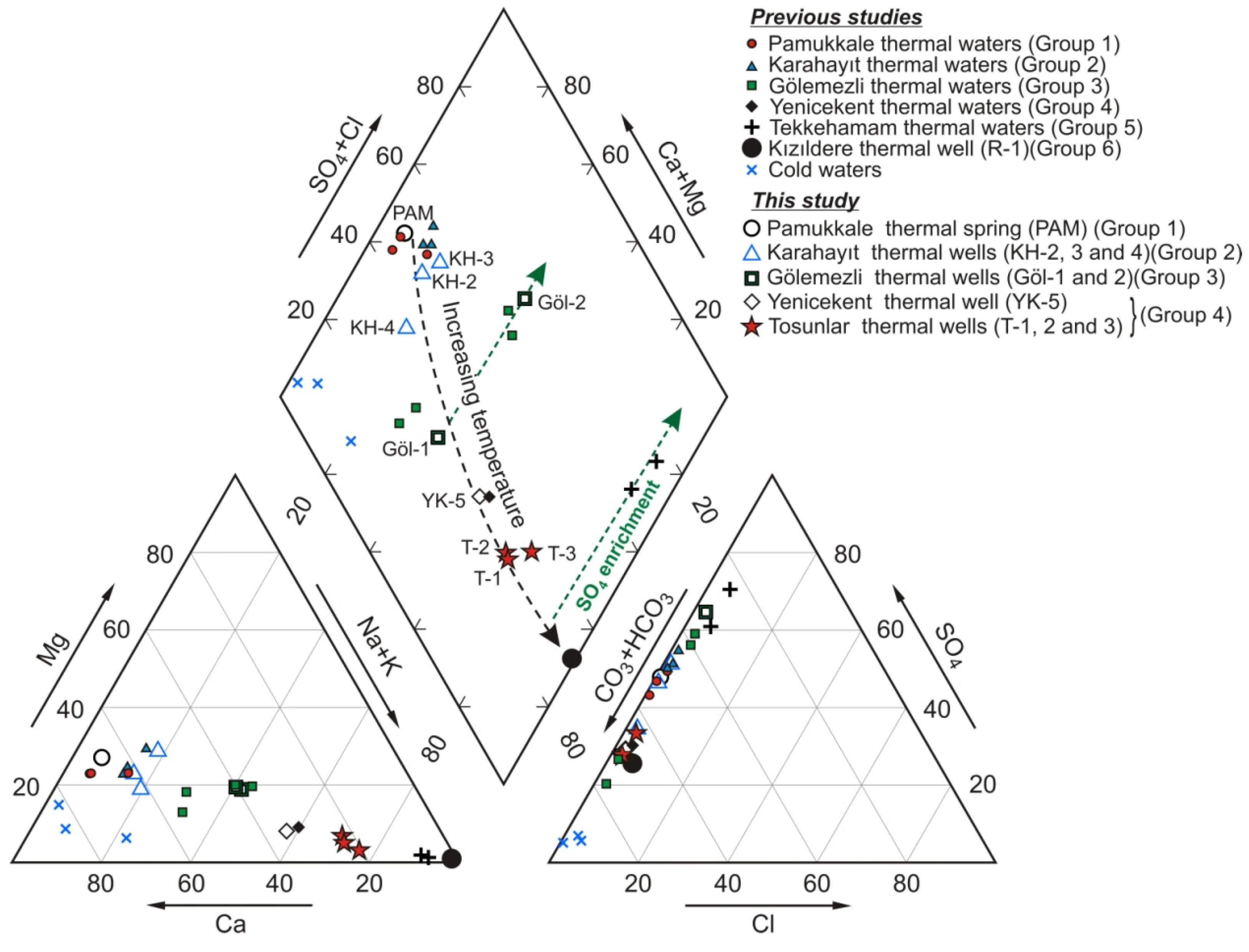
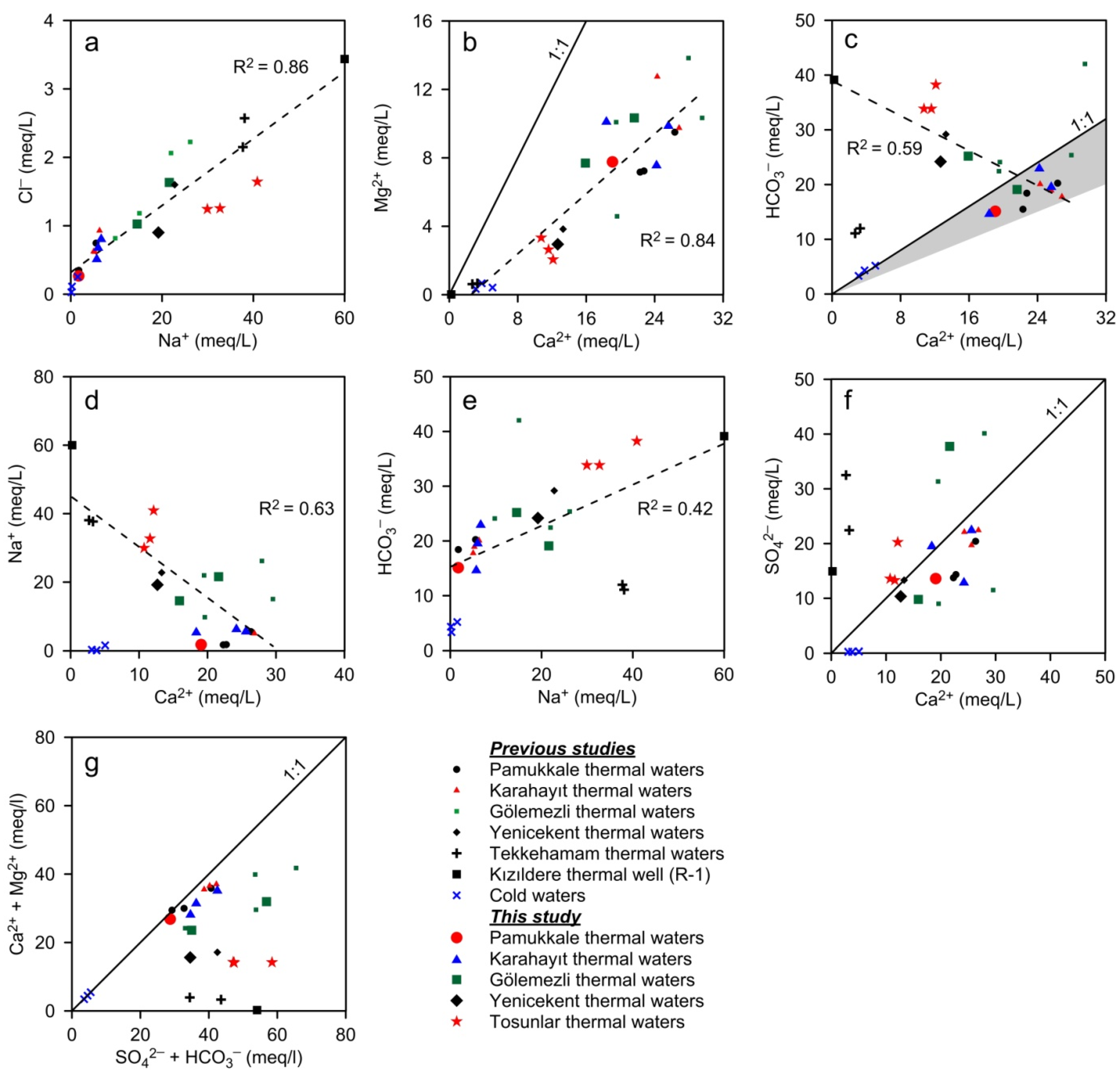
- Ca-Mg-HCO3-SO4 type thermal waters: This type of thermal spring water is unique to PGF. These waters, which precipitate the white travertine, are distinguished from thermal waters in other areas by their low temperature and low electrical conductivity and slightly acidic nature. Measurements made between 2002 and 2018 showed that the temperatures of these springs and other thermal springs in the area (Figure 4a) varied between 31.5 and 35.1 °C [23]. The thermal springs and private well waters in the plain have slightly lower temperatures falling in the range of 20 to 32 °C. The EC and pH values of PGF thermal waters vary within a narrow range between 2300 and 2390 µS/cm, and 6.10 and 6.37, respectively (Table 2). The dominant cation is Ca2+ (381–455 mg/L, 19.1–22.8 meq/L) and the dominant anion is HCO3− (932–1124 mg/L, 15.1–18.4 meq/L) (Figure 5 and Figure 6). This type of water has the lowest Na+, K+, Cl−, F−, Li+, B and SiO2 contents among other types of thermal waters (Table 2).
- Ca-Mg-SO4-HCO3 type thermal waters: KGF thermal well waters characterize this type. The temperatures of this group of waters are between 47.0 and 60.7 °C, EC values are from 2500 to 3080 µS/cm and pH varies from 6.25 to 7.70. The dominant cation and dominant anion are Ca2+ and SO42− although SO42− concentrations are generally slightly higher than HCO3− (in meq/L). The EC is similar to PGF thermal waters; however, Mg2+, Na+, K+, Cl−, Li+, B and SiO2 values are higher (Table 2, Figure 6). KH-4, one of the thermal wells in the KGF field, is of Ca-SO4-HCO3 type and distinguished by higher Na+, HCO3− and less Mg2+ and SO42− contents (Table 2).
- Ca-Na-HCO3-SO4 and Ca-Na-SO4-HCO3 type thermal waters: This type of thermal spring water is unique to GGF. Most of the thermal springs in GGF have dried up after the intense drilling, and the temperature of one active spring (Eski Hamam) was measured as 48 °C (Figure 4(c3)). Göl-1 and DG-3 well waters are of Ca-Na-HCO3-SO4 type. The temperatures of these thermal well waters are between 53.3 and 63.0 °C, EC is from 2930 to 3780 μS/cm and pH is in the range from 6.69 to 7.32. Ca-Na-SO4-HCO3 type Göl-2 and DG-1 thermal well waters have higher temperatures (62.0–69.0 °C; 88 °C at bottom-hole in DG-1 [51]) and EC (4030–4730 μS/cm). Na+, Cl− and SO42−, F−, Li+, B and SiO2 values are higher and HCO3− contents are lower than Ca-Na-SO4-HCO3 type waters (Table 2, Figure 5 and Figure 6).
- Na-Ca-HCO3-SO4 type thermal waters: YGF and TGF thermal well waters are included in this group. The bottom-hole temperatures of the TGF waters, sampled for the first time in this study, vary between 88 and 149 °C [73] and the discharge temperatures are from 84 to 98 °C. YGF waters (YK-1 and YK-5) have lower temperature (~50–60 °C) and EC than TGF (T-1, T-2 and T-3) waters and lower B and SiO2 values except Ca2+ and Mg2+ (Table 2).
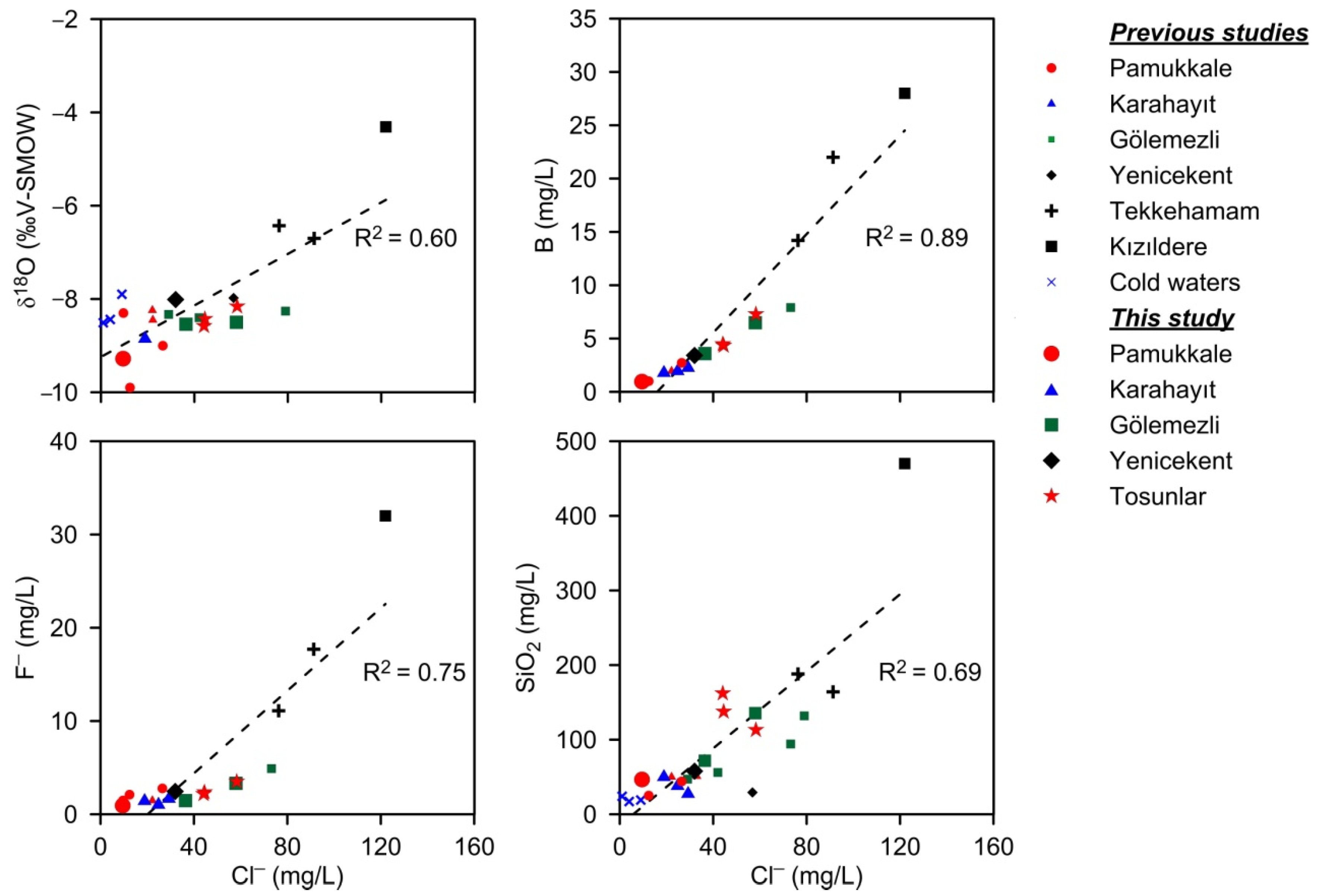
- Na-SO4-HCO3 type thermal waters: Thermal springs and well waters in TeGF fall into this group. The temperatures of these thermal waters are reported between 23.4 and 168 °C [24,71,74]. Deep wells opened by private companies produced thermal waters with temperatures of about 170 °C. In these waters (in meq/L), 85% of the cations consist of Na+ (up to 876 mg/L), and 60–70% of the anions are comprised of SO42− (up to 1560 mg/L). These thermal waters have the lowest HCO3− contents among other group waters. Except for the KıGF samples, Cl−, F−, Li+, B and SiO2 values are significantly higher than other group waters (Table 2).
- Na-HCO3-SO4 type thermal waters: The KıGF thermal waters constitute this group, which are characterized by high temperature (137–245 °C) and EC values (3010–5520 μS/cm) and generally alkaline (pH: 6.3–8.7) thermal fluids. In this group, waters with temperature above 200 °C (24 thermal wells) have the lowest Ca2+ (<10.3 mg/L) and Mg2+ (<1.79 mg/L), and the highest Na+ (1035–1538 mg/L), K+ (83–347 mg/L), alkalinity (1806–2990 mg/L), Cl− (93.2−254 mg/L), F− (8–33 mg/L), Li+ (2.6–5.7 mg/L), B (11.1–31.0 mg/L) and SiO2 (239–697 mg/L) contents [26]. The physicochemical properties of R-1 thermal well water (Table 2) best represent the KıGF waters.

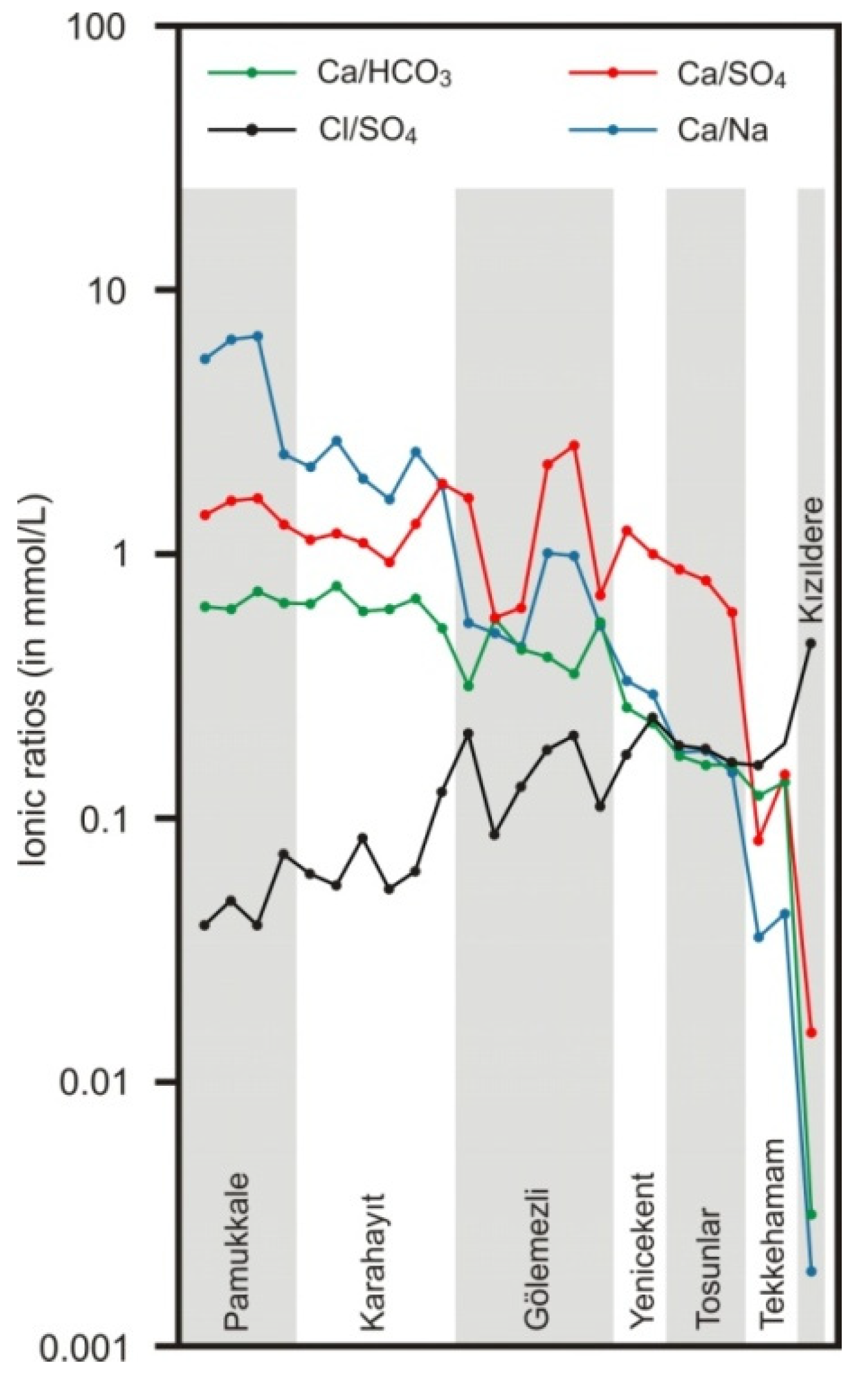
5.1.1. Factors Affecting the Chemical Composition of Waters
- Gypsum dissolution within Neogene formations (particularly Sazak formation). In the Gölemezli and Tekkehamam fields, the Neogene cover above the Menderes massif is quite thick. For example, in the MDO-1 well drilled at Tekkehamam to a depth of 2401 m, the cover thickness is 1950 m (Sazak formation progresses between 814 and 1143 m) [74].
- Oxidation of H2S (or SO2). The gas component of thermal fluids in the Kızıldere field is composed of 0.008 to 0.052% H2S [26]. H2S possibly occurs in the Tekkehamam and Gölemezli fields. Oxidation of H2S results in the addition of large amounts of sulfate to the water in these fields. The elevated sulfate concentrations in the Tekkehamam springs could also have resulted from further oxidation of H2S or SO2 by the oxygen-rich groundwaters which might precipitate native sulfur [19].
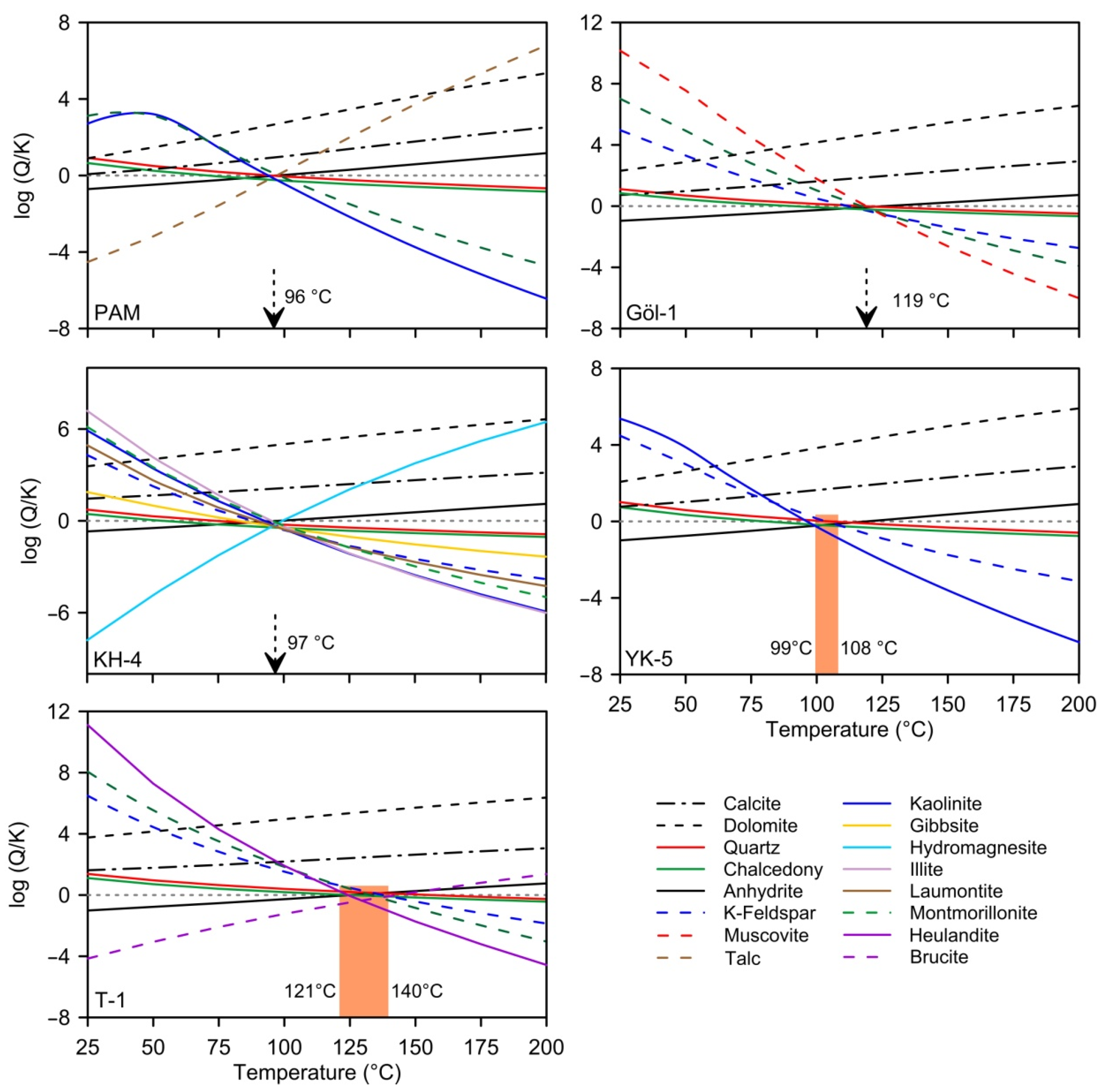
5.1.2. Mineral Saturation
5.2. Isotope Composition
5.2.1. Oxygen–Hydrogen Isotopes
| Site | Sample ID | δ18O | δD | d-Excess | T | δ13C | δ34S(SO4) | δ18O(SO4) | 14C | Age * | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (VSMOW) | (VSMOW) | (VSMOW) | (TU) | (VPDB) | (VCDT) | (VSMOW) | (pmC) | (YBP) | (YBP) | ||
| This study | |||||||||||
| Pamukkale | PAM | −9.28 | −59.75 | 14.49 | 2.16 | 5.68 | 16.82 | 13.94 | na | na | na |
| Karahayıt | KH-3 | −8.81 | −57.69 | 12.79 | 2.13 | 5.45 | 17.11 | 13.73 | 1.104 | 35,954 | 36,202 |
| KH-4 | −8.57 | −55.63 | 12.93 | na | na | na | na | na | na | na | |
| Gölemezli | Göl-1 | −8.54 | −57.62 | 10.70 | 0.54 | 7.65 | 16.43 | 12.64 | 0.617 | 40,613 | 40,878 |
| Göl-2 | −8.50 | −60.56 | 7.44 | 0.02 | 3.79 | 20.04 | 15.71 | 0.676 | 39,909 | 40,143 | |
| Yenicekent | YK-5 | −8.01 | −55.17 | 8.91 | 0.05 | 6.32 | 14.10 | 10.65 | 0.930 | 37,408 | 37,662 |
| Tosunlar | T-1 | −8.43 | −59.35 | 8.09 | 0.71 | 2.55 | 16.46 | 11.33 | 5.879 | 22,538 | 22,764 |
| T-2 | −8.58 | −60.82 | 7.82 | na | na | na | na | na | na | ||
| T-3 | −8.16 | −59.57 | 5.71 | 0.54 | 2.20 | 16.59 | 10.64 | 1.578 | 33,107 | 33,328 | |
| Rainwater | RW | na | na | na | 5.64 | na | na | na | na | na | na |
| Previous studies | |||||||||||
| Pamukkale | PAM a | −9.90 | −58.70 | 20.50 | 3.50 | na | 14.30 | 16.74 | na | na | na |
| Beltes b | −8.30 | −58.90 | 7.50 | na | na | na | na | na | na | na | |
| Çukurbağ b | −9.00 | −58.70 | 13.30 | na | na | na | na | na | na | na | |
| Karahayıt | KH-2 c | −8.45 | −56.49 | 11.11 | na | 9.76 | na | na | na | na | na |
| KH-3 d | −8.24 | −53.00 | 12.93 | na | na | na | na | na | na | na | |
| Gölemezli | DG-3 e | −8.33 | −57.41 | 9.23 | 0.40 | 6.98 | na | na | na | na | na |
| Göl-1 e | −8.40 | −57.80 | 9.40 | 0.23 | 6.18 | na | na | na | na | na | |
| Göl-2 e | −8.26 | −60.46 | 5.62 | 0.21 | 5.11 | na | na | na | na | na | |
| Yenicekent | YK-1 f | −7.98 | −56.25 | 7.59 | na | 6.80 | na | na | na | na | na |
| Tekkehamam | İnaltı a, g | −6.70 | −57.50 | −3.90 | 0.10 | 0.90 | 11.10 | 18.69 | 2.130 | - | 34,898 |
| W-3 h | −6.43 | −53.32 | −1.88 | 0.00 | na | na | na | na | na | na | |
| Kızıldere | R-1 i | −4.10 | −51.30 | −18.50 | 0.33 | 1.26 | na | na | 2.270 | 31,034 | |
| Cold waters | CS-1 c | −8.44 | −54.70 | 12.82 | na | −10.38 | na | na | na | na | na |
| CS-2 c | −8.51 | −54.19 | 13.89 | 2.73 | −9.71 | na | na | na | na | na | |
| CS-3 c | −7.90 | −54.13 | 9.07 | na | −8.16 | na | na | na | na | na |
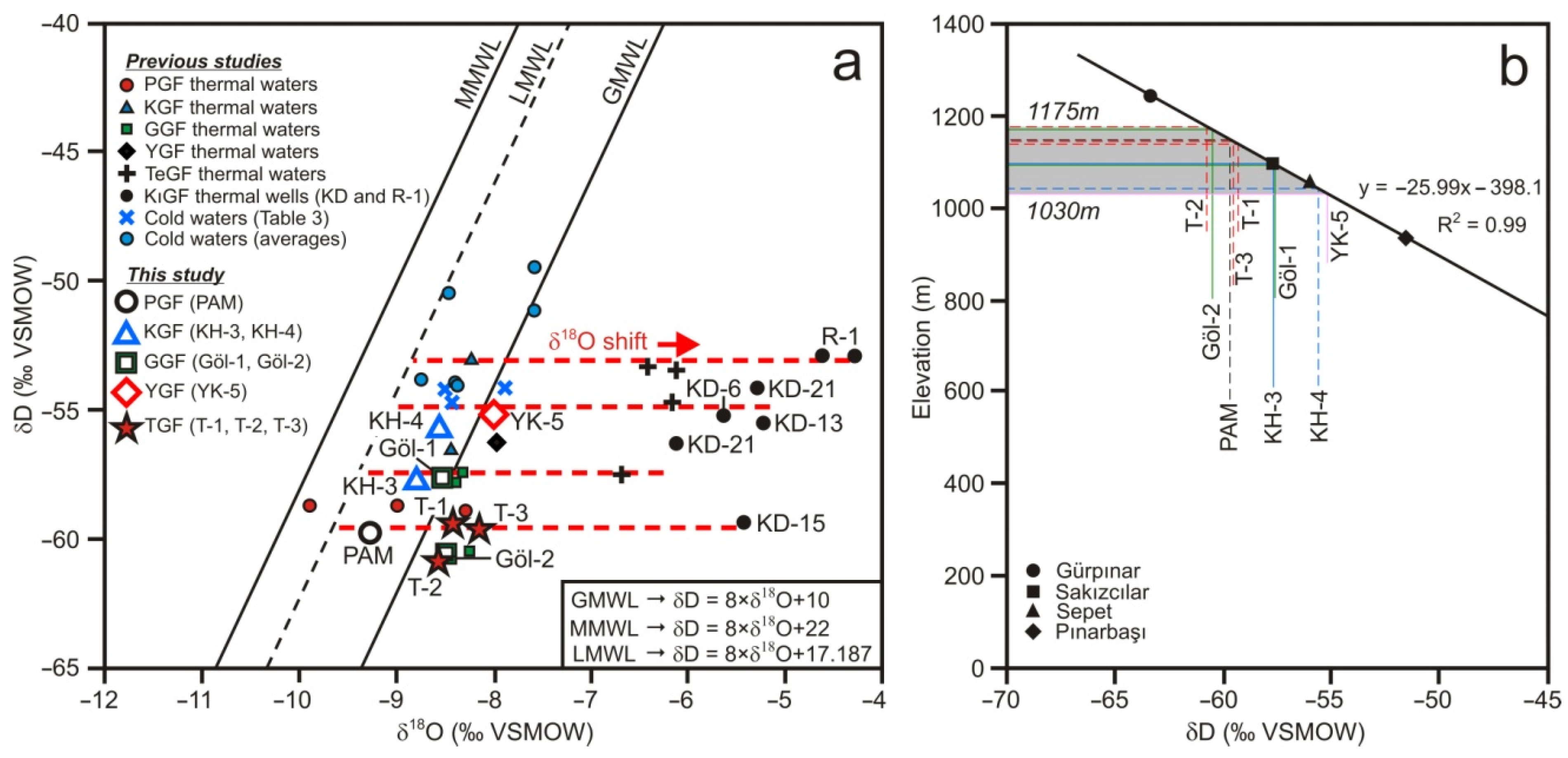
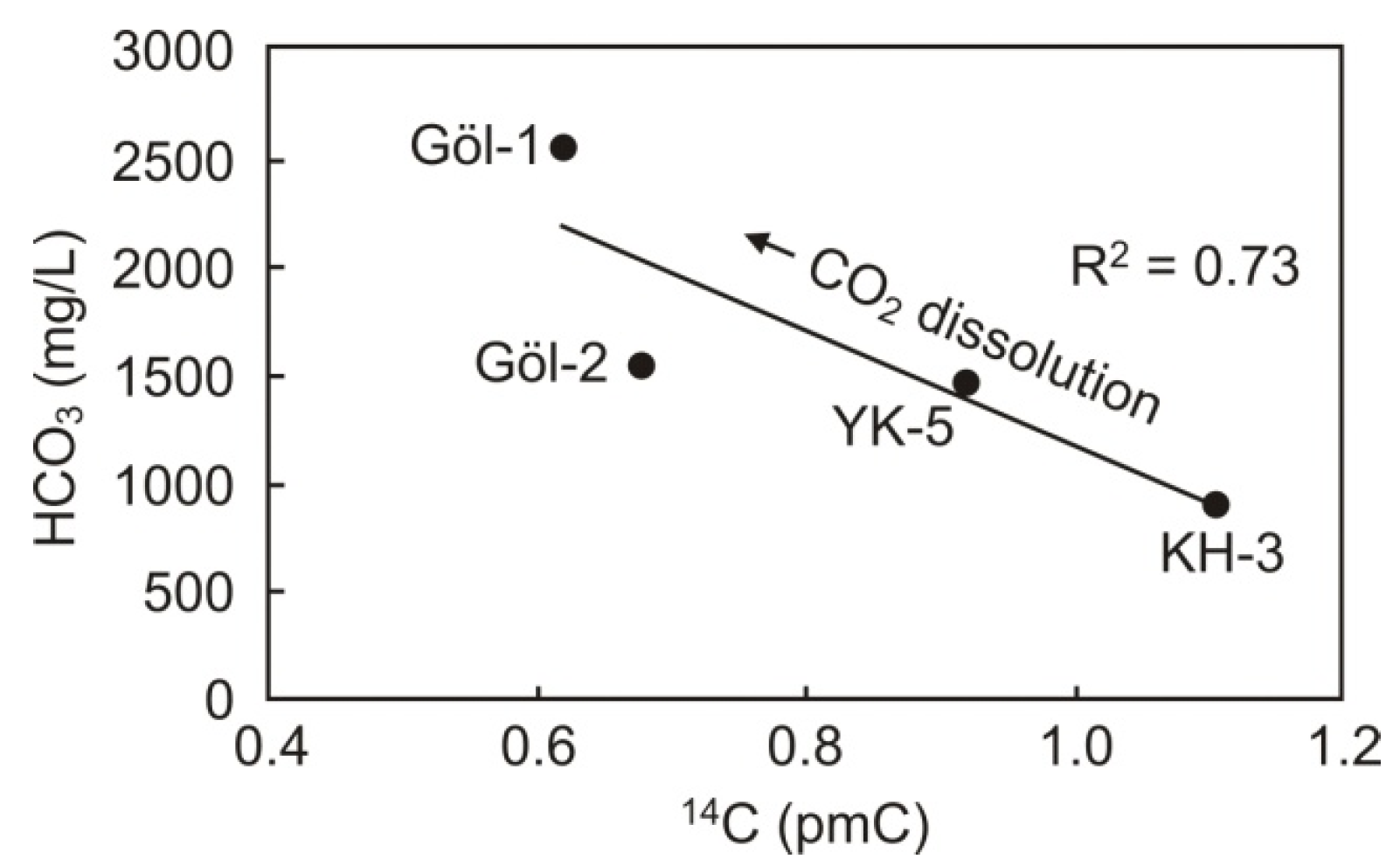
5.2.2. Water Circulation
- : Initial activity of parent radionuclide
- : Activity of parent radionuclide at time t (measured value)
- λ: Decay constant (for 14C:5730 years).
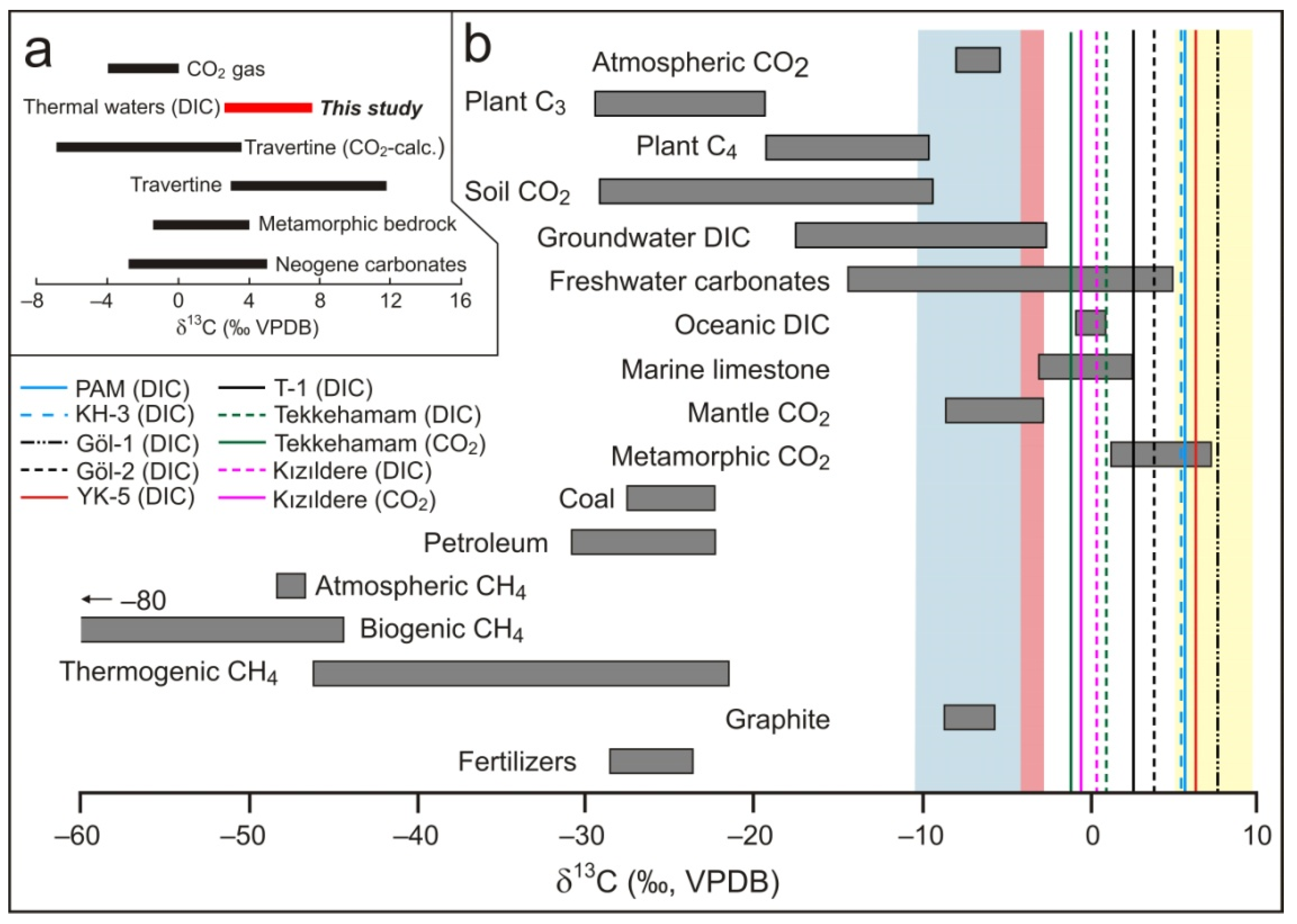
5.2.3. δ13C
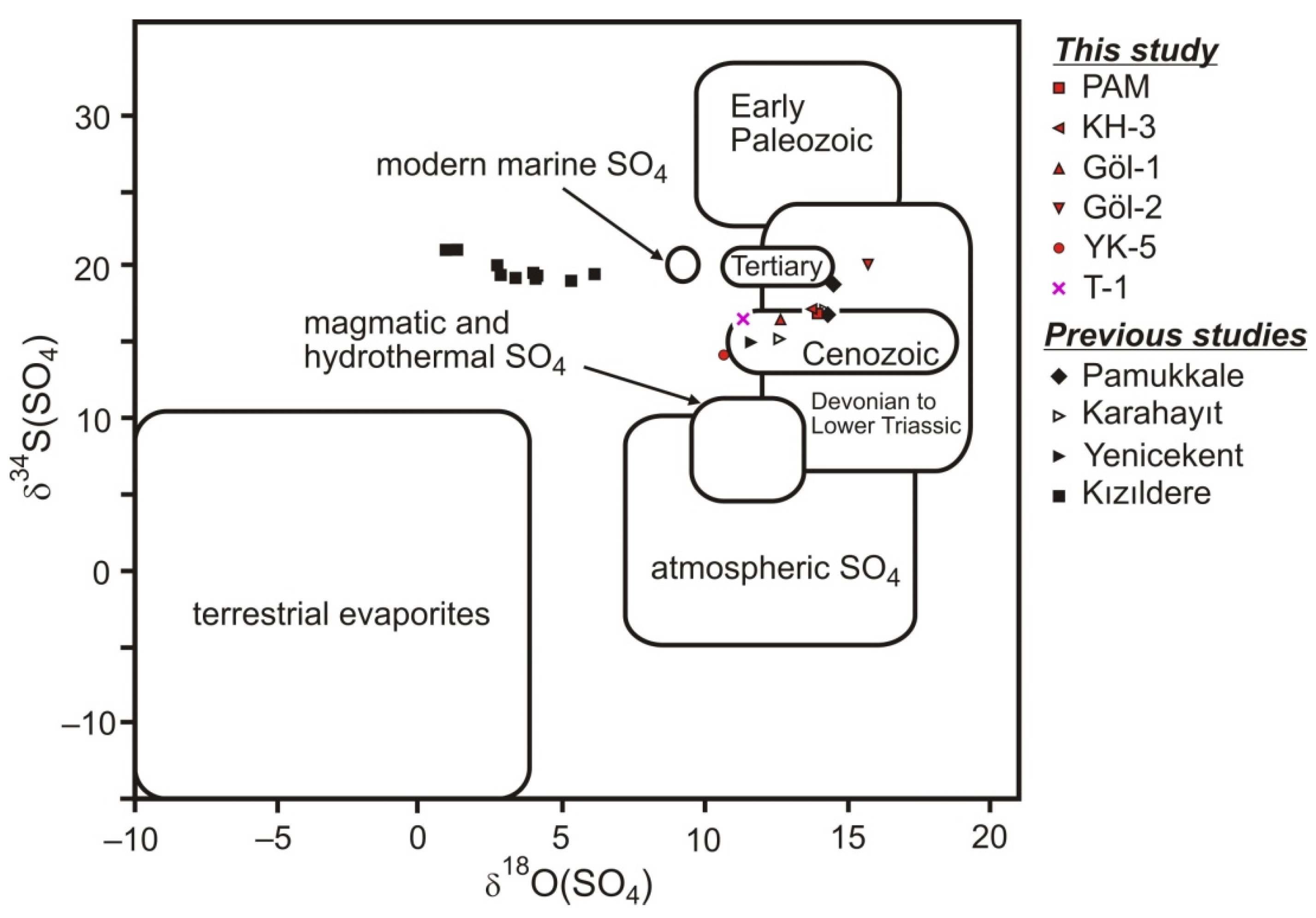
5.2.4. Sulfur and Oxygen Isotopes on Sulfate
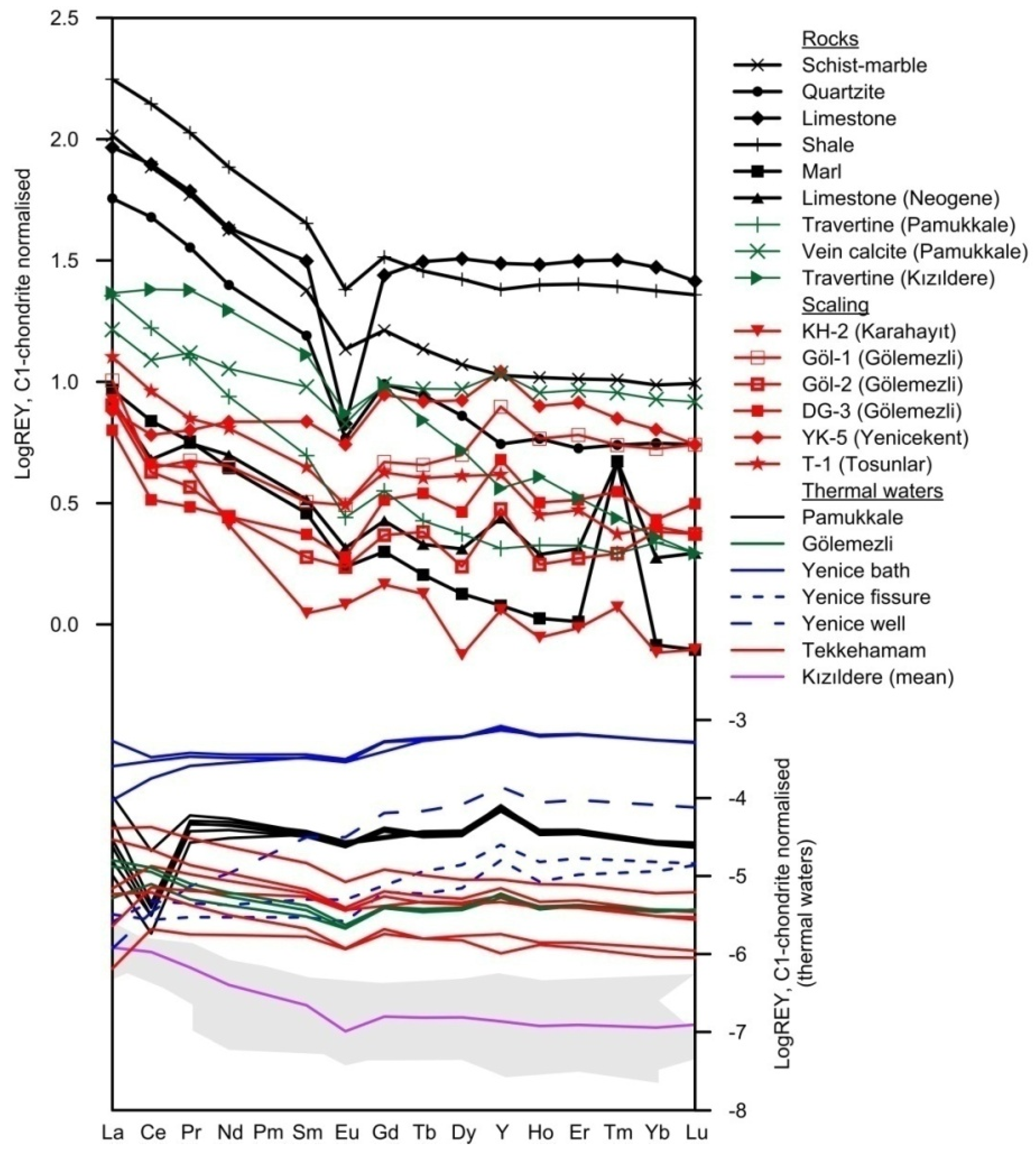
5.3. Rare Earth Element Fractionation
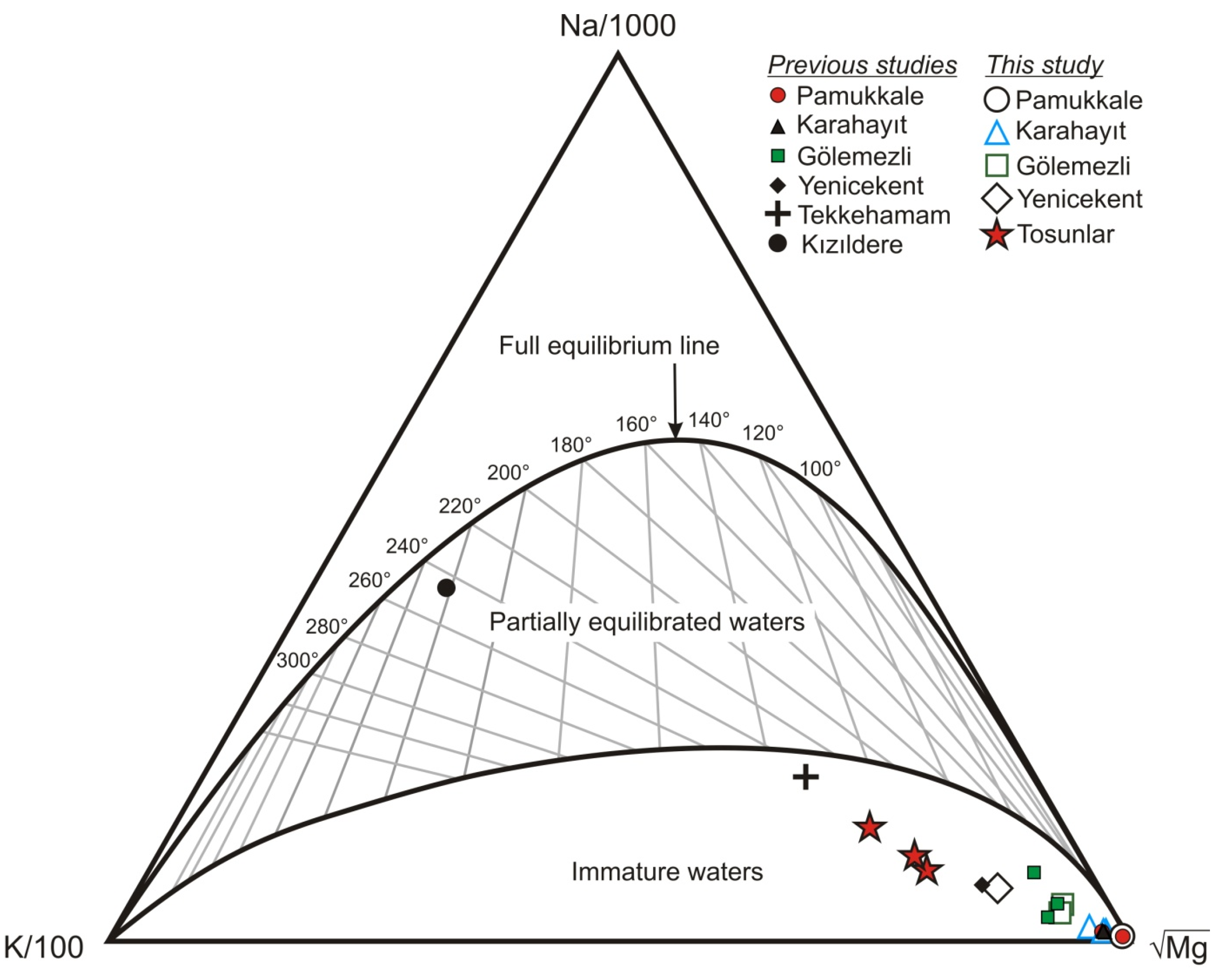
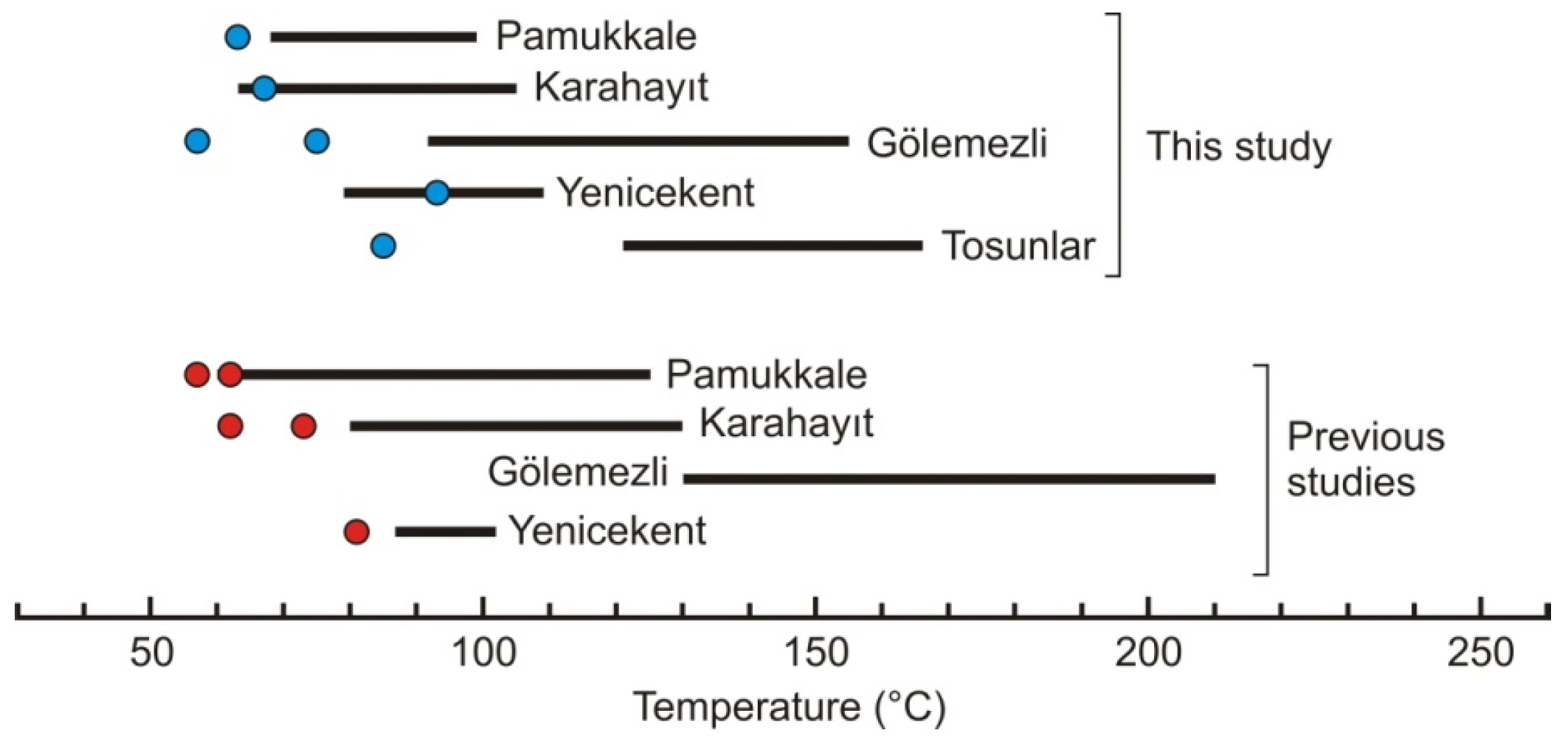
5.4. Geothermometry
5.5. Fluid Characterization
5.6. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, E. Geothermal energy technology and current status: An overview. Renew. Sustain. Energy Rev. 2002, 6, 3–65. [Google Scholar] [CrossRef]
- Soltani, M.; Kashkooli, F.M.; Dehghani-Sanij, A.R.; Nokhosteen, A.; Ahmadi-Joughi, A.; Gharali, K.; Mahbaz, S.B.; Dusseault, M.B. A comprehensive review of geothermal energy evolution and development. Int. J. Green Energy 2019, 16, 971–1009. [Google Scholar] [CrossRef]
- Nicholson, K. Geothermal Fluids, Chemistry and Exploration Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 978-3-642-77846-9. [Google Scholar]
- Altunel, E. Morphological features, ages and neotectonic significance of Pamukkale travertines. Bull. Miner. Res. Explor. 1996, 118, 47–64. (In Turkish) [Google Scholar]
- Utku, M. Etkinlik ve yığınsal etkinlik dönemlerine göre Denizli depremlerinin analizi. Maden Tetk. Ve Aram. Derg. 2009, 138, 9–34. [Google Scholar]
- Kaypak, B.; Gökkaya, G. 3-D imaging of the upper crust beneath the Denizli geothermal region by local earthquake tomography, western Turkey. J. Volcanol. Geoth. Res. 2012, 211–212, 47–60. [Google Scholar] [CrossRef]
- Yılmaz, Y. Some thoughts on the morphotectonic development of the Denizli region. Bull. Miner. Res. Explor. 2022, 169, 1–15. [Google Scholar] [CrossRef]
- Şengör, A.M.C.; Yılmaz, Y. Tethyan evolution of Turkey—A plate tectonic approach. Tectonophysics 1981, 75, 181–241. [Google Scholar] [CrossRef]
- Bozkurt, E. Neotectonics of Turkey—A synthesis. Geodin. Acta 2001, 14, 3–30. [Google Scholar] [CrossRef]
- Mutlu, H.; Güleç, N. Hydrogeochemical outline of thermal waters and geothermometry applications in Anatolia (Turkey). J. Volcanol. Geotherm. Res. 1998, 85, 495–515. [Google Scholar] [CrossRef]
- Şimşek, Ş. Geothermal model of Denizli, Sarayköy-Buldan area. Geothermics 1985, 14, 393–417. [Google Scholar] [CrossRef]
- Aydın, H.; Karakuş, H.; Mutlu, H. Hydrogeochemistry of geothermal waters in eastern Turkey: Geochemical and isotopic constraints on water-rock interaction. J. Volcanol. Geotherm. Res. 2020, 390, 106708. [Google Scholar] [CrossRef]
- MTA Genel Müdürlüğü. Türkiye Jeotermal Enerji Potansiyeli ve Arama Çalışmaları. Available online: https://www.mta.gov.tr/v3.0/arastirmalar/jeotermal-enerji-arastirmalari (accessed on 8 March 2025).
- Enerji Piyasası Düzenleme Kurulu, 2023 Yılı Elektrik Piyasası Gelişim Raporu. Available online: https://www.epdk.gov.tr/Detay/Icerik/4-14475/2023-yili-sektor-raporlari-yayinlanmistir (accessed on 8 March 2025).
- Lund, J.W.; Toth, A.N. Direct utilization of geothermal energy 2020 worldwide review. Geothermics 2021, 90, 101915. [Google Scholar]
- Hepbaşlı, A.; Özgener, L. Development of geothermal energy utilization in Turkey: A review. Renew. Sustain. Energy Rev. 2004, 8, 433–460. [Google Scholar]
- Seyitoğlu, G.; Scott, B. Late Cenozoic crustal extension and basin formation in west Turkey. Geol. Mag. 1991, 128, 155–166. [Google Scholar] [CrossRef]
- Gürer, Ö.F. A new look at the origin of N-S trending young basins of western Anatolia. Bull. Miner. Res. Explor. 2023, 170, 117–146. [Google Scholar] [CrossRef]
- Karakuş, H.; Şimşek, Ş. Tracing deep thermal water circulation systems in the E–W trending Büyük Menderes Graben, western Turkey. J. Volcanol. Geoth. Res. 2013, 252, 38–52. [Google Scholar] [CrossRef]
- Alçiçek, H.; Bülbül, A.; Alçiçek, M.C. Hydrogeochemistry of the thermal waters from the Yenice Geothermal Field (Denizli Basin, Southwestern Anatolia, Turkey). J. Volcanol. Geoth. Res. 2016, 309, 118–138. [Google Scholar] [CrossRef]
- Alçiçek, H.; Bülbül, A.; Brogi, A.; Liotta, D.; Ruggieri, G.; Capezzuoli, E.; Meccheri, M.; Yavuzer, İ.; Alçiçek, M.C. Origin, evolution and geothermometry of the thermal waters in the Gölemezli Geothermal Field, Denizli Basin (SW Anatolia, Turkey). J. Volcanol. Geoth. Res. 2018, 349, 1–30. [Google Scholar] [CrossRef]
- Alçiçek, H.; Bülbül, A.; Yavuzer, İ.; Alçiçek, M.C. Hydrogeochemical and isotopic assessment and geothermometry applications in relation to the Karahayıt Geothermal Field (Denizli Basin, SW Anatolia, Turkey). Hydrogeol. J. 2019, 27, 1791–1816. [Google Scholar] [CrossRef]
- Alçiçek, H.; Bülbül, A.; Yavuzer, İ.; Alçiçek, M.C. Origin and evolution of the thermal waters from the Pamukkale Geothermal Field (Denizli Basin, SW Anatolia, Turkey): Insights from hydrogeochemistry and geothermometry. J. Volcanol. Geoth. Res. 2019, 372, 48–70. [Google Scholar] [CrossRef]
- Avşar, Ö.; Altuntaş, G. Hydrogeochemical evaluation of Umut geothermal field (SW Turkey). Environ. Earth Sci. 2017, 76, 582. [Google Scholar] [CrossRef]
- Kele, S.; Özkul, M.; Gökgöz, A.; Fórizs, I.; Baykara, M.O.; Alçiçek, M.C.; Németh, T. Stable isotope geochemical and facies study of Pamukkale travertines: New evidences of low-temperature non-equilibrium calcite-water fractionation. Sediment. Geol. 2011, 238, 191–212. [Google Scholar] [CrossRef]
- Tut Haklıdır, F.S.; Şengün, R.; Aydın, H. Characterization and comparison of geothermal fluids geochemistry within the Kızıldere Geothermal Field in Turkey: New findings with power capacity expanding studies. Geothermics 2021, 94, 102110. [Google Scholar] [CrossRef]
- Dewey, J.F.; Şengör, A.M.C. Aegean and surrounding regions: Complex multiplate and continuum tectonics in a convergent zone. Geol. Soc. Am. Bull. 1979, 90, 84–92. [Google Scholar] [CrossRef]
- Yılmaz, Y.; Genç, S.C.; Gürer, O.F.; Bozcu, M.; Yılmaz, K.; Karacık, Z.; Altunkaynak, S.; Elmas, A. When did the western Anatolian grabens begin to develop? In Tectonics and Magmatism in Turkey and the Surrounding Area; Bozkurt, E., Winchester, J.A., Piper, J.D.A., Eds.; Geological Society of London: London, UK, 2000; Volume 173, pp. 353–384. ISBN 978-186-239-064-5. [Google Scholar]
- Hetzel, R.; Ring, U.; Akal, C.; Troesch, M. Miocene NNE directed extensional unroofing an the Menderes Massif, Southwestern Turkey. J. Geol. Soc. Lond. 1995, 152, 639–654. [Google Scholar] [CrossRef]
- Koçyiğit, A.; Yusufoğlu, H.; Bozkurt, E. Evidence from the Gediz Graben for episodic two-stage extension in Western Turkey. J. Geol. Soc. 1999, 156, 605–616. [Google Scholar] [CrossRef]
- Seyitoğlu, G.; Tekeli, O.; Çemen, İ.; Şen, Ş.; Işık, V. The role of the flexural rotation/rolling hinge model in the tectonic evolution of the Alasehir graben, western Turkey. Geol. Mag. 2002, 139, 15–26. [Google Scholar] [CrossRef]
- Westaway, R. Neogene evolution of the Denizli region of Western Turkey. J. Struct. Geol. 1993, 15, 37–53. [Google Scholar] [CrossRef]
- Altunel, E.; Karacabak, V. Determination of horizontal extension from fissure ridge travertines: A case study from the Denizli Basin, southwestern Turkey. Geodin. Acta 2005, 18, 333–342. [Google Scholar] [CrossRef]
- Alçiçek, H.; Varol, B.; Özkul, M. Sedimentary facies, depositional environments and palaeogeographic evolution of the Neogene Denizli Basin of SW Anatolia, Turkey. Sediment. Geol. 2007, 202, 596–637. [Google Scholar] [CrossRef]
- Koçyiğit, A. The Denizli graben-horst system and the eastern limit of western Anatolian continental extension: Basin-fill, structure, deformational mode, throw amount and episodic evolutionary history, SW Turkey. Geodin. Acta 2005, 18, 167–208. [Google Scholar] [CrossRef]
- Alçiçek, H. Sedimentological Investigations of Neogene Deposits of the Denizli Basin (Sarayköy-Buldan Area, SW Turkey). Ph.D. Thesis, Ankara University, Ankara, Türkiye, 2007. [Google Scholar]
- Altunel, E. Active Tectonics and the Evolution of Quaternary Travertines at Pamukkale, Western Turkey. Ph.D. Thesis, University of Bristol, Bristol, UK, 1994. [Google Scholar]
- Uysal, I.T.; Feng, Y.; Zhao, J.; Altunel, E.; Weatherley, D.; Karabacak, V.; Cengiz, O.; Golding, S.D.; Lawrence, M.G.; Collerson, K.D. U-series dating and geochemical tracing of late Quaternary travertine in co-seismic fissures. Earth Planet Sci. Lett. 2007, 257, 450–462. [Google Scholar] [CrossRef]
- De Filippis, L.; Faccenna, C.; Billi, A.; Anzalone, E.; Brilli, M.; Özkul, M.; Soligo, M.; Tuccimei, P.; Villa, M. Growth of fissure ridge travertines from geothermal springs of Denizli Basin, western Turkey. Geol. Soc. Am. Bull. 2012, 124, 1629–1645. [Google Scholar] [CrossRef]
- Özkul, M.; Kele, S.; Gökgöz, A.; Shen, C.C.; Jones, B.; Baykara, M.O.; Fόrizs, I.; Németh, T.; Chang, Y.; Alçiçek, M.C. Comparison of the Quaternary travertine sites in the Denizli Extensional Basin based on their depositional and geochemical data. Sediment. Geol. 2013, 294, 179–204. [Google Scholar] [CrossRef]
- Altunel, E.; Barka, A. Evaluation of archaeoseismic damages at Hierapolis. Geol. Bull. Turk. 1996, 39, 65–74. (In Turkish) [Google Scholar]
- Hancock, P.L.; Chalmers, R.M.L.; Altunel, E.; Çakır, Z.; Becher-Hancock, A. Creation and destruction of travertine monumental stone by earthquake faulting at Hierapolis, Turkey. In The Archaeology of Geological Catastrophes; McGuire, W.G., Griffiths, D.R., Hancock, P.L., Stewart, I.S., Eds.; The Geological Society of London: London, UK, 2000; pp. 1–14. ISBN 978-186-239-062-1. [Google Scholar]
- Piccardi, L. The AD 60 Denizli Basin earthquake and the apparition of Archangel Michael at Colossae (Aegean Turkey). J. Geol. Soc. Lond. 2007, 273, 95–105. [Google Scholar] [CrossRef]
- Kumsar, H.; Aydan, Ö.; Şimşek, C.; D’Andria, F. Historical earthquakes that damaged Hierapolis and Laodikeia antique cities and their implications for earthquake potential of Denizli basin in western Turkey. Bull. Eng. Geol. Environ. 2016, 75, 519–536. [Google Scholar] [CrossRef]
- İlkışık, O.M. Regional heat flow in western Anatolia using silica temperature estimates from thermal springs. Tectonophysics 1995, 244, 175–184. [Google Scholar] [CrossRef]
- Bilim, F.; Akay, T.; Aydemir, A.; Koşaroğlu, S. Curie point depth, heat-flow and radiogenic heat production deduced from the spectral analysis of the aeromagnetic data for geothermal investigation on the Menderes Massif and the Aegean Region, western Turkey. Geothermics 2016, 60, 44–57. [Google Scholar] [CrossRef]
- Şimşek, Z. Tekkehamam Jeotermal Alanı Gradyent Sondajları Değerlendirilmesi; Report No. 6236; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1978.
- Demirörer, M. Denizli-Sarayköy Jeotermik Enerji Aramalari Kizildere-Tekkehamam ve Karakova Jeotermal Gradyent Etüdleri Rapor-I; Report No. 4141; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1986.
- Ürgün, S. Denizli Kızıldere Jeotermal Alanındaki Çalışmaların Kompilasyonu; Report No: 5919; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1976.
- Bozkurt, E. Timing of extension on the Büyük Menderes Graben, Western Turkey, and its tectonic implications. In Tectonics and Magmatism in Turkey and the Surrounding Area; Bozkurt, E., Winchester, J.A., Piper, J.D.A., Eds.; Geological Society of London: London, UK, 2000; Volume 173, pp. 385–403. ISBN 978-186-239-064-5. [Google Scholar]
- Akkuş, İ.; Akıllı, H.; Ceyhan, S.; Dilemre, A.; Tekin, Z. Geothermal Inventory of Turkey; General Directorate of Mineral Research and Exploration of Turkey (AB Ofset): Ankara, Türkiye, 2005; No. 201; ISBN 975-8964-36-4.
- Şimşek, Ş. Hydrogeological and isotopic survey of geothermal fields in the Buyuk Menderes graben, Turkey. Geothermics 2003, 32, 669–678. [Google Scholar] [CrossRef]
- Sun, S. Denizli-Uşak Arasının Jeolojisi ve Linyit Olanakları; Report No. 9985; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1990.
- Konak, N.; Şenel, M. Geological Map of Turkey in 1/500.000 Scale: Denizli Sheet; Publication of Mineral Research and Exploration Directorate of Turkey (MTA): Ankara, Türkiye, 2002.
- Gökgöz, A.; Yılmazlı, İ.E.; Güngör, İ.; Yavuzer, İ. Hydrogeology and environmental study at the Karahayıt geothermal field (Western Turkey). In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Kocairi, C. Denizli Metropolitan Municipality Karahayıt KH-4 Geothermal Production Drilling Well Completion Report; Fensu Sondaj: Konya, Türkiye, 2019. [Google Scholar]
- Demirel, V.; Kahraman, S. Denizli Belediyesi Adına Yapılan Denizli-Gölemezli (DG-3, DG-4, DG-5) Kuyularına ait Kuyu Bitirme Raporu; Report No: 10660; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 2003.
- Demirel, V.; Tamgaç, Ö.F. Denizli-Buldan-Yenicekent Jeolojik Etüdü ve Yenicekent (YK-1, YK-2 ve YK-3) Sondajları Kuyu bitirme Raporu; MTA Report; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 2004.
- Çelik, H. Buldan-Yenicekent (Denizli) Resource Protection Area Study Report Regarding Operation License No. 30 (J-460); G.S.E. Enerji Sanayii ve Ticaret Limited.Şirketi: Ankara, Türkiye, 2017. [Google Scholar]
- Subay, E. Hydrogeological and Hydrogeochemical Investigation of Tosunlar Geothermal Field (Denizli). Master’s Thesis, Pamukkale University, Denizli, Türkiye, 2019. [Google Scholar]
- Şimşek, Ş. Denizli-Sarayköy-Buldan alanının jeolojisi ve jeotermal enerji olanakları. İ.Ü. Yer Bil. Fak. Yayın Organı 1984, 3, 145–162. [Google Scholar]
- UKAM. Pamukkale Koruma Amaçlı İmar Planında Öngörülen Travertenlerin Korunması ve Geliştirilmesi için Proje Hizmetleri; Hacettepe University International Research and Application Center for Karst Water Resources (UKAM): Ankara, Türkiye, 1994. [Google Scholar]
- ÇED ve Çevre İzinleri Şube Müdürlüğü. Denizli İli 2020 Yılı Çevre Durum Raporu; Çevre ve Şehircilik İl Müdürlüğü: Denizli, Türkiye, 2020. Available online: https://webdosya.csb.gov.tr/db/ced/icerikler/2020_den-zl-_-cdr-20211018151306.pdf (accessed on 8 March 2025).
- Şimşek, Ş.; Günay, G.; Elhatip, H.; Ekmekçi, M. Environmental protection of geothermal waters and travertines at Pamukkale, Turkey. Geothermics 2000, 29, 557–572. [Google Scholar] [CrossRef]
- Gökgöz, A. Hydrogeology of Pamukkale-Karahayıt-Gölemezli Hydrothermal Karst. Ph.D. Thesis, Süleyman Demirel University, Isparta, Türkiye, 1994. [Google Scholar]
- Bülbül, A. Hydrogeology of Kamara and Çizmeli (Yenice-Buldan) hot and mineral waters. Master’s Thesis, Pamukkale University, Denizli, Türkiye, 2000. [Google Scholar]
- Gökgöz, F. Pamukkale-Gölemezli (Denizli) Source Protection Area Study Report Regarding Geothermal Resource Operation License No. 34 (J-433/A); The Denizli Metropolitan Municipality: Denizli, Türkiye, 2020. [Google Scholar]
- Akdemir, M.; Hançer, M.; Karaş, M.; Kıroba, M.O. Geothermal Energy Possibilities of Buldan/Yenicekent (Denizli) Field and Surroundings with Geothermal Operation License No. 30 (J-460); Geology, Tectonic and Geophysical Survey Report; MSC Jeoteknik Sondajcılık Sanayı Ticaret Limited Şirketi: İzmir, Türkiye, 2020. [Google Scholar]
- Uysallı, H.; Keskin, B. Denizli–Sarayköy KD-I, KD-II, TH-I, KD-I/A, KD-III, IV, VI, IX, XII, XIII, XIV derin jeotermik sondajları bitirme raporu; Report No. 4491; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1971.
- Şimşek, Ş.; Yıldırım, N.; Gülgör, A. Developmental and environmental effects of the Kızıldere geothermal power project, Turkey. Geothermics 2005, 34, 239–256. [Google Scholar] [CrossRef]
- Yıldırım, N.; Güner, İ.N. Isotopic and hydrogeochemical properties of waters encountered in the geothermal area, eastern Menderes graben. In Proceedings of the Hidrojeolojide İzotop Tekniklerinin Kullanılması Sempozyumu, Adana, Türkiye, 21–25 October 2002; pp. 79–97. [Google Scholar]
- Özkul, M.; Kele, S.; Gökgöz, A.; Forisz, I.; Alçiçek, M.C.; Eros, A. Stable Isotopic Studies on Travertines for Paleoenvironmental and Paleoclimatic Reconstruction: A Comparative Study on the Genesis of Occurrences in Turkey and Hungary; Project (106Y207); TÜBİTAK-NKHT: Ankara, Türkiye, 2009.
- Güvendi, M. Sarayköy-Tosunlar (Denizli) Revised Resource Protection Area Study Report Regarding Operation License No. 23; Akça Enerji Arşivi: Denizli, Turkey, 2014; pp. 40–50. [Google Scholar]
- Karamanderesi, İ.H.; Ölçenoğlu, K. Geology of the Denizli Sarayköy (Gerali) Geothermal Field, Western Anatolia, Turkey. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: London, UK, 2005; ISBN 978-041-536-428-7. [Google Scholar]
- Schoeller, H. Les échanges de bases dans les eaux souterraines; trois exemples es Tunisie. Bull. Soc. Geol. Fr. 1934, 4, 389–420. [Google Scholar]
- Şahinci, A. Doğal Suların Jeokimyası; Reform Matbaası: İzmir, Türkiye, 1991. (In Turkish) [Google Scholar]
- Mayo, A.L.; Loucks, M.D. Solute and isotopic geochemistry and ground water flow in the central Wasatch Range. Utah. J. Hydrol. 1995, 172, 31–59. [Google Scholar] [CrossRef]
- Gürgey, K.; Simoneit, B.R.T.; Batı, Z.; Karamanderesi, İ.H.; Varol, B. Origin of petroliferous bitumen from the Büyük Menderes–Gediz geothermal graben system, Denizli-Sarayköy, western Turkey. Appl. Geochem. 2007, 22, 1393–1415. [Google Scholar] [CrossRef]
- Baudrand, M.; Aloisi, G.; Martineau, F.; Fourel, F.; Lecuyer, C.; Pancost, R.; Blanc-Valleron, M.M.; Rouchy, J.M.; Aref, M.A.M.; Grossi, V. Microbial diagenesis of gypsum. In Proceedings of the Goldschmidt Conference, Knoxville, TN, USA, 13–18 June 2010. [Google Scholar]
- Arnórsson, S. The use of mixing models and chemical geothermometers for estimating underground temperature in geothermal systems. J. Volcanol. Geoth. Res. 1985, 23, 299–335. [Google Scholar] [CrossRef]
- Dilsiz, C. Conceptual hydrodynamic model of the Pamukkale hydrothermal field, southwestern Turkey, based on hydrochemical and isotopic data. Hydrogeol. J. 2006, 14, 562–572. [Google Scholar] [CrossRef]
- İnan, A.B. The seasonal investigation of the chemical and isotopic composition of the Pamukkale thermal spring (Denizli). Master’s Thesis, Pamukkale University, Denizli, Türkiye, 2024. [Google Scholar]
- Kumsar, H.; Aydan, Ö.; Tano, H.; Ulusay, R.; Çelik, S.B.; Kaya, M.; Karaman, M. An On-Line Monitoring System of Multi-Parameter Changes of Geothermal Systems Related to Earthquake Activity in Western Anatolia in Turkey. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Boğaziçi Üniversitesi Kandilli Rasathanesi BDTİM Deprem Sorgulama Sistemi. Available online: http://koeri.boun.edu.tr/sismo/zeqdb/ (accessed on 16 March 2025).
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report, 99–4259; US Geological Survey: Reston, VA, USA, 1999.
- Şamilgil, E.; Arda, O. Laboratory Analyses Result of Scale Sample in Kızıldere; Report No. 9527; General Directorate of Mineral Research and Exploration of Turkey (MTA): Ankara, Türkiye, 1977.
- Giese, L.B.; Pekdeğer, A.; Dahms, E. Thermal fluids and scalings in the geothermal power plant of Kızıldere, Turkey. In Proceedings of the 9th International Symposium on Water-Rock Interaction, Taupo, New Zealand, 30 March–3 April 1998; pp. 625–628. [Google Scholar]
- Tarcan, G.; Özen, T.; Gemici, Ü.; Çolak, M.; Karamanderesi, İ.H. Geochemical assessment of mineral scaling in Kızıldere geothermal field, Turkey. Environ. Earth Sci. 2016, 75, 1317. [Google Scholar] [CrossRef]
- Filiz, Ş. Investigation of the important geothermal areas by using C, H, O isotopes. In Proceedings of the Seminar on Utilization of Geothermal Energy for Electric Power Production and Space Heating, UN Economic Commission for Europe, EP/SEM.9/R.3, Florence, Italy, 14–17 May 1984; 13p. [Google Scholar]
- Yaman, D. Origin of high boron contents of the geothermal waters of the Menderes Massif continental rift zones. Ph.D. Thesis, Süleyman Demirel University, Isparta, Türkiye, 2005. [Google Scholar]
- Uzun, E. Formation, history and preservation of travertines in Pamukkale (Denizli). Master’s Thesis, Süleyman Demirel University, Isparta, Türkiye, 2017. [Google Scholar]
- Özgür, N. Geochemical signature of the Kızıldere geothermal field, Western Anatolia, Turkey. Int. Geol. Rev. 2002, 44, 153–163. [Google Scholar] [CrossRef]
- Khorshtd, T. Hydrogeochemical and hydrogeological investigation of the Yenicekent Gölemezli geothermal field. Master’s Thesis, Dokuz Eylül University, İzmir, Türkiye, 2013. [Google Scholar]
- Dilaver, A.T.; Aydın, B.; Özyurt, N.N.; Bayarı, C.S. Isotope Contents of Türkiye Precipitation (2012–2016); General Directorate of State Hydraulic Works (DSI): Ankara, Türkiye, 2018. Available online: https://www.mgm.gov.tr/FILES/arastirma/izotopyagis/izotopYagisDegerlendirme.pdf (accessed on 8 March 2025).
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Gat, J.R.; Carmi, I. Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea area. J. Geophys. Res. 1970, 75, 3039–3048. [Google Scholar] [CrossRef]
- Güner, İ.N.; Yıldırım, N. Hydrochemical modelling and determination of 14C ages of geothermal waters in Kızıldere and Tekkehamam fields (Sarayköy-Denizli). In Proceedings of the II. Ulusal Hidrolojide İzotop Teknikleri Sempozyumu, İzmir, Türkiye, 26–30 September 2005; pp. 297–308. (In Turkish). [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; ISBN 978-156-670-249-2. [Google Scholar]
- Yaman, D.; Özgür, N. Hydrogeological, hydrogeochemical and isotopic properties of geothermal fields of Kızıldere and its vicinity. In Proceedings of the II. Ulusal Hidrolojide İzotop Teknikleri Sempozyumu, İzmir, Türkiye, 26–30 September 2005; pp. 1–10. (In Turkish). [Google Scholar]
- Önhon, E.; Ertan, I.; Güler, S.; Nazik, M.; Kaplan, A. Research on the Origin of the Karst Waters in Yukarı Çürüksu Plain Using Isotope Techniques; Final Report; General Directorate of State Hydraulic Works (DSI): Ankara, Türkiye, 1989.
- Carreira, P.M.; Marques, J.M.; Carvalho, M.R.; Nunes, D.; Antunes da Silva, M. Carbon isotopes and geochemical processes in CO2-rich cold mineral water, N-Portugal. Environ. Earth Sci. 2014, 71, 2941–2953. [Google Scholar] [CrossRef]
- Güner, İ.N.; Yıldırım, N. Important points to consider in the sampling and calculation methods of the geothermal 14C age studies, case study: Pamukkale thermal springs. In Proceedings of the 75 Geological Congress of Turkey, Ankara, Türkiye, 10–14 April 2023; p. 313. [Google Scholar]
- Yıldırım, G.; Mutlu, H.; Karabacak, V.; Uysal, I.T.; Dirik, K.; Temel, A.; Yüce, G.; Zhao, J.X. Temporal changes in geochemical-isotopic systematics of the late Pleistocene Akkaya travertines (Turkey)—Implications for fluid flow circulation and seismicity. Geochemistry 2020, 80, 125630. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, S.; Xu, J.; Yuan, C.; Zhang, M.; Wang, C. Geochemical evolution of geothermal waters in the Pearl River Delta region, South China: Insights from water chemistry and isotope geochemistry. J. Hydrol. 2024, 51, 101670. [Google Scholar] [CrossRef]
- Ercan, T.; Matsuda, J.; Nagano, K.; Kita, I. Noble gas isotopic compositions in gas and water samples from Anatolia. In Proceedings of the International Symposium on the Geology of the Black Sea Region, Ankara, Turkey, 7–11 September 1992; General Directorate of Mineral Research and Exploration of Turkey (MTA) and Chamber of Geological Engineers: Ankara, Turkey, 1995; pp. 197–206. [Google Scholar]
- Mutlu, H.; Güleç, N.; Hilton, D.R. Helium-carbon relationships in geothermal fluids of western Anatolia, Turkey. Chem. Geol. 2008, 247, 305–321. [Google Scholar] [CrossRef]
- Süer, S.; Wiersberg, T.; Güleç, N.; Grassa, F. Stable Isotope Evaluation of Geothermal Gases from the Kızıldere and Tekke Hamam Geothermal Fields, Western Anatolia, Turkey. Geoscience 2022, 12, 452. [Google Scholar] [CrossRef]
- Brogi, A.; Alçiçek, M.C.; Yalçıner, C.C.; Capezzuoli, E.; Liotta, D.; Meccheri, M.; Rimondi, V.; Ruggieri, G.; Gandin, A.; Boschi, C.; et al. Hydrothermal fluids circulation and travertine deposition in an active tectonic setting: Insights from the Kamara geothermal area (western Anatolia, Turkey). Tectonophysics 2016, 680, 211–232. [Google Scholar] [CrossRef]
- Panichi, C.; Tongiorgi, E. Carbon isotopic composition of CO2 from springs, fumaroles, mofettes and travertines of central and southern Italy: A preliminary prospection method of geothermal areas. In Proceedings of the Second United Nations Symposium on the Development and Use of Geothermal Resources, San Francisco, CA, USA, 20–29 May 1975; U. S. Energy Research and Development Administration: Washington, DC, USA, 1976; pp. 815–825. [Google Scholar]
- Rizzo, A.L.; Uysal, T.; Mutlu, H.; Ünal-İmer, E.; Dirik, K.; Yüce, G.; Caracausi, A.; Italiano, F.; Misseri, M.; Temel, A.; et al. Geochemistry of Fluid Inclusions in Travertines from Western and Northern Turkey: Inferences on the Role of Active Faults in Fluids Circulation. Geochem. Geophy. Geosy. 2019, 20, 5473–5498. [Google Scholar] [CrossRef]
- Koralay, T.; Kılınçarslan, S. Minero-petrographic and isotopic characterization of two antique marble quarries in the Denizli region (western Anatolia, Turkey). Period. Mineral. 2015, 84, 263–288. [Google Scholar] [CrossRef]
- Zöldföldi, J. 5000 Years Marble History in Troia and the Troad. Petroarchaeological Study on the Provenance of White Marbles in West Anatolia. Ph.D. Thesis, Eberhard Karls Universität: Tübingen, Germany, 2011. [Google Scholar]
- Brilli, M.; Giustini, F.; Conte, A.M.; Mercadal, P.L.; Quarta, G.; Plumed, H.R.; Scardozzi, G.; Belardi, G. Petrography, geochemistry and cathodoluminescence of ancient white marble from quarries in the southern Phrygia and northern Caria regions of Turkey: Considerations on provenance discrimination. J. Archaeol. Sci. 2015, 4, 124–142. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany, 1980; ISBN 978-038-709-917-0. [Google Scholar]
- Javoy, M.; Pineau, F.; Delorme, H. Carbon and nitrogen isotopes in the mantle. Chem. Geol. 1986, 57, 41–62. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P.; Savasçın, Y.; Conrad, M. Rare earth elements, yttrium and Pb isotope ratios in thermal spring and well waters of West Anatolia, Turkey: A hydrochemical study of their origin. Chem. Geol. 2004, 206, 97–118. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- De Carlo, E.H.; Green, W.J. Rare earth elements in the water column of Lake Vanda, McMurdo Dry Valleys, Antarctica. Geochim. Cosmochim. Acta 2002, 66, 1323–1333. [Google Scholar] [CrossRef]
- Rankama, K.; Sahama, T.G. Geochemistry; University of Chicago Press: Chicago, IL, USA, 1950; ISBN 978-022-670-395-4. [Google Scholar]
- Tanaka, K.; Kawabe, I. REE abundance in ancient seawater inferred from marine limestone and experimental REE partition coefficients between calcite and aqueous solution. Geochem. J. 2006, 40, 425–435. [Google Scholar] [CrossRef]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Longman Scientific and Technical: London, UK, 1993. [Google Scholar]
- Möller, P.; Dulski, P.; Morteani, G. Partitioning of rare earth elements, yttrium, and some major elements among source rocks, liquid and steam of Larderello–Travale Geothermal Field, Tuscany (Central Italy). Geochim. Cosmochim. Acta 2003, 67, 171–183. [Google Scholar] [CrossRef]
- Möller, P. Rare earth elements and yttrium distribution in water-rock interactions: Field observations and experiments. In Water–Rock Interaction; Stober, I., Bucher, K., Eds.; Kluwer Academic Publishing: Dordrecht, The Netherlands, 2002; pp. 97–123. [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Truesdell, A.H. Summary of Section III: Geochemical Techniques in Exploration; Lawrence Berkeley Labortory: Berkeley, CA, USA, 1976; pp. 54–80.
- Fournier, R.O. A revised equation for the Na-K geothermometer. Geotherm. Resour. Counc. Trans. 1979, 3, 221–224. [Google Scholar]
- Kharaka, Y.K.; Mariner, R.H. Chemical geothermometers and their application to formation waters from sedimentary basins. In Thermal History of Sedimentary Basins; Methods and Case Histories; Naeser, N.D., McCulloh, T.H., Eds.; Springer: New York, NY, USA, 1989; pp. 99–117. ISBN 978-038-796-702-8. [Google Scholar]
- Giggenbach, W.; Gonfiantini, R.; Jangi, B.L.; Truesdell, A.H. Isotopic and chemical composition of Parbati valley geothermal discharges, N.W. Himalaya, India. Geothermics 1983, 12, 199–222. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. An empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Fournier, R.O.; Potter, R.W. Magnesium correction to Na-K-Ca geothermometer. Geochim. Cosmochim. Acta 1979, 43, 1543–1550. [Google Scholar] [CrossRef]
- Lloyd, R.M. Oxygen isotope behaviour in the sulfate-water system. J. Geophys. Res. 1968, 73, 6099–6110. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria-derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Mutlu, H. Chemical geothermometry and fluid–mineral equilibria for the Ömer-Gecek thermal waters, Afyon area, Turkey. J. Volcanol. Geoth. Res. 1998, 80, 303–321. [Google Scholar] [CrossRef]
- Mizutani, Y.; Rafter, T.A. Oxygen isotopic composition of sulphates. Part 3. Oxygen isotopic fractionation in the bisulfate ion-water system. N. Z. J. Sci. 1969, 12, 54–59. [Google Scholar]
- Fritz, P.; Basharmal, G.M.; Drimmie, R.J.; Ibsen, J.; Qureshi, R.M. Oxygen isotope exchange between sulphate and water during bacterial reduction of sulphate. Chem. Geol. 1989, 79, 99–105. [Google Scholar] [CrossRef]
- Boschetti, T.; Cortecci, G.; Toscani, L.; Iacumin, P. Sulfur and oxygen isotope compositions of Upper Triassic sulfates from northern Apennines (Italy): Paleogeographic and hydrogeochemical implications. Geol. Acta 2011, 9, 129–147. [Google Scholar] [CrossRef]
- Farley, K.A.; Neroda, E. Noble gases in the Earth’s mantle. Annu. Rev. Earth Planet Sci. 1998, 26, 189–218. [Google Scholar] [CrossRef]
- Güleç, N.; Hilton, D.R.; Mutlu, H. Helium isotope variations in Turkey: Relationship to tectonics, volcanism and recent seismic activities. Chem. Geol. 2002, 187, 129–142. [Google Scholar] [CrossRef]
- Wiersberg, T.; Süer, S.; Güleç, N.; Erzinger, J.; Parlaktuna, M. Noble gas isotopes and the chemical composition of geothermal gases from the eastern part of the Büyük Menderes Graben (Turkey). J. Volcanol. Geotherm. Res. 2011, 208, 112–121. [Google Scholar] [CrossRef]
- Akan, S.B.; Şimşek, Ş. Study of the gas discharges in the Denizli–Pamukkale Geothermal area (SW-Turkey). In Proceedings of the International Conference on Water Problems in the Mediterranean Countries, Lefkoşa, Cyprus, 17–21 November 1997; pp. 207–215. [Google Scholar]

| Site | Sample ID | T °C | EC | pH | Ca2+ | Mg2+ | Na+ | K+ | Cl− | SO42− | HCO3− | F− | Li+ | Al | B | Ba | Sr | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This study | ||||||||||||||||||
| PGF | PAM | 33.9 | 2390 | 6.19 | 381 | 93 | 40 | 5.1 | 9.5 | 653 | 932 | 0.9 | 0.13 | 0.004 | 0.96 | 0.019 | 6.32 | 46.6 |
| KGF | KH-2 | 56.0 | 3000 | 6.35 | 512 | 119 | 138 | 24 | 25 | 1089 | 1208 | 1.2 | 0.27 | 0.010 | 2.13 | 0.062 | 10.68 | 40.6 |
| KH-3 | 60.7 | 3060 | 7.01 | 367 | 122 | 131 | 23 | 19 | 948 | 908 | 1.6 | 0.33 | 0.011 | 1.97 | 0.053 | 9.43 | 52.7 | |
| KH-4 | 53.4 | 3150 | 7.28 | 484 | 92 | 153 | 33 | 29 | 630 | 1414 | 1.9 | 0.31 | 0.026 | 2.44 | 0.084 | 5.98 | 30 | |
| GGF | Göl-1 | 63.3 | 3780 | 7.32 | 318 | 93 | 335 | 56 | 36 | 470 | 1537 | 1.5 | 0.32 | 0.024 | 3.59 | 0.079 | 7.12 | 71.7 |
| Göl-2 | 69.0 | 4470 | 6.85 | 432 | 124 | 496 | 56 | 58 | 1812 | 1165 | 3.3 | 1.52 | 0.012 | 6.48 | 0.037 | 11.30 | 136 | |
| YGF | YK-5 | 61.5 | 3140 | 6.84 | 254 | 35 | 442 | 70 | 32 | 497 | 1476 | 2.5 | 0.66 | 0.017 | 3.42 | 0.056 | 5.11 | 57.9 |
| TGF | T-1 | 97.0 | 3850 | 7.48 | 232 | 32 | 752 | 123 | 45 | 638 | 2063 | 2.4 | 0.59 | 0.020 | 4.33 | 0.104 | 7.50 | 138 |
| T-2 | 98.0 | 3770 | 7.77 | 215 | 40 | 688 | 132 | 44 | 651 | 2063 | 2.2 | 0.61 | 0.012 | 4.46 | 0.122 | 8.09 | 162 | |
| T-3 | 84.1 | 4820 | 7.27 | 243 | 25 | 940 | 139 | 58 | 971 | 2332 | 3.5 | 1.54 | 0.030 | 7.28 | 0.069 | 7.16 | 113 | |
| Previous studies | ||||||||||||||||||
| PGF | PAM a,b | 35.0 | 2300 | 6.37 | 455 | 87 | 40 | 5.9 | 12 | 688 | 1124 | 2.1 | 0.10 | 0.043 | 1.00 | 0.028 | 6.09 | 25 |
| Beltes b | 34.7 | 2340 | 6.10 | 446 | 86 | 39 | 5.2 | 9.6 | 660 | 945 | 1.5 | 0.14 | 0.046 | 1.04 | 0.024 | 6.37 | 47.8 | |
| Çukurbağ b | 56.1 | 3010 | 6.90 | 527 | 114 | 128 | 26 | 27 | 980 | 1235 | 2.8 | 0.32 | 0.084 | 2.72 | 0.061 | 7.62 | 44 | |
| KGF | KH-2 b | 59.5 | 2710 | 7.70 | 537 | 117 | 115 | 24 | 22 | 1078 | 1085 | 1.5 | 0.32 | 0.073 | 1.95 | 0.060 | 10.70 | 50 |
| KH-2 c | 47.0 | 2500 | 6.25 | 486 | 153 | 145 | 29 | 33 | 1062 | 1225 | na | na | na | na | na | na | 51 | |
| KH-3 d | 58.2 | 3050 | 6.52 | 512 | 119 | 121 | 25 | 22 | 946 | 1155 | na | na | na | na | na | na | na | |
| GGF | DG-1 a | 62.0 | 4030 | 6.60 | 390 | 121 | 505 | 55 | 73 | 1504 | 1369 | 4.9 | 1.50 | na | 7.90 | na | na | 94.1 |
| DG-3 e | 53.0 | 2930 | 6.70 | 393 | 55 | 224 | 56 | 29 | 432 | 1470 | na | na | na | na | na | na | 47 | |
| Göl-1 e | 58.5 | 3760 | 6.69 | 591 | 124 | 346 | 76 | 42 | 552 | 2564 | na | na | na | na | na | na | 56 | |
| Göl-2 e | 65.0 | 4730 | 6.34 | 559 | 166 | 602 | 71 | 79 | 1936 | 1548 | na | na | na | na | na | na | 132 | |
| YGF | YK-1 f | 51.5 | 3080 | 6.65 | 266 | 46 | 524 | 94 | 57 | 639 | 1780 | na | na | na | na | na | na | 46 |
| TekGF | İnaltı a | 96.0 | 4170 | 7.71 | 54 | 7.4 | 876 | 84 | 91 | 1560 | 676 | 17.7 | 1.30 | na | 22.0 | na | na | 164 |
| W-3 g | 99.1 | 4080 | 9.38 | 66 | 7.9 | 867 | 107 | 76 | 1077 | 732 | 11.1 | 3.89 | 1.019 | 14.2 | 0.677 | na | 188 | |
| KıGF | R-1 h,i,* | 242.0 | 5570 | 8.40 | 4.7 | 0.2 | 1380 | 162 | 122 | 717 | 2388 | 32 | 4.40 | na | 28.0 | na | na | 470 |
| Cold waters | CS-1 c | 11.5 | 390 | 7.62 | 62 | 4 | 6 | 1.2 | 4 | 12 | 203 | na | na | na | na | na | na | 17 |
| CS-2 c | 13.4 | 380 | 7.68 | 76 | 8 | 3 | 0.5 | 1 | 12 | 264 | na | na | na | na | na | na | 24 | |
| CS-3 c | 8.7 | 460 | 8.03 | 101 | 5 | 35 | 3.4 | 9 | 15 | 316 | na | na | na | na | na | na | 19 | |
| Field | Well | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KGF | KH-2 | 2.0 | 2.8 | 0.42 | 1.2 | 0.17 | 0.07 | 0.3 | 0.05 | 0.19 | 0.05 | 0.16 | 0.03 | 0.13 | 0.02 | 1.8 |
| GGF | Göl-1 | 2.4 | 2.7 | 0.45 | 2.1 | 0.49 | 0.18 | 0.96 | 0.17 | 1.27 | 0.33 | 1.00 | 0.14 | 0.90 | 0.14 | 12.4 |
| Göl-2 | 1.9 | 2.6 | 0.35 | 1.3 | 0.29 | 0.10 | 0.48 | 0.09 | 0.44 | 0.10 | 0.31 | 0.05 | 0.41 | 0.06 | 4.7 | |
| DG-3 | 1.5 | 2.0 | 0.29 | 1.3 | 0.36 | 0.11 | 0.67 | 0.13 | 0.74 | 0.18 | 0.54 | 0.09 | 0.46 | 0.08 | 7.5 | |
| YGF | YK-5 | 1.8 | 3.7 | 0.60 | 3.2 | 1.05 | 0.32 | 1.82 | 0.31 | 2.13 | 0.45 | 1.36 | 0.18 | 1.08 | 0.14 | 17.3 |
| TGF | T-1 | 3.0 | 5.6 | 0.67 | 3.0 | 0.68 | 0.18 | 0.87 | 0.15 | 1.04 | 0.16 | 0.49 | 0.06 | 0.43 | 0.06 | 6.5 |
| Geothermometer | Calibration | PAM | KH-2 | KH-3 | KH-4 | Göl-1 | Göl-2 | YK-5 | T-1 | T-2 | T-3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmeasured | 33.9 | 56.0 | 60.7 | 53.4 | 63.3 | 69.0 | 61.5 | 97.0 | 98.0 | 84.1 | |
| SiO2 1–Chalcedony | 68 | 62 | 75 | 48 | 91 | 130 | 79 | 131 | 143 | 118 | |
| SiO2 1—Quartz No steam loss | 99 | 92 | 104 | 79 | 119 | 155 | 109 | 156 | 166 | 144 | |
| SiO2 1—Quartz Max. steam loss at 100 °C | 99 | 94 | 105 | 83 | 118 | 148 | 109 | 148 | 157 | 139 | |
| Na-K 2 | 215 | 254 | 246 | 287 | 251 | 201 | 244 | 247 | 270 | 233 | |
| Na-K 3 | 239 | 268 | 262 | 292 | 266 | 228 | 260 | 263 | 280 | 253 | |
| Mg-Li 4 | 27 | 38 | 41 | 43 | 44 | 74 | 69 | 68 | 66 | 95 | |
| Na-Li 4 | 213 | 183 | 197 | 185 | 145 | 210 | 168 | 136 | 142 | 173 | |
| K-Mg 5 | 30 | 58 | 55 | 68 | 81 | 77 | 99 | 116 | 115 | 124 | |
| Na-K-Ca 6,7 | 16 | 61 | 64 | 73 | 55 * | 52 * | 107 * | 121 * | 104 * | 141 * | |
| 18O(SO4-H2O) 8 | 63 | - | 67 | - | 75 | 57 | 93 | 85 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gökgöz, A.; Mutlu, H.; Subay, E. Comparative Assessment of Chemical and Isotopic Composition of Geothermal Fluids in the Eastern Part of the Büyük Menderes Graben (Western Türkiye). Water 2025, 17, 961. https://doi.org/10.3390/w17070961
Gökgöz A, Mutlu H, Subay E. Comparative Assessment of Chemical and Isotopic Composition of Geothermal Fluids in the Eastern Part of the Büyük Menderes Graben (Western Türkiye). Water. 2025; 17(7):961. https://doi.org/10.3390/w17070961
Chicago/Turabian StyleGökgöz, Ali, Halim Mutlu, and Erdem Subay. 2025. "Comparative Assessment of Chemical and Isotopic Composition of Geothermal Fluids in the Eastern Part of the Büyük Menderes Graben (Western Türkiye)" Water 17, no. 7: 961. https://doi.org/10.3390/w17070961
APA StyleGökgöz, A., Mutlu, H., & Subay, E. (2025). Comparative Assessment of Chemical and Isotopic Composition of Geothermal Fluids in the Eastern Part of the Büyük Menderes Graben (Western Türkiye). Water, 17(7), 961. https://doi.org/10.3390/w17070961





