Modeling Nitrogen Migration Characteristics in Cool-Season Turf Grass Soils via HYDRUS-2D
Abstract
1. Introduction
2. Materials and Methods
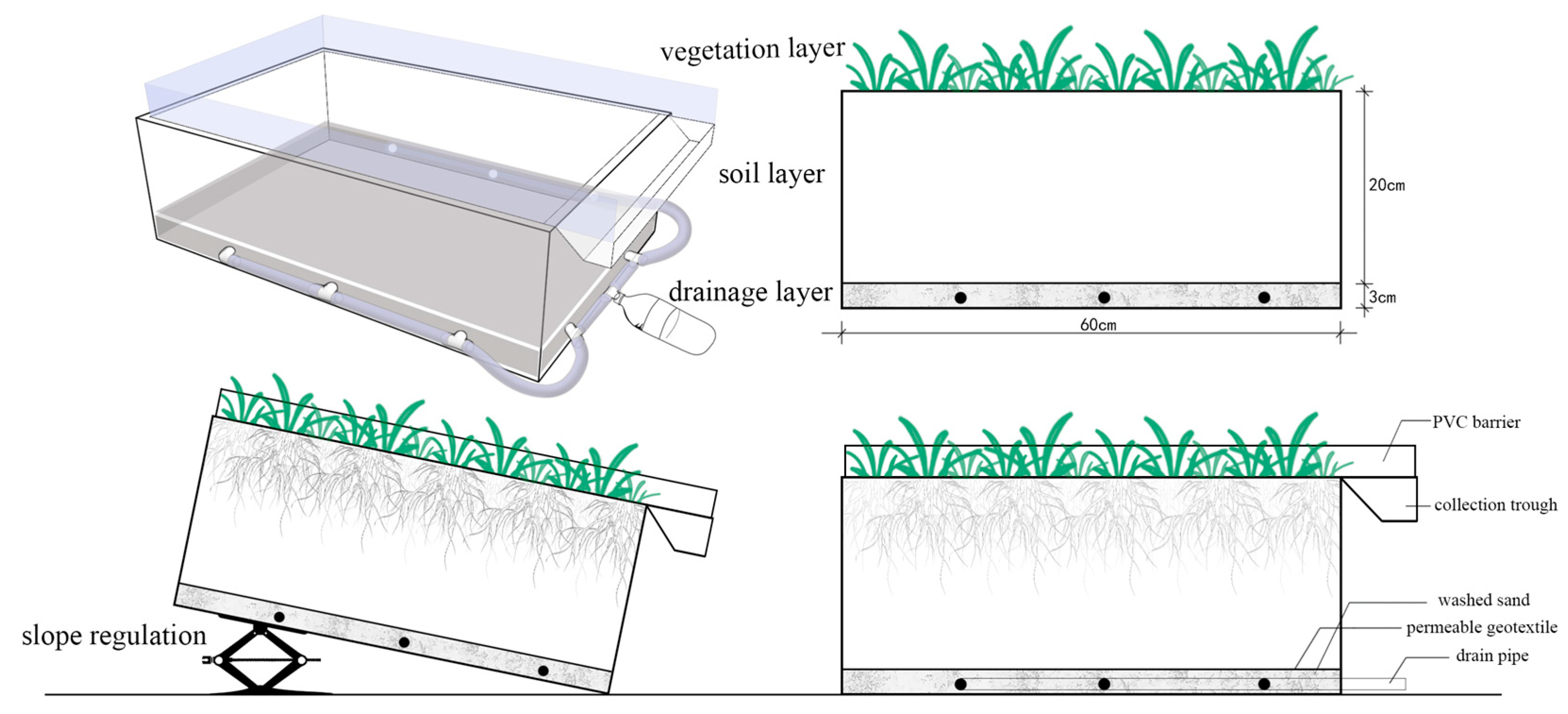
2.1. Grow Box Simulation Test
2.1.1. Soil Sample Collection and Analysis
2.1.2. Design of the Test Device
2.1.3. Test Methods
2.2. HYDRUS-2D Model Construction
2.2.1. Basic Equations of the Model
Soil Moisture Movement Equation
Solute Transport Equation
Root Water Uptake Equation
2.2.2. Modeling Soil Nitrogen Transport in the Grow Boxes
Initial and Boundary Conditions
Time-Step Information and Finite Element Mesh Dissections
Model Parameters and Adjustments
Model Calibration
3. Results and Discussion
3.1. Model Results and Validation
3.1.1. Model Parameterization
3.1.2. Validation of Model Results
3.2. Nitrogen Distribution and Trends on Slopes
3.2.1. Correlation of Initial Soil Nitrogen Distribution Differences Across Slope Gradients and Nitrogen Rates
Analysis of Longitudinal Soil Nitrogen Differences and Slope Gradient Correlations
Analysis of Lateral Differences in Soil Nitrogen and Slope Gradient Correlation
Correlations Between Nitrogen Application and the Amount of Cross-Sectional and Longitudinal Differences in Nitrogen on Slope Gradients
3.2.2. Characterization of Soil Nitrogen Dynamics During the Conservation Cycle
Changes in Soil Content Under Different Slope Gradients
Changes in Soil Content Under Different Slope Gradients
3.3. Changes in Cumulative Nitrogen Flux on Slope Gradients
3.3.1. Simulating and
3.3.2. Relationship of Slope Gradient and Nitrogen Application Rate with Cumulative Nitrogen Flux
3.4. Limitations and Prospects
4. Conclusions
- The optimized HYDRUS-2D model simulated the soil nitrogen content on all slopes well, with reasonable R2 values and RMSEs. The R2 value for the nitrogen content at each point in the soil profile was greater than 0.9, and the RMSE was less than 5. This level of accuracy not only validates the model’s applicability under field conditions with complex hydrological patterns but also provides a reliable tool for environmental managers to assess nitrogen leaching risks and optimize fertilizer application strategies.
- With the increment of slope gradient, the horizontal disparity of soil nitrogen content enlarges, whereas the vertical disparity diminishes. The augmentation of nitrogen application dosage exacerbates the variations in the horizontal and vertical discrepancies between ammonium nitrogen and nitrate nitrogen. In the treatment, N5 (0.312 g), the horizontal differential quantity of nitrate nitrogen is 6.9 times greater than that of ammonium nitrogen, and the vertical differential quantity is 7 times greater. The amount of nitrate nitrogen application exhibits a positive correlation with the horizontal and vertical disparities. Specifically, the longitudinal differential quantity at the slope crest demonstrates the maximum correlation with the amount of nitrogen application (0.71). Conversely, the amount of ammonium nitrogen application shows a negative correlation with the horizontal and vertical disparities, and the longitudinal differential quantity at the slope bottom presents the strongest negative correlation with the amount of nitrogen application (−0.78).
- During the management and maintenance periods, the contents of nitrate nitrogen and ammonium nitrogen in the soil first tended to increase but then tended to decrease. The nitrate nitrogen content increased rapidly at the initial stage of nitrogen application and then decreased gradually. The trends were similar under different slope gradients, but the peak time points were different. In the early stage of nitrogen application, the ammonium nitrogen content reached its peak slowly. The effects of nitrogen application and slope gradient on the changes in nitrate and ammonium nitrogen contents were significant, and the effects of slope gradient on the changes gradually weakened with time. These insights inform best management practices—split fertilizer applications should be prioritized on steep slopes (>8°) to synchronize nutrient availability with crop uptake periods. Long-term monitoring networks incorporating real-time soil moisture sensors could effectively track these dynamic shifts.
- The slope exerts a statistically significant influence on the cumulative flux of nitrate nitrogen (p < 0.05), whereas its significance regarding the cumulative flux of ammonium nitrogen is relatively low (p > 0.05). The quantity of nitrogen application manifests a pronounced effect on the cumulative flux of nitrogen. The cumulative fluxes of ammonium nitrogen and nitrate nitrogen exhibit analogous variation tendencies. With the augmentation of the slope, the overall flux experiences a reduction and reaches the nadir at the i3 slope, which aligns with the conclusions of related research. The observed minimum flux at the i3 slope (15.4°) aligns with previous studies but introduces a critical threshold for slope engineering—land managers should consider constructing contour trenches above this gradient to mitigate nitrogen loss.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics. National Bureau of Statistics: Beijing, China, 2006. Available online: https://www.stats.gov.cn/ (accessed on 20 February 2025).
- National Bureau of Statistics. China Statistical Yearbook 2004; China Stat Press: Beijing, China, 2004.
- Jones, J.B.; Wolf, B.; Mills, H.A. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro Publishing: Athens, GA, USA, 1996. [Google Scholar]
- Wen, W.; Lin, Y. From the emergence and development of urban park systems: A look at the construction of urban green space systems in China’s cities during the 1920s and 1930s. Chin. Landsc. Archit. 2023, 39, 133–138. [Google Scholar]
- Marciano, D.; Ricciardi, V.; Maddalena, G.; Massafra, A.; Fassolo, E.M.; Masiero, S.; Bianco, P.A.; Failla, O.; De Lorenzis, G.; Toffolatti, S.L. Influence of Nitrogen on Grapevine Susceptibility to Downy Mildew. Plants 2023, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Ros, G.H.; Chen, Y.; Shao, Q.; Young, M.D.; Zhang, F.; de Vries, W. Global mean nitrogen recovery efficiency in croplands can be enhanced by optimal nutrient, crop and soil management practices. Nat. Commun. 2023, 14, 5747. [Google Scholar] [PubMed]
- Shen, C.; Li, Q.; An, Y.; Zhou, Y.; Zhang, Y.; He, F.; Chen, L.; Liu, C.; Mao, W.; Wang, X.; et al. The transcription factor GNC optimizes nitrogen use efficiency and growth by up-regulating the expression of nitrate uptake and assimilation genes in poplar. J. Exp. Bot. 2022, 73, 4778–4792. [Google Scholar]
- Sun, Y.; Dai, J.; Zhou, W. Overview and reflections on the development of the lawn industry. Chin. Landsc. Archit. 1998, 2, 34–36. [Google Scholar]
- Shuliang, H. Overview and progress of modern turfgrass science. China Grassl. 1994, 6, 57–63. [Google Scholar]
- Shortle, J.; Abler, D. Environmental Policies for Agricultural Pollution Control; CABI Digital Library: Wallingford, UK, 2001. [Google Scholar]
- Simunek, J.J.; Hopmans, J.W. Modeling compensated root water and nutrient uptake. Ecol. Model. 2009, 220, 505–521. [Google Scholar]
- Genuchten, M.T.V. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar]
- Qi, Z.; Feng, H.; Zhao, Y.; Zhang, T.; Zhang, Z. Spatial distribution and simulation of soil moisture and salinity under mulched drip irrigation combined with tillage in an arid saline irrigation district, northwest China. Agric. Water Manag. 2018, 201, 219–231. [Google Scholar]
- Grecco, K.L.; Miranda, J.H.; Silveira, L.K.; Genuchten, M.T.V. HYDRUS-2D simulations of water and potassium movement in drip irrigated tropical soil container cultivated with sugarcane. Agric. Water Manag. 2019, 221, 334–347. [Google Scholar]
- Ranjbar, A.; Rahimikhoob, A.; Ebrahimian, H.; Varavipour, M. Simulation of nitrogen uptake and distribution under furrows and ridges during the maize growth period using HYDRUS-2D. Irrig. Sci. 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Shafeeq, P.M.; Aggarwal, P.; Krishnan, P.; Rai, V.; Pramanik, P.; Das, T.K. Modeling the temporal distribution of water, ammonium-N, and nitrate-N in the root zone of wheat using HYDRUS-2D under conservation agriculture. Math. Res. Lett. 2020, 27, 2197–2216. [Google Scholar]
- Shen, X.; Xia, W.; Cirenlamu Li, Y. The impact of Allylthiourea, a nitrification inhibitor, on soil nitrification and microorganisms under short-term cultivation. Acta Pedol. Sin. 2021, 58, 1552–1563. [Google Scholar]
- Xu, X.; Ban, R.; Jin, Y.; Zhang, Y.; Gao, Y.; Xu, H. Research on the characteristics of nitrogen transport in agricultural fields in the plain river network area based on the HYDRUS-1D model. J. Agric. Environ. Sci. 2022, 41, 400–410. [Google Scholar]
- Fan, Y.; Zhao, T.; Bai, G.; Liu, W. Horizontal micro-irrigation wetting body simulation with HYDRUS-2D and analysis of influencing factors. Trans. Chin. Soc. Agric. Eng. 2018, 34, 10. [Google Scholar]
- Matteau, J.P.; Gumiere, S.J.; Gallichand, J.; Létourneau, G.; Khiari, L.; Gasser, M.O.; Michaud, A. Coupling of a nitrate production model with HYDRUS to predict nitrate leaching. Agric. Water Manag. 2019, 213, 616–626. [Google Scholar] [CrossRef]
- Feddes, R.A. Simulation of field water use and crop yield. Soil Sci. 1978, 129, 193. [Google Scholar]
- Huan, D.; Fei, D.; Ruijie, S.; Feng, W.; Xuefeng, S.; Wuyun, Z. Simulation of water and fertilizer transport in full-film double-ridge trench corn with HYDRUS-2D/3D and root response. J. Agric. Mech. Res. 2022, 53, 9. [Google Scholar]
- Gong, Y.; Li, X.; Xie, P.; Fu, H.; Nie, L.; Li, J.; Li, Y. The migration and accumulation of typical pollutants in the growing media layer of bioretention facilities. Environ. Sci. Pollut. Res. 2023, 30, 44591–44606. [Google Scholar]
- Baram, S.; Smart, D.R.; Hopmans, J.W.; Kandelous, M.; Harter, T. Estimating nitrate leaching to groundwater from orchards: Comparing crop nitrogen excess, deep vadose zone data-driven estimates, and HYDRUS modeling. Vadose Zone J. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Han, M.Y.; Hu, J.H.; Sang, Z.J.; Sun, Y.N. Morris and orthogonal test based sensitivity analysis of SWMM model parameters. Hydropower Autom. Dam Monit. 2024, 45, 11–18. [Google Scholar]
- Liao, R.T.; Xu, Z.X.; Ye, C.L.; Zuo, B.B.; Xiang, D.F.; Su, X.Y. Sensitivity analysis of stormwater management model parameters. J. Hydroelectr. Eng. 2022, 41, 11–21. [Google Scholar]
- Azad, N.; Behmanesh, J.; Rezaverdinejad, V.; Abbasi, F.; Navabian, M. Evaluation of fertigation management impacts of surface drip irrigation on reducing nitrate leaching using numerical modeling. Environ. Sci. Pollut. Res. 2019, 26, 36499–36514. [Google Scholar]
- Liu, W. Pesticide Environmental Chemistry; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Chaplot, V.A.M.; Le Bissonnais, Y. Runoff features for interrill erosion at different rainfall intensities, slope lengths, and gradients in an agricultural loessial hillslope. Soil Sci. Soc. Am. J. 2003, 67, 844–851. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Wang, B.; Hou, B.; Du, G.; Si, H. Analysis of the adsorption and fixation process of ammonium nitrogen in arable soil by biochar based on molecular dynamics simulation. Sci. Total Environ. 2024, 930, 172815. [Google Scholar]
- King, K.W.; Balogh, J.C.; Harmel, R.D. Nutrient flux in storm water runoff and baseflow from managed turf. Environ. Pollut. 2007, 150, 321–328. [Google Scholar]









| Depth | Soil Bulk Density | Clay | Silt | Sand | ||
|---|---|---|---|---|---|---|
| [cm] | [g·cm−3] | [mg·cm−3] | [mg·cm−3] | [%] | [%] | [%] |
| 0–10 | 1.547 | 10.163 | 6.064 | 25.76 | 21.16 | 53.08 |
| 10–20 | 1.622 | 13.439 | 3.485 | 29.63 | 21.34 | 49.03 |
| Lawn Maintenance Quota | Primary Maintenance | Secondary Maintenance | Tertiary Maintenance | |||
|---|---|---|---|---|---|---|
| Warm-Season Turf Grass | Cool-Season Turf Grass | Warm-Season Turf Grass | Cool-Season Turf Grass | Warm-Season Turf Grass | Cool-Season Turf Grass | |
| urea [kg] | 6.600 | 13.20 | 4.400 | 8.800 | 4.400 | 8.800 |
| Water [m3] | 66.20 | 93.76 | 56.18 | 81.23 | 51.16 | 71.22 |
| Water Movement Parameters | Unit (of Measure) | Range of Values | Solute Transport Parameters | Unit (of Measure) | Range of Values |
|---|---|---|---|---|---|
| 0.1–0.5 | 1–1.5 | ||||
| 0–0.1 | 1–30 | ||||
| 0.02–0.05 | 0.1–6 | ||||
| 100–1440 | 4–20 | ||||
| -- | 1.08–2.79 | 0 |
| Parameters | Unit (of Measure) | Range of Values |
|---|---|---|
| 0.00072–0.04008 | ||
| 0.01992–0.92 | ||
| 0.0096–0.24 | ||
| 0.0035–0.004 |
| Type | Range of Values | Sensitivity Level |
|---|---|---|
| 1 | highly sensitive | |
| 2 | sensitive | |
| 3 | slightly sensitive | |
| 4 | Insensitive |
| Parameters | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.981 | sensitive | 0.566 | sensitive | 1.016 | highly sensitive | 1.665 | highly sensitive | ||
| 0.028 | insensitive | 0.017 | insensitive | 0.461 | sensitive | 0.483 | sensitive | ||
| 0.113 | slightly sensitive | 0.0512 | slightly sensitive | 4.948 | highly sensitive | 6.129 | highly sensitive | ||
| 0 | insensitive | 0 | insensitive | 0.719 | sensitive | 2.268 | highly sensitive | ||
| Influential Factors | Cumulative Flux | Cumulative Flux | ||
|---|---|---|---|---|
| F | p | F | p | |
| Slope Gradient | 2.559 | 0.053 | 2.708 | 0.044 |
| Nitrogen Application | 353.388 | 0.000 | 238.097 | 0.000 |
| Slope | |||||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 20 | Day 30 | Day 40 | |
| i0 | 15.21 ± 0.21 | 116.53 ± 1.53 | 216.36 ± 2.49 | 298.45 ± 3.03 | 362.65 ± 7.18 |
| i1 | 14.15 ± 0.22 | 103.87 ± 1.50 | 192.11 ± 2.49 | 265.02 ± 3.03 | 321.86 ± 6.70 |
| i2 | 11.93 ± 0.21 | 87.35 ± 1.53 | 162.20 ± 2.49 | 223.73 ± 3.04 | 272.45 ± 5.99 |
| i3 | 9.04 ± 0.21 | 66.48 ± 1.53 | 123.43 ± 2.49 | 170.35 ± 3.04 | 207.03 ± 6.53 |
| Slope | |||||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 20 | Day 30 | Day 40 | |
| i0 | 57.04 ± 0.68 | 323.55 ± 3.54 | 493.37 ± 5.06 | 599.84 ± 5.71 | 673.02 ± 12.92 |
| i1 | 55.79 ± 0.70 | 305.02 ± 3.45 | 463.98 ± 5.06 | 564.21 ± 5.70 | 632.89 ± 12.05 |
| i2 | 49.53 ± 0.66 | 270.65 ± 3.53 | 412.92 ± 5.06 | 502.13 ± 5.70 | 564.18 ± 10.76 |
| i3 | 46.50 ± 0.65 | 252.38 ± 3.53 | 385.87 ± 5.06 | 468.31 ± 5.70 | 524.22 ± 11.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Du, Y.; Liu, L.; Su, W.; Tu, K.; Li, Y.; Liu, Y. Modeling Nitrogen Migration Characteristics in Cool-Season Turf Grass Soils via HYDRUS-2D. Water 2025, 17, 943. https://doi.org/10.3390/w17070943
Li R, Du Y, Liu L, Su W, Tu K, Li Y, Liu Y. Modeling Nitrogen Migration Characteristics in Cool-Season Turf Grass Soils via HYDRUS-2D. Water. 2025; 17(7):943. https://doi.org/10.3390/w17070943
Chicago/Turabian StyleLi, Rui, Yueying Du, Longfei Liu, Wangxin Su, Ke Tu, Yonghua Li, and Yang Liu. 2025. "Modeling Nitrogen Migration Characteristics in Cool-Season Turf Grass Soils via HYDRUS-2D" Water 17, no. 7: 943. https://doi.org/10.3390/w17070943
APA StyleLi, R., Du, Y., Liu, L., Su, W., Tu, K., Li, Y., & Liu, Y. (2025). Modeling Nitrogen Migration Characteristics in Cool-Season Turf Grass Soils via HYDRUS-2D. Water, 17(7), 943. https://doi.org/10.3390/w17070943






