Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Crop Materials
2.2. Preparations and Analysis of Water Samples
2.3. Experimental Procedure and Measurement
2.4. Statistical Analysis
3. Results
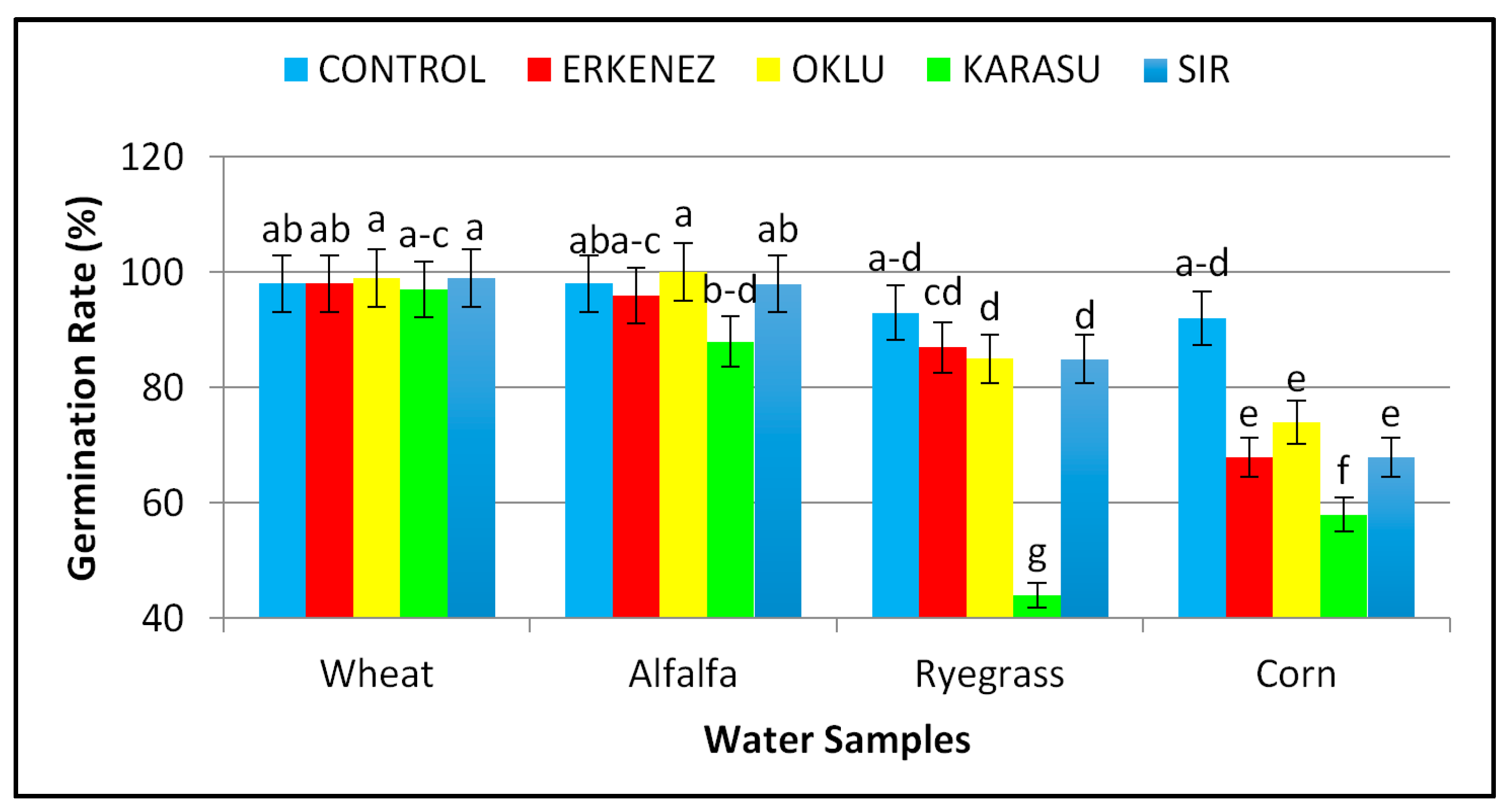
3.1. Germination Rate (%)
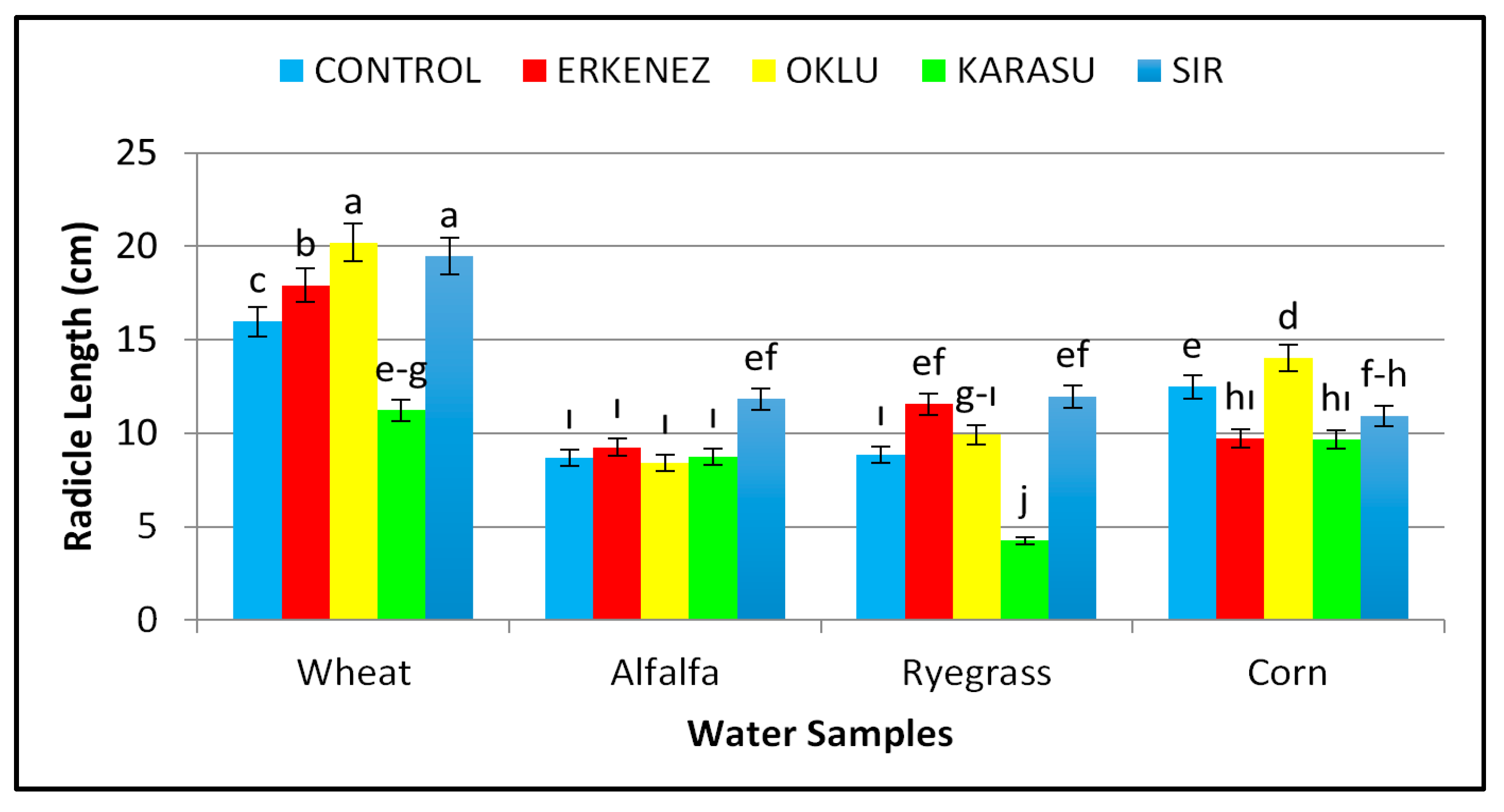
3.2. Radicle Length (cm)
3.3. Plumule Length (cm)
3.4. Seedling Length (cm)
3.5. Seedling Fresh Weight (g)
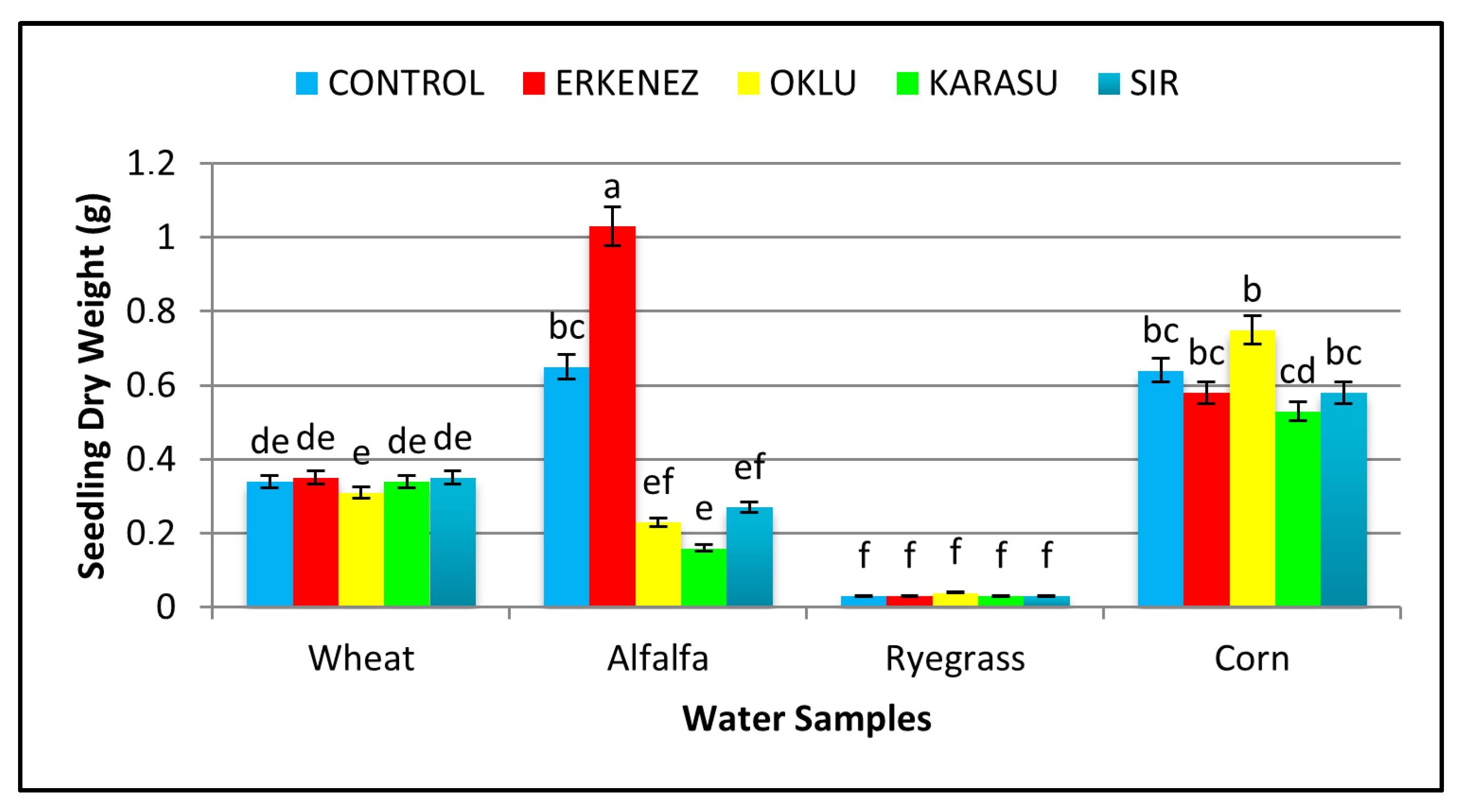
3.6. Seedling Dry Weight (g)
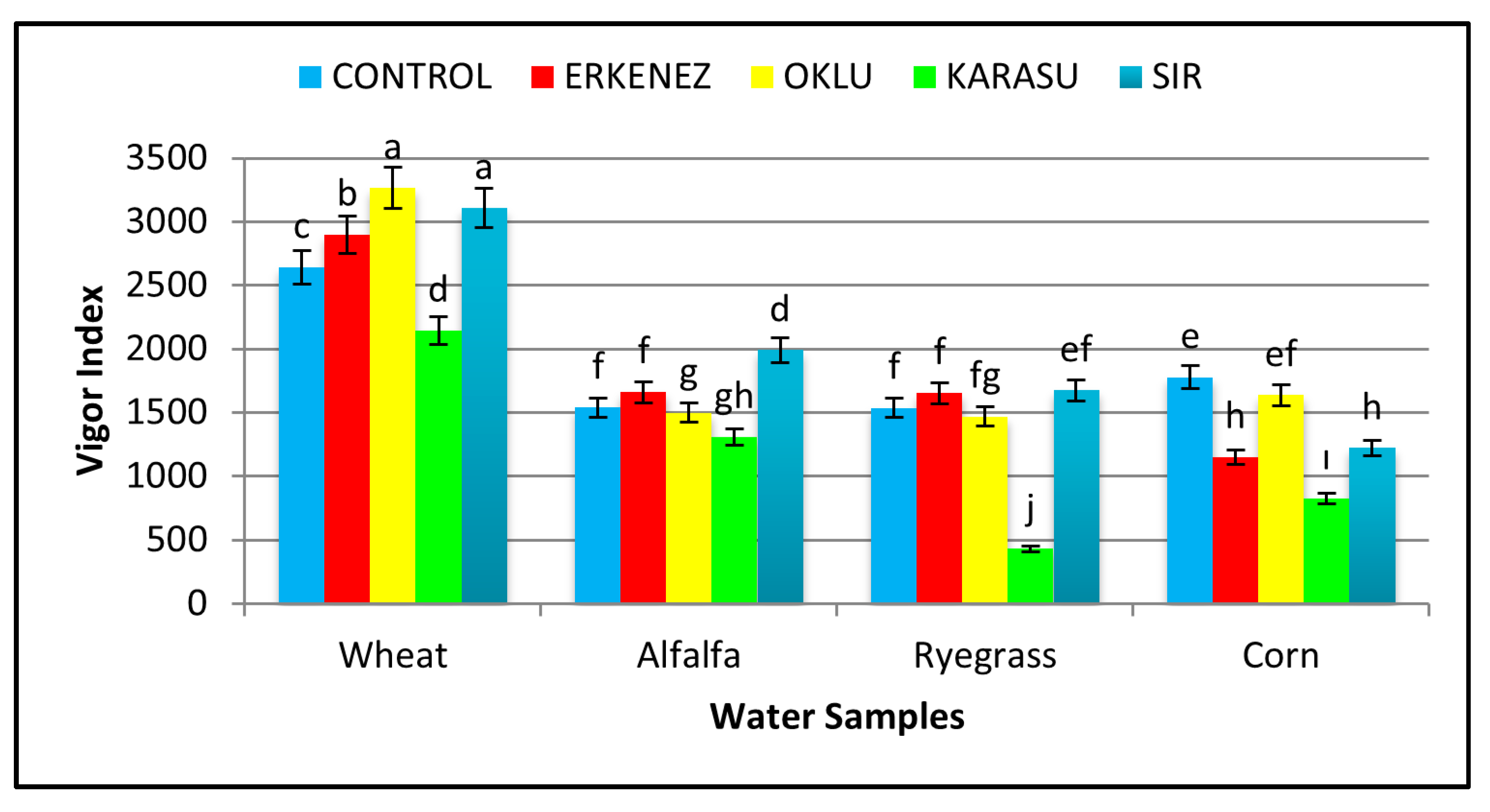
3.7. Vigor Index
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aksoy, A.; Demir, N.; Kaymak, H.Ç.; Sarı, M.M. Seed Sector of Turkey in Terms of Sustainable Agriculture. Atatürk Univ. J. Agric. Fac. 2017, 48, 133–138. [Google Scholar]
- SUSTAINABLE FOOD SYSTEMS COUNTRY REPORT, Turkiye, 2021. Available online: https://www.tarimorman.gov.tr/ABDGM/Belgeler/Uluslararas%C4%B1%20Kurulu%C5%9Flar/NATIONAL%20PATHWAY%20OF%20TURKEY_29%20Kas%C4%B1m.pdf (accessed on 8 February 2024).
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (psnps) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of germination time on the compositional, functional and antioxidant properties of whole wheat malt and its end-use evaluation in cookie-making. Food Chem. 2021, 349, 129125. [Google Scholar] [PubMed]
- Beta, T.; Qiu, Y.; Liu, Q.; Borgen, A.; Apea-Bah, F.B. Purple wheat (Triticum sp.) seeds. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–125. [Google Scholar]
- Lemmens, E.; De Brier, N.; Goos, P.; Smolders, E.; Delcour, J.A. Steeping and germination of wheat (Triticum aestivum L.). I. Unlocking the impact of phytate and cell wall hydrolysis on bio-accessibility of iron and zinc elements. J. Cereal Sci. 2019, 90, 102847. [Google Scholar] [CrossRef]
- Yan, M.; Xue, C.; Xiong, Y.; Meng, X.; Li, B.; Shen, R.; Lan, P. Proteomic dissection of the similar and different responses of wheat to drought, salinity and submergence during seed germination. J. Proteom. 2020, 220, 103756. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E.; Horgan-Kobelski, T.; Riggs, C. Adaptive transgenerational plasticity in an annual plant: Grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 2012, 52, 77–88. [Google Scholar]
- Nautiyal, P.C.; Sivasubramaniam, K.; Dadlani, M. Seed Dormancy and Regulation of Germination. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023; pp. 39–66. [Google Scholar]
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Fu, F.F.; Peng, Y.S.; Wang, G.B.; El-Kassaby, Y.A.; Cao, F.L. Integrative analysis of the metabolome and transcriptome reveals seed germination mechanism in Punica granatum L. J. Integr. Agric. 2021, 20, 132–146. [Google Scholar] [CrossRef]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination; The Arabidopsis Book/American Society of Plant Biologists: Rockville, MD, USA, 2008. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1994; p. 421. [Google Scholar]
- Manz, B.; Mü, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef]
- Masubelele, N.H.; Dewitte, W.; Menges, M.; Maughan, S.; Collins, C.; Huntley, R.; Nieuwland, J.; Scofield, S.; Murray, J.A.H. D-Type Cyclins Activate Division in the Root Apex to Promote Seed Germination in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 15694–15699. [Google Scholar] [CrossRef]
- Ozden, E.; Light, M.E.; Demir, I. Alternating temperatures increase germination and emergence in relation to endogenous hormones and enzyme activities in aubergine seeds. S. Afr. J. Bot. 2021, 139, 130–139. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Balla, I.; Gyuricza, C.; Eser, A.; Tarnawa, Á. The Effect of Temperature and Water Stresses on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Sustainability 2022, 14, 3887. [Google Scholar] [CrossRef]
- Abido, W.A.E.; Zsombik, L. Effect of water stress on germination of some hungarian wheat landraces varieties. Shengtai Xuebao/Acta Ecol. Sin. 2020, 38, 422–428. [Google Scholar] [CrossRef]
- Aderounmu, A.F.; Nkemnkeng, F.J.; Anjah, G.M. Effects of seed provenance and growth media on the growth performance of Vitellaria paradoxa c.f. gaertn. Int. J. Biol. Chem. Sci. 2020, 14, 2659–2669. [Google Scholar] [CrossRef]
- Ogundele, D.T.; Oladejo, A.A.; Oke, C.O. Effect of pharmaceutical effluent on the growth of crops in Nigeria. Chem. Sci. Intern J. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Orhon, D.; Kabdasli, I.; Germirli Babuna, F.; Sozen, S.; Dulkadiroglu, H.; Dogruel, S.; Karahan, O.; Ve Insel, G. Wastewater reuse for the minimization of fresh water demand in coastal reasselected cases from the textile finishing industry. J. Environ. Sci. Health A 2003, 38, 1641–1657. [Google Scholar] [CrossRef]
- Can, Y. Water Use in Textile Sector and Waste Water Management; Akademik Platform: Trondheim, Norway, 2014. [Google Scholar]
- Huang, S.; Li, Q.; Yang, Y.; Yuan, C.; Ouyang, K.; You, P. Risk assessment of heavy metals in soils of a lead-zinc mining area in Hunan Province (China). J. Chem. Chemical Eng. 2017, 66, 173–178. [Google Scholar] [CrossRef]
- Dindaroğlu, T.; Babur, E.; Laz, B. Determination of the ecology and heavy metal tolerance limits of Alyssum Pateri subsp. Pateri growing on ultramafic soils. J. Soil Sci. Plant Nut. 2019, 7, 110–120. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Peerzada, P.H.; Javed, A.M.; Dawood, M.H.; Hussain, N.M.; Ahmad, S. Growth and development dynamics in agronomic crops under environmental stress. In Agronomic Crops; Springer: Singapore, 2019; pp. 83–114. [Google Scholar]
- Uslu, O.S.; Babur, E.; Alma, M.H.; Solaiman, Z.M. Walnut shell biochar increases seed germination and early growth of seedlings of fodder crops. Agriculture 2020, 10, 427. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Belimov, A.A.; Safronova, V.I.; Tsyganov, V.E.; Borisov, A.Y.; Kozhemyakov, A.P.; Stepanok, V.V.; Tikhonovich, I.A. Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.). Euphytica 2003, 131, 25–35. [Google Scholar] [CrossRef]
- Tsamo, C.; Alanyuy, S.M.; Zama, E.F.; Tamu, C.C. Germination and survival of maize and beans seeds: Effects of ırrigation with NaCl and heavy metals contaminated water. Open J. Appl. Sci. 2022, 12, 769–792. [Google Scholar] [CrossRef]
- Munzuroğlu, O.; Geçkil, H. Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Arch. Environ. Contam. Toxicol. 2002, 43, 203–213. [Google Scholar] [CrossRef]
- El-Ghamery, A.A.; El-Kholy, M.A.; Abou El-Yousser, M.A. Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 537, 29–41. [Google Scholar] [CrossRef]
- King-Díaz, B.; Montes-Ayala, J.; Escartín-Guzmán, C.; Castillo-Blum, S.E.; Iglesias-Prieto, R.; Lotina-Hennsen, B.; Barba-Behrens, N. Cobalt (LI) coordination compounds of ethyl 4-methyl-5-imidazolecarboxylate: Chemical and biochemical characterization on photosynthesis and seed germination. Bioinorg. Chem. Appl. 2005, 3, 93–108. [Google Scholar] [CrossRef]
- Drazkiewicz, M.; Baszynski, T. Growth parameters and photosynthetic pigments in leaf segments of Zea mays exposed to cadmium, as related to protection mechanisms. J. Plant Physiol. 2005, 162, 1013–1021. [Google Scholar] [CrossRef]
- Jarup, L. Hazard of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Lea, P.J. Toxic metals in plants. Braz. J. Plant Physiol. 2005, 17, 1. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Taylor, G.J.; Foy, C.D. Differential uptake and toxicity of ionic and chelated copper in Triticum aestivum. Canad. J. Bot. 1985, 63, 1271–1275. [Google Scholar] [CrossRef]
- Wheeler, D.M.; Power, I.L.; Edmeades, D.C. Effect of various metal ions on growth of two wheat lines known to differ in aluminium tolerance. Plant Soil 1993, 155/156, 489–492. [Google Scholar] [CrossRef]
- Shafique, F.; Ali, Q.; Malik, A. Effects of Heavy Metal Toxicity on Maize Seedlings Growth Traits. Biol. Clin. Sci. Res. 2020, e027. [Google Scholar]
- Siddiqui, A.H.; Tabrez, S.; Ahmad, M. Validation of plant-based bioassays for the toxicity testing of Indian waters. Environ. Monit. Assess. 2011, 179, 241–253. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA, AWWA, WPCF: New York, NY, USA, 1999. [Google Scholar]
- Anonymous. 1991. Available online: https://webdosya.csb.gov.tr/db/destek/icerikler/ek4-20191127123641.pdf (accessed on 18 February 2025).
- Kacar, B.; Inal, A. Plant Analysis; Nobel Publication No. 1241; Nobel Publication Distribution Ltd.: Ankara, Turkey, 2008; 892p. [Google Scholar]
- SAS. SAS 9.3 User Guide, Copyright © 2013; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill: New York, NY, USA, 1980; p. 481. [Google Scholar]
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Sabry, G.E. The Importance of Using High Quality Seeds in Agriculture Systems. Agric. Res. Tech. Open Access J. 2018, 15, 555961. [Google Scholar]
- Mircea, D.M.; Estrelles, E.; Al Hassan, M.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species. Plants 2023, 12, 1260. [Google Scholar] [CrossRef]
- Dindaroglu, T.; Babur, E.; Battaglia, M.; Seleiman, M.; Uslu, O.S.; Roy, R. Impact of Depression Areas and Land-Use Change in the Soil Organic Carbon and Total Nitrogen contents in a Semi-Arid Karst Ecosystem. CERNE 2021, 27, e-102980. [Google Scholar] [CrossRef]
- Hadinezhad, P.; Payamenur, V.; Mohamadi, J.; Ghaderifar, F. The effect of priming on seed germination and seedling growth in Quercus castaneifolia. Seed Sci. Technol. 2013, 41, 121–124. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Lee, S.H.; Kang, K.Y.; Lee, J.J.; Kim, P.J.; Yoon, H.S.; Kim, J.S.; Lee, B.H. Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 2007, 67, 1182–1193. [Google Scholar] [PubMed]
- Suvendran, S.; Johnson, D.; Acevedo, M.; Smithers, B.; Xu, P. Effect of Irrigation Water Quality and Soil Compost Treatment on Salinity Management to Improve Soil Health and Plant Yield. Water 2024, 16, 1391. [Google Scholar] [CrossRef]
- Tadros, M.J.; AL-Mefleh, N.; Mohawesh, O. Effect of irrigation water qualities on Leucaena leucocephala germination and early growth stage. Int. J. Environ. Sci. Technol. 2012, 9, 281–286. [Google Scholar]
- Pang, H.C.; Li, Y.Y.; Yang, J.S.; Laing, Y.S. Effect of brackish water irrigation and straw mulching on soil salinity and crop yields under monsoonal climatic conditions. Agric. Water Manag. 2009, 97, 1971–1977. [Google Scholar]
- Talebi, S.; Kalat, S.N.; Darban, A.S. The study effects of heavy metals on germination characteristics and proline content of Triticale (Triticoseale Wittmack). Inter. J. Farm. Allied. Sci. 2014, 3, 1080–1087. [Google Scholar]
- Khajeh-Hosseini, M.; Powell, A.A.; Bingham, I.J. The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar]
- Rahman Khan, M.; Mahmud Khan, M. Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust. J. Basic Appl. Sci. 2010, 4, 1036–1046. [Google Scholar]
- Singh, D.; Nath, K.; Kumar Sharma, Y. Response of wheat seed germination and seedling growth under copper stress. J. Environ. Biol. 2007, 28, 409–414. [Google Scholar]
- Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Gomez, E.; Tiermann, K.J.; Parsons, J.G.; Carrillo, G. Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environ. Poll. 2002, 119, 291–301. [Google Scholar]
- Azmat, R.; Haider, S.; Askari, S. Phyotoxicity of Pb: I. Effect of Pb on germination, growth, morphology and histomorphology of Phaseolus mungo and Lens culinaris. Pak. J. Biol. Sci. 2006, 9, 979–984. [Google Scholar]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium: Toxicity and tolerance in plants. J. Environ. Biol. 2009, 30, 165–174. [Google Scholar] [PubMed]
- Skórzyńska-Polit, E.; Drążkiewicz, M.; Krupa, Z. Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol. Plant 2010, 32, 169–175. [Google Scholar]
- John, R.; Gadgil, K.; Sharma, G. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Inter. J. Plant Prod. 2009, 3, 66–70. [Google Scholar]
- Blum, W.H. Cadmium uptake by higher plants. In Proceedings of the Extended Abstracts from the Fourth International Conference on the Biogeochemistry of Trace Elements, Berkeley, CA, USA, 23–26 June 1997; University of California. pp. 109–110. [Google Scholar]
- An, Y.J. Soil ecotoxicity assessment using cadmium sensitive plants. Environ. Pollut. 2004, 127, 21–26. [Google Scholar]
- Pan, J.W.; Zhu, M.Y.; Chen, H. Aluminum-induced cell death in root-tip cells of barley. Environ. Exp. Bot. 2001, 46, 71–79. [Google Scholar] [CrossRef]
- Radha, J.; Srivastava, S.; Solomon, S.; Shrivastava, A.K.; Chandra, A. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiol. Plant 2010, 32, 979–986. [Google Scholar]
- Shukla, A.K.; Prasad, S.; Srivastava, S.K.; Singh, S.P.; Singh, R.P. Allelopathic effect of thatch grass (Imperata cylindrica L.) on various Kharif and Rabi season crops and weeds. Indian J. Weed Sci. 2003, 35, 163–166. [Google Scholar]
- Maity, M.; Chatterjee, G.; Banerjee, R. Water Quality Demonstrates Detrimental Effects on Two Different Seeds during Germination. Curr. Agri. Res. J. 2019, 7, 377–389. [Google Scholar]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz J. Plant Physiol. 2005, 17, 21–24. [Google Scholar]
- Pal, M.; Horváth, E.; Janda, T.; Paldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar]
- Eshghi, S.; Mahmoodabadi, M.R.; Abdi, G.R.; Jamali, B. Zeolite Ameliorates the Adverse Effect of Cadmium Contamination on Growth and Nodulation of Soybean Plant (Glycine max L.). J. Biol. Environ. Sci. 2010, 4, 43–50. [Google Scholar]
- He, J.; Ren, Y.; Cheng, Z.; Jiang, D. Effects of cadmium stress on seed germination, seedling growth and seed amylase activities in rice (Oryza sativa). Rice Sci. 2008, 15, 319–325. [Google Scholar]
- Athar, R.; Ahmad, M. Heavy metal toxicity effect on plant growth and metal uptake by wheat, and on free-living Azotobacter. Water Air. Soil Poll. 2002, 180, 138–165. [Google Scholar]
- Zeng, L.S.; Liao, M.; Chen, L.C.; Huang, Y.C. Effects of lead contamination on soil enzymatic activities, microbial biomass and rice physiological indices in soil-lead-rice (Oryza sativa L.) system. Ecotoxicol. Environ. Saf. 2007, 67, 67–74. [Google Scholar]
- Oncel, I.; Kele, Y.; Ustun, A.S. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Sci. Technol. 2000, 29, 1232–1238. [Google Scholar]
- Özkay, F.; Kiran, S.; Taş, B.I.; Kuşvuran, C.S. Effects of Copper, Zinc, Lead and Cadmium Applied with Irrigation Water on Some Eggplant Plant Growth Parameters and Soil Properties. Turk. J. Agri. Natur. Sci. 2014, 1, 377–383. [Google Scholar]
- Channappagoudar, B.B.; Jalager, B.R.; Biradar, N.R. Allelopathic effect of aqueous extracts of weed species on germination and seedling growth of some crops. Karnataka J. Agric. Sci. 2005, 18, 916–920. [Google Scholar]
- Shaikh, I.R.; Shaikh, P.R.; Shaikh, R.A.; Shaikh, A.A. Phytotoxic effects of heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L.) seed germination and seedlings growth in black cotton soil of Nanded. Res. J. Chemical. Sci. 2013, 3, 14–23. [Google Scholar]
- Laz, B.; Babur, E.; Akpınar, D.M.; Avgın, S.S. Determination of biotic and abiotic pests observed in Kahramanmaraş-Elmalar green belt Annex-3 plantation area. KSÜ Agric. Nat. J. 2018, 21, 926–935. [Google Scholar]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 752708, 12. [Google Scholar]
- Reddy, A.M.; Kumar, S.G.; Jyonthsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead Induced Changes in Antioxidant Metabolism of Horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and Bengalgram (Cicemr arietinum L.). Chemosphere 2002, 60, 97–104. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar]
- Vassilev, A.; Vangronsveld, J.; Yordanov, I. Cadmium Phytoremediation: Present State, Biological Background and Research Needs. Bulg. J. Plant Physiol. 2002, 28, 68–95. [Google Scholar]
- Kalyanaraman, S.B.; Sivagurunathan, P. Effect of cadmium, copper and zinc on the growth of blackgram. J. Plant Nutr. 1993, 16, 2029–2042. [Google Scholar] [CrossRef]
- Bewley, R.J.F.; Stotzky, G. Effects of cadmium and zinc on microbial activity in soil; Influence of clay minerals. Part II: Metal added simultaneously. Sci. Tot. Environ. 1983, 31, 57–69. [Google Scholar]
- Salgare, S.A.; Acharekar, C. Effect of industrial pollution on growth and content of certain weeds. J. Nat. Conserve. 1992, 4, 1–6. [Google Scholar]

| Karasu Creek | Erkenez Creek | Oklu Creek | Sır Dam | |
|---|---|---|---|---|
| Latitude | 37°31′3.45″ K | 37°32′25.56″ K | 37°33′49.39″ K | 37°34′30.35″ K |
| Longitude | 36°56′0.85″ D | 36°55′13.30″ D | 36°54′42.67″ D | 36°48′6.19″ D |
| Elements | Maximum Possible Amount per Unit (kg ha−1) | Allowable Maximum Concentrations | |
|---|---|---|---|
| Boundary Values in Cases of Continuous Irrigation for All Kinds of Soil (mg L−1) | When Watering Less than 24 Years in Clay Soils with a pH Value Between 6.0–8.5 (mg L−1) | ||
| Arsenic (As) | 90 | 0.1 | 2.0 |
| Cadmium (Cd) | 09 | 0.01 | 0.05 |
| Chrome (Cr) | 09 | 0.1 | 1.0 |
| Copper (Cu) | 190 | 0.2 | 5.0 |
| Iron (Fe) | 4600 | 5.0 | 20.0 |
| Lead (Pb) | 4600 | 5.0 | 10.0 |
| Nickel (Ni) | 920 | 0.2 | 2.0 |
| AMC | KC | OC | EC | SD | |
|---|---|---|---|---|---|
| Cu (mg L−1) | 0.2 | 0.98 | 1.627 | 0.945 | 1.218 |
| Fe (mg L−1) | 5.0 | 18.69 | 0.555 | 2.395 | 8.89 |
| Pb (mg L−1) | 5.0 | 0.00015 | 0.00056 | 4.5 | 0.013 |
| Cr (mg L−1) | 5.0 | 0.097 | 0.00038 | 0.02 | 0.00013 |
| As (mg L−1) | 1.0 | 0.206 | 0.171 | 0.165 | 0.132 |
| Ni (mg L−1) | 0.5 | 0.00328 | 0.04 | 0.00067 | 0.00067 |
| Cd (mg L−1) | 0.005 | 0.00068 | 0.00069 | 0.099 | 0.115 |
| GR (%) | RL (cm) | PL (cm) | SL (cm) | SFW (g) | SDW (g) | VI | ||
|---|---|---|---|---|---|---|---|---|
| ** | ** | ** | ** | ** | ** | ** | ||
| Species | Wheat | 98 a | 16.96 a | 11.63 a | 28.58 a | 1.68 b | 0.34 c | 2812 a |
| Alfalfa | 96 a | 9.38 c | 7.25 b | 16.64 b | 1.46 b | 0.47 b | 1598 b | |
| Ryegrass | 78 b | 9.31 c | 6.97 b | 16.28 b | 0.28 c | 0.04 d | 1352 c | |
| Corn | 72 b | 11.36 b | 6.63 b | 17.99 b | 4.66 a | 0.62 a | 1322 c | |
| LSD | 6.97 | 1.76 | 0.88 | 2.17 | 0.68 | 0.09 | 232.60 | |
| ** | ** | ** | ** | ns | ** | ** | ||
| Irrigation waters | Control | 95 a | 11.50 a | 8.16 a | 19.66 a | 2.17 | 0.42 ab | 1874 a |
| Erkenez Creek | 87 a | 12.11 a | 8.55 a | 20.67 a | 2.02 | 0.50 a | 1841 a | |
| Oklu Creek | 89 a | 13.14 a | 8.55 a | 21.69 a | 2.45 | 0.34 bc | 1967 a | |
| Karasu Creek | 71 b | 8.46 b | 6.60 b | 15.06 b | 1.61 | 0.27 c | 1177 b | |
| Sır Dam | 87 a | 13.55 a | 8.73 a | 22.28 a | 1.84 | 0.31 bc | 1997 a | |
| LSD | 7.98 | 1.97 | 0.98 | 2.43 | 0.76 | 0.11 | 260.10 | |
| Mean | 86 | 11.75 | 8.12 | 19.87 | 2.02 | 0.37 | 1771 | |
| CV % | 12.77 | 23.64 | 17.04 | 17.24 | 53.28 | 41.27 | 20.74 |
| GR (%) | RL (cm) | PL (cm) | SL (cm) | SFW (g) | SDW (g) | VI | ||
|---|---|---|---|---|---|---|---|---|
| ** | * | ns | ns | ns | ** | * | ||
| Wheat | Control | 98 ab | 15.99 c | 11.03 | 27.01 | 1.51 | 0.34 de | 2643 c |
| Erkenez Creek | 98 ab | 17.93 b | 11.63 | 29.56 | 1.62 | 0.36 de | 2899 b | |
| Oklu Creek | 99 a | 20.19 a | 12.83 | 33.02 | 2.04 | 0.32 e | 3266 a | |
| Karasu Creek | 97 abc | 11.22 efg | 10.76 | 21.98 | 1.55 | 0.34 de | 2143 d | |
| Sır Dam | 99 a | 19.46 a | 11.88 | 31.34 | 1.67 | 0.35 de | 3107 a | |
| Alfalfa | Control | 98 ab | 8.69 i | 7.00 | 15.68 | 1.37 | 0.65 bc | 1538 f |
| Erkenez Creek | 96 abc | 9.23 i | 8.06 | 17.31 | 1.83 | 1.04 a | 1659 ef | |
| Oklu Creek | 100 a | 8.42 i | 6.57 | 14.98 | 1.34 | 0.23 ef | 1498 fg | |
| Karasu Creek | 88 bcd | 8.73 i | 6.18 | 14.91 | 1.09 | 0.17 ef | 1306 gh | |
| Sır Dam | 98 ab | 11.83 ef | 8.48 | 20.30 | 1.67 | 0.27 e | 1988 d | |
| Italian Ryegrass | Control | 93 abcd | 8.86 i | 7.65 | 16.51 | 0.33 | 0.04 f | 1536 f |
| Erkenez Creek | 87 cd | 11.56 ef | 7.39 | 18.95 | 0.26 | 0.04 f | 1653 ef | |
| Oklu Creek | 85 d | 9.91 ghi | 7.40 | 17.31 | 0.36 | 0.05 f | 1467 fg | |
| Karasu Creek | 44 g | 4.25 j | 4.70 | 8.95 | 0.21 | 0.03 f | 429 j | |
| Sır Dam | 85 d | 11.98 ef | 7.69 | 19.67 | 0.23 | 0.04 f | 1672 ef | |
| Corn | Control | 92 abcd | 12.48 e | 6.97 | 19.45 | 5.48 | 0.65 bc | 1778 e |
| Erkenez Creek | 68 e | 9.71 hi | 7.14 | 16.85 | 4.36 | 0.59 bc | 1149 h | |
| Oklu Creek | 74 e | 14.03 d | 7.41 | 21.44 | 6.08 | 0.76 b | 1636 ef | |
| Karasu Creek | 58 f | 9.65 hi | 4.77 | 14.41 | 3.58 | 0.53 cd | 827 i | |
| Sır Dam | 68 e | 10.93 fgh | 6.87 | 17.80 | 3.78 | 0.58 bc | 1218 h | |
| LSD | 9.09 | 1.37 | 0.46 | 1.60 | 0.38 | 0.18 | 186.90 | |
| Mean | 86 | 11.75 | 8.12 | 19.87 | 2.02 | 0.37 | 1771 | |
| CV % | 12.77 | 23.64 | 17.04 | 17.24 | 53.28 | 41.27 | 20.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uslu, Ö.S.; Gedik, O.; Kaya, A.R.; Erol, A.; Babur, E.; Khan, H.; Seleiman, M.F.; Wasonga, D.O. Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops. Water 2025, 17, 892. https://doi.org/10.3390/w17060892
Uslu ÖS, Gedik O, Kaya AR, Erol A, Babur E, Khan H, Seleiman MF, Wasonga DO. Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops. Water. 2025; 17(6):892. https://doi.org/10.3390/w17060892
Chicago/Turabian StyleUslu, Ömer Süha, Osman Gedik, Ali Rahmi Kaya, Adem Erol, Emre Babur, Haroon Khan, Mahmoud F. Seleiman, and Daniel O. Wasonga. 2025. "Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops" Water 17, no. 6: 892. https://doi.org/10.3390/w17060892
APA StyleUslu, Ö. S., Gedik, O., Kaya, A. R., Erol, A., Babur, E., Khan, H., Seleiman, M. F., & Wasonga, D. O. (2025). Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops. Water, 17(6), 892. https://doi.org/10.3390/w17060892












