Analysis of Carbon Density Distribution Characteristics in Urban Wetland Ecosystems: A Case Study of Shanghai Fish and Dishui Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Research Methods
2.2.1. Field Survey Method
2.2.2. Direct Measurement Method
2.2.3. Elemental Analysis Method
2.2.4. Remote Sensing Data Acquisition and Inversion
3. Results and Discussion
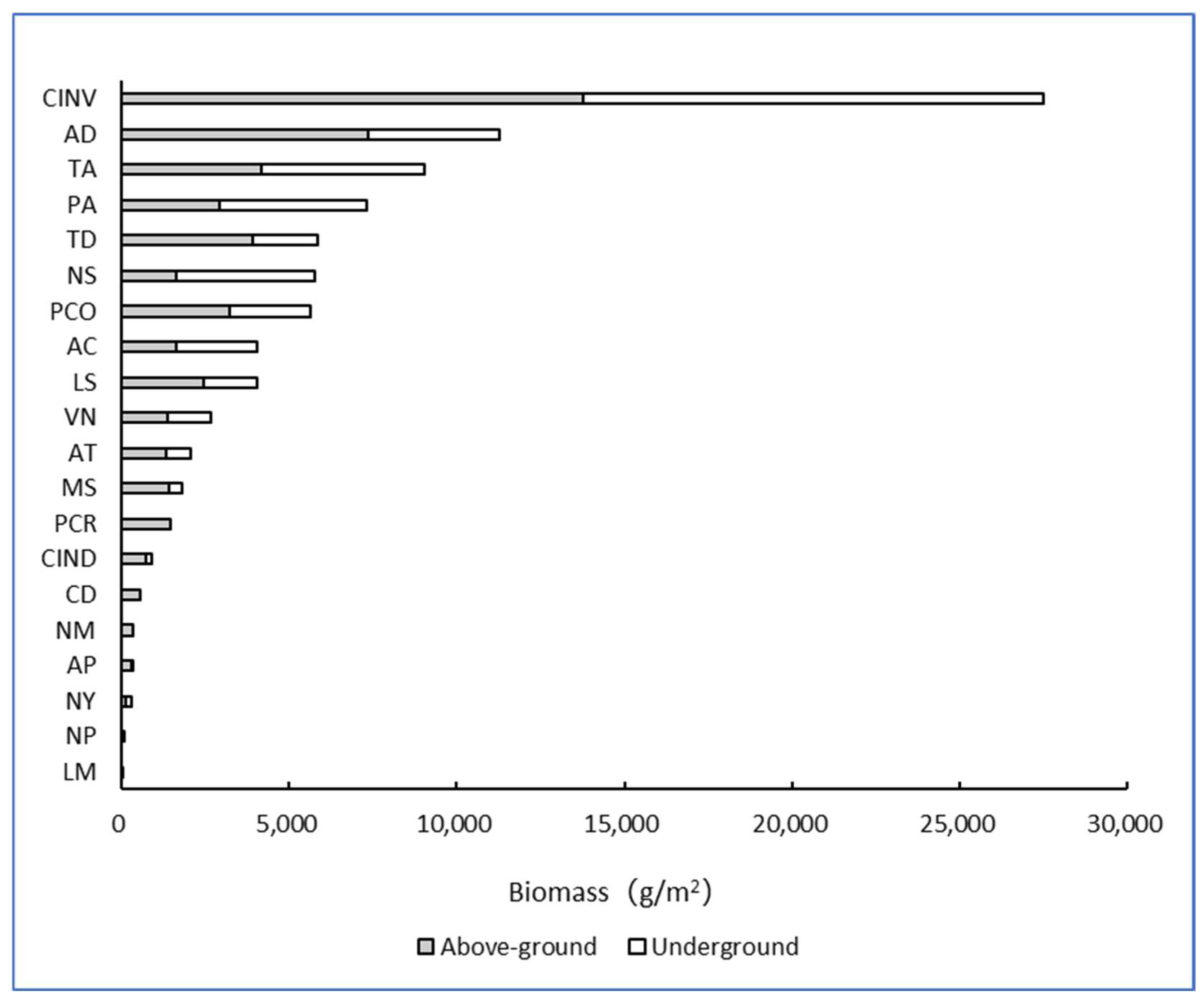
3.1. Biomass of Common Wetland Plants in Shanghai
3.2. Differences in Biomass Distribution Among Different Types of Wetland Plants
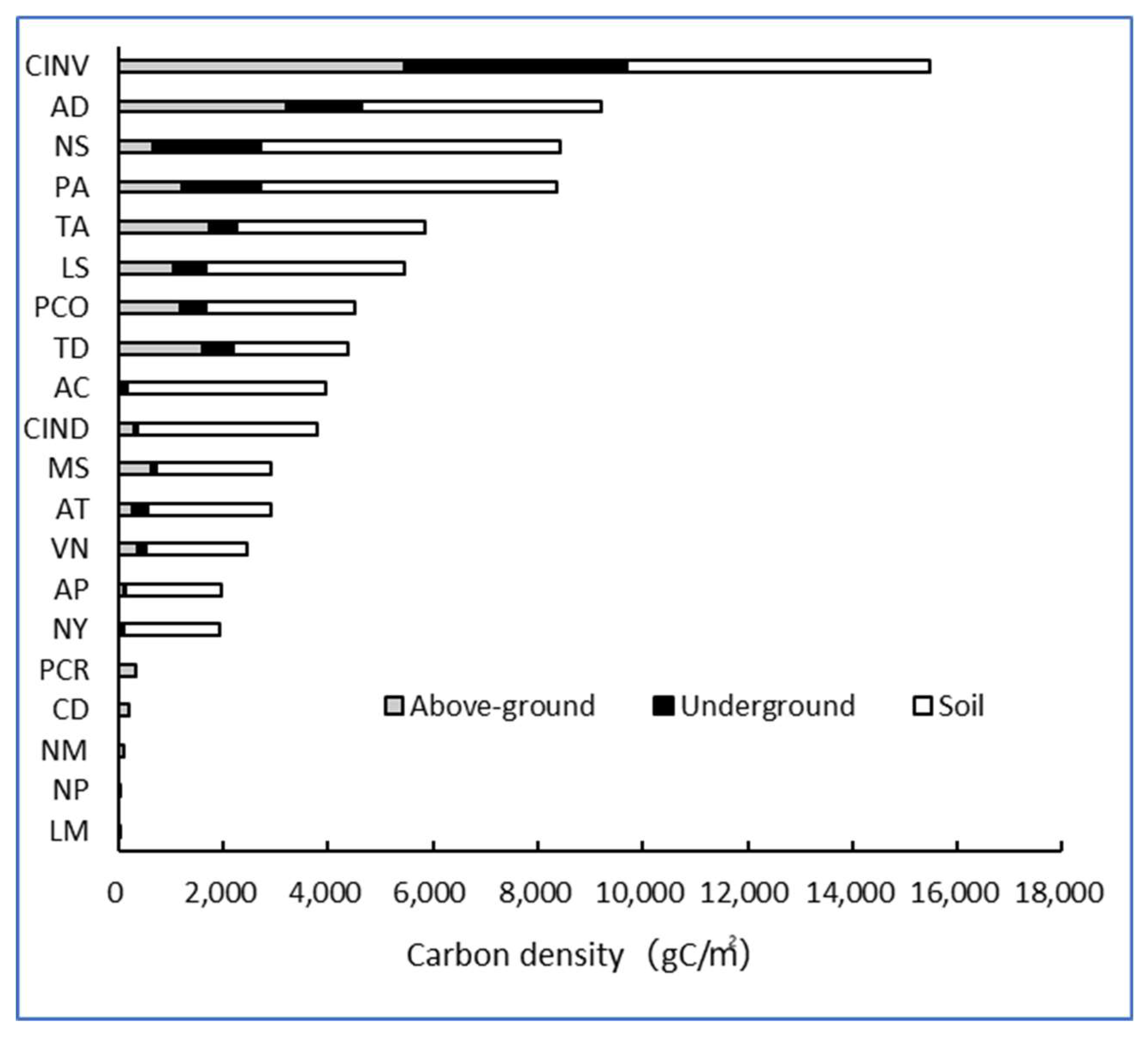
3.3. Carbon Content and Carbon Density Distribution Patterns in Plants
3.3.1. Differences in Carbon Content and Carbon Density Distribution Among Different Plants
3.3.2. Differences in Carbon Content and Carbon Density Distribution Among Different Plant Types
3.3.3. Comparison of Soil Carbon Content and Carbon Density
3.3.4. Analysis of Differences in Overall Carbon Storage Capacity of Plants
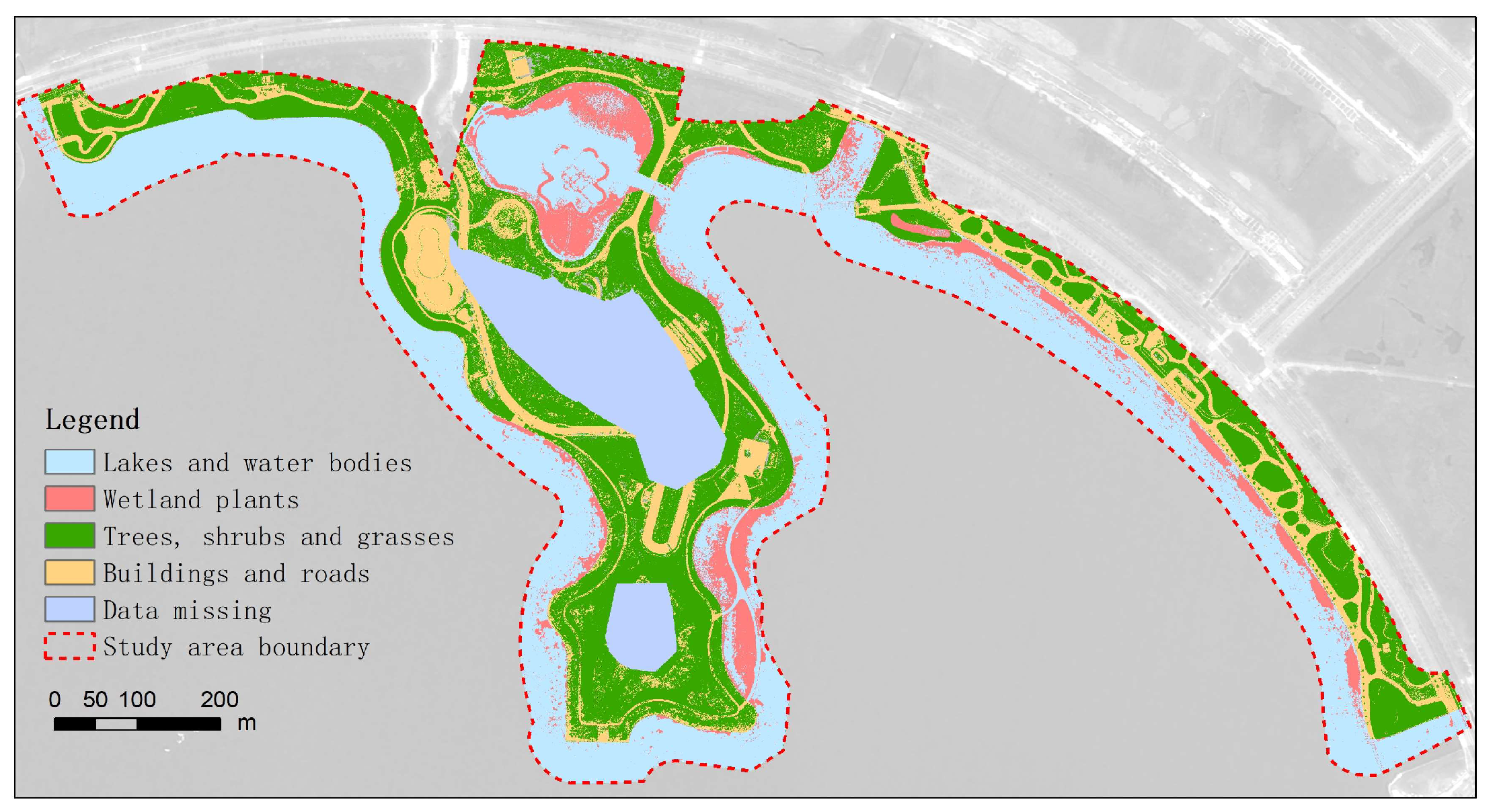
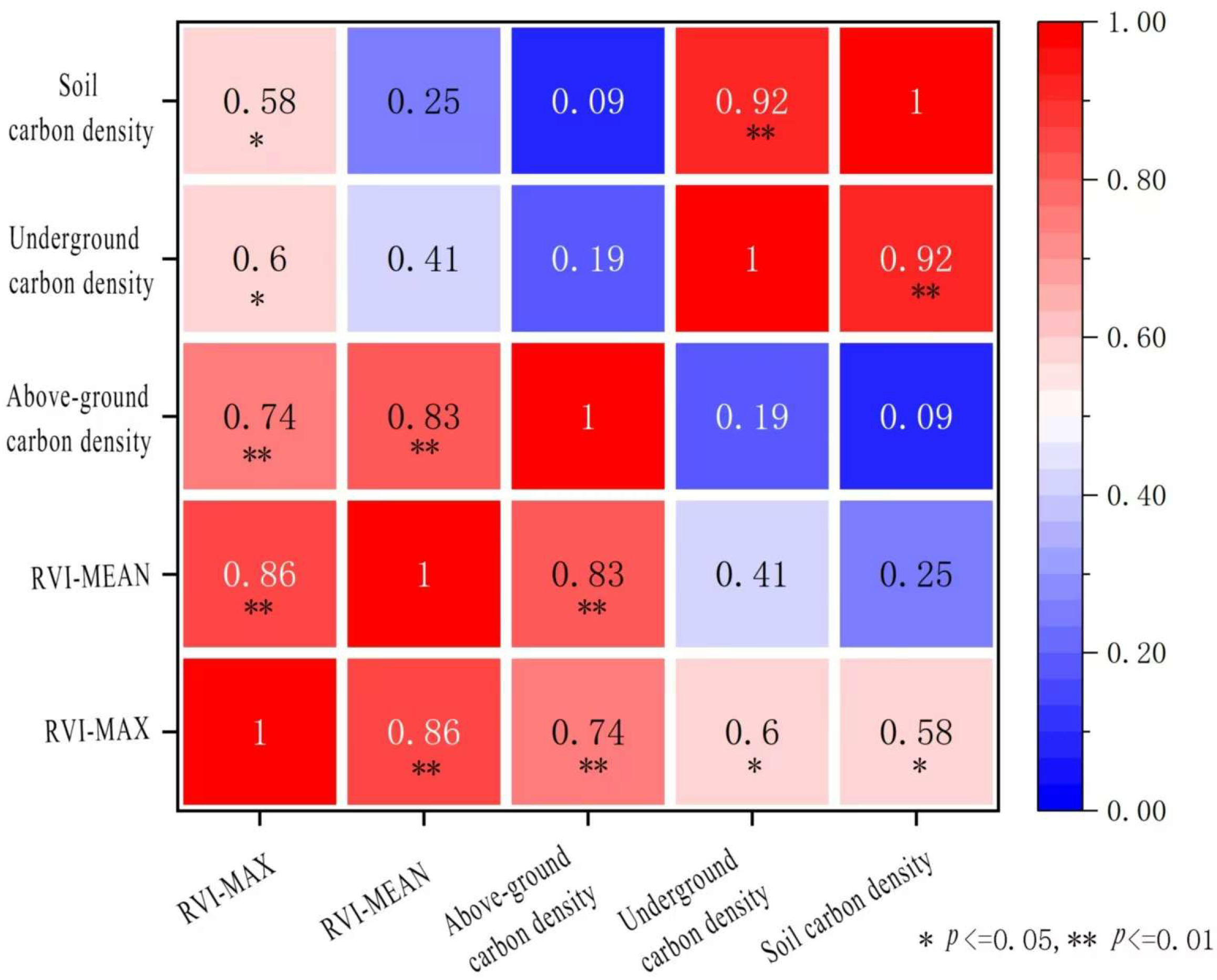
3.4. Remote Sensing Information Extraction of Vegetation in Artificial Wetlands and Distribution Characteristics of Wetland Vegetation
3.4.1. Vegetation Extraction Methods
3.4.2. Analysis of Spatial Distribution Characteristics of Wetland Vegetation
- (1)
- Shanghai Fish Artificial Wetland
- (2)
- North Island of Dishui Lake Artificial Wetland
3.5. Analysis of Carbon Sequestration Pattern Differences in Two Artificial Wetlands Across Different Periods
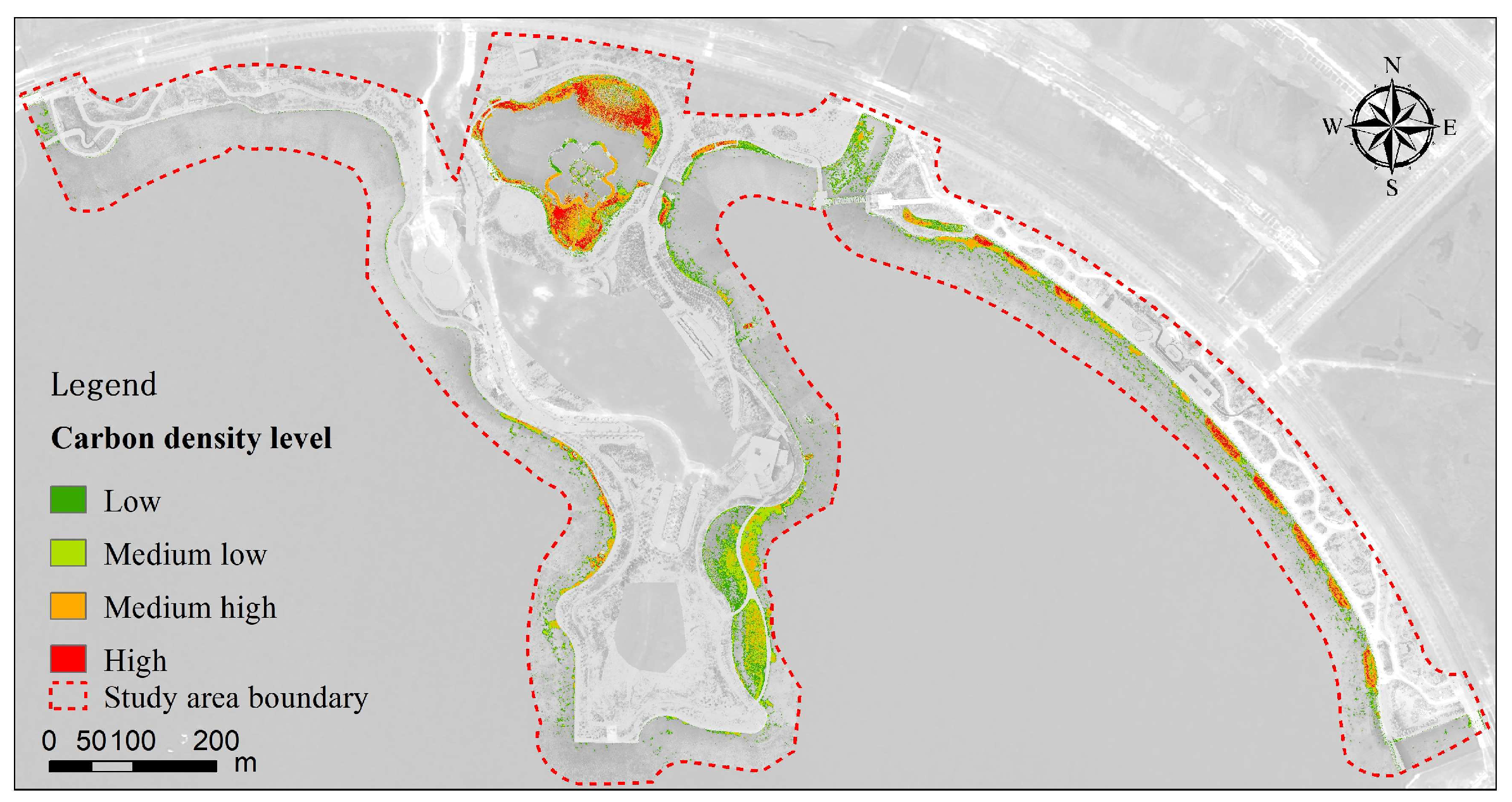
3.5.1. Current Carbon Density Patterns
- (1)
- Spatial Characteristics of Aboveground Carbon Density
- (2)
- Spatial Characteristics of Underground Carbon Density
- (3)
- Spatial Characteristics of Soil Carbon Density
- (4)
- Overall Spatial Characteristics of Carbon Density
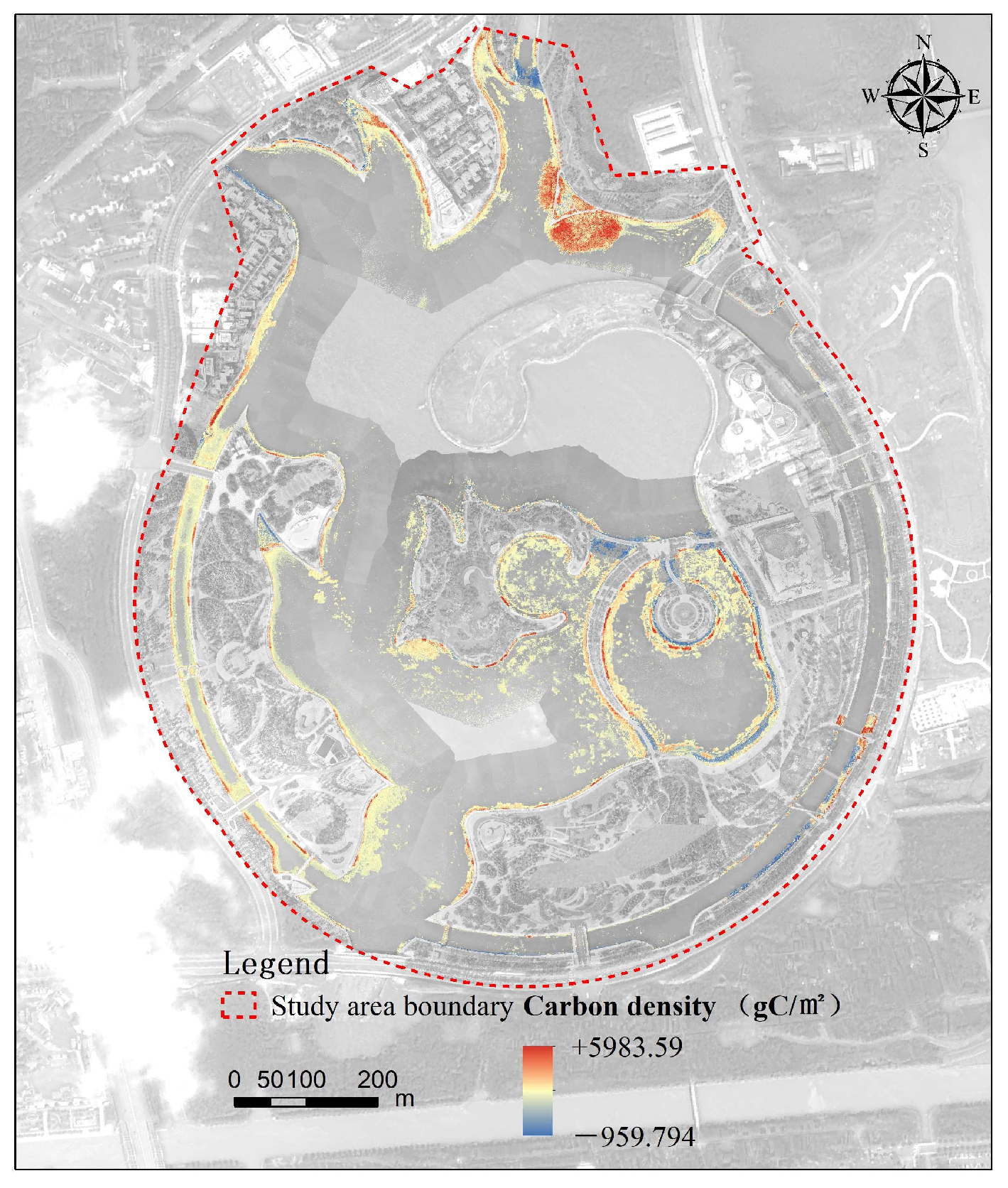
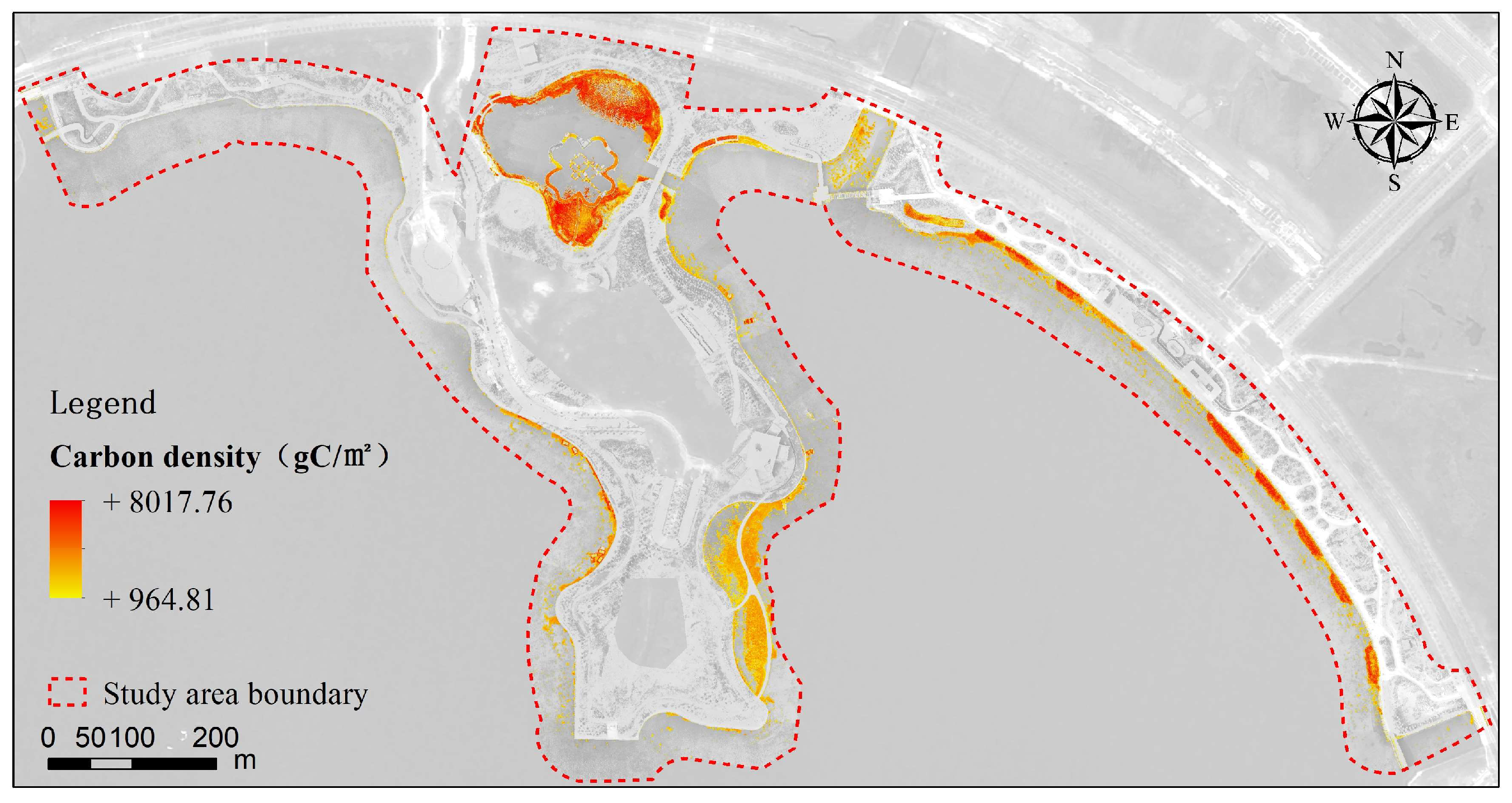
3.5.2. Changes in Carbon Sequestration Patterns
4. Conclusions
- (1)
- There were significant differences in the biomass of 20 common wetland plant species in Shanghai, and the ratio of aboveground biomass and underground biomass also varied. Emergent plants had the largest biomass, with Cyperus involucratus having the highest biomass at 27.5 kg/m2, while floating plants had the smallest biomass, with Lemna minor being the lowest at 14.8 g/m2. These differences in biomass were mainly attributed to the type of plant and the development of their root systems. For instance, Cyperus involucratus and Arundo donax gain an advantage in wetland environments through their developed root systems, while plants like Lemna minor and Nymphoides peltate show lower biomass due to their relatively small root systems. The pattern of carbon content in the plants showed little variation in aboveground carbon content, primarily concentrated around 40%, whereas their underground carbon content showed significant variability, ranging from 12.05% to 50.30%. The soil carbon content of the wetland plants was generally low, with Phragmites australis having the highest at 28.50‰ and Vallisneria natans having the lowest at 8.60‰. Most of the carbon assimilated by wetland plants is stored in the wetland soil as sediment, making the carbon density in the soil far higher than in the plants, with the plant carbon density being most significantly influenced by biomass. Cyperus involucratus, Arundo donax, Phragmites australis, Nelumbo sp., and Typha angustifolia rank among the top five plants with the highest carbon density, while Lemna minor, Nymphoides peltate, Najas marina, Ceratophyllum demersum, and Potamogeton crispus are among the five with the lowest carbon density. Overall, the emergent plants exhibited the strongest carbon sequestration capacity, while the floating plants were the weakest. The carbon density of the emergent plants was 287 times greater than that of the floating plants, 2.9 times that of the floating-leaved plants, and 2.6 times that of the submerged plants. Emergent plants play a particularly crucial role in carbon sequestration in wetland ecosystems, closely linked to their well-developed root systems and strong adaptability.
- (2)
- In these two wetlands, the proportion of wetland vegetation coverage was lower, and wetland vegetation was not the primary surface cover type. The total area of wetland vegetation in Shanghai Fish and the north island of Dishui Lake accounted for approximately 9.69% and 7.55%, respectively. The spatial distribution of wetland vegetation exhibited significant heterogeneity. In the artificial wetland of Shanghai Fish, it was mainly distributed in the northern and central–western regions, while in the north island of Dishui Lake, it was primarily located in the northern inner lake, the southern east coast, and the western east coast. The distribution patterns of wetland vegetation in both wetlands exhibited both strip-like and patch-like characteristics. This distribution pattern reflects the influence of wetland vegetation and water level (affecting water availability and nutrient distribution) on carbon density.
- (3)
- From 2018 to 2023, the overall carbon density in both artificial wetlands showed a significant increase, indicating that ecological restoration and management efforts in the wetlands had achieved positive results, significantly enhancing their carbon sequestration capacity. In 2018, the average carbon density in Shanghai Fish was approximately 1235 gC/m2, while the overall average carbon density in 2023 was 1563 gC/m2. Dishui Lake had no wetland plants in 2018, so its carbon density was 0, but by 2023, the overall average carbon density had reached 1823 gC/m2. Between them, the highest carbon density increase in Shanghai Fish was 5984 gC/m2, while the highest increase in the north island of Dishui Lake was 8018 gC/m2. In Shanghai Fish, from 2018 to 2023, the overall area of the carbon stock zone increased by about 78,023 m2, with the area of the carbon sequestration zone reaching 100,293 m2, distributed throughout the wetland, while the area of the carbon stock reduction zone was 2536 m2. Overall, Dishui Lake showed an increase in carbon sequestration, particularly significant in the northern and nearshore areas of Shanghai Fish and in the eastern to northern lakeshore areas of the northern island of Dishui Lake. This study demonstrates that wetland management and protection play a positive role in enhancing carbon sequestration capacity, providing important scientific evidence for the future protection and management of wetland ecosystems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.L.; An, S.Q.; Zeng, Q.; Zhu, K.Q.; Zhang, K.F.; Xia, Y.X.; Zhao, H. Current status of wetland resources and spatial arrangement of wetland protection in Shanghai. Wetl. Sci. Manag. 2021, 17, 73–76. [Google Scholar]
- Li, J.; Jiang, M.; Pei, J.; Fang, C.M.; Li, B.; Nie, M. Convergence of carbon sink magnitude and water table depth in global wetlands. Ecol. Lett. 2023, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, W.; Peng, K.; Wu, Z.F.; Ling, Z.Y.; Li, Z. Assessment of the impact of wetland changes on carbon storage in coastal urban agglomerations from 1990 to 2035 in support of SDG15.1. Sci. Total Environ. 2023, 873, 162824. [Google Scholar] [CrossRef]
- Deng, W.B.; Liu, X.Y.; Hu, H.B.; Liu, Z.Q.; Ge, Z.W.; Xia, C.P.; Wang, P.; Liang, L.; Zhu, Z.Y.; Sun, Y.; et al. Carbon storages and densities of different ecosystems in Changzhou City, China: Subtropical forests, urban green spaces, and wetlands. Forests 2024, 15, 303. [Google Scholar] [CrossRef]
- Pal, S.; Chattopadhyay, B.; Datta, S.; Mukhopadhyay, S.K. Potential of wetland macrophytes to sequester carbon and assessment of seasonal carbon input into the East Kolkata Wetland ecosystem. Wetlands 2017, 37, 497–512. [Google Scholar] [CrossRef]
- Turmel-Courchesne, L.; Davies, M.A.; Guêné-Nanchen, M.; Strack, M.; Rochefort, L. Rewetting increases vegetation cover and net growing season carbon uptake under fen conditions after peat-extraction in Manitoba, Canada. Sci. Rep. 2023, 13, 12345. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, R.; Wang, Y.; Huang, J. Evolution characteristics of wetland landscape pattern and its impact on carbon sequestration in Wuhan from 2000 to 2020. Land 2023, 12, 582. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.Z.; Yan, Z.Z.; Chen, X.Z.; He, Y.L.; Guo, W.Y.; Sun, P.Y. Biomass and carbon storage of Phragmites australis and Spartina alterniflora in the Jiuduansha salt marsh wetland of the Yangtze River Estuary. Chin. J. Appl. Ecol. 2013, 24, 2129–2134. [Google Scholar]
- Lolu, A.J.; Ahluwalia, A.S.; Sidhu, M.C.; Reshi, Z.A. Carbon sequestration potential of macrophytes and seasonal carbon input assessment into the Hokersar wetland, Kashmir. Wetlands 2019, 39, 453–472. [Google Scholar] [CrossRef]
- Mei, X.Y.; Zhang, X.F. Research on carbon storage and carbon sequestration function of typical wetland vegetation in the Yangtze River Estuary-A case study of the reed belt in Chongming Dongtan. Chin. J. Eco-Agric. 2008, 02, 269–272. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, H.Q.; Fang, Y.; Li, K. Carbon sequestration capacity of plant communities in coastal wetlands of Chongming Island. Chin. J. Ecol. 2014, 33, 915–921. [Google Scholar]
- Xu, S.J.; Xu, Z.C. Research on carbon sink function of wetland vegetation in Guangzhou. Wetl. Sci. 2015, 13, 190–196. [Google Scholar]
- Yu, Q.Z. Preliminary Study on Carbon Storage of Wetland Vegetation in Nansi Lake. Master’s Thesis, Shandong Normal University, Jinan, China, 2011. [Google Scholar]
- Zhang, L.; Guo, Z.H.; Li, Z.Y. Progress in research on carbon storage and carbon sinks in mangrove wetlands. Chin. J. Appl. Ecol. 2013, 24, 1153–1159. [Google Scholar]
- Euliss, N.H., Jr.; Gleason, R.A.; Olness, A.; McDougal, R.L.; Murkin, H.R.; Robarts, R.D.; Bourbonniere, R.A.; Warner, B.G. North American prairie wetlands are important nonforested land-based carbon storage sites. Sci. Total Environ. 2006, 361, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.; Lee, S.H.; Kang, H. Estimation of carbon storage in coastal wetlands and comparison of different management schemes in South Korea. J. Ecol. Environ. 2019, 43, 8. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Carbon sequestration in two created riverine wetlands in the Midwestern United States. J. Environ. Qual. 2013, 42, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.W.; Yan, J.F.; Hu, Y.; Gao, L.Y.; Wu, P.F.; Wang, L. Spatial distribution patterns of annual soil carbon accumulation and carbon storage in the Jiuduansha wetland of the Yangtze River estuary. Environ. Monit. Assess. 2019, 191, 750. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, J.; Xue, L.; Craft, C. Distribution of organic carbon storage in different salt-marsh plant communities: A case study at the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2020, 243, 106900. [Google Scholar] [CrossRef]
- Rogerson, R.; Horgan, D.; Roberts, J. Integrating artificial urban wetlands into communities: A pathway to carbon zero? Front. Built Environ. 2021, 7, 777383. [Google Scholar] [CrossRef]
- Doughty, C.L.; Langley, J.A.; Walker, W.S.; Feller, I.C.; Schaub, R.; Chapman, S.K. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuar. Coasts. 2016, 39, 385–396. [Google Scholar] [CrossRef]
- Wu, G.F.; Liu, Y.L.; Ji, W.T. Estimating aboveground biomass of submerged vegetation using Landsat 5 TM imagery: A case study of Jiangxi Poyang Lake National Nature Reserve, China. Geomat. Inf. Sci. Wuhan Univ. 2006, 31, 953–957. [Google Scholar]
- Villa, J.A.; Mitsch, W.J. Carbon sequestration in different wetland plant communities in the Big Cypress Swamp region of southwest Florida. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 17–28. [Google Scholar] [CrossRef]
- Mueller, P.; Ladiges, N.; Jack, A.; Schmiedl, G.; Kutzbach, L.; Jensen, K.; Nolte, S. Assessing the long-term carbon-sequestration potential of the semi-natural salt marshes in the European Wadden Sea. Ecosphere 2019, 10, e2556. [Google Scholar] [CrossRef]
- Inamdar, D.; Kalacska, M.; Leblanc, G.; Arroyo-Mora, J. Implementation of the directly-georeferenced hyperspectral point cloud. MethodsX 2021, 8, 101429. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zhang, C. Response mechanism of the thermal environment in the karst rocky desertification areas from the perspective of settlement transition. Urban Clim. 2024, 55, 101984. [Google Scholar] [CrossRef]
- Jia, X.L.; Han, H.T.; Feng, Y.; Song, P.H.; He, R.Z.; Liu, Y.; Wang, P.; Zhang, K.H.; Du, C.Y.; Ge, S.D.; et al. Scale-dependent and driving relationships between spatial features and carbon storage and sequestration in an urban park of Zhengzhou, China. Sci. Total Environ. 2023, 894, 164916. [Google Scholar] [CrossRef]
- Hassan, M.A.; Júnior, S.A.; Silva, T.S. Root-to-shoot ratio variations in wetland plants: Implications for biomass allocation and wetland management. Ecol. Indic. 2021, 128, 107–119. [Google Scholar]
- Jin, Q.; Zhang, Y.; Wang, J. Biomass dynamics and carbon sequestration potential in constructed wetlands: A case study of emergent macrophytes. Environ. Sci. Pollut. Res. 2021, 28, 11578–11590. [Google Scholar]
- Lee, H.; Kim, K.S. Wetland plant strategies for aboveground and belowground biomass distribution under varying water levels. Aquat. Bot. 2022, 177, 103486. [Google Scholar]
- Wang, L.; Xu, X.; Zhao, Y. The role of floating plants in wetland ecosystems: A synthesis of biomass distribution and ecological functions. Wetl. Ecol. Manag. 2023, 31, 287–300. [Google Scholar]
- Zhou, Q.; Liu, G.; Yan, Y. Comparative study of biomass and carbon storage in different wetland plant communities. Ecol. Eng. 2022, 181, 106647. [Google Scholar]
- Wang, Y.; Bao, H.; Kavana, D.J.; Li, Y.; Xu, W. Effects of vegetation types and soil properties on regional soil carbon and nitrogen in Salinized Reservoir Wetland, Northeast China. Plants. 2023, 12, 3767. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Kumar, S.; Sarma, H.P.; Devi, A. Total organic carbon, heavy metal content and metal bioaccumulation in a freshwater wetland of Indo-Burmese province, India. Environ. Pollut. 2021, 270, 116118. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Mao, D.; Xi, Y.; Wang, Z. Northeast China holds huge wetland soil organic carbon storage: An estimation from 819 soil profiles and random forest algorithm. Soil Biol. Biochem. 2023, 160, 108–118. [Google Scholar] [CrossRef]
- Xiong, J.; Shao, X.; Yuan, H.; Liu, E.; Wu, M. Carbon, nitrogen, and phosphorus stoichiometry and plant growth strategy as related to land-use in Hangzhou Bay Coastal Wetland, China. Front. Plant Sci. 2022, 13, 946949. [Google Scholar] [CrossRef]
- Bennett, J.D.; Chambers, L.G. Wetland soil carbon storage exceeds uplands in an urban natural area (Florida, USA). Soil Res. 2022, 60, 531–544. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Shen, M.; Che, S. Study on the optimization of carbon sequestration in Shanghai’s urban artificial wetlands: The cases of Shanghai Fish and Dishui Lake. Land 2024, 13, 2148. [Google Scholar] [CrossRef]
- Yang, R.R.; Fang, J.H.; Cao, Q.Q.; Zhao, D.; Dong, J.Y.; Wang, R.Q.; Liu, J. The content, composition, and influencing factors of organic carbon in the sediments of two types of constructed wetlands. Environ. Sci. Pollut. Res. 2021, 28, 49206–49219. [Google Scholar] [CrossRef]
- Mander, Ü.; Maddison, M.; Valach, A.C.; Soosaar, K.; Kill, K.; Kasak, K. High methane emissions as trade-off for phosphorus removal in surface flow treatment wetlands. Aquat. Bot. 2024, 190, 103719. [Google Scholar] [CrossRef]
- Petrovska, I.; Dimov, L. Accuracy assessment of unsupervised land cover classification. Sci. J. Civ. Eng. 2020, 9, 83–88. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Li, D.; Xu, G.; Guo, Y. Spatial heterogeneity of vegetation extent and the response to water level fluctuations and micro-topography in Poyang Lake, China. Ecol. Indic. 2021, 124, 107420. [Google Scholar] [CrossRef]
- Huang, W.; Hu, T.; Mao, J.; Montzka, C.; Bol, R.; Wan, S.; Dai, H. Hydrological drivers for the spatial distribution of wetland herbaceous communities in Poyang Lake. Remote. Sens. 2022, 14, 4870. [Google Scholar] [CrossRef]
- Fu, H.; Zhou, Y.; Yuan, G.; Peng, H.; Wu, A.; Li, W.; Jeppesen, E. Environmental and spatial drivers for wetland plant communities in a freshwater lake: Reduced coupling of species and functional turnover. Ecol. Eng. 2021, 159, 106092. [Google Scholar] [CrossRef]
- Li, Z.Q.; Sun, W.C.; Chen, H.Y.; Xue, B.L.; Yu, J.S.; Tian, Z.F. Interannual and seasonal variations of hydrological connectivity in a large shallow wetland of north china estimated from landsat 8 images. Remote. Sens. 2021, 13, 1214. [Google Scholar] [CrossRef]
- An, X.; Jin, W.; Long, X.; Chen, S.; Qi, S.; Zhang, M. Spatial and temporal evolution of carbon stocks in Dongting Lake wetlands based on remote sensing data. Geocarto Int. 2022, 37, 14983–15009. [Google Scholar] [CrossRef]
- Shen, X.J.; Jiang, M.; Lu, X.G.; Liu, X.T.; Liu, B.; Zhang, J.Q.; Wang, X.W.; Tong, S.T.; Lei, G.C.; Wang, S.Z.; et al. Aboveground biomass and its spatial distribution pattern of herbaceous marsh vegetation in China. Sci. China Earth Sci. 2021, 64, 1115–1125. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, Q.; Chen, M.; Wu, Q.; Feng, Z.; Wang, Q.; Hu, Q. Root C, N and C stoichiometry of 22 plant species in Nanji wetlands of Poyang Lake. Acta Ecol. Sin. 2020, 40, 864–873. [Google Scholar]
- Xia, S.P.; Song, Z.L.; van Zwieten, L.; Guo, L.D.; Yu, C.X.; Wang, W.Q.; Li, Q.; Hartley, I.P.; Yang, Y.; Liu, H.; et al. Storage, patterns and influencing factors for soil organic carbon in coastal wetlands of China. Glob. Change Biol. 2022, 28, 6065–6085. [Google Scholar] [CrossRef]
- Wang, T.; Mu, C.C.; Sun, Z.Q.; Li, M.L.; Wang, W.J.; Xu, W.; Zhao, H.M. Variation of carbon source and sink along the environmental gradient from lakeside to highlands in Yuanchi swamp wetlands, Changbai Mountains, China. Chin. J. Appl. Ecol. 2023, 34, 2363–2373. [Google Scholar]
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Wang, B.; Mu, C.C.; Lu, H.C.; Li, N.; Zhang, Y.; Ma, L. Ecosystem carbon storage and sink/source of temperate forested wetlands in Xiaoxing’anling, northeast China. J. For. Res. 2021, 33, 839–849. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Mander, Ü. Wetlands and carbon revisited. Ecol. Eng. 2018, 114, 1–6. [Google Scholar] [CrossRef]





| Plant Name | Carbon Density (gC/m2) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Aboveground Live Carbon Density | Litter Carbon Density | Total Aboveground Carbon Density | Underground Root Carbon Density | Total Vegetation Carbon Density | Soil Carbon Density | Soil Depth (cm) | ||
| Phragmites australis | 416 | 41 | 457 | 1551 | 2008 | 1049 | 30 | 8 |
| Spartina alterniflora | 663 | 56 | 720 | 1418 | 2138 | 583 | ||
| Scirpus mariqueter | 66 | - | 66 | 245 | 312 | 344 | 30 | 8 |
| Phragmites australis | - | - | 680 | 340 | 1020 | - | - | 11 |
| Spartina alterniflora | - | - | 935 | 175 | 1100 | - | - | |
| Scirpus mariqueter | - | - | 260 | 70 | 330 | - | - | |
| Phragmites australis | 800–1240 | 50–100 | 850–1340 | 260–1070 | 1100–2410 | - | - | 10 |
| Cyperus papyrus | - | - | 17 | 91 | 108 | 2446 | 100 | 19 |
| Nelumbo sp. | - | - | 79–202 | 927–1668 | 1006–1870 | - | - | 13 |
| No. | Name | Aboveground Carbon Content (%) | Underground Carbon Content (%) | Aboveground Carbon Density (gC/m2) | Underground Carbon Density (gC/m2) |
|---|---|---|---|---|---|
| 1 | Alternanthera philoxeroides | 40.56 | 35.77 | 118 | 8.8 |
| 2 | Canna indica | 39.04 | 34.19 | 283 | 66 |
| 3 | Lythrum salicaria | 41.94 | 39.79 | 1035 | 621 |
| 4 | Typha angustifolia | 41.35 | 22.72 | 1729 | 537 |
| 5 | Nelumbo sp. | 38.91 | 50.30 * | 641 | 2064 |
| 6 | Nymphoides peltate | 37.21 | / | 34 | / |
| 7 | Acorus calamus | 39.59 | 39.14 | 64 | 92 |
| 8 | Nymphaea | 38.90 | 30.94 ** | 58 | 43 ** |
| 9 | Lemna minor | 34.46 | / | 5.1 | / |
| 10 | Arundo donax | 43.65 | 36.19 | 3211 | 1421 |
| 11 | Cyperus involucratus | 39.67 | 30.90 ** | 5455 | 4249 ** |
| 12 | Najas marina | 31.89 | / | 101 | / |
| 13 | Pontederia cordata | 37.09 | 19.81 | 1195 | 483 |
| 14 | Vallisneria natans | 26.43 | 12.05 | 369 | 153 |
| 15 | Potamogeton crispus | 23.18 | / | 335 | / |
| 16 | Miscanthus sinensis | 42.96 | 32.23 | 612 | 118 |
| 17 | Thalia dealbata | 41.52 | 28.24 | 1619 | 556 |
| 18 | Phragmites australis | 40.86 | 34.28 * | 1202 | 1502 * |
| 19 | Acorus tatarinowii | 38.65 | 20.21 | 270 | 274 |
| 20 | Ceratophyllum demersum | 34.08 | / | 197 | / |
| Plant Type | Emergent Plants | Free-Floating Plants | Floating-Leaved Plants | Submerged Plants |
|---|---|---|---|---|
| Carbon content of aboveground part (%) | 40.43 | 35.84 | 39.79 | 28.90 |
| Carbon content of underground part (%) | 32.02 | / | 33.35 | 12.05 |
| Aboveground carbon density (gC/m2) | 1390 | 20 | 81 | 253 |
| Underground carbon density (gC/m2) | 456 | / | 33 | 30 |
| Items | Emergent Plants | Floating Plants | Floating-Leaved Plants | Submerged Plants |
|---|---|---|---|---|
| Soil carbon content (‰) | 15.71 | / | 10.70 | 8.60 |
| Soil carbon density (gC/m2) | 3805 | / | 1824 | 1936 |
| Plant Name | RVI-MAX | RVI-MEAN | Aboveground Carbon Density (gC/m2) | Underground Carbon Density (gC/m2) | Soil Carbon Density (gC/m2) |
|---|---|---|---|---|---|
| Potamogeton crispus | 4.81 | 1.25 | 335 | / | / |
| Najas marina | 5.51 | 3.22 | 110 | / | / |
| Lemna minor | 6.55 | 2.45 | 5 | / | / |
| Ceratophyllum demersum | 6.94 | 3.55 | 197 | / | / |
| Vallisneria natans | 8.29 | 4.12 | 369 | 153 | 1936 |
| Acorus tatarinowii | 9.60 | 3.03 | 270 | 274 | 2352 |
| Alternanthera philoxeroides | 11.12 | 5.41 | 118 | 9 | 1824 |
| Miscanthus sinensis | 16.92 | 5.87 | 612 | 118 | 2181 |
| Typha angustifolia (Shanghai Fish) | 20.73 | 6.74 | 756 | 259 | 3572 |
| Phragmites australis | 20.92 | 9.01 | 1202 | - | - |
| Arundo donax | 21.41 | 8.40 | - | 1421 | - |
| Nymphaea (Dishui Lake) | 22.11 | 9.88 | - | 48 | 1824 |
| Typha angustifolia (Dishui Lake) | 25.71 | 9.67 | 1729 | 537 | - |
| Thalia dealbata | 27.69 | 9.36 | 1619 | 556 | 2191 |
| Nymphaea (Shanghai Fish) | 30.01 | 6.32 | - | 43 | 1824 |
| Pontederia cordata | 32.91 | 13.10 | 1195 | - | 2826 |
| Nelumbo sp. | 38.04 | 8.65 | 641 | 2064 | 5723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yu, J.; Shen, M.; Che, S. Analysis of Carbon Density Distribution Characteristics in Urban Wetland Ecosystems: A Case Study of Shanghai Fish and Dishui Lake. Water 2025, 17, 650. https://doi.org/10.3390/w17050650
Wang J, Yu J, Shen M, Che S. Analysis of Carbon Density Distribution Characteristics in Urban Wetland Ecosystems: A Case Study of Shanghai Fish and Dishui Lake. Water. 2025; 17(5):650. https://doi.org/10.3390/w17050650
Chicago/Turabian StyleWang, Jin, Jingren Yu, Manjuan Shen, and Shengquan Che. 2025. "Analysis of Carbon Density Distribution Characteristics in Urban Wetland Ecosystems: A Case Study of Shanghai Fish and Dishui Lake" Water 17, no. 5: 650. https://doi.org/10.3390/w17050650
APA StyleWang, J., Yu, J., Shen, M., & Che, S. (2025). Analysis of Carbon Density Distribution Characteristics in Urban Wetland Ecosystems: A Case Study of Shanghai Fish and Dishui Lake. Water, 17(5), 650. https://doi.org/10.3390/w17050650







