The Role of Climate Warming and Thermal Stratification in the Ecological Success of Diaphanosoma brachyurum in Lake Maggiore
Abstract
1. Introduction
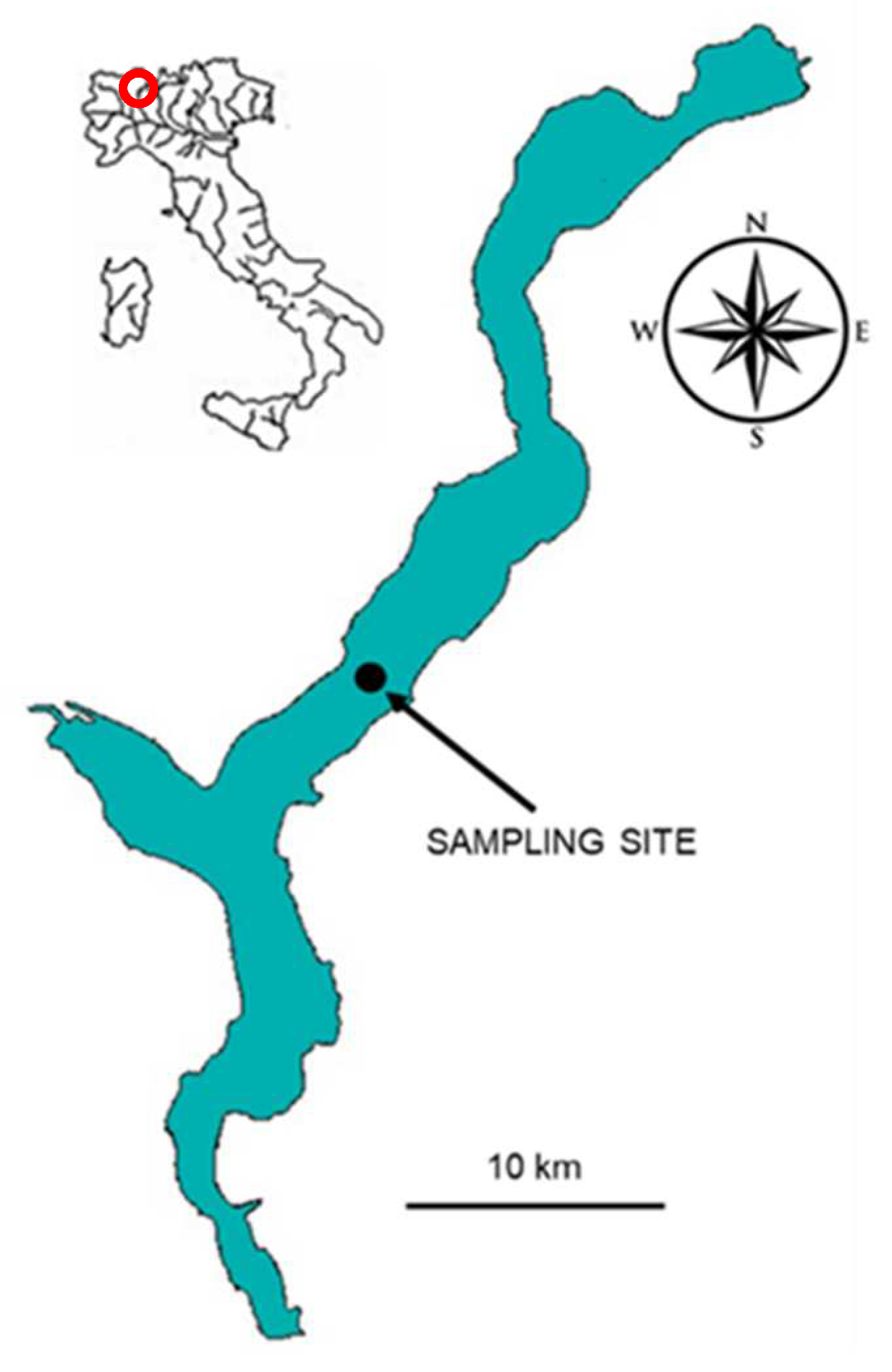
2. Materials and Methods
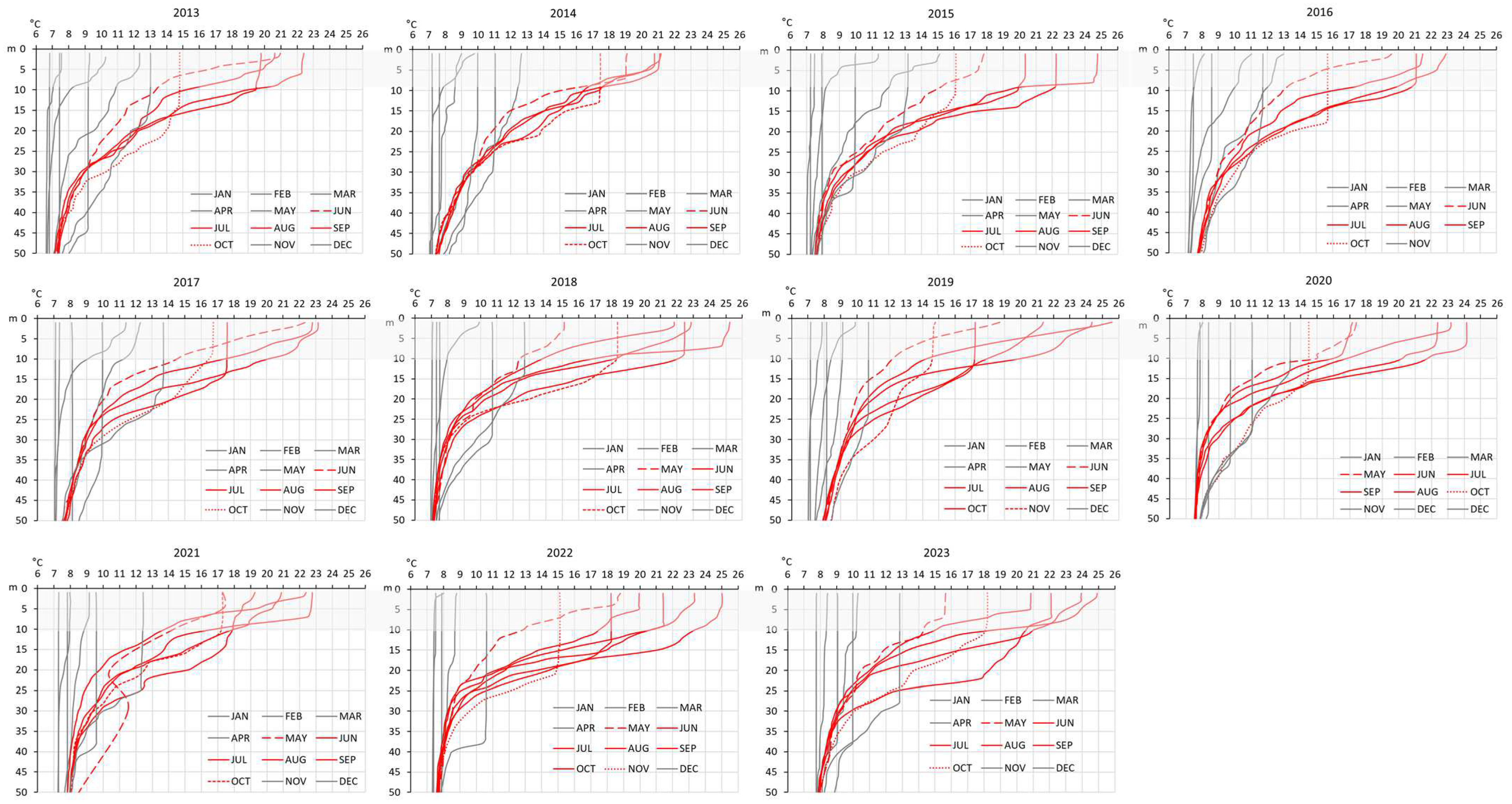
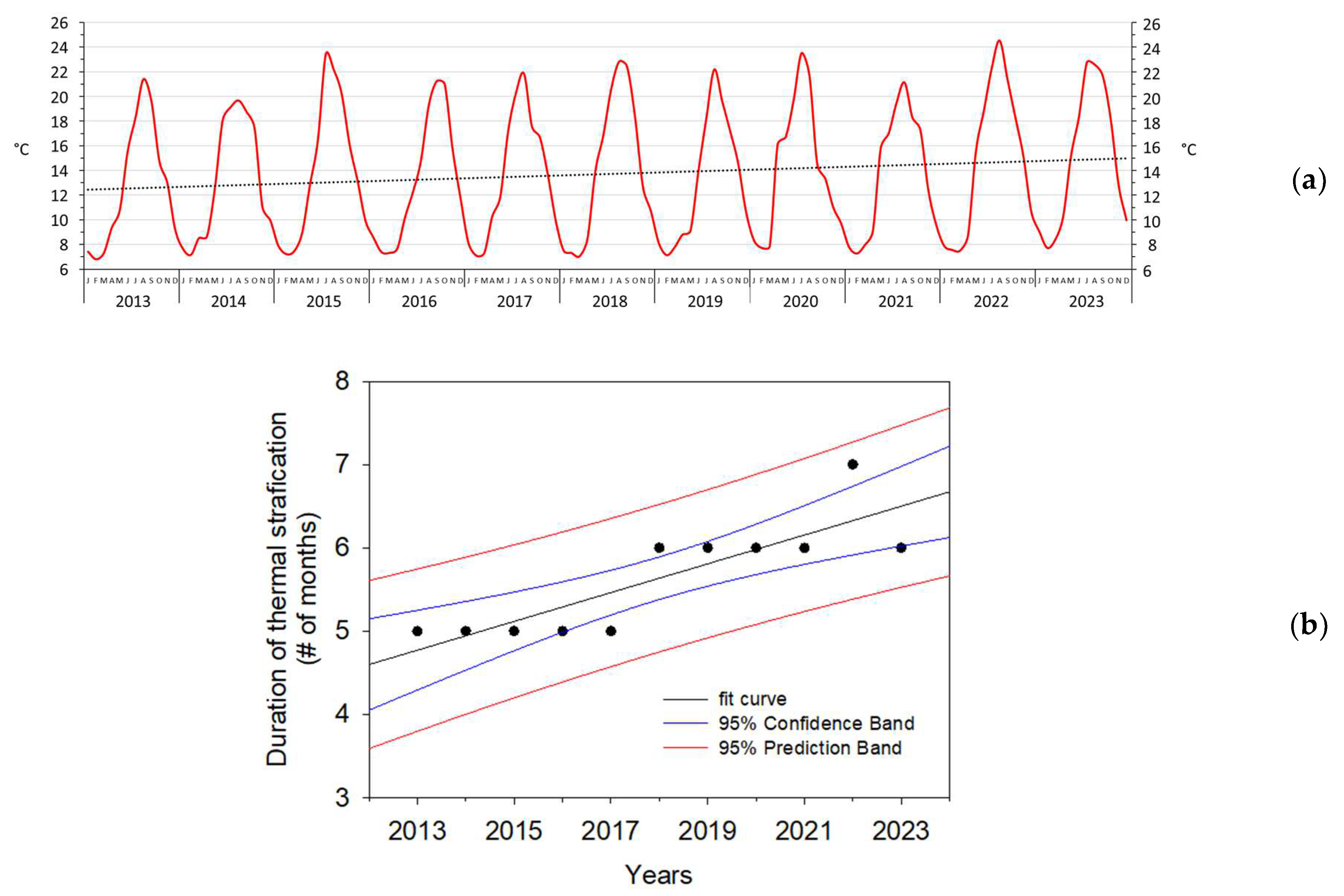
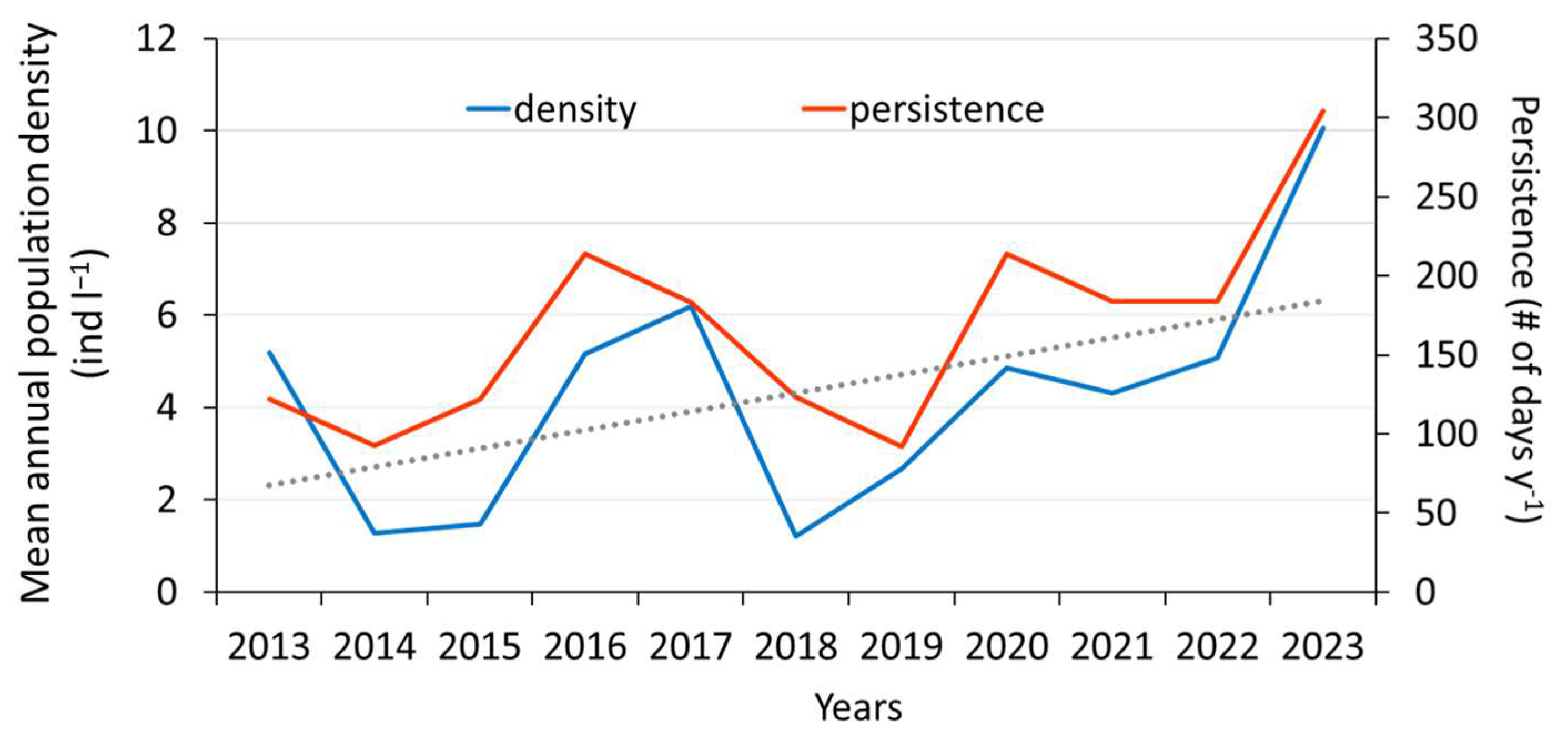
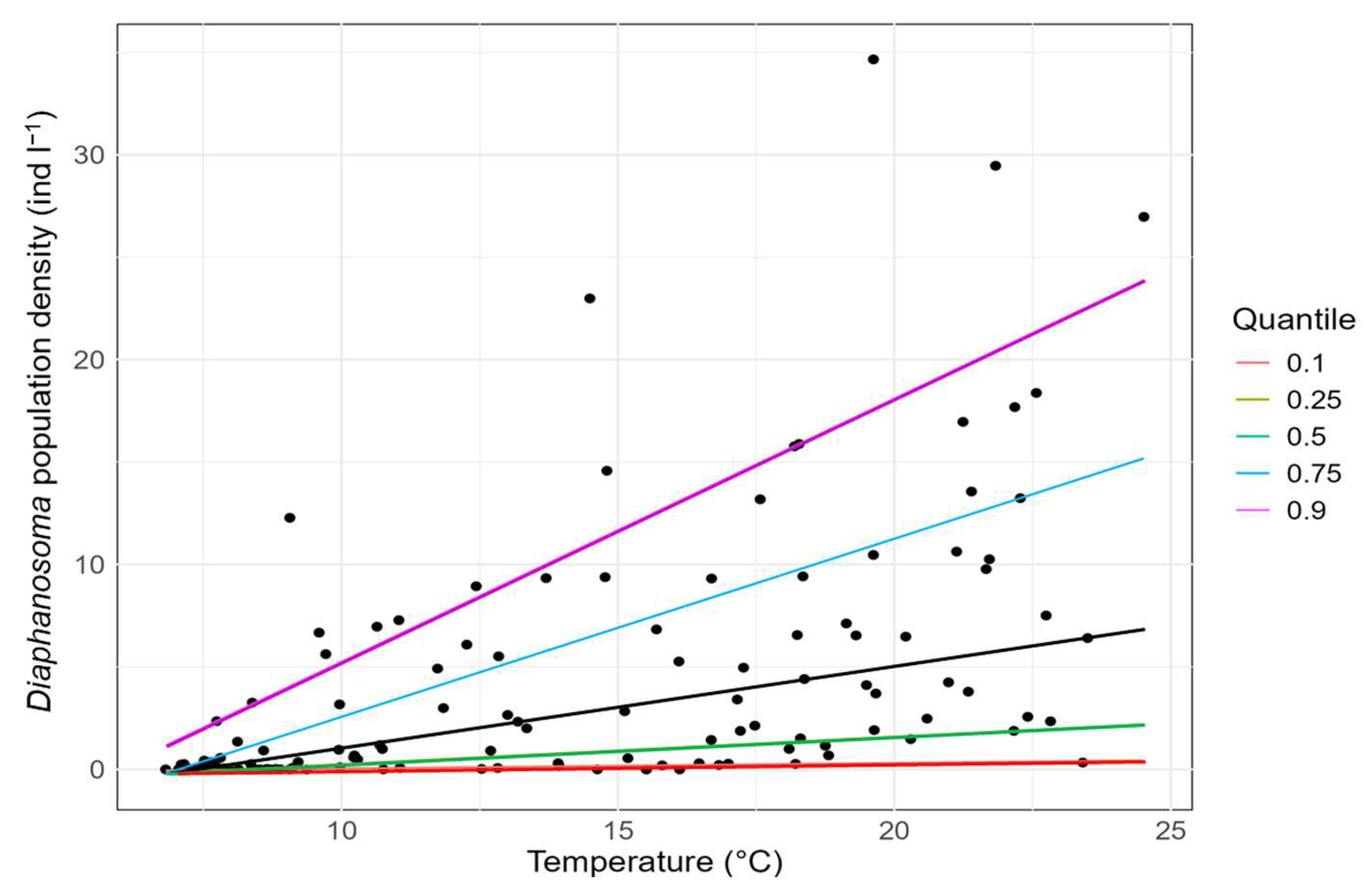
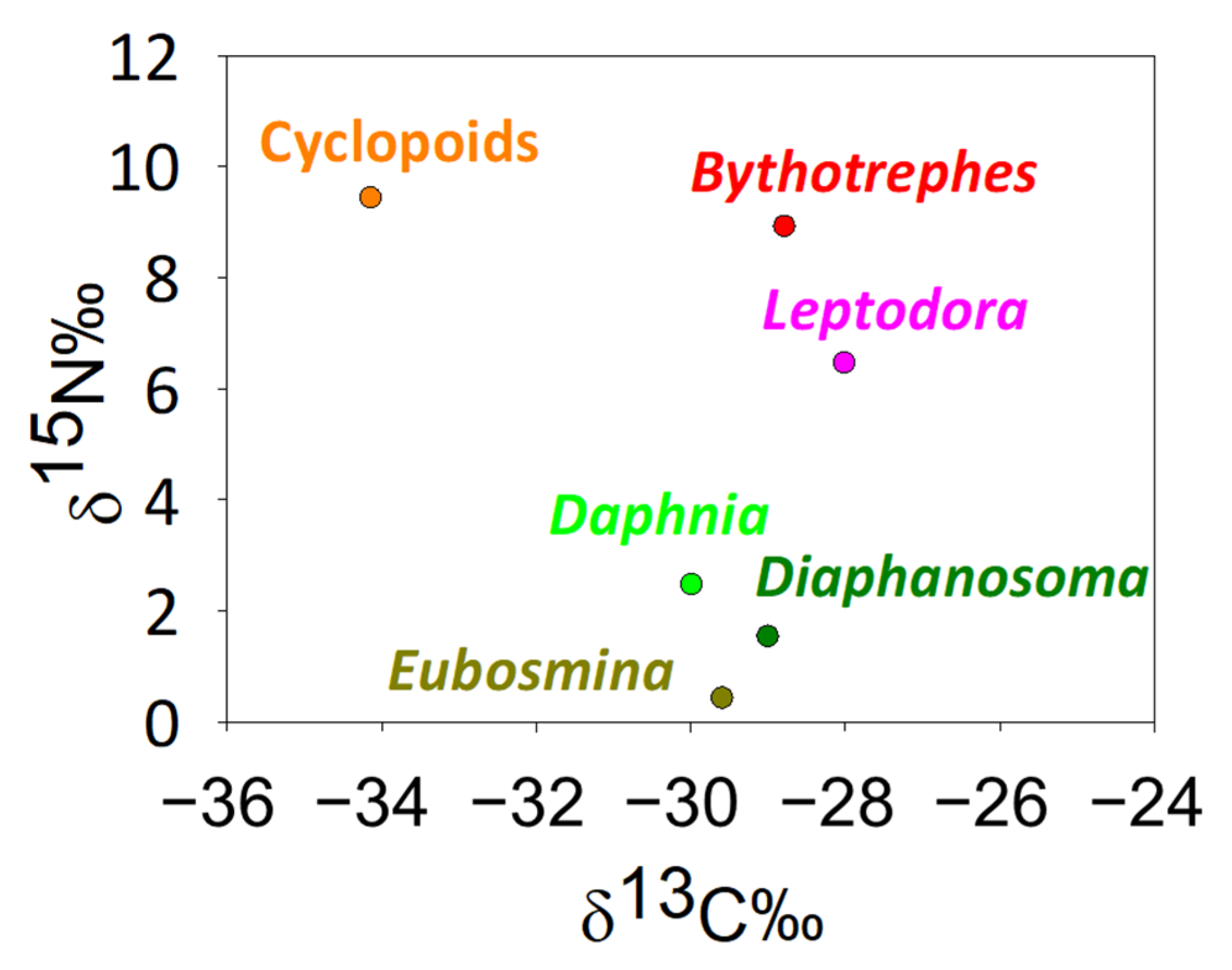
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Winder, M.; Schindler, D.E. Climatic effects on the phenology of lake processes. Glob. Change Biol. 2004, 10, 1844–1856. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evolut. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Peeters, F.; Straile, D.; Lorke, A.; Livingstone, D.M. Earlier onset of the spring phytoplankton bloom in lakes of the temperate zone in a warmer climate. Glob. Change Biol. 2007, 13, 1898–1909. [Google Scholar] [CrossRef]
- Thomas, D.W.; Blondel, J.; Perret, P.; Lambrechts, M.M.; Speakman, J.R. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 2001, 291, 2598–2600. [Google Scholar] [CrossRef]
- Post, E.; Forchhammer, M.C.; Stenseth, N.C.; Callaghan, T.V. The timing of life–history events in a changing climate. Proc. R. Soc. Biol. Sci. Ser. B 2001, 268, 15–23. [Google Scholar] [CrossRef]
- Visser, M.E.; Holleman, L.J. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Biol. Sci. Ser. B 2001, 268, 289–294. [Google Scholar] [CrossRef]
- Manca, M.; Torretta, B.; Comoli, P.; Amsinck, S.L.; Jeppesen, E. Major changes in trophic dynamics in large, deep sub-alpine Lake Maggiore from 1940s to 2002: A high resolution comparative palaeo-neolimnological study. Freshw. Biol. 2007, 52, 2256–2269. [Google Scholar] [CrossRef]
- Caroni, R.; Piscia, R.; Free, G.; Manca, M. Interpreting Seasonal Patterns and Long-Term Changes of Zooplankton in a Deep Subalpine Lake Using Stable Isotope Analysis. Water 2023, 15, 3143. [Google Scholar] [CrossRef]
- Han, B.P.; Yin, J.; Lin, X.; Dumont, H.J. Why is Diaphanosoma (Crustacea: Ctenopoda) so common in the tropics? Influence of temperature and food on the population parameters of Diaphanosoma dubium, and a hypothesis on the nature of tropical cladocerans. Hydrobiologia 2011, 668, 109–115. [Google Scholar] [CrossRef]
- Dumont, H.J.; Han, B.P.; Guo, F.F.; Chen, H.; Cheng, D.; Liu, P.; Xu, L.; Sanoamuang, L.-O.; Rietzler, A.C.; Xu, S.; et al. Toward a phylogeny and biogeography of Diaphanosoma (Crustacea: Cladocera). Aquat. Ecol. 2021, 55, 1207–1222. [Google Scholar] [CrossRef]
- Dumont, H.J. On the diversity of the Cladocera in the tropics. Hydrobiologia 1994, 272, 27–38. [Google Scholar] [CrossRef]
- Hanazato, T.; Yasuno, M. Effect of temperature in the laboratory studies on growth, egg development and first parturition of five species of Cladocera. Jpn. J. Limnol. 1985, 46, 185–191. [Google Scholar] [CrossRef]
- Verbitskii, V.B.; Verbitskaya, T.I.; Malysheva, O.A. Population dynamics of Daphnia longispina (OF Müller, 1785) and Diaphanosoma brachyurum (Lievin, 1848) (Crustacea, Cladocera) under stable and graded temperature regimes. Biol. Bull. 2009, 36, 66–73. [Google Scholar] [CrossRef]
- CNR IRSA. Sede di Verbania. In Ricerche Sull’evoluzione Del Lago Maggiore. Aspetti Limnologici. Programma Triennale 2022–2024; Campagna 2023; Commissione Internazionale per la Protezione delle Acque Italo-Svizzere, Ed.; CNR IRSA: Rome, Italy, 2024; 112p. [Google Scholar]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Joo, G.J. Spatio-temporal distribution of Diaphanosoma brachyurum (Cladocera: Sididae) in freshwater reservoir ecosystems: Importance of maximum water depth and macrophyte beds for avoidance of fish predation. J. Limnol. 2015, 74, 403–413. [Google Scholar] [CrossRef]
- Morabito, G.; Manca, M.; Ruggiu, D. Seasonal dynamics of planktonic communities in Lago Maggiore and clear-water phase during 1993. In Proceedings of the Atti XII Congresso AIOL, Isola di Vulcano, Italy, 18–21 September 1996; pp. 265–274. [Google Scholar]
- Doulka, E.; Kehayias, G. Seasonal vertical distribution and diel migration of zooplankton in a temperate stratified lake. Biologia 2011, 66, 308–319. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Morabito, G.; Volta, P.; Rogora, M.; Yan, N.D.; Manca, M. Climate warming restructures an aquatic food web over 28 years. Glob. Change Biol. 2020, 26, 6852–6866. [Google Scholar] [CrossRef]
- De Bernardi, R. Methods for the estimation of zooplankton abundance. In A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; IBP Handbook; Wiley: Chichester, UK, 1984; Volume 17, pp. 59–86. [Google Scholar]
- Zhdanova, S.M. Diaphanosoma mongolianum Ueno, 1938 (Cladocera: Sididae) in Lakes of Yaroslavl Oblast (Russia). Inland Water Biol. 2018, 11, 145–152. [Google Scholar] [CrossRef]
- Vijverberg, J.; Koelewijn, H.P. Size dependent mortality and production of Diaphanosoma brachyurum (Lieven) in an eutrophic lake. Verh. Int. Ver. Theor. Angew. Limnol. 1991, 24, 2768–2771. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Fenocchi, A.; Rogora, M.; Sibilla, S.; Ciampittiello, M.; Dresti, C. Forecasting the evolution in the mixing regime of a deep subalpine lake under climate change scenarios through numerical modelling (Lake Maggiore, Northern Italy/Southern Switzerland). Clim. Dyn. 2018, 51, 3521–3536. [Google Scholar] [CrossRef]
- Dresti, C.; Rogora, M.; Fenocchi, A. Hypolimnetic oxygen depletion in a deep oligomictic lake under climate change. Aquat. Sci. 2023, 85, 4. [Google Scholar] [CrossRef]
- Schindler, D.W.; Beaty, K.G.; Fee, E.J.; Cruikshank, D.R.; DeBruyn, E.R.; Findlay, D.L.; Linsey, G.A.; Shearer, J.A.; Stainton, M.P.; Turner, M.A. Effects of climatic warming on lakes of the central boreal forest. Science 1990, 250, 967–970. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Webster, K.E.; Assel, R.A.; Bowser, C.J.; Dillon, P.J.; Eaton, J.G.; Evans, H.E.; Fee, E.J.; Hall, R.I.; Mortsch, L.R.; et al. Potential effects of climate changes on aquatic systems: Laurentian Great Lakes and Precambrian Shield Region. Hydrol. Process. 1997, 11, 825–871. [Google Scholar] [CrossRef]
- Straile, D. Meteorological forcing of plankton dynamics in a large and deep continental European lake. Oecologia 2000, 122, 44–50. [Google Scholar] [CrossRef]
- Gerten, D.; Adrian, R. Species-specific changes in the phenology and peak abundance of freshwater copepods in response to warm summers. Freshw. Biol. 2002, 47, 2163–2173. [Google Scholar] [CrossRef]
- Arhonditsis, G.B.; Brett, M.T.; Degasperi, C.L.; Schindler, D.E. Meteorological forcing of thermal dynamics in Lake Washington (USA). Limnol. Oceanogr. 2004, 49, 256–270. [Google Scholar] [CrossRef]
- Geller, W.; Müller, H. The filtration apparatus of Cladocera: Filter mesh-sizes and their implications on food selectivity. Oecologia 1981, 49, 316–321. [Google Scholar] [CrossRef]
- Herzig, A. Temperature and life cycle strategies of Diaphanosoma brachyurum: An experimental study on development, growth, and survival. Arch. Hydrobiol. 1984, 101, 143–178. [Google Scholar]
- Ruggiu, D.; Morabito, G.; Panzani, P.; Pugnetti, A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy). In Phytoplankton and Trophic Gradients, Proceedings of the 10th Workshop of the International Association of Phytoplankton Taxonomy & Ecology (IAP), held in Granada, Spain, 21–29 June 1996; Springer: Dordrecht, The Netherlands, 1988; pp. 243–247. [Google Scholar]
- Kamenir, Y.; Morabito, G. Lago Maggiore oligotrophication as seen from the long-term evolution of its phytoplankton taxonomic size structure. J. Limnol. 2009, 68, 146–161. [Google Scholar] [CrossRef]
- Caroni, R.; Piscia, R.; Manca, M. Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy). Water 2025, 17, 511. [Google Scholar] [CrossRef]
- Matveev, V.F. Effect of competition on the demography of planktonic cladocerans—Daphnia and Diaphanosoma. Oecologia 1987, 74, 468–477. [Google Scholar] [CrossRef]
- Adamczuk, M. Predation follows competition in depth selection behaviour of Cladocera in a deep lake (E Poland). Biol. Lett. 2009, 46, 29–36. [Google Scholar] [CrossRef]
- Nagata, T. Filter mesh-sizes of Daphnia longispina and its filtering rates on natural bacteria. Mem. Fac. Sci. Kyoto Univ. Ser. Biol 1985, 10, 109–114. [Google Scholar]
- Gliwicz, Z.M. Studies on the feeding of pelagic zooplankton in lakes with varying trophy. Ekol. Pol. Ser. A. 1969, 12, 663–708. [Google Scholar]
- De Bernardi, R.; Giussani, G.; Manca, M. Cladocera: Predators and prey. In Cladocera: Proceedings of the Cladocera Symposium, Budapest 1985; Springer: Dordrecht, The Netherlands, 1987; pp. 225–243. [Google Scholar]
- Pothoven, S.A. The influence of ontogeny and prey abundance on feeding ecology of age-0 Lake Whitefish (Coregonus clupeaformis) in southeastern Lake Michigan. Ecol. Freshw. Fish 2020, 29, 103–111. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Jones, R.I.; Grey, J.; Sleep, D.; Quarmby, C. An assessment, using stable isotopes, of the importance of allochthonous organic carbon sources to the pelagic food web in Loch Ness. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 105–110. [Google Scholar] [CrossRef]
- Fulton, R.S.; Paerl, H.W. Effects of the blue-green alga Microcystis aeruginosa on zooplankton competitive relations. Oecologia 1988, 76, 383–389. [Google Scholar] [CrossRef]
- Nandini, S.; Sarma, S.S.S. Experimental studies on zooplankton-toxic cyanobacteria interactions: A review. Toxics 2023, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.P.; Carpenter, E.J.; Capone, D.G. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol. Oceanogr. 2002, 47, 1617–1628. [Google Scholar] [CrossRef]
- De Bernardi, R.; Canali, S. Population dynamics of pelagic cladocerans in Lago Maggiore. Mem. Ist. ital. Idrobiol. 1975, 32, 365–392. [Google Scholar]
- Lampert, W.; Fleckner, W.; Rai, H.; Taylor, B.E. Phytoplankton control by grazing zooplankton: A study on the spring clear-water phase 1. Limnol. Oceanogr. 1986, 31, 478–490. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. NatL. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; Quintana, X.D.; Jensen, E.; Nathansen, L.W.; Trochine, C.; Meerhoff, M.; Gascó, S.; Jeppesen, E. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: Implications for effects of climate change. Limnol. Oceanogr. 2010, 55, 1697–1711. [Google Scholar] [CrossRef]
- Moore, M.; Folt, C. Zooplankton body size and community structure: Effects of thermal and toxicant stress. Trends Ecol. Evol. 1993, 8, 178–183. [Google Scholar] [CrossRef]
- Zohary, T.; Flaim, G.; Sommer, U. Temperature and the size of freshwater phytoplankton. Hydrobiologia 2021, 848, 143–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscia, R.; Caroni, R.; Dresti, C.; Manca, M. The Role of Climate Warming and Thermal Stratification in the Ecological Success of Diaphanosoma brachyurum in Lake Maggiore. Water 2025, 17, 768. https://doi.org/10.3390/w17050768
Piscia R, Caroni R, Dresti C, Manca M. The Role of Climate Warming and Thermal Stratification in the Ecological Success of Diaphanosoma brachyurum in Lake Maggiore. Water. 2025; 17(5):768. https://doi.org/10.3390/w17050768
Chicago/Turabian StylePiscia, Roberta, Rossana Caroni, Claudia Dresti, and Marina Manca. 2025. "The Role of Climate Warming and Thermal Stratification in the Ecological Success of Diaphanosoma brachyurum in Lake Maggiore" Water 17, no. 5: 768. https://doi.org/10.3390/w17050768
APA StylePiscia, R., Caroni, R., Dresti, C., & Manca, M. (2025). The Role of Climate Warming and Thermal Stratification in the Ecological Success of Diaphanosoma brachyurum in Lake Maggiore. Water, 17(5), 768. https://doi.org/10.3390/w17050768








