Regenerable Biochar Catalyst from Biogas Residue for Peroxymonosulfate Activation in Bisphenol A-Containing Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BR-Based Biochar Preparation
2.3. BPA Degradation by Peroxymonosulfate Activation Test and Catalyst Stability Evaluation
2.4. Structural Characterizations
2.5. Analytical Methods
3. Results and Discussion
3.1. Catalytic Performance of BR-Based Biochar Catalysts
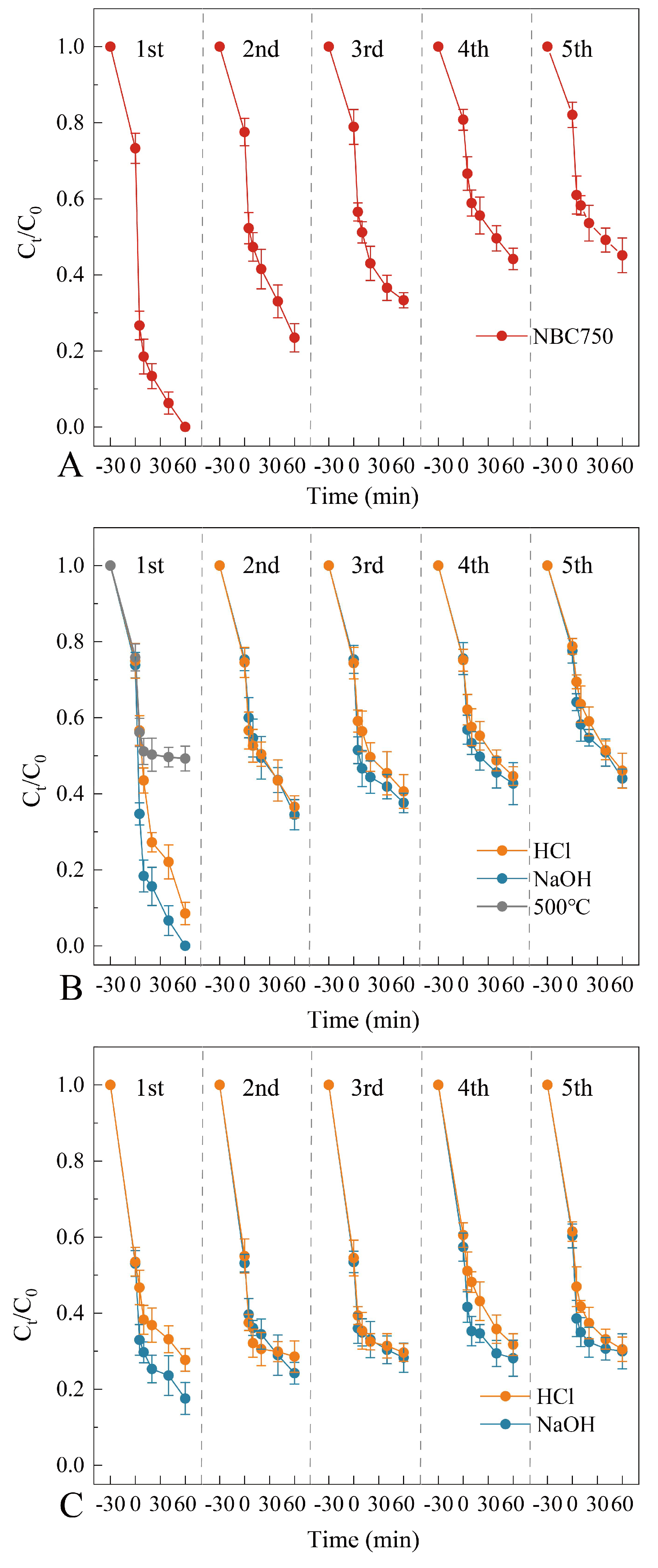
3.2. Stability Tests and Reactivation of BR-Based Biochar Catalysts
3.3. Role of Radical and Non-Radical Process in BR-Based Biochar Catalysts in PMS System
3.4. Characteristic Changes in BR-Based Biochar Catalysts in PMS System
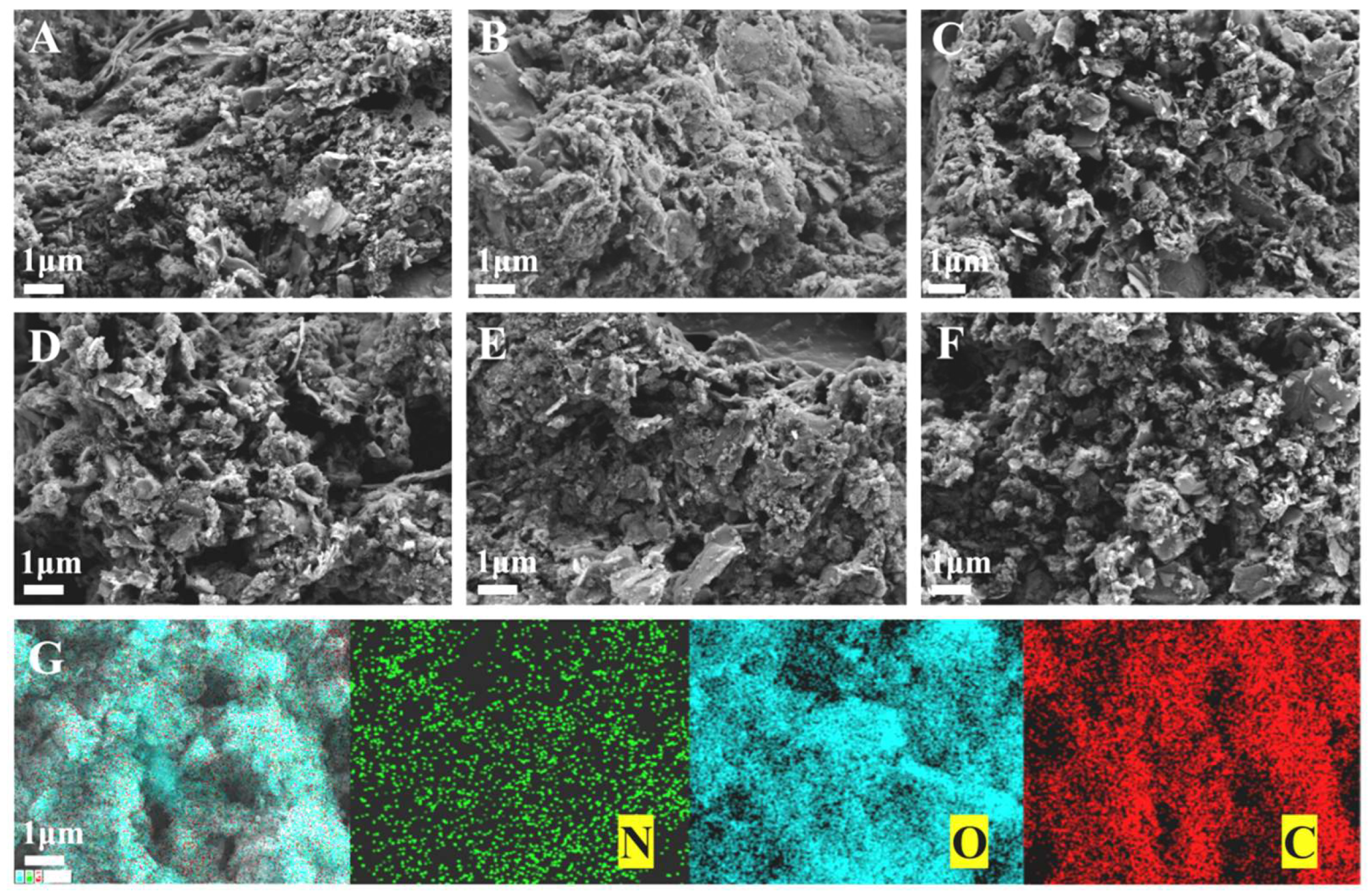
3.4.1. Surface Morphology and Porosity Analysis
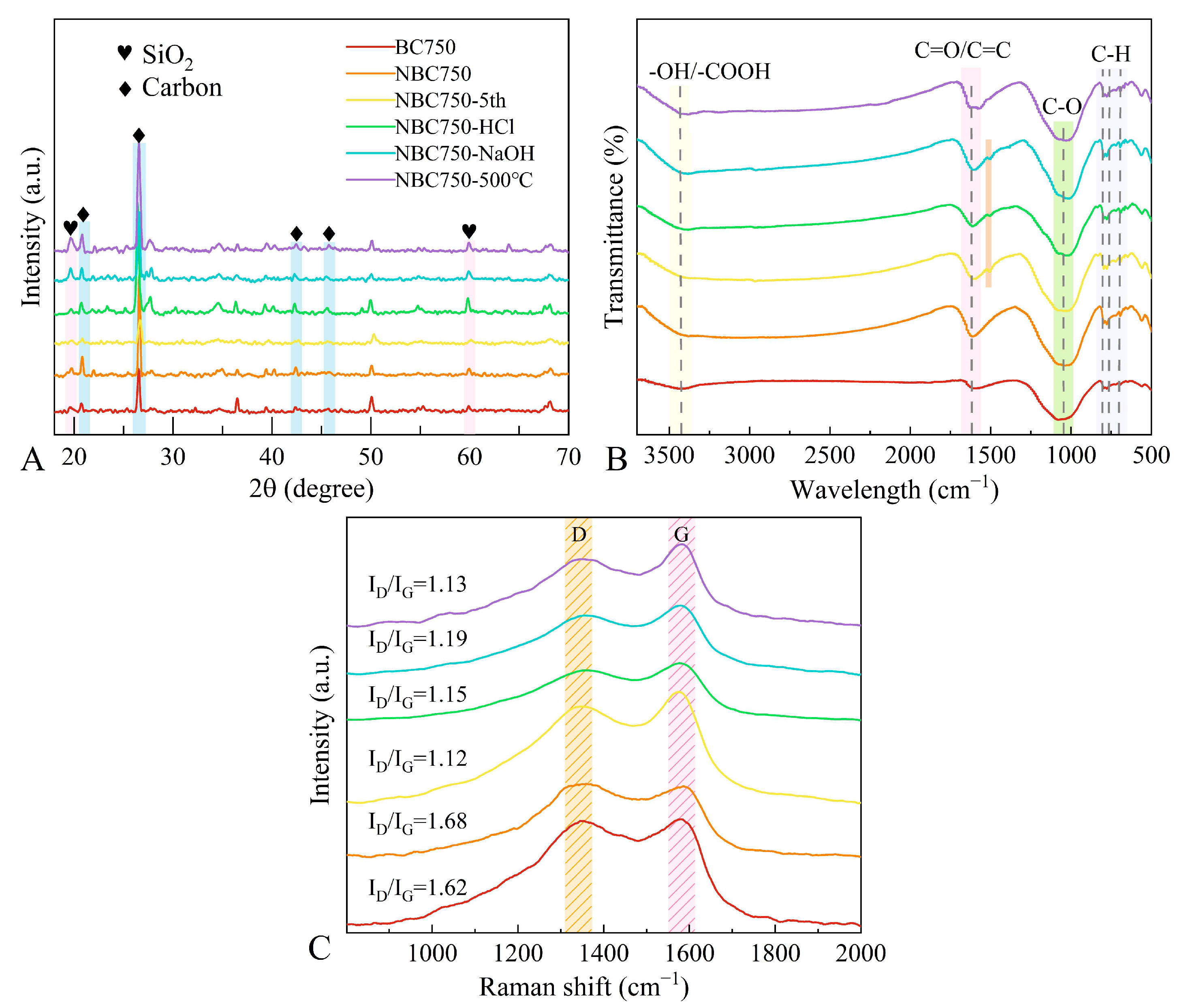
3.4.2. Molecular Structure Analysis
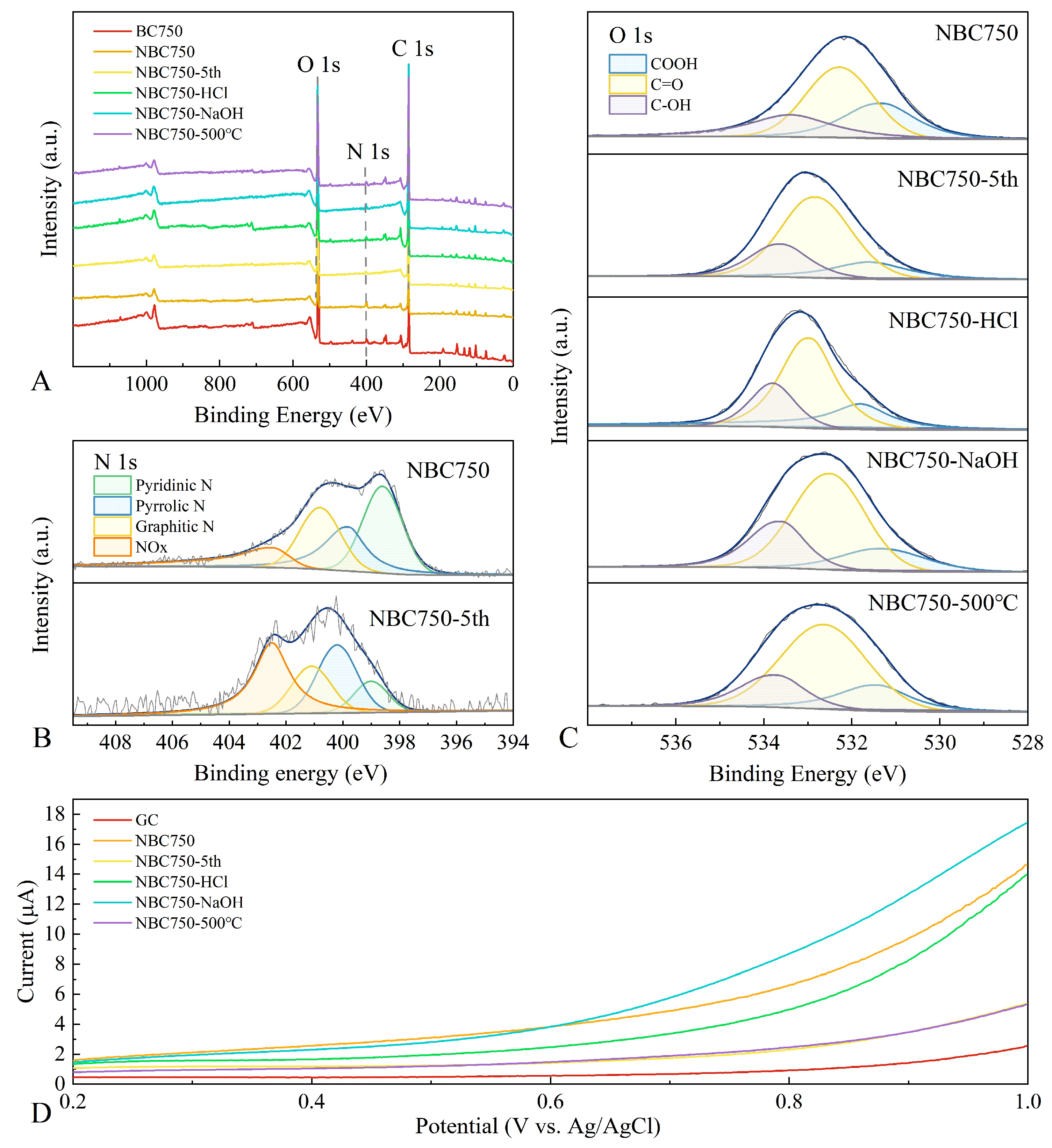
3.4.3. Chemical Components on the BR-Based Biochar Catalyst Surface
3.4.4. Mechanism of Catalytic and Regeneration of the BR-Based Biochar Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Sun, Z.F.; Pan, X.W.; Tan, J.Y.; Yang, S.S.; Wu, J.T.; Chen, C.; Yuan, Y.; Ren, N.Q. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. X 2023, 18, 12. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qin, Y.J.; Chen, B.Q.; Wu, C.L.; Zheng, S.H.; Chen, R.L.; Yang, S.H.; Yang, L.; Liu, Z.J. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ. Res. 2020, 188, 9. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, Y.D.; Yang, Z.K.; Nagarajan, D.; Chang, J.S.; Ren, N.Q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kummu, M.; Guillaume, J.H.A.; De Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- Xiao, H.P.; Li, K.; Zhang, D.Q.; Tang, Z.H.; Niu, X.J.; Yi, L.Z.; Lin, Z.; Fu, M.L. Environmental, energy, and economic impact assessment of sludge management alternatives based on incineration. J. Environ. Manag. 2022, 321, 115848. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Q.; Liang, N.; Ji, P.X.; Lu, M.; Wu, M.; Oleszczuk, P.; Pan, B.; Xing, B.S. The promoted degradation of biochar-adsorbed 2,4-dichlorophenol in the presence of Fe(III). J. Hazard. Mater. 2023, 458, 131774. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Chen, M.F.; Sun, C.T.; Han, Y.Y.; Xu, L.L.; Zhao, Y.M. Synergistic enhancement in hydrodynamic cavitation combined with peroxymonosulfate fenton-like process for bpa degradation: New insights into the role of cavitation bubbles in regulation reaction pathway. Water Res. 2025, 268, 13. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Guo, Y.J.; Ma, C.Y.; Gao, Z.Y.; Wu, M.Z.; Shen, C.C.; Xu, Z.H. Insights into mechanism of peroxymonosufate activation by Mo single-atom catalysts: Singlet oxygen evolution and role of Mo-N coordination. J. Environ. Manag. 2024, 358, 120846. [Google Scholar] [CrossRef]
- Jiang, S.F.; Ling, L.L.; Chen, W.J.; Liu, W.J.; Li, D.C.; Jiang, H. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Hou, S.Y.; Hu, H.L.; Fu, Q.; Xiao, T.Y.; Xie, J.Q.; Chan, S.H.; He, M.J.; Miao, B.; Zhang, L. Undaria pinnatifida (wakame)-derived Fe, N co-doped graphene-like hierarchical porous carbon as highly efficient catalyst for activation of peroxymonosulfate (PMS) toward degradation of tetracycline (TC). Sep. Purif. Technol. 2024, 333, 13. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Liu, F.; Yin, J.L.; Li, W.; Dai, X.Q.; Zhou, P.; Liu, Y.; Lai, B. Immobilizing cobalt-iron bimetal on Al2O3 by electroless plating-calcination for peroxymonosulfate activation: Performance, mechanistic and practicality. Chem. Eng. J. 2024, 488, 11. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.M.; Pan, Y.L.; Su, C.; Duan, X.G.; Sun, H.Q.; Liu, S.M.; Wang, S.B.; Shao, Z.P. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Huang, S.M.; Wang, T.; Chen, K.; Mei, M.; Liu, J.X.; Li, J.P. Engineered biochar derived from food waste digestate for activation of peroxymonosulfate to remove organic pollutants. Waste Manag. 2020, 107, 211–218. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 26. [Google Scholar] [CrossRef]
- He, C.; Wang, J.B.; Wang, C.R.; Zhang, C.H.; Hou, P.; Xu, X.Y. Catalytic ozonation of bio-treated coking wastewater in continuous pilot- and full-scale system: Efficiency, catalyst deactivation and in-situ regeneration. Water Res. 2020, 183, 116090. [Google Scholar] [CrossRef]
- Kong, X.T.; Garg, S.; Chen, G.F.; Waite, D. Investigation of the deactivation and regeneration of an Fe2O3/Al2O3·SiO2 catalyst used in catalytic ozonation of coal chemical industry wastewater. J. Hazard. Mater. 2023, 451, 131194. [Google Scholar] [CrossRef]
- Yang, S.J.; Qiu, X.J.; Jin, P.K.; Dzakpasu, M.; Wang, X.C.C.; Zhang, Q.H.; Zhang, L.; Yang, L.; Ding, D.H.; Wang, W.D.; et al. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Hou, J.F.; Xu, L.X.; Han, Y.X.; Tang, Y.Q.; Wan, H.Q.; Xu, Z.Y.; Zheng, S.R. Deactivation and regeneration of carbon nanotubes and nitrogen-doped carbon nanotubes in catalytic peroxymonosulfate activation for phenol degradation: Variation of surface functionalities. RSC Adv. 2019, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.R.; Tong, W.H.; Li, Y.L.; Xie, Y.; Chen, Y.D.; Wen, Z.Q.; Feng, S.F.; Wang, X.Q.; Li, P.Y.; Wang, Y.B.; et al. Hydrothermal route-enabled synthesis of sludge-derived carbon with oxygen functional groups for bisphenol A degradation through activation of peroxymonosulfate. J. Hazard. Mater. 2020, 388, 121801. [Google Scholar] [CrossRef]
- Cheng, C.; Li, J.P.; Wen, Y.Z.; Wang, J.L.; Jin, C.Y.; Sun, C.L.; Wang, H.L.; Wei, H.Z.; Yang, X.J. Deactivation mechanism of Fe/Al2O3 catalyst during the ozonation of reverse osmosis concentrates (ROCs): Effect of silicate. Chem. Eng. J. Adv. 2020, 1, 8. [Google Scholar] [CrossRef]
- Wang, G.L.; Chen, S.; Quan, X.; Yu, H.T.; Zhang, Y.B. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 2017, 115, 730–739. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Chen, M.; Yang, C.X.; Yu, M.H.; Han, M.Y.; Meng, Z.H.; Zhao, T.; Niu, J.R.; Mu, S.T.; Zhang, J.; Ma, J.J.; et al. N-doped carbon achieved by pyrolyzing urea and active carbon for the capacitive deionization coupled PMS oxidation system. Desalination 2024, 587, 12. [Google Scholar] [CrossRef]
- Edwin, N.N.; Garcia, T.; Solsona, B.; Taylor, S.H. The influence of cerium to urea preparation ratio of nanocrystalline ceria catalysts for the total oxidation of naphthalene. Catal. Today 2008, 137, 373–378. [Google Scholar] [CrossRef]
- Chen, Z.G.; Lei, C.; Yao, L.L.; Mo, Y.; Li, J.X.; Qu, H.W.; Zhou, Z.; Luo, W. Synergistic pyrolysis of rice and chili straw under N2/CO2 atmosphere: Nutritional elements (N/P/K) migration and transformation from straw to pyrolysis products. Energy 2025, 316, 13. [Google Scholar] [CrossRef]
- Xiao, T.; Zhou, P.K.; Liu, Y.; Zhang, K.K.; Liu, F.Y.; Guo, G.; Ni, F.Q.; Deng, Y. Impact of pyrolysis temperature on heavy metals environmental risk in biochar derived from co-pyrolysis of Alternanthera philoxeroides and sludge. J. Environ. Chem. Eng. 2024, 12, 9. [Google Scholar] [CrossRef]
- Zhao, C.H.; Shao, B.B.; Yan, M.; Liu, Z.F.; Liang, Q.H.; He, Q.Y.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.G.; Gao, J.; Shen, Y.; Feng, X.H.; Yu, Z.J.; Tan, X.Y.; Liu, S.M.; Wang, S.B. Roles of structure defect, oxygen groups and heteroatom doping on carbon in nonradical oxidation of water contaminants. Water Res. 2020, 185, 116244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lan, X.; Peng, W.Y.; Wang, Z.H. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef]
- Tang, W.Q.; Liu, Y.Y.; You, Q.L.; Yang, X.F.; Liao, G.Y.; Wang, D.S.; Yan, Y.; Shang, Q.G. Constructing a 3D ordered macroporous cobalt monoatomic catalyst for efficient SMX degradation via PMS activation. J. Environ. Chem. Eng. 2024, 12, 12. [Google Scholar] [CrossRef]
- Oh, W.D.; Lim, T.T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Zhu, K.M.; Wang, X.S.; Geng, M.Z.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.L.; Yuan, X.H.; Yu, Z.W.; Zhang, H.; Duan, X.G.; Wang, S.B. Activation of Peroxydisulfate on Carbon Nanotubes: Electron-Transfer Mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Li, W.Z.; Feng, J.; Zhao, Y.; Zhang, H.X.; Ren, Y.M. Nitrogen-doped biochar/MnO2 as an efficient PMS activator for synergistic BPA degradation via non-free radical pathways in the water. J. Environ. Chem. Eng. 2024, 12, 112446. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 17. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Q.; Wang, Z.Q.; Hua, S.; Ding, D.H.; Cai, T.M.; Zhang, R.H. Pyrrolic N-rich biochar without exogenous nitrogen doping as a functional material for bisphenol A removal: Performance and mechanism. Appl. Catal. B Environ. 2021, 291, 120093. [Google Scholar] [CrossRef]
- Fan, H.L.; Wang, J.X.; Wu, P.P.; Zheng, L.; Xiang, J.F.; Liu, H.L.; Han, B.X.; Jiang, L. Hydrophobic ionic liquid tuning hydrophobic carbon to superamphiphilicity for reducing diffusion resistance in liquid-liquid catalysis systems. Chem 2021, 7, 1852–1869. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas Solid Systems with Special Reference To the Determination of Surface-Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Song, W.; Yu, Z.H.; Li, H.; Ji, Y.Q.; Cao, L.L.; Ren, L.Y.; Li, X.G.; Li, Y.F.; Xu, X.; Yan, L.G. Insights into the factors influencing the oxidation of antibiotic pollutants in nitrogen-doped biochar/PMS system: The roles of physicochemical properties and reaction pathways. Chem. Eng. J. 2024, 498, 155601. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Liu, W.J.; Nie, C.Y.; Li, W.L.; Ao, Z.M.; Wang, S.B.; An, T.C. Oily sludge derived carbons as peroxymonosulfate activators for removing aqueous organic pollutants: Performances and the key role of carbonyl groups in electron-transfer mechanism. J. Hazard. Mater. 2021, 414, 125552. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.M.; Chen, B.L. Adsorption of Polycyclic Aromatic Hydrocarbons by Graphene and Graphene Oxide Nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Li, S.J.; Wulan, B.R.; Yan, J.M.; Jiang, Q. Anchoring PdCu Amorphous Nanocluster on Graphene for Electrochemical Reduction of N2 to NH3 under Ambient Conditions in Aqueous Solution. Adv. Energy Mater. 2018, 8, 6. [Google Scholar] [CrossRef]
- Wu, C.X.; Li, L.F.; Zhou, H.; Ai, J.; Zhang, H.T.; Tao, J.L.; Wang, D.S.; Zhang, W.J. Effects of chemical modification on physicochemical properties and adsorption behavior of sludge-based activated carbon. J. Environ. Sci. 2021, 100, 340–352. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Hu, Z.T.; Sun, Y.M.; Webster, R.D.; Li, S.Z.; Lim, T.T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.L.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Li, M.; Li, D.Y.; Guan, Z.Y.; Xu, Q.Q.; Shi, Y.T.; Xia, D.S. Carboxy-functionalized sludge-derived biochar for efficiently activating peroxymonosulfate to degrade bisphenol A. Sep. Purif. Technol. 2022, 297, 121525. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Yang, X.; Wei, H.; Liu, X.; Wang, P.; Duan, N.; Lin, M. Regenerable Biochar Catalyst from Biogas Residue for Peroxymonosulfate Activation in Bisphenol A-Containing Wastewater Treatment. Water 2025, 17, 744. https://doi.org/10.3390/w17050744
Pan Y, Yang X, Wei H, Liu X, Wang P, Duan N, Lin M. Regenerable Biochar Catalyst from Biogas Residue for Peroxymonosulfate Activation in Bisphenol A-Containing Wastewater Treatment. Water. 2025; 17(5):744. https://doi.org/10.3390/w17050744
Chicago/Turabian StylePan, Yating, Xue Yang, Haijuan Wei, Xiang Liu, Pan Wang, Nina Duan, and Miao Lin. 2025. "Regenerable Biochar Catalyst from Biogas Residue for Peroxymonosulfate Activation in Bisphenol A-Containing Wastewater Treatment" Water 17, no. 5: 744. https://doi.org/10.3390/w17050744
APA StylePan, Y., Yang, X., Wei, H., Liu, X., Wang, P., Duan, N., & Lin, M. (2025). Regenerable Biochar Catalyst from Biogas Residue for Peroxymonosulfate Activation in Bisphenol A-Containing Wastewater Treatment. Water, 17(5), 744. https://doi.org/10.3390/w17050744





