Reconstructing the Historical Density, Size, and Age Structure of the Noble Pen Shell (Pinna nobilis) Population: Insights from Malo Jezero Lagoon, Mljet National Park (Adriatic Sea)
Abstract
1. Introduction
2. Materials and Methods
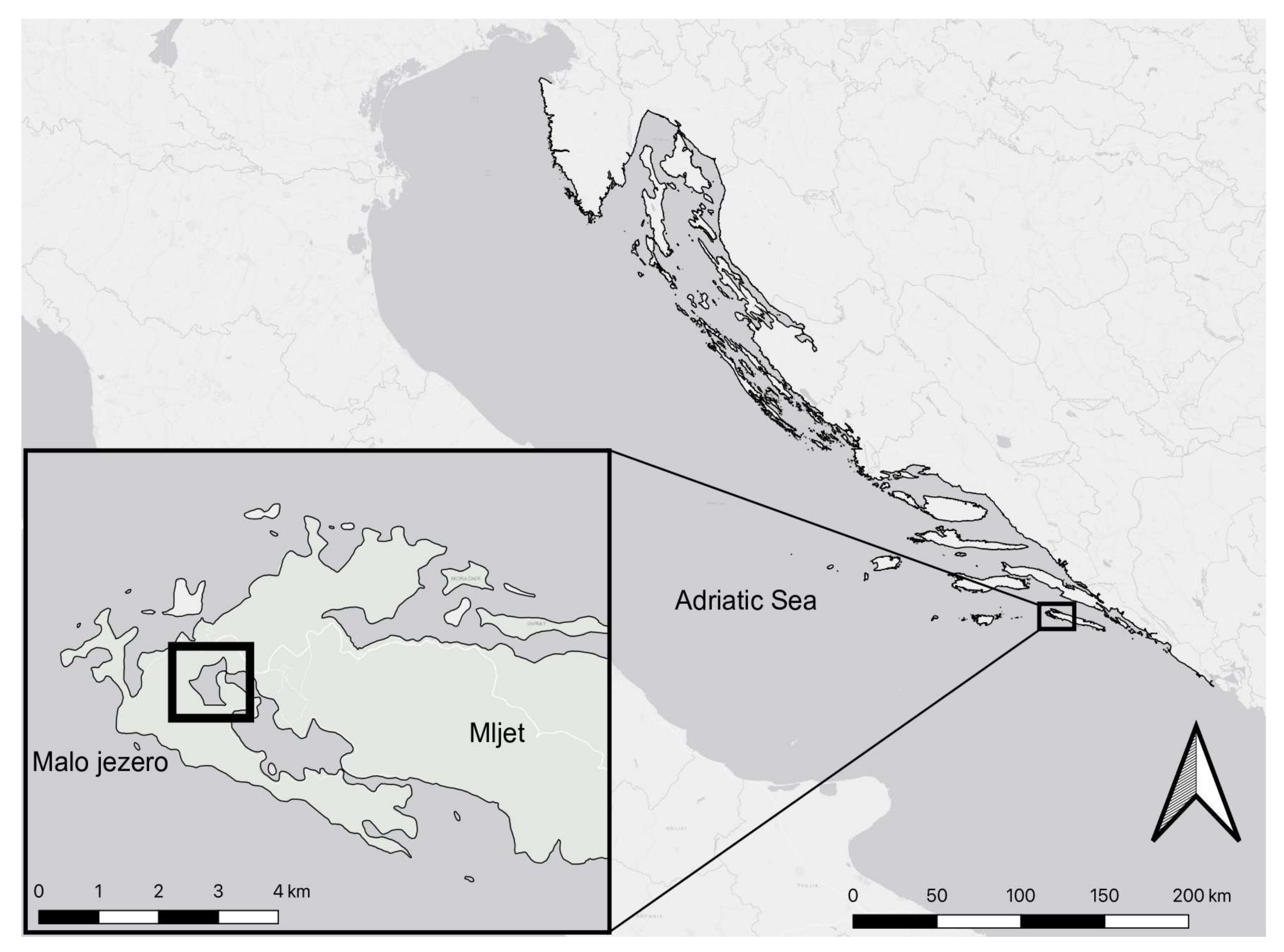
2.1. Study Location
2.2. Sampling Design and Density Measurement
2.3. Size and Age Estimation
2.4. Statistical Analysis
3. Results
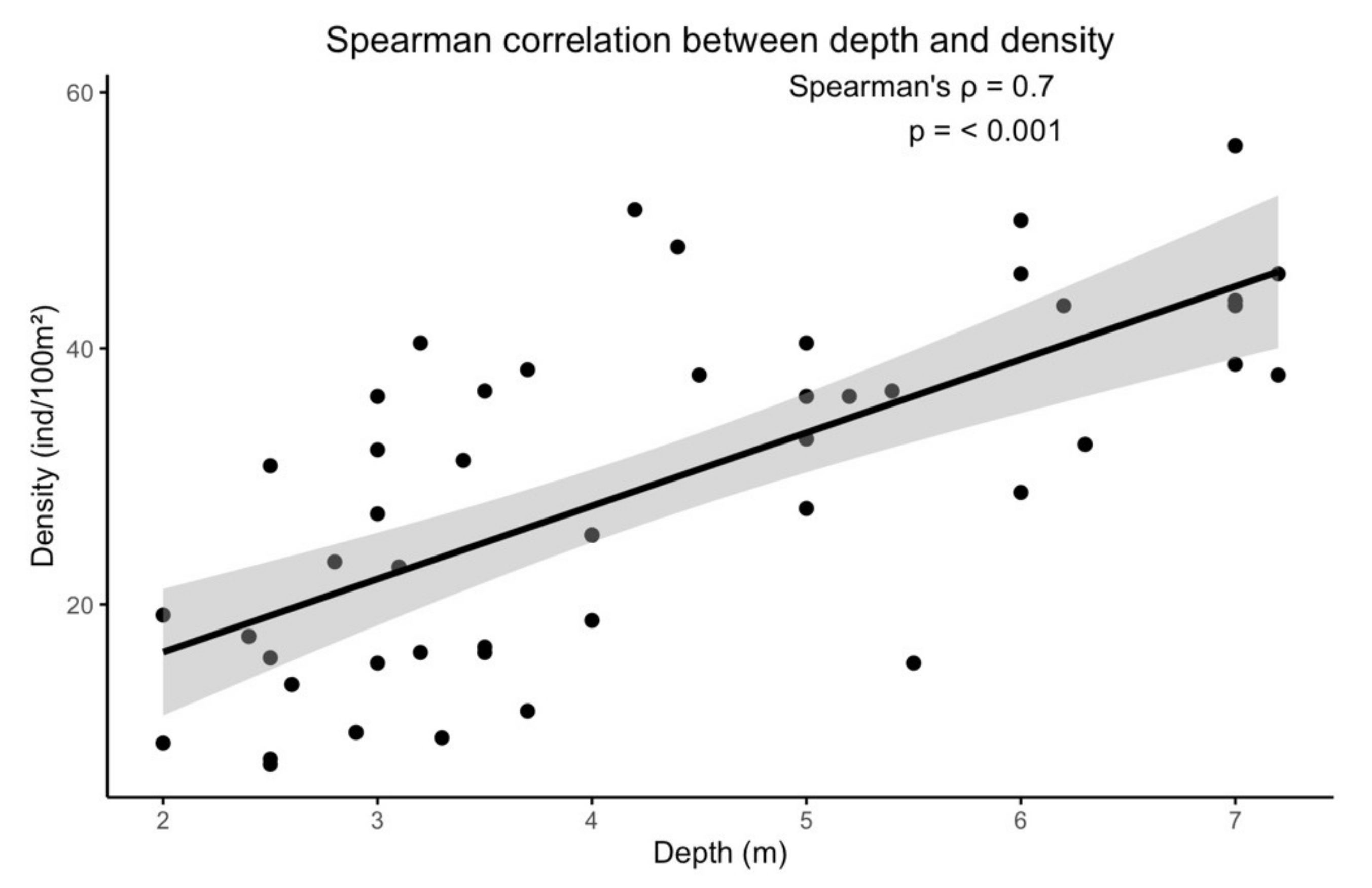
3.1. Density
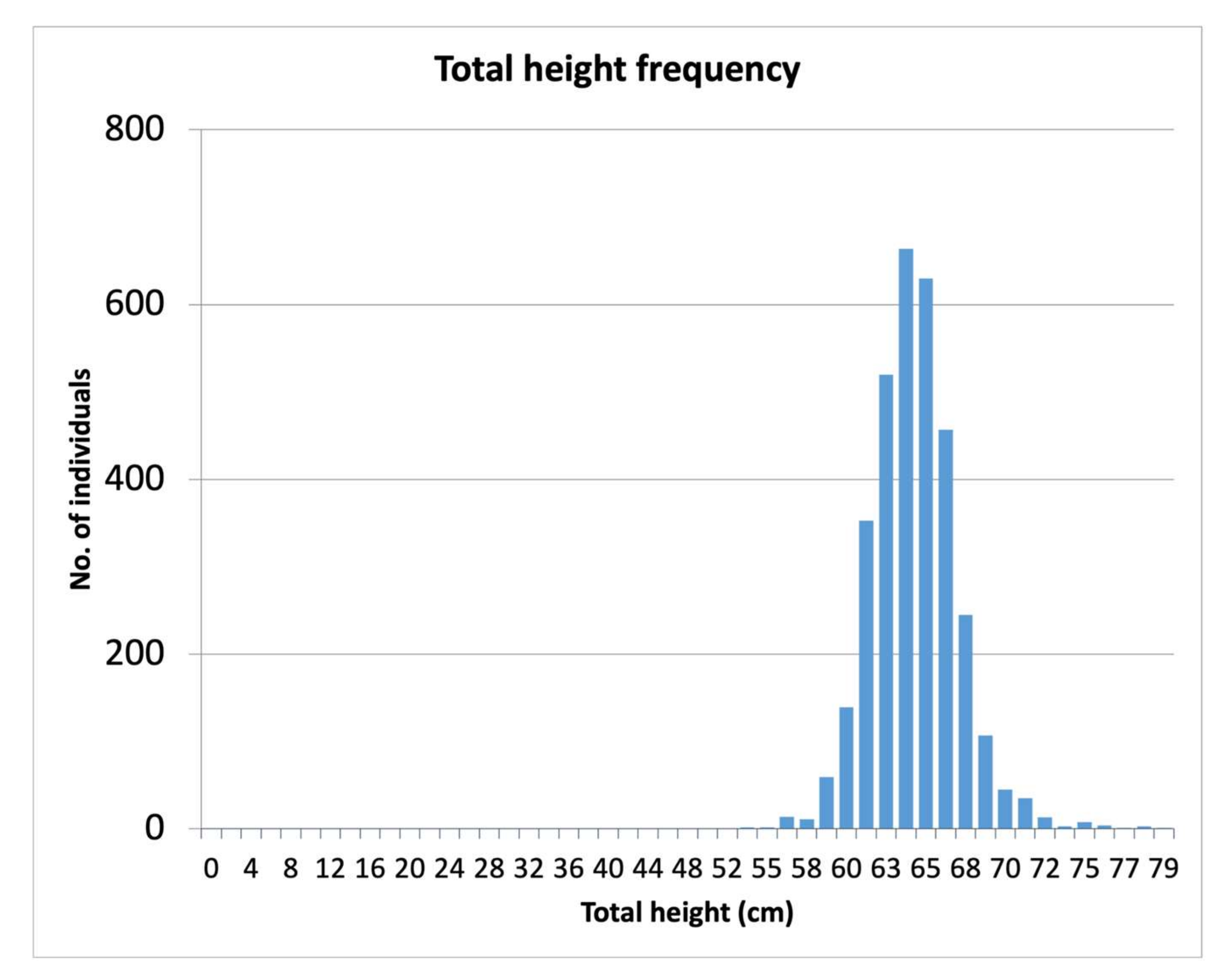
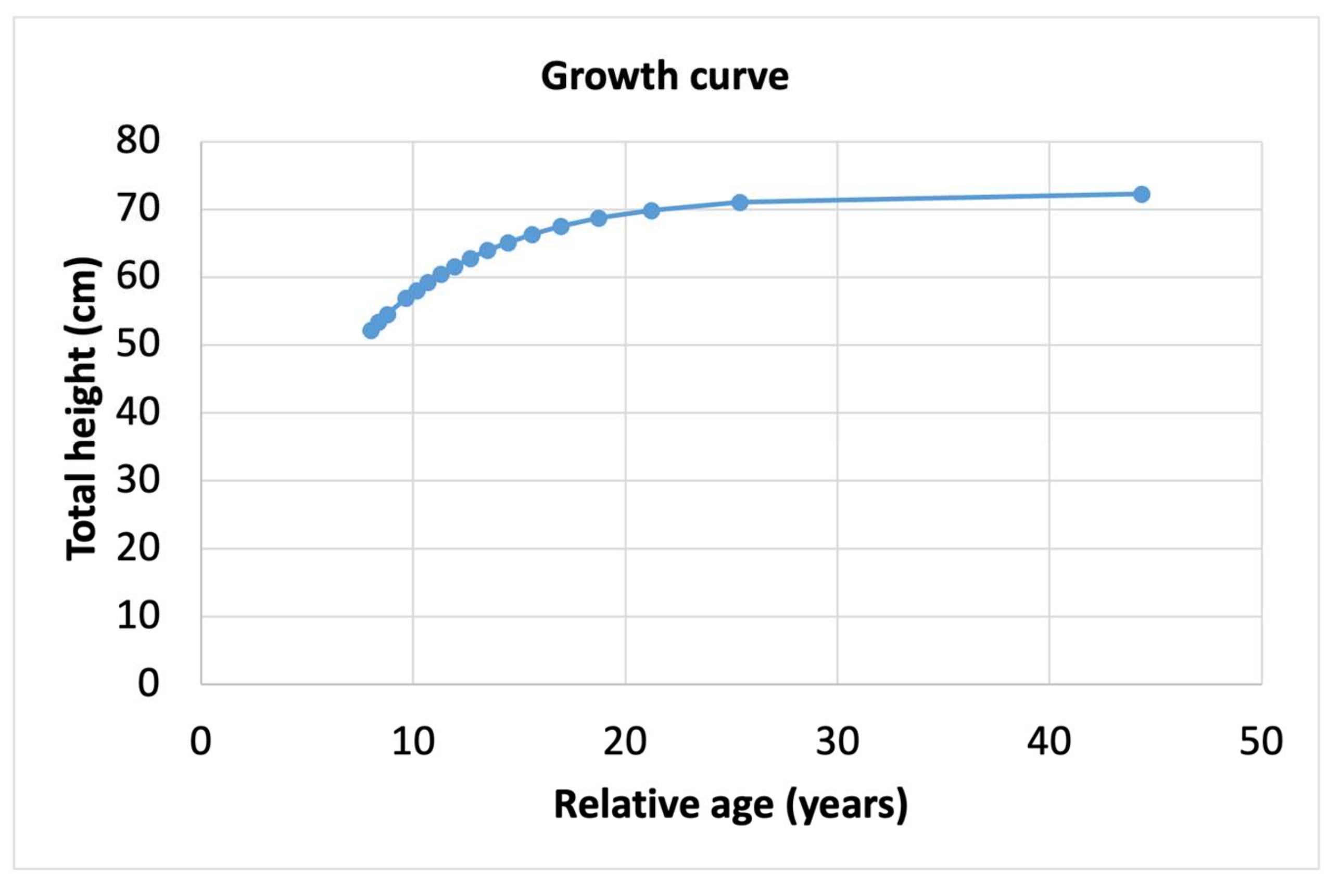
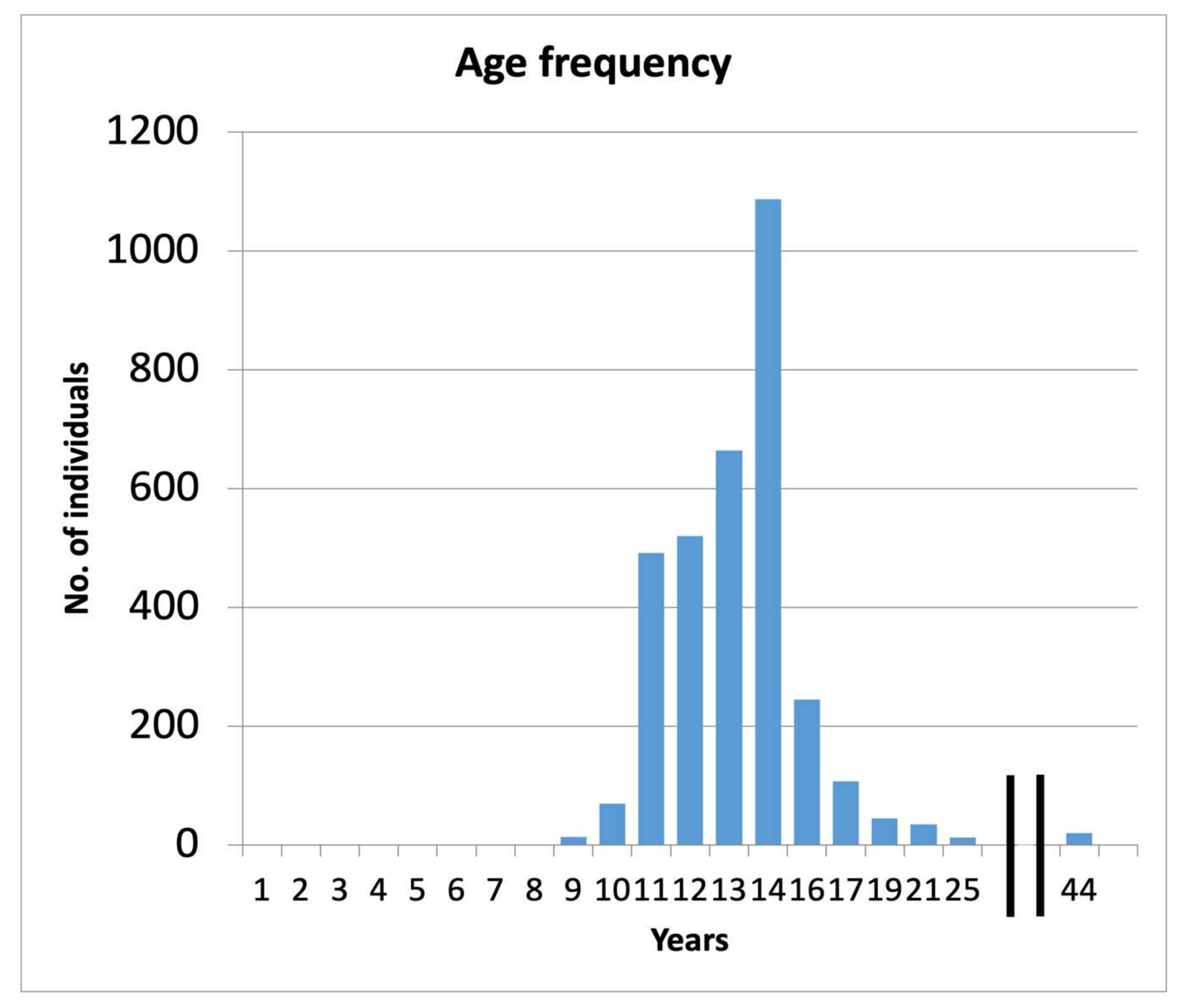
3.2. Size and Age Distribution
4. Discussion
4.1. Density
4.2. Size and Age Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, C.A.; Peharda, M.; Kennedy, H.; Kennedy, P.; Onofri, V. Age, growth rate and season of recruitment of Pinna nobilis (L) in the Croatian Adriatic determined from Mg: Ca and Sr: Ca shell profiles. J. Exp. Mar. Biol. Ecol. 2004, 299, 1–16. [Google Scholar] [CrossRef]
- Šiletić, T.; Peharda, M. Population study of the fan shell Pinna nobilis L. in Malo and Veliko Jezero of the Mljet National Park (Adriatic Sea). Sci. Mar. 2003, 67, 91–98. [Google Scholar] [CrossRef]
- Coppa, S.; Guala, I.; de Lucia, G.A.; Massaro, G.; Bressan, M. Density and distribution patterns of the endangered species Pinna nobilis within a Posidonia oceanica meadow in the Gulf of Oristano (Italy). J. Mar. Biol. Assoc. 2010, 90, 885–894. [Google Scholar] [CrossRef]
- Natalotto, A.; Sureda, A.; Maisano, M.; Spanò, N.; Mauceri, A.; Deudero, S. (Biomarkers of environmental stress in gills of Pinna nobilis (Linnaeus 1758) from Balearic Island. Ecotoxicol. Env. Saf. 2015, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rabaoui, L.; Tlig-Zouari, S.; Katsanevakis, S.; Ben Hassine, O.K. Modelling population density of Pinna nobilis (Bivalvia) on the eastern and southeastern coast of Tunisia. J. Molluscan Stud. 2010, 76, 340–347. [Google Scholar] [CrossRef]
- Rouanet, E.; Trigos, S.; Vicente, N. From youth to death of old age: The 50-year story of a Pinna nobilis fan mussel population at Port-Cros Island (Port-Cros National Park, Provence, Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park. 2015, 29, 209–222. [Google Scholar]
- Peyran, C.; Morage, T.; Nebot-Colomer, E.; Iwankow, G.; Planes, S. Unexpected residual habitats raise hope for the survival of the fan mussel Pinna nobilis along the Occitan coast (Northwest Mediterranean Sea). Endanger. Species Res. 2022, 48, 123–137. [Google Scholar] [CrossRef]
- Zavodnik, D.; Hrs-Brenko, M.; Legac, M. Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic Sea. In Les Espèces Marines à Protéger en Méditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; Gis Posidonie Publ.: Marseille, France, 1991; pp. 169–178. [Google Scholar]
- Richardson, C.A.; Kennedy, H.; Duarte, C.M.; Kennedy, D.P.; Proud, S.V. Age and growth of the fan mussel Pinna nobilis from south-east Spanish Mediterranean seagrass (Posidonia oceanica) meadows. Mar. Biol. 1999, 133, 205–212. [Google Scholar] [CrossRef]
- Katsanevakis, S. Growth and mortality rates of the fan mussel Pinna nobilis in Lake Vouliagmeni (Korinthiakos Gulf, Greece): A generalized additive modelling approach. Mar. Biol. 2007, 152, 1319–1331. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Hernandis, S.; Mourre, B.; Fraile-Nuez, E.; Álvarez, E.; Deudero, S.; Albentosa, M.; Vázquez-Luis, M. No recruits for an ageing population: First signs of probable population extinction in one of the last reservoirs of the Critically Endangered species Pinna nobilis. J. Nat. Conserv. 2024, 79, 126600. [Google Scholar] [CrossRef]
- Addis, P.; Secci, M.; Brundu, G.; Manunza, A.; Corrias, S.; Cau, A. Density, size structure, shell orientation and epibiontic colonization of the fan mussel Pinna nobilis L. 1758 (Mollusca: Bivalvia) in three contrasting habitats in an estuarine area of Sardinia (W Mediterranean). Sci. Mar. 2009, 73, 143–152. [Google Scholar] [CrossRef]
- Deudero, S.; Vázquez-Luis, M.; Álvarez, E. Human stressors are driving coastal benthic long-lived sessile fan mussel Pinna nobilis population structure more than environmental stressors. PLoS ONE 2015, 10, e0134530. [Google Scholar] [CrossRef] [PubMed]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An emergency situation for pen shells in the Mediterranean: The Adriatic Sea, one of the last Pinna nobilis shelters, is now affected by a mass mortality event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef]
- Šarić, T.; Župan, I.; Aceto, S.; Villari, G.; Palić, D.; De Vico, G.; Carella, F. Epidemiology of noble pen shell (Pinna nobilis L. 1758) mass mortality events in Adriatic Sea is characterised with rapid spreading and acute disease progression. Pathogens 2020, 9, 776. [Google Scholar] [CrossRef]
- Pensa, D.; Fianchini, A.; Grosso, L.; Ventura, D.; Cataudella, S.; Scardi, M.; Rakaj, A. Population status, distribution and trophic implications of Pinna nobilis along the South-eastern Italian coast. NPJ Biodivers. 2022, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. The IUCN Red. List. of Threatened Species 2019; IUCN Red List: London, UK, 2019. [Google Scholar] [CrossRef]
- Peharda, M.; Vilibić, I. Modelling the recruitment effect in a small marine protected area: The example of saltwater lakes on the Island of Mljet (Adriatic Sea). Acta Adriat. 2008, 49, 25–35. [Google Scholar]
- Benović, A.; Lučić, D.; Onofri, V.; Peharda, M.; Carić, M.; Jasprica, N.; Bobanović-Čolić, S. Ecological characteristics of the Mljet Islands seawater lakes (South Adriatic Sea) with special reference to their resident populations of medusae. Sci. Mar. 2000, 64, 197–2206. [Google Scholar] [CrossRef]
- García-March, J.R.; Nardo, V. Protocol to study and monitor Pinna nobilis populations within marine protected areas. In Management Tool Developed Under the MedPAN—Interreg IIIC Project; Malta Environment and Planning Authority (MEPA): Floriana, Malta, 2006. [Google Scholar]
- Čižmek, H.; Zubak Čižmek, I.; Čolić, B. The Total Height of Noble Pen Shell (Pinna nobilis) in the Isolated Population of Malo Jezero Lagoon, Mljet MPA (Adriatic Sea): A New Equation. Rapp. Et. Procès Verbaux Des. Réunions-Comm. Int. Pour Explor. Sci. De. La. Mer. Méditerranée 2024, 43, 7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 19 January 2025).
- Posit Team. Posit Workbench: Integrated Development Environment for R and Python, Version 2023.12.1 Build 402, “Ocean Storm” Release; Posit, PBC: Boston, MA, USA, 2024. Available online: https://posit.co (accessed on 19 January 2025).
- QGIS Development Team. QGIS Geographic Information System (Version 3.22.5-Białowieża) 2002, Open-Source Geospatial Foundation Project. Available online: http://qgis.org (accessed on 19 January 2025).
- Peharda, M.; Hrs-Brenko, M.; Onofri, V.; Lučić, D.; Benović, A. A visual census of bivalve distributions in the saltwater lake Malo jezero (Mljet National Park, South Adriatic Sea). Acta Adriat. 2002, 43, 65–75. [Google Scholar]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The pen shell, Pinna nobilis: A review of population status and recommended research priorities in the Mediterranean Sea. Adv. Mar. Biol. 2015, 71, 109–160. [Google Scholar] [PubMed]
- Draganović, E. Litoralne biocenoze mljetskih jezera i problemi njihove zaštite. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 1980. [Google Scholar]
- Šiletić, T. Marine fauna of Mljet National Park (Adriatic Sea, Croatia). 5. Mollusca: Bivalvia. Nat. Croat. 2006, 15, 109. [Google Scholar]
- Peharda, M.; Hrs-Brenko, M.; Bogner, D.; Lučić, D.; Onofri, V.; Benović, A. Spatial distribution of live and dead bivalves in saltwater lake Malo jezero (Mljet National Park). Period. Biol. 2002, 104, 115–122. [Google Scholar]
- Kersting, D.K.; García-March, J.R. Long-term assessment of recruitment, early stages and population dynamics of the endangered Mediterranean fan mussel Pinna nobilis in the Columbretes Islands (NW Mediterranean). Mar. Env. Res. 2017, 130, 282–292. [Google Scholar] [CrossRef] [PubMed]
- García-March, J.R.; Hernandis, S.; Vázquez-Luis, M.; Prado, P.; Deudero, S.; Vicente, N.; Tena-Medialdea, J. Age and growth of the endangered fan mussel Pinna nobilis in the western Mediterranean Sea. Mar. Env. Res. 2020, 153, 104795. [Google Scholar] [CrossRef] [PubMed]
- Claramonte, L.; Álvarez, E.; Hidalgo, M.; Deudero, S.; Vázquez-Luis, M. Demographic regulation processes in Pinna nobilis population subunits: Implications for restocking. Estuar. Coast. Shelf Sci. 2024, 306, 108894. [Google Scholar] [CrossRef]
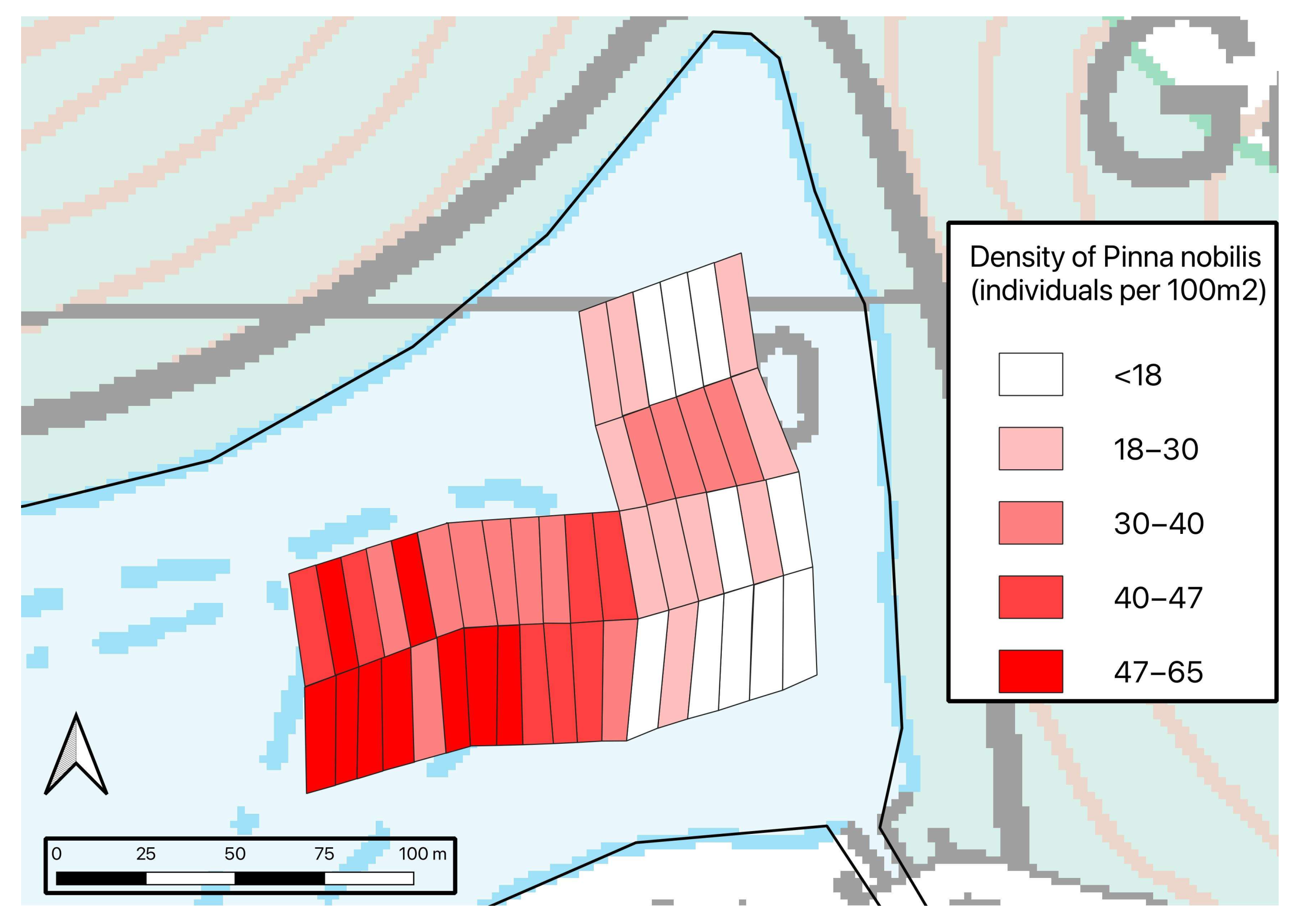
| Transect Code | Count (per 240 m2) | Density (ind/100 m2) | Average Depth (m) |
|---|---|---|---|
| 1A | 42 | 17.5 | 2.4 |
| 1B | 33 | 13.75 | 2.6 |
| 1C | 38 | 15.83 | 2.5 |
| 1D | 24 | 10 | 2.9 |
| 1E | 55 | 22.92 | 3.1 |
| 1F | 39 | 16.25 | 3.2 |
| 2A | 56 | 23.33 | 2.8 |
| 2B | 65 | 27.08 | 3.0 |
| 2C | 77 | 32.08 | 3.0 |
| 2D | 87 | 36.25 | 3.0 |
| 2E | 75 | 31.25 | 3.4 |
| 2F | 61 | 25.42 | 4.0 |
| 3A | 22 | 9.17 | 2.0 |
| 3B | 46 | 19.17 | 2.0 |
| 3C | 37 | 15.42 | 3.0 |
| 3D | 40 | 16.67 | 3.5 |
| 3E | 61 | 25.42 | 4.0 |
| 3F | 45 | 18.75 | 4.0 |
| 4A | 19 | 7.92 | 2.5 |
| 4B | 18 | 7.5 | 2.5 |
| 4C | 23 | 9.58 | 3.3 |
| 4D | 28 | 11.67 | 3.7 |
| 4E | 39 | 16.25 | 3.5 |
| 4F | 37 | 15.42 | 5.5 |
| 5A | 115 | 47.92 | 4.4 |
| 5B | 122 | 50.83 | 4.2 |
| 5C | 88 | 36.67 | 3.5 |
| 5D | 97 | 40.42 | 3.2 |
| 5E | 92 | 38.33 | 3.7 |
| 5F | 74 | 30.83 | 2.5 |
| 6A | 87 | 36.25 | 5.0 |
| 6B | 88 | 36.67 | 5.4 |
| 6C | 79 | 32.92 | 5.0 |
| 6D | 66 | 27.5 | 5.0 |
| 6E | 97 | 40.42 | 5.0 |
| 6F | 91 | 37.92 | 4.5 |
| 7A | 69 | 28.75 | 6.0 |
| 7B | 104 | 43.33 | 6.2 |
| 7C | 78 | 32.5 | 6.3 |
| 7D | 93 | 38.75 | 7.0 |
| 7E | 134 | 55.83 | 7.0 |
| 7F | 91 | 37.92 | 7.2 |
| 8A | 120 | 50 | 6.0 |
| 8B | 87 | 36.25 | 5.2 |
| 8C | 110 | 45.83 | 6.0 |
| 8D | 105 | 43.75 | 7.0 |
| 8E | 110 | 45.83 | 7.2 |
| 8F | 104 | 43.33 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čižmek, H.; Čolić, B.; Zubak Čižmek, I. Reconstructing the Historical Density, Size, and Age Structure of the Noble Pen Shell (Pinna nobilis) Population: Insights from Malo Jezero Lagoon, Mljet National Park (Adriatic Sea). Water 2025, 17, 663. https://doi.org/10.3390/w17050663
Čižmek H, Čolić B, Zubak Čižmek I. Reconstructing the Historical Density, Size, and Age Structure of the Noble Pen Shell (Pinna nobilis) Population: Insights from Malo Jezero Lagoon, Mljet National Park (Adriatic Sea). Water. 2025; 17(5):663. https://doi.org/10.3390/w17050663
Chicago/Turabian StyleČižmek, Hrvoje, Barbara Čolić, and Ivana Zubak Čižmek. 2025. "Reconstructing the Historical Density, Size, and Age Structure of the Noble Pen Shell (Pinna nobilis) Population: Insights from Malo Jezero Lagoon, Mljet National Park (Adriatic Sea)" Water 17, no. 5: 663. https://doi.org/10.3390/w17050663
APA StyleČižmek, H., Čolić, B., & Zubak Čižmek, I. (2025). Reconstructing the Historical Density, Size, and Age Structure of the Noble Pen Shell (Pinna nobilis) Population: Insights from Malo Jezero Lagoon, Mljet National Park (Adriatic Sea). Water, 17(5), 663. https://doi.org/10.3390/w17050663






