Pb2+ Adsorption, Performance, and Response Surface Optimization of Hydroxyapatite Nanowire Sodium Alginate Aerogel (HSA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
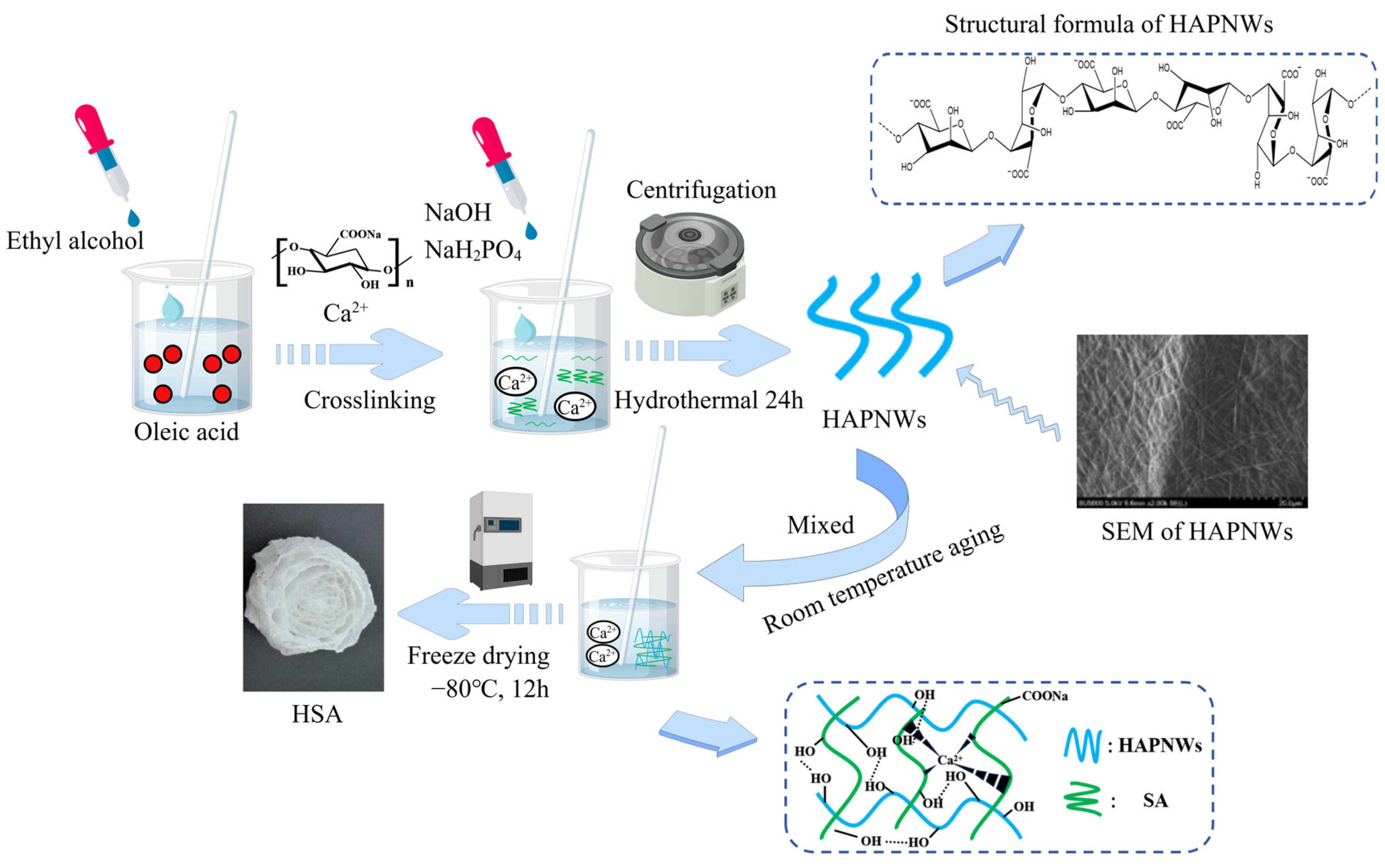
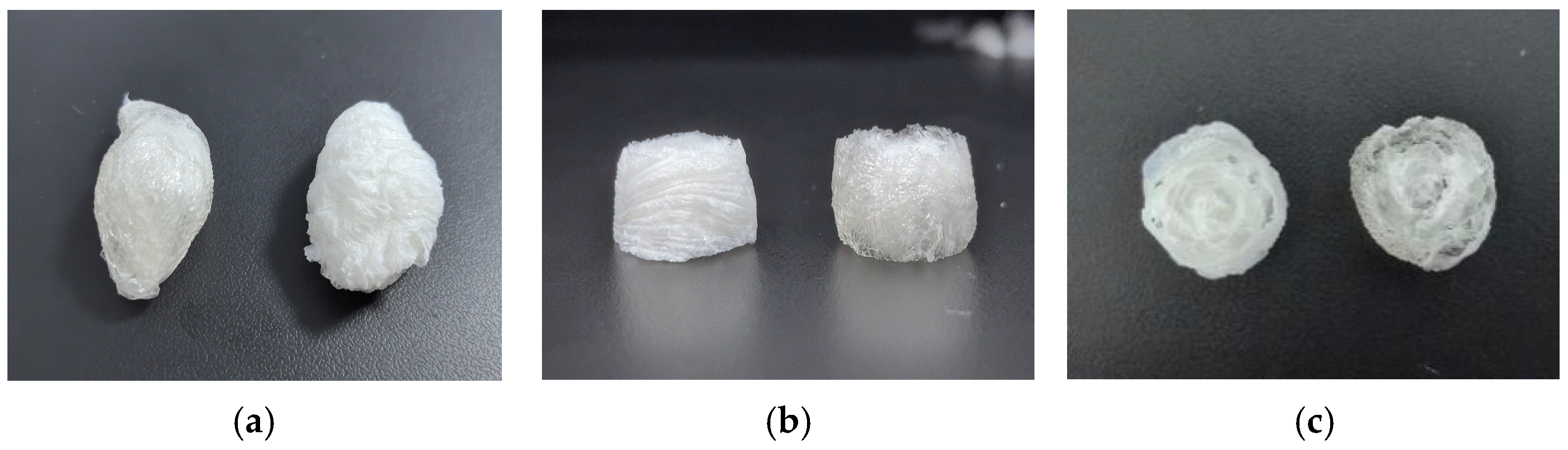
2.2. HSA Preparation
2.3. Adsorption Testing
2.4. Response Surface Optimization Experiment
2.5. Aerogel Characterization
3. Result and Discussion
3.1. Characterization Analysis
3.1.1. SEM Analysis
3.1.2. Crystal Pattern Analysis
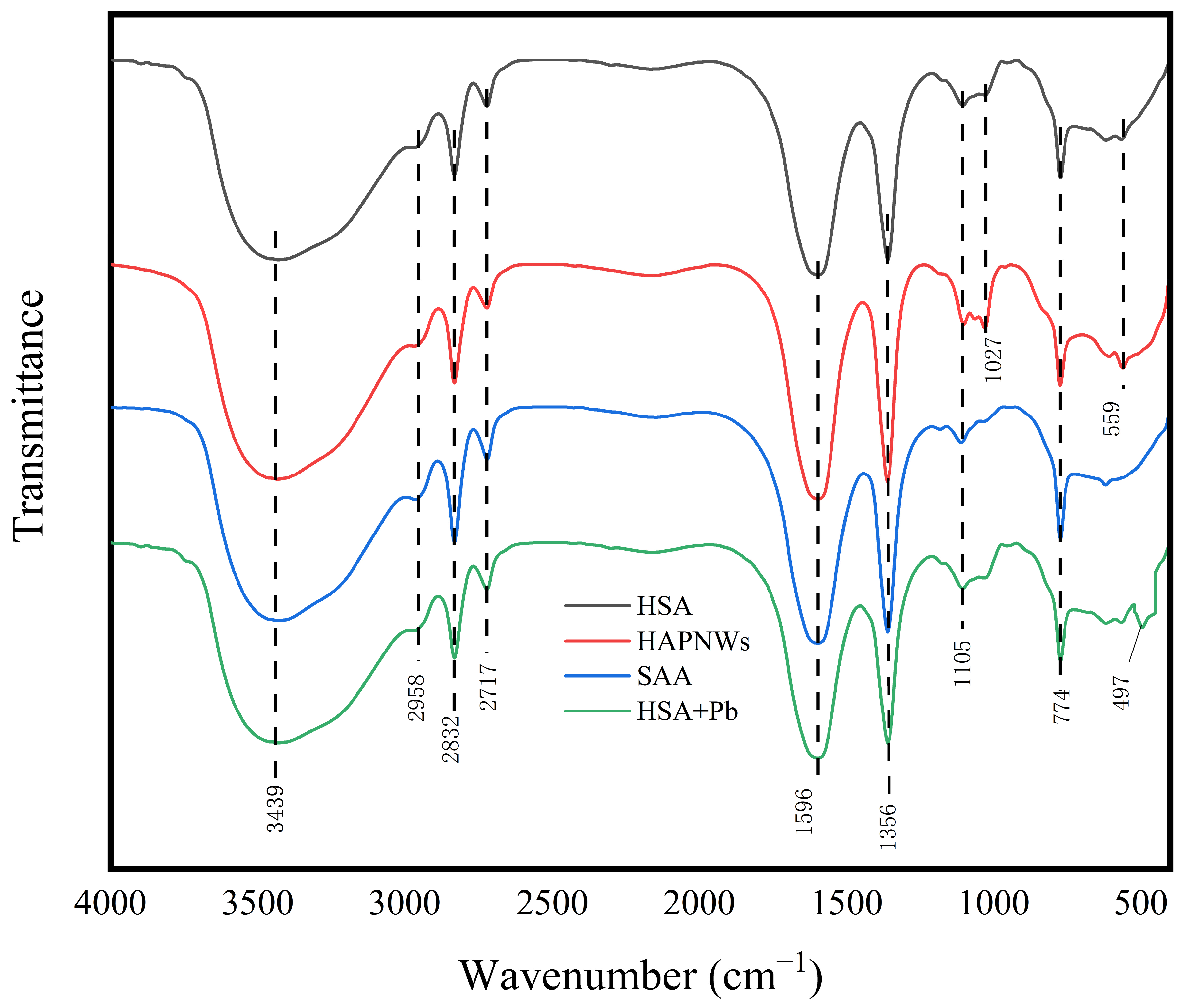
3.1.3. Infrared Functional Group Analysis
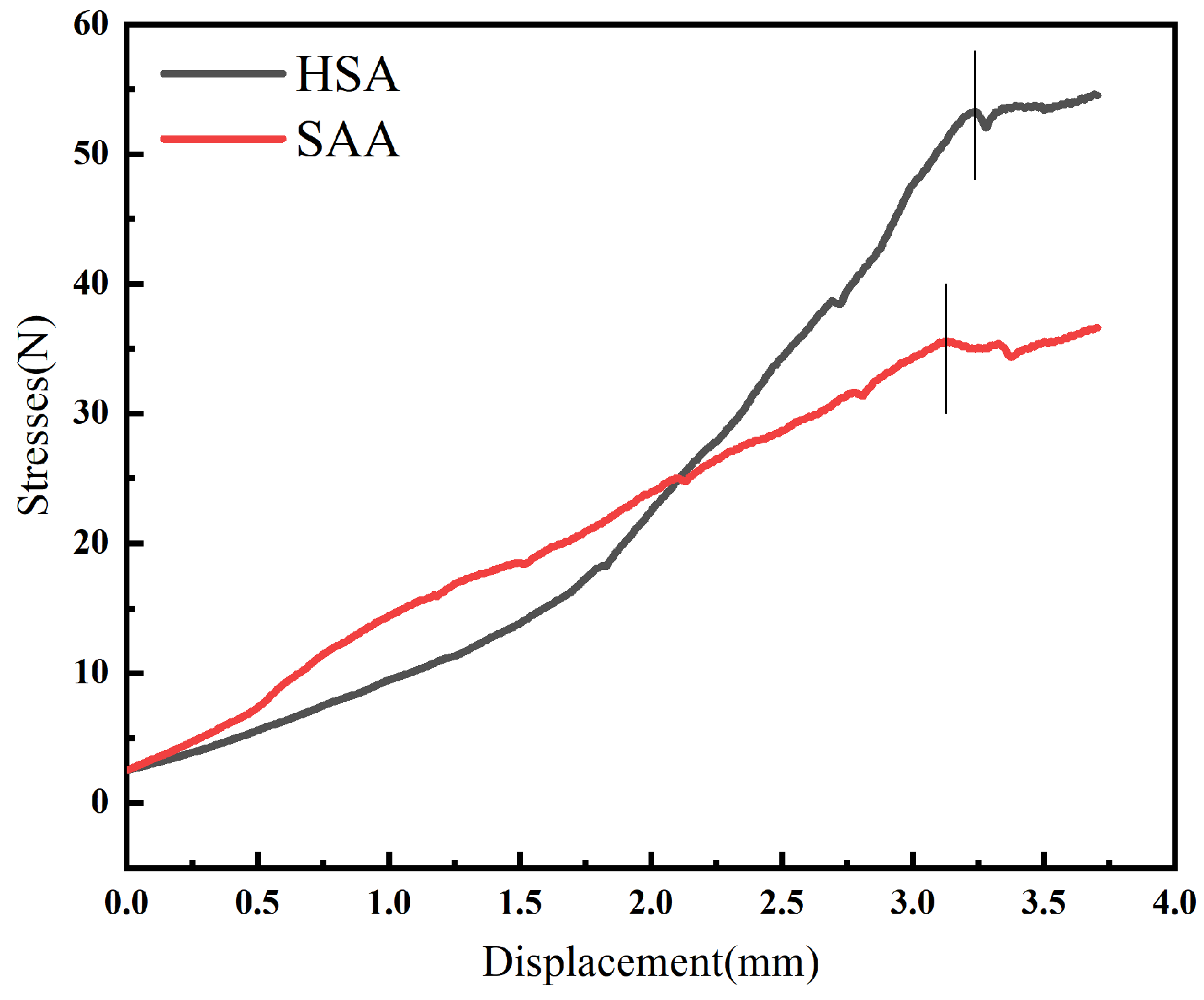
3.1.4. Mechanical Pressure Test Analysis
3.2. Factors Affecting Adsorption Experiments
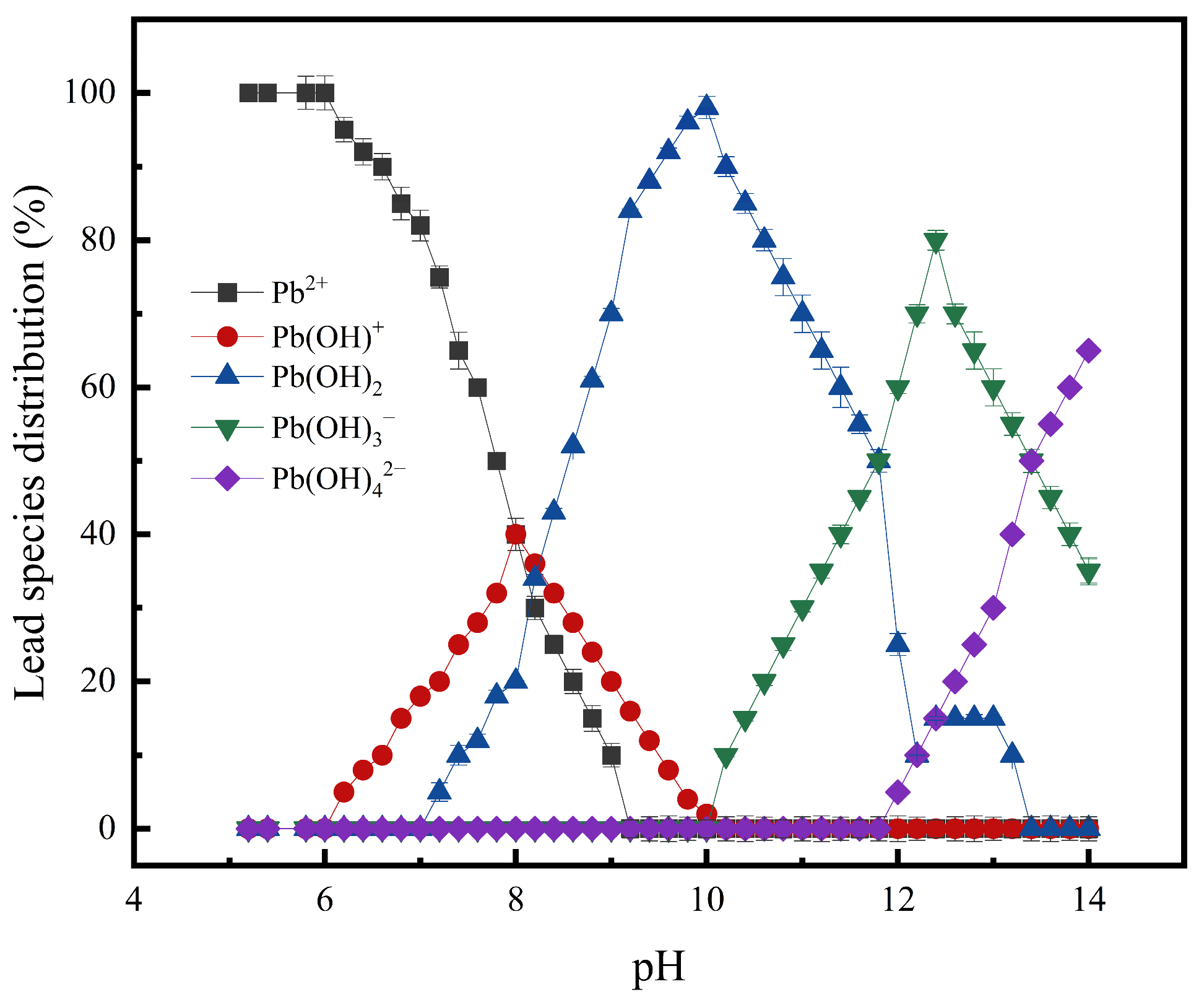
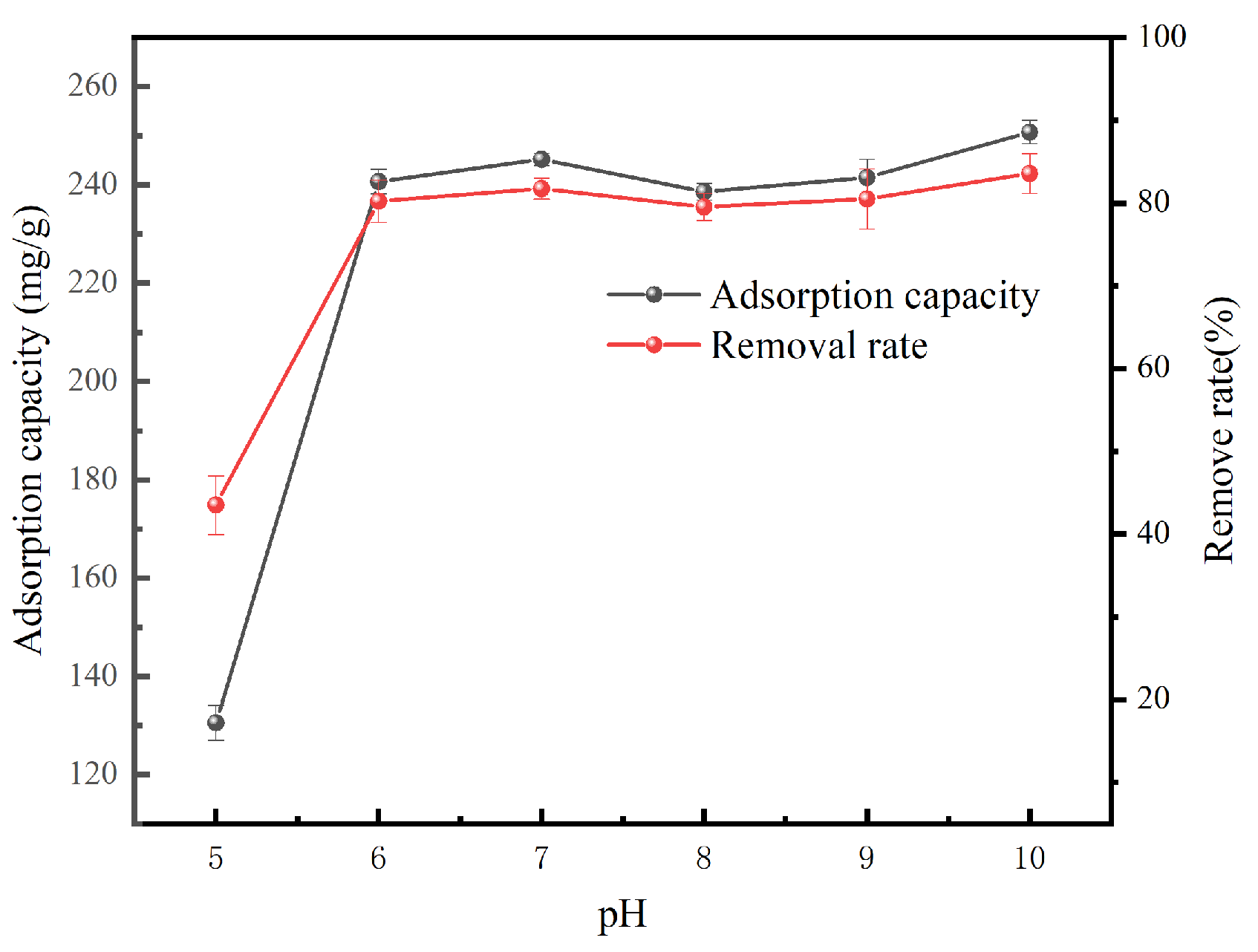
3.2.1. Effect of pH
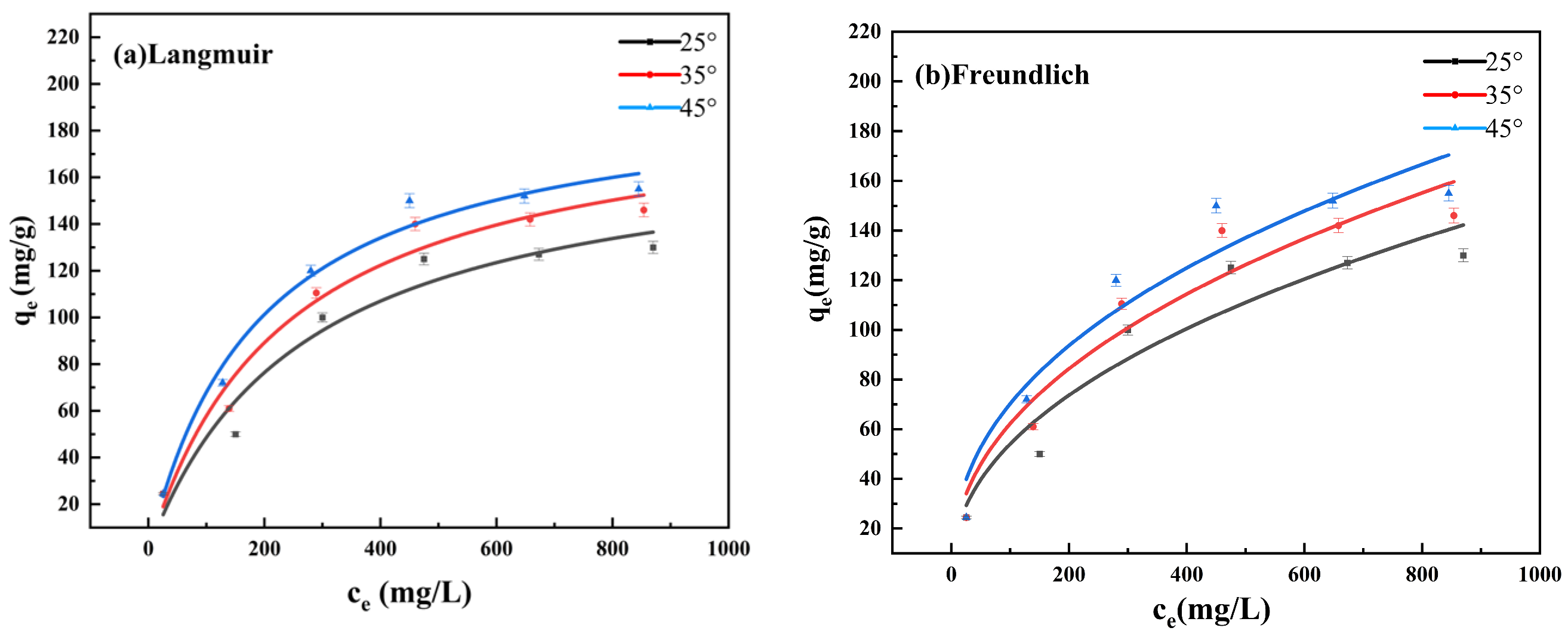
3.2.2. Effects of Concentration and Adsorption Isotherm Analysis
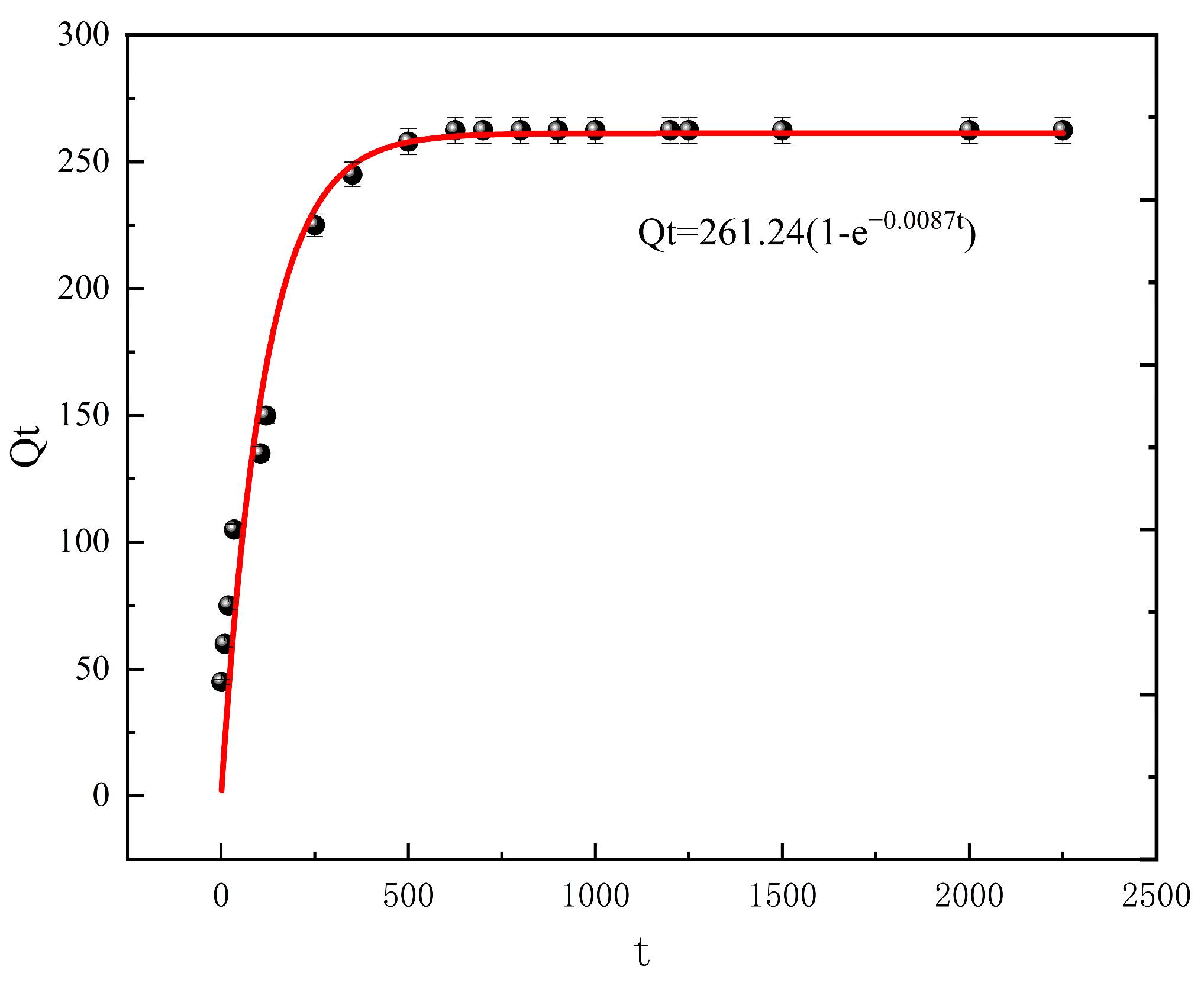
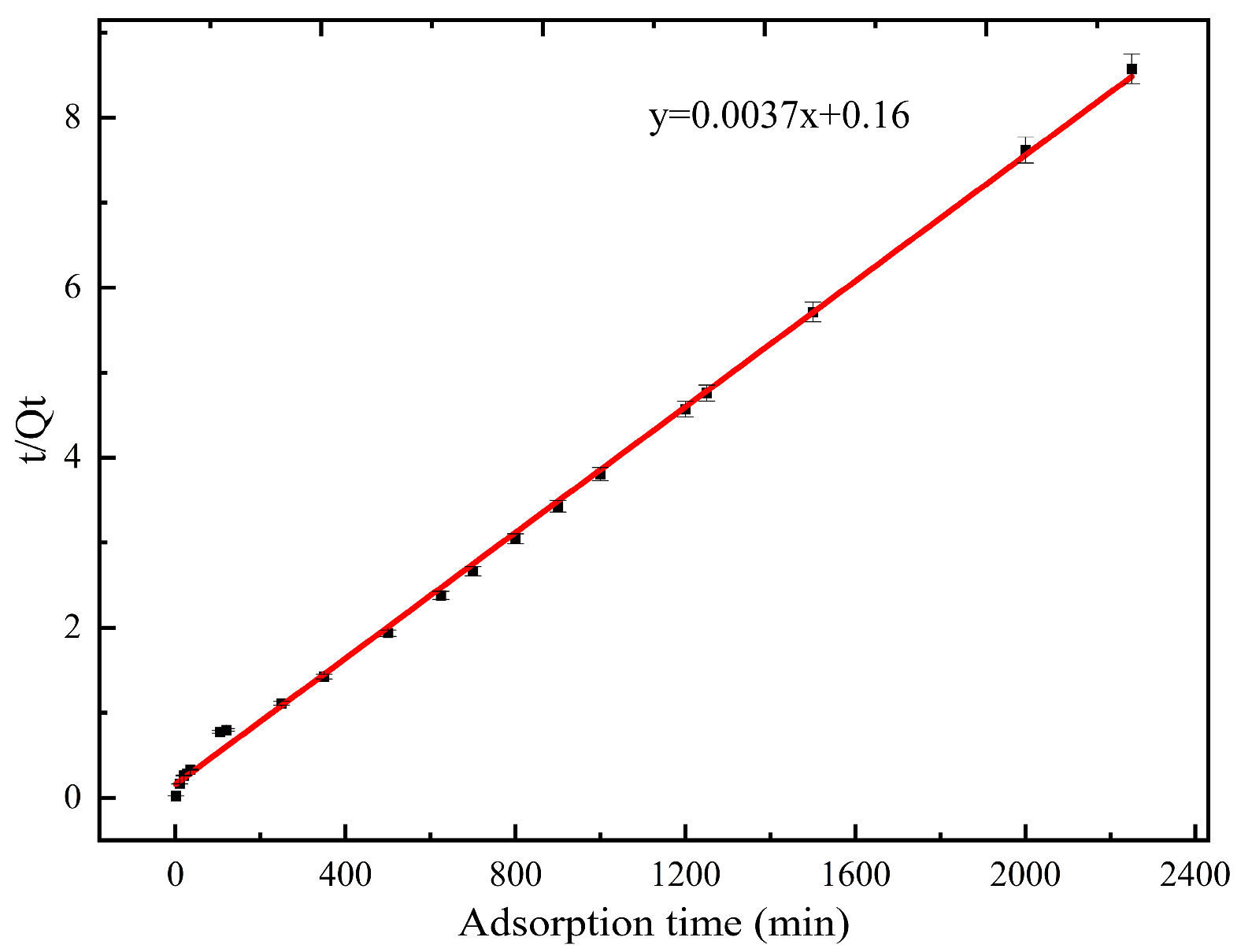
3.2.3. Effect of Time and Adsorption Kinetics
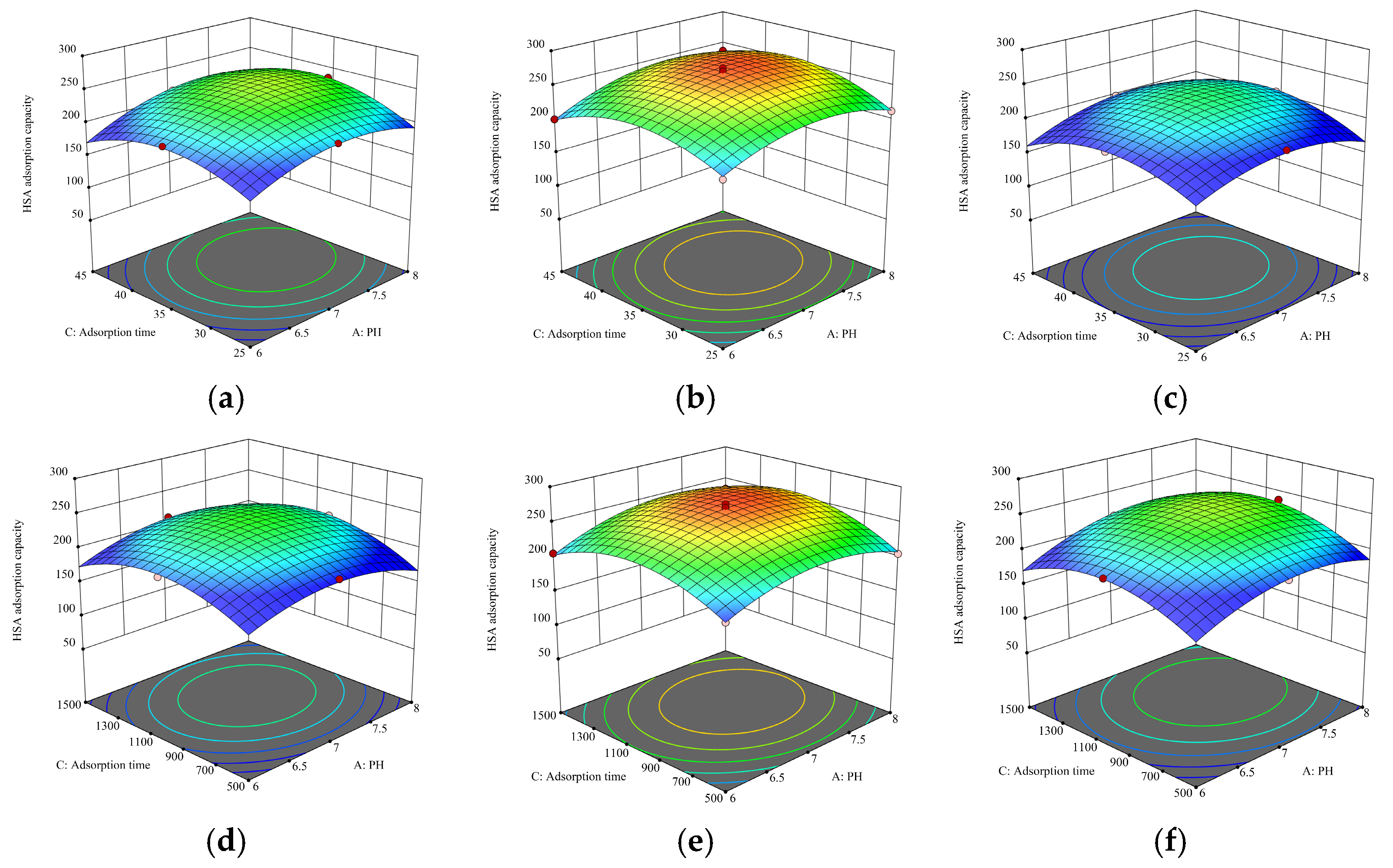
3.3. Optimization of HSA Adsorption Conditions Using Response Surface Methodology
3.3.1. Experimental Model Fitting
3.3.2. Analysis of Response Surface Results
3.3.3. Validation of Results
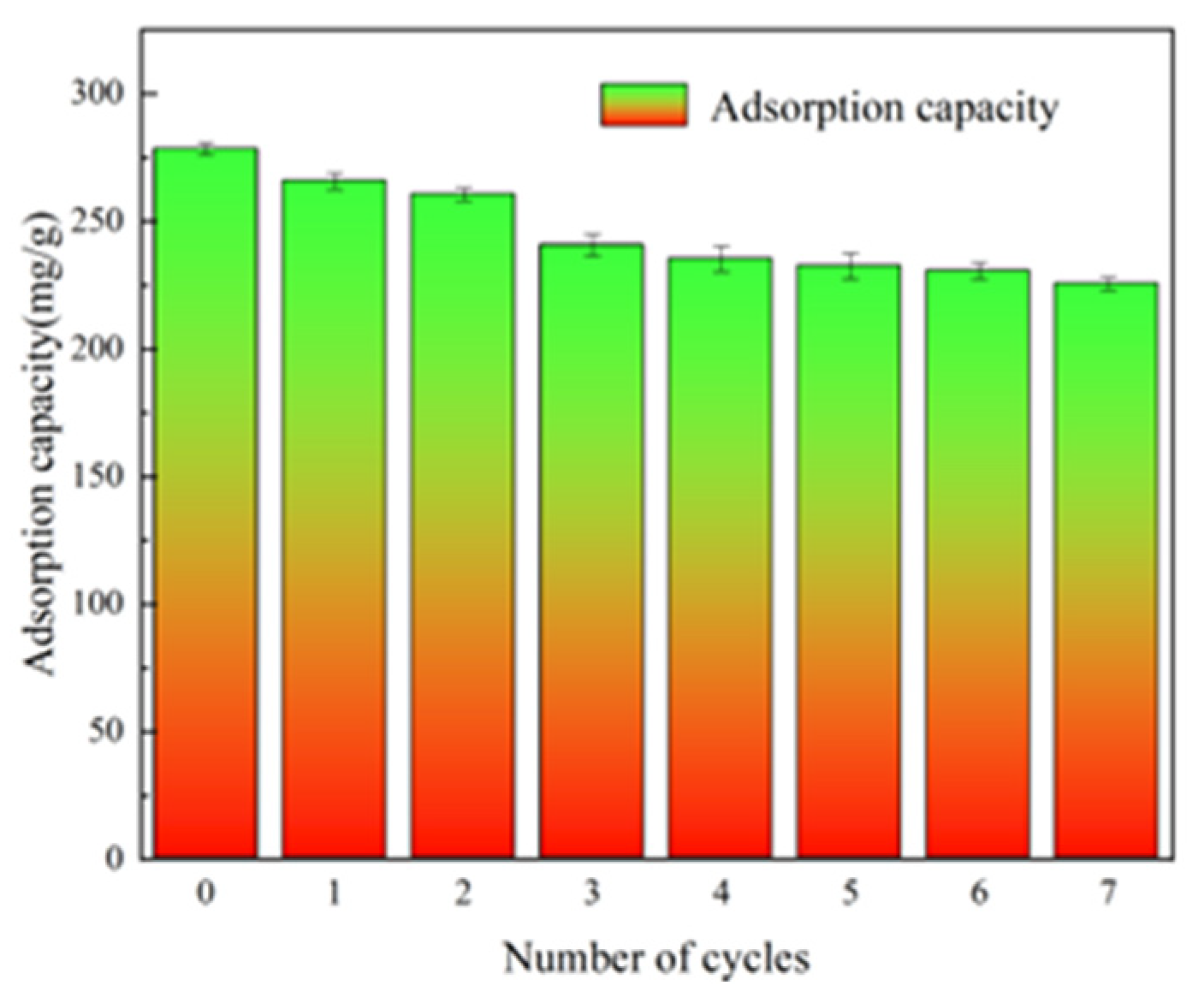
3.3.4. Reuse Efficiency of Adsorbent
3.3.5. Mechanism Speculation
3.3.6. A Comparison of the Used Sorbent Material with Other Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Han, X.; Cai, H.; Wu, X.; Yuan, Y.; Zhang, Y. Aramid nanofibers/WS2 nanosheets co-assembled aerogels for efficient and stable Pb (II) adsorption in harsh environments. Chem. Eng. J. 2022, 450, 138268. [Google Scholar] [CrossRef]
- Luo, J.; Fan, C.; Zhou, X. Functionalized graphene oxide/carboxymethyl chitosan composite aerogels with strong compressive strength for water purification. J. Appl. Polym. Sci. 2021, 138, e50065. [Google Scholar] [CrossRef]
- Rizk, H.E.; Imam, D.M.; Attallah, M.F. Potential Fast and Promising Separation for Valuable Ions of Biomedical Interest. Russ. J. Appl. Chem. 2023, 96, 385–394. [Google Scholar] [CrossRef]
- Liao, J.; Ru, X.; Xie, B.; Zhang, W.; Wu, H.; Wu, C.; Wei, C. Multi-phase distribution and comprehensive ecological risk assessment of heavy metal pollutants in a river affected by acid mine drainage. Ecotoxicol. Environ. Saf. 2017, 141, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, L.; Amri, S.; Bensouilah, M.; Ouzrout, R. Heavy Metal Resistance AND Metallothionein Induction in Bacteria Isolated from Seybouse River, Algeria. Appl. Ecol. Environ. Res. 2020, 18, 1721–1737. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Msagati, T.A.M.; Popoola, E.O. A Novel Approach for the Removal of Lead (II) Ion from Wastewater Using Mucilaginous Leaves of Diceriocaryum eriocarpum Plant. Sustainability 2015, 7, 14026–14041. [Google Scholar] [CrossRef]
- Li, L.P.; Zhang, Y.Q.; Ippolito, J.A.; Xing, W.Q.; Qiu, K.Y.; Yang, H. Lead smelting effects heavy metal concentrations in soils, wheat, and potentially humans. Environ. Pollut. 2020, 257, 113641. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Guo, C.M.; Lv, Z.J.; Jiang, H.J.; Li, S.Y.; Lu, Y.; Zhang, Z. From animal to cell model: Pyroptosis targeted-fibrosis is a novel mechanism of lead-induced testicular toxicity. Food. Chem. Toxicol. 2023, 178, 113886. [Google Scholar] [CrossRef]
- Abdel-Raheem, K.H.M.; Khalil, M.M.; Abdelhady, A.A.; Tan, L. Anthropogenic-induced environmental and ecological changes in the Nile Delta over the past half-century. Sci. Total. Environ. 2024, 926, 171941. [Google Scholar] [CrossRef]
- Vellingiri, B.; Suriyanarayanan, A.; Selvaraj, P.; Abraham, K.S.; Pasha, M.Y.; Winster, H.; Gopalakrishnan, A.V.; Singaravelu, G.; Reddy, J.K.; Ayyadurai, N.; et al. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 2022, 301, 134625. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Enviro. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid. Interfac. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lu, L.; Li, Y.; Han, W.; Gao, A.; Zhao, S.; Cui, J.; Zhang, G. Robust and Multifunctional 3D Graphene-Based Aerogels Reinforced by Hydroxyapatite Nanowires for Highly Efficient Organic Solvent Adsorption and Fluoride Removal. Acs. Appl. Mater. Inter. 2022, 14, 25385–25396. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Zhu, Y.-J.; Chen, F.; Wu, J. Solvothermal synthesis of submillimeter ultralong hydroxyapatite nanowires using a calcium oleate precursor in a series of monohydroxy alcohols. Ceram. Int. 2015, 41, 6098–6102. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Zhang, P.; Song, L.; Wang, X.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohyd. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Rong, N.N.; Chen, C.C.; Ouyang, K.W.; Zhang, K.J.; Wang, X.R.; Xu, Z.Y. Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep. Purif. Technol. 2021, 274, 119120. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Fedotov, P.S.; Dzhenloda, R.K.; Dampilova, B.V.; Doroshkevich, S.G.; Karandashev, V.K. Unexpected behavior of Zn, Cd, Cu, and Pb in soils contaminated by ore processing after 70 years of burial. Environ. Chem. Lett. 2018, 16, 637–645. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohyd. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Guo, T.; Pei, Y.; Tang, K.Y.; He, X.C.; Huang, J.B.; Wang, F. Mechanical and drug release properties of alginate beads reinforced with cellulose. J. Appl. Polym. Sci. 2017, 134, 44495. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zheng, P.; Zheng, H.; Chen, Z.; Liu, Z.; Li, P.; Zhao, X.; Peng, Q.; He, X. Lightweight and Mechanically Robust MXene/Polyimide/Silver Nanowire Composite Aerogels for Flexible Piezoresistive Sensors. Acs. Appl. Nano. Mater. 2023, 6, 19098–19107. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Tao, H.-C.; Li, S.; Zhang, L.-J.; Chen, Y.-Z.; Deng, L.-P. Magnetic chitosan/sodium alginate gel bead as a novel composite adsorbent for Cu(II) removal from aqueous solution. Environ. Geochem. Health. 2019, 41, 297–308. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, F.; Wang, Y.F.; Zhang, W.Z.; Peng, X.L.; Pepe, F. Silica modified calcium alginate-xanthan gum hybrid bead composites for the removal and recovery of Pb (II) from aqueous solution. Chem. Eng. J. 2013, 234, 33–42. [Google Scholar] [CrossRef]
- Attallah, M.F.; Youssef, M.A.; Imam, D.M. Preparation of novel nano composite materialsfrom biomass waste and their sorptive characteristics for certain radionuclides. Radiochim. Acta 2020, 108, 137–149. [Google Scholar] [CrossRef]
- Imam, D.M.; Youssef, M.A.; Attallah, M.F. Promising framework of nanocomposite materials: Synthesis and radio-lanthanides labeling for nuclear medicine application. J. Radioanal. Nucl. Chem. 2020, 323, 749–761. [Google Scholar] [CrossRef]
- Solomon Chuka, N.; Adanma Uzoma, O.; Echezona Nnaemeka, O.; Arinze Everest, C.; Godknows Chibueze, I. The effect of flux composition on tensile strength of re-engineered E308H-16 electrode. Adv. Mech. Eng. 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohyd. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishikawa, O.; Matsui, T.; Honma, S.; Kokado, H. Fourier transform infrared spectroscopy study of dopants of polypyrrole. J. Phys. Chem. 1991, 95, 6011–6014. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, L. Infrared spectra of the M(NO)n(M = Sn, Pb; n=1,2) and PbNO−molecules. Inorg. Chem. 2006, 45, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Neeli, S.T.; Ramsurn, H.; Ng, C.Y.; Wang, Y.; Lu, J. Removal of Cr (VI), As (V), Cu (II), and Pb (II) using cellulose biochar supported iron nanoparticles: A kinetic and mechanistic study. J. Environ. Chem. Eng. 2020, 8, 103886. [Google Scholar] [CrossRef]
- Wang, S.-J.; Bu, H.; Chen, H.-J.; Hu, T.; Chen, W.-Z.; Wu, J.-H.; Hu, H.-J.; Lin, M.-Z.; Li, Y.; Jiang, G.-B. Floatable magnetic aerogel based on alkaline residue used for the convenient removal of heavy metals from wastewater. Chem. Eng. J. 2020, 399, 125760. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhou, X.; Li, S. Efficient Removal of Heavy Metal Ions in Wastewater by Using a Novel Alginate-EDTA Hybrid Aerogel. Appl. Sci. 2019, 9, 547. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+and Cu2+ from water. Int. J. Biol. Macromol. 2018, 107, 741–747. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, P.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef]
- Deze, E.G.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K. Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: Effect of porosity in Cu2+ and Cd2+ ion sorption. Chem. Eng. J. 2012, 209, 537–546. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Zhao, X.; Wang, Z. Highly efficient removal of copper ions from water by using a novel alginate-polyethyleneimine hybrid aerogel. Int. J. Biol. Macromol. 2019, 138, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Deng, H.; Qiao, N.; Zhang, D.; Lin, H.; Chen, Y. Modified graphene oxide composite aerogels for enhanced adsorption behavior to heavy metal ions. J. Environ. Chem. Eng. 2021, 9, 106008. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr (VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.; Ryu, J.; Kim, H.; Hong, H.-J.; Kim, S. Simultaneous removal of radioactive cesium and strontium from seawater using a highly efficient Prussian blue-embedded alginate aerogel. J. Environ. Manag. 2021, 297, 113389. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M. Highly efficient and selective adsorption of heavy metal ions by hydrazide-modified sodium alginate. Carbohyd. Polym. 2022, 276, 118797. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Chen, L.; Hao, X.; Tao, E.; Li, Y. Pore size adjustment of sodium alginate-based composite aerogel spheres by zirconia for efficient selective removal of Cu (II). Chem. Eng. J. 2023, 464, 142547. [Google Scholar] [CrossRef]
- Su, H.; Qiu, W.; Deng, T.; Zheng, X.; Wang, H.; Wen, P. Fabrication of physically multi-crosslinked sodium alginate/carboxylated-chitosan/montmorillonite-base aerogel modified by polyethyleneimine for the efficient adsorption of organic dye and Cu (II) contaminants. Sep. Purif. Technol. 2024, 330, 125321. [Google Scholar] [CrossRef]




| Model and Parameter | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| T | KL | Qm | R2 | KF | 1/n | R2 |
| 25 °C | 0.0037 | 178.51 | 0.9419 | 6.8834 | 0.4474 | 0.9014 |
| 35 °C | 0.0042 | 194.60 | 0.9668 | 8.2141 | 0.4396 | 0.9153 |
| 45 °C | 0.0052 | 198.24 | 0.9801 | 10.3659 | 0.4155 | 0.9109 |
| Model and Parameter | Pseudo-Primary Adsorption Kinetic Model | Pseudo-Secondary Adsorption Kinetic Model | ||||
|---|---|---|---|---|---|---|
| T | K1 | Qe | R2 | K2 | Qe | R2 |
| Value | 0.0087 | 261.24 | 0.9444 | 0.0001 | 270.27 | 0.9986 |
| Symbolic | Considerations | Unit | Level | |
|---|---|---|---|---|
| Low Value | High Value | |||
| A | pH | / | 6 | 8 |
| B | Temperature | °C | 25 | 45 |
| C | Adsorption time | min | 500 | 1500 |
| Serial Number | A (pH) | B (Temperature/°C) | C (Adsorption Time/min) | Q (HAS Adsorption/mg·g−1) |
|---|---|---|---|---|
| 1 | 6 | 45 | 1000 | 200.854 |
| 2 | 6 | 35 | 500 | 194.523 |
| 3 | 6 | 35 | 1500 | 205.751 |
| 4 | 8 | 35 | 1500 | 234.581 |
| 5 | 7 | 25 | 500 | 196.654 |
| 6 | 8 | 45 | 1000 | 237.852 |
| 7 | 7 | 25 | 1500 | 210.288 |
| 8 | 8 | 25 | 1000 | 213.052 |
| 9 | 8 | 35 | 500 | 205.354 |
| 10 | 7 | 45 | 500 | 198.752 |
| 11 | 7 | 45 | 1500 | 214.380 |
| 12 | 6 | 25 | 1000 | 199.582 |
| 13 | 7 | 35 | 1000 | 263.524 |
| 14 | 7 | 35 | 1000 | 268.254 |
| 15 | 7 | 35 | 1000 | 270.698 |
| 16 | 7 | 35 | 1000 | 275.652 |
| 17 | 7 | 35 | 1000 | 270.257 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 14,964.25 | 9 | 1662.69 | 74.31 | <0.0001 | significant |
| A | 1015.40 | 1 | 1015.40 | 45.38 | 0.0003 | |
| B | 130.10 | 1 | 130.10 | 5.81 | 0.0467 | |
| C | 607.56 | 1 | 607.56 | 27.15 | 0.0012 | |
| AB | 138.39 | 1 | 138.39 | 6.18 | 0.0418 | |
| AC | 80.99 | 1 | 80.99 | 3.62 | 0.0988 | |
| BC | 0.9940 | 1 | 0.9940 | 0.0444 | 0.8391 | |
| A2 | 2825.36 | 1 | 2825.36 | 126.27 | <0.0001 | |
| B2 | 4030.11 | 1 | 4030.11 | 180.11 | <0.0001 | |
| C2 | 4787.71 | 1 | 4787.71 | 213.96 | <0.0001 | |
| Residual | 156.63 | 7 | 22.38 | |||
| Lack of Fit | 79.67 | 3 | 26.56 | 1.38 | 0.3699 | not significant |
| Pure Error | 76.96 | 4 | 19.24 | |||
| Cor Total | 15,120.89 | 16 | ||||
| C.V.% = 2.08% R2 = 0.9896 Adjusted R2 = 0.9763 | ||||||
| Factor | Coefficient Estimate | Df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 269.68 | 9 | 2.12 | 264.67 | 274.68 | 1 |
| A | 11.27 | 1 | 1.67 | 7.31 | 15.22 | 1 |
| B | 4.03 | 1 | 1.67 | 0.0781 | 7.99 | 1 |
| C | 8.71 | 1 | 1.67 | 4.76 | 12.67 | 1 |
| AB | 5.88 | 1 | 2.37 | 0.2892 | 11.47 | 1 |
| AC | 4.50 | 1 | 2.37 | −1.09 | 10.09 | 1 |
| BC | 0.4985 | 1 | 2.37 | −5.09 | 6.09 | 1 |
| A2 | −25.90 | 1 | 2.31 | −31.36 | −20.45 | 1.01 |
| B2 | −30.94 | 1 | 2.31 | −36.39 | −25.49 | 1.01 |
| C2 | −33.72 | 1 | 2.31 | −39.17 | −28.27 | 1.01 |
| Material | Modified Materials | Pollutant | pH | Time | Temperature | Adsorbent Dosage | Pollutant Concentration | Adsorption Capacity | References |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxyapatite nanowire sodium alginate aerogel (HSA) | HAPNWs | Pb(II) | 6.0–8.0 | 500~1500 min | 25~45 °C | 0.3 g, 20 mL | 1000 mg/L | 278.4 mg/g | This study |
| Magnetic sodium alginate–alkaline residue aerogel (Fe3O4/SA-AR) | 5 wt% alkaline residue solution, Fe3O4 | Cd (II) | 5.0 | 24 h | 25 °C | 0.3 g, 50 mL | 20 mg/L | 38.8 mg/g | [35] |
| Calcium alginate-disodium ethylenediaminetetra-acetate dihydrate hybrid aerogel (Alg-EDTA) | EDC (0.2 g), NHS (0.06 g), and EDA (0.05 g) | Cd (II) | 4.0–6.5 | 6 h | 25 °C | 100 mg Alg-EDTA, 50 mL | 1.5 mM | 177.3 mg/g | [36] |
| Ethylenediamine-modified calcium alginate aerogel (ECAA) | Ethylenediamine | Pb(II) | 4.5 | 7 h | 25 °C | 50 mg ECAA/50 mL | 1.5 mmol/L | 219.3 mg/g | [37] |
| Ethylenediamine-modified calcium alginate aerogel (ECAA) | Ethylenediamine | Cu(II) | 4.5 | 7 h | 25 °C | 50 mg ECAA/50 mL | 1.5 mmol/L | 87.8 mg/g | [37] |
| Sodium alginate/graphene oxide with GO content of 4 wt% (SAGO-4) | Graphite, CaCO3, and D-glucono-ɑ-lactone | Cu(II) | 5.0 | 3 h | 30 °C | monolithic SAGO aerogel with GO content of 4 wt% | 500 mg/L | 98 mg/g | [38] |
| Sodium alginate/graphene oxide with GO content of 4 wt% (SAGO-4) | Graphite, CaCO3, and D-glucono-ɑ-lactone | Pb(II) | 6.0 | 4 h | 30 | monolithic SAGO aerogel with GO content of 4 wt% | 500 mg/L | 267.4 mg/g | [38] |
| Calcium alginate porous aerogel beads | sc-CO2 | Cu(II) | 4.5 | 6 h | 25 °C | 0.1 g/100 mL | 100 mL CuSO4·5H2O and 3CdSO4·8H2O | 126.82 mg/g | [39] |
| Calcium alginate porous aerogel beads | sc-CO2 | Cd (II) | 4.5 | 6 h | 25 °C | 0.1 g/100 mL | 100 mL CuSO4·5H2O and 3CdSO4·8H2O | 244.55 mg/g | [39] |
| Enhanced strength-toughness alginate composite fiber | Polystyrene colloidal particles, graphene oxide | Pb(II) | 3.0–7.0 | 40 min | 25 °C | 50 mg/50 mL | 1.5 mM Pb2+ | 368.2 mg/g | [40] |
| Enhanced strength-toughness alginate composite fiber | Polystyrene colloidal particles, graphene oxide | Cu(II) | 3.0–7.0 | 40 min | 25 °C | 50 mg/50 mL | 1.5 mM Cu2+ | 98.1 mg/g | [40] |
| Enhanced strength-toughness alginate composite fiber | Polystyrene colloidal particles, graphene oxide | Cd(II) | 3.0–7.0 | 40 min | 25~60 °C | 50 mg/50 mL | 1.5 mM Cd2+ | 183.6 mg/g | [40] |
| Alginate/melamine/chitosan aerogel | Chitosan and melamine | Pb(II) | 5.5 | 100 min | 298 K | 20 mg/20 mL | 100 mg/L Pb(NO3)2 | 1331.6 mg/g | [17] |
| Alginate-polyethyleneimine hybrid aerogel (Alg-PEI) | Polyethyleneimine | Cu(II) | 4.0 | 40 h | 25 °C | 100 mg/50 mL | 0.1 mM Cu2+ | 214.4 mg/g | [41] |
| Sodium alginate, graphene oxide and β-cyclodextrin (SA/GO-βCD) | β-Cyclodextrin, Graphene oxide | Cu(II) | 6.0 | 4 h | 30 °C | 1 g/L | 400 mg/L Cu2+ | 198.18 mg/g | [42] |
| Sodium alginate, graphene oxide and β-cyclodextrin (SA/GO-βCD) | β-Cyclodextrin, graphene oxide | Cu(II) | 6.0 | 4 h | 30 °C | 1 g/L | 400 mg/L Cu2+ | 243.91 mg/g | [42] |
| MXene/PEI modified sodium alginate aerogel (MPA) | Polyethyleneimine, amino functionalized Ti3C2Tx | Cr(VI) | 2.0 | 3 h | 318 K | 10 mg/50 mL | 500 mg/L Cr(VI) | 550.3 mg/g | [43] |
| Prussian blue-embedded alginate aerogel | Prussian blue | Hg2+ | 6.0 | 5 h | 298.15 K | 50 mg | 0.499 mmol/L Hg2+ | 8.633 mmol/g | [44] |
| Prussian blue-embedded alginate aerogel | Prussian blue | Sr2+ | 6.5 | 12 h | 25 °C | 4 mg/150 mL | Na+: 12,000 mg/L, K+: 400 mg/L, Ca2+: 400 mg/L, Mg2+: 1200 mg/L | 24.1 mg/g | [44] |
| Dialdehyde sodium alginate grafted adipic acid dihydrazide (DSA-AAD@Ca2+) | Dialdehyde sodium alginate, grafted adipic acid dihydrazide | Pb(II) | 5.0 | 5 h | 298.15 K | 50 mg | Pb2+: 0.483 mmol/L | 1.968 mmol/g | [45] |
| Dialdehyde sodium alginate grafted adipic acid dihydrazide (DSA-AAD@Ca2+) | Dialdehyde sodium alginate, grafted adipic acid dihydrazide | Cd(II) | 5.0 | 5 h | 298.15 K | 50 mg | Cd2+: 0.889 mmol/L | 5.062 mmol/g | [45] |
| Dialdehyde sodium alginate grafted adipic acid dihydrazide (DSA-AAD@Ca2+) | Dialdehyde sodium alginate, grafted adipic acid dihydrazide | Cu(II) | 5.0 | 5 h | 298.15 K | 50 mg | Cu2+: 1.573 mmol/L | 4.068 mmol/g | [45] |
| Grapheneoxide–zirconium oxide/sodium alginate (GZS) | GO nanosheets, sodium alginate, ZrO2 | Cu(II) | 6.0 | 1.5 h | 60 °C | 0.3 g | / | 132.57 mg/g | [46] |
| Sodium alginate/carboxylated chitosan/montmorillonite (MSC-P) | polyethyleneimine (PEI) | Cu(II) | 2.0~6.0 | 12 h | 25 °C | 0.5 g/L | 100 mg/L Cu(II) | 203.99 mg/g | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Yang, Z.; Liu, R.; Zhang, Z.; Chen, G.; Zhou, Z.; Xu, L. Pb2+ Adsorption, Performance, and Response Surface Optimization of Hydroxyapatite Nanowire Sodium Alginate Aerogel (HSA). Water 2025, 17, 631. https://doi.org/10.3390/w17050631
Cao W, Yang Z, Liu R, Zhang Z, Chen G, Zhou Z, Xu L. Pb2+ Adsorption, Performance, and Response Surface Optimization of Hydroxyapatite Nanowire Sodium Alginate Aerogel (HSA). Water. 2025; 17(5):631. https://doi.org/10.3390/w17050631
Chicago/Turabian StyleCao, Weiyuan, Zixuan Yang, Ren Liu, Zilin Zhang, Guokuan Chen, Zilin Zhou, and Liwei Xu. 2025. "Pb2+ Adsorption, Performance, and Response Surface Optimization of Hydroxyapatite Nanowire Sodium Alginate Aerogel (HSA)" Water 17, no. 5: 631. https://doi.org/10.3390/w17050631
APA StyleCao, W., Yang, Z., Liu, R., Zhang, Z., Chen, G., Zhou, Z., & Xu, L. (2025). Pb2+ Adsorption, Performance, and Response Surface Optimization of Hydroxyapatite Nanowire Sodium Alginate Aerogel (HSA). Water, 17(5), 631. https://doi.org/10.3390/w17050631





