Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Setup and Procedures
2.2.1. Aquaculture Seawater Treatment
2.2.2. Simulated Experiment of FLO Degradation
2.3. Analytical Methods
2.3.1. Quantitation of Antibiotics
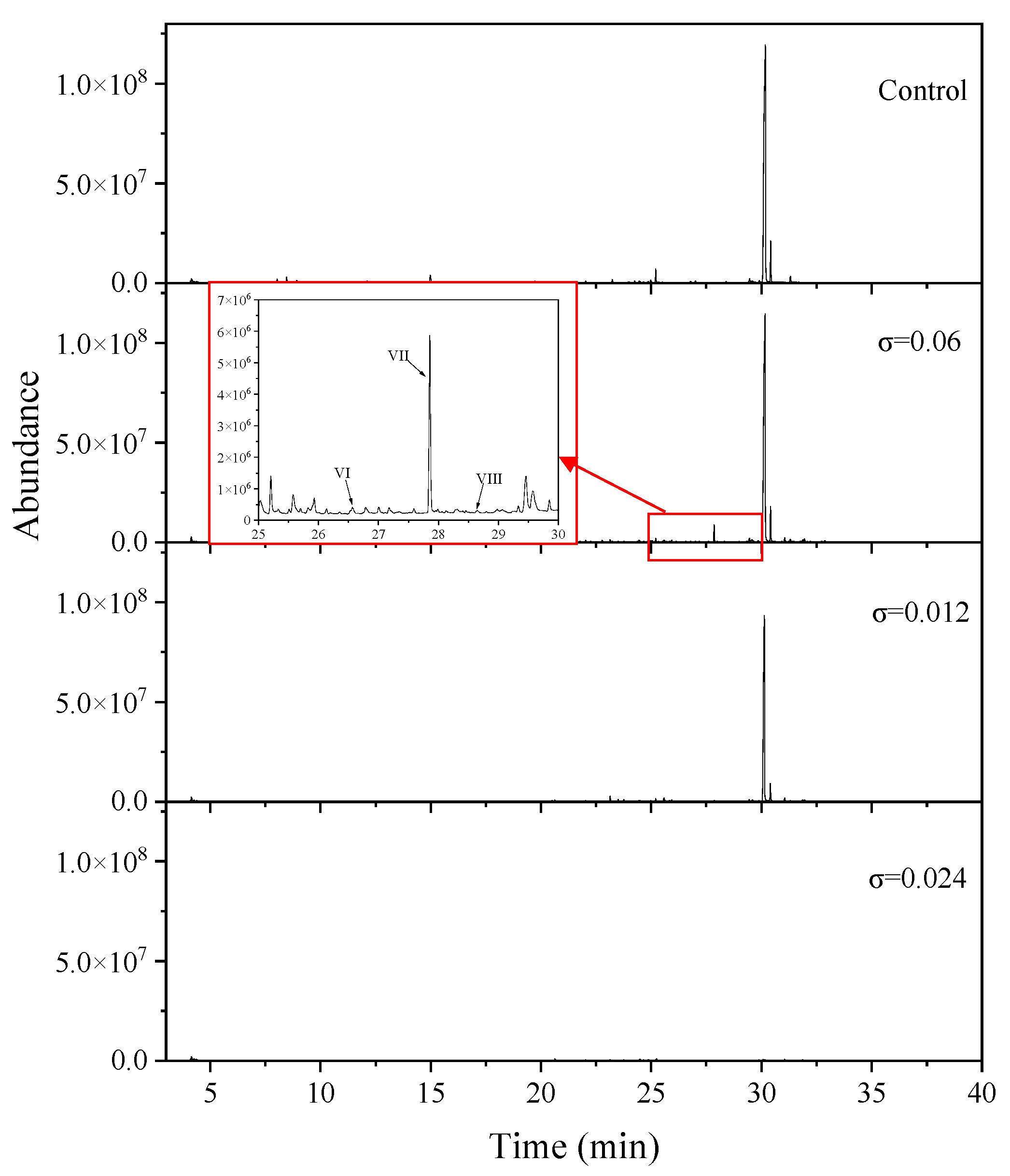
2.3.2. Identification of Intermediates During FLO Degradation
2.3.3. Determination of Water Quality
3. Results
3.1. Degradation of Antibiotics During Aquaculture Seawater Treatment
3.2. The Effect of OAHC-Based System on the Water Quality
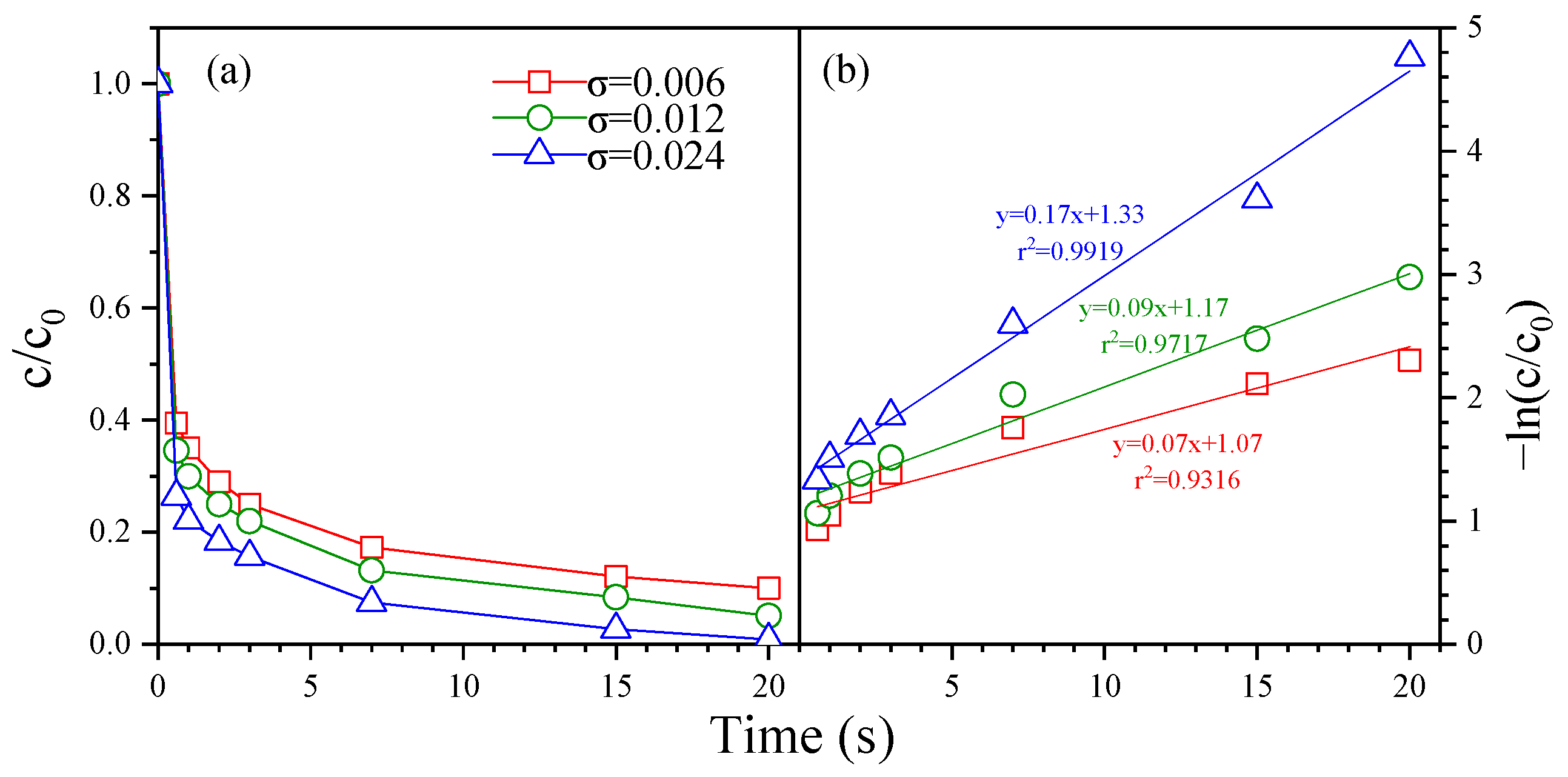
3.3. Kinetic of FLO Degradation
3.4. Mechanism of FLO Degradation
4. Discussion
5. Conclusions
- (1)
- Based on the combination of strong ionization discharge and hydraulic cavitation, the OAHC-based system exhibits excellent degradation efficiency for antibiotics within a reaction time of 20 s.
- (2)
- Following treatment by the OAHC-based system, all water quality parameters met the limits specified in the Chinese Sea Water Quality Standard, and the DBPs complied with the China National Standards for drinking water quality.
- (3)
- The degradation process of FLO can be divided into two stages: the initial direct degradation by ROS generated during the miscible process under hydraulic cavitation, followed by continuous oxidation promoted by residual ozone in the water.
- (4)
- The FLO degradation in the OAHC-based system can be attributed to three primary pathways: (a) substitution reaction at highly electronegative sites; (b) cleavage of the C-N bond; and (c) the electrophilic attacking at the site of the benzene ring.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARBs | Antibiotic-resistant bacteria |
| AOPs | Advanced oxidation processes |
| CHL | Chloramphenicol |
| CTC | Chlorotetracycline |
| FLO | Florfenicol |
| GC | Gas chromatography |
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| OAHC | Ozone assisted with hydrodynamic cavitation |
| OAS | Oxygen activated species |
| OTC | Oxytetracycline |
| ROS | Reactive oxygen species |
| SDZ | Sulfadiazine |
| SMX | Sulfamethoxazole |
| SMZ | Sulfamerazine |
| SPE | Solid-phase extraction |
| TC | Tetracycline |
References
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable Intensification of Aquaculture through Nutrient Recycling and Circular Economies: More Fish, Less Waste, Blue Growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-138763-4.
- Henriksson, P.J.G.; Belton, B.; Jahan, K.M.-; Rico, A. Measuring the Potential for Sustainable Intensification of Aquaculture in Bangladesh Using Life Cycle Assessment. Proc. Natl. Acad. Sci. USA 2018, 115, 2958–2963. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; An, L.; Xu, X.; Du, W.; Dai, R. A Review of Antibiotics in Surface Water and Their Removal by Advanced Electrocoagulation Technologies. Sci. Total Environ. 2024, 906, 167737. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.L. Occurrence of Antibiotics in the Aquatic Environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- McCorquodale-Bauer, K.; Grosshans, R.; Zvomuya, F.; Cicek, N. Critical Review of Phytoremediation for the Removal of Antibiotics and Antibiotic Resistance Genes in Wastewater. Sci. Total Environ. 2023, 870, 161876. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Liu, Y.; Cui, Y.; Li, Y.; Yang, Z. Distribution, Residue Level, Sources, and Phase Partition of Antibiotics in Surface Sediments from the Inland River: A Case Study of the Xiangjiang River, South-Central China. Environ. Sci. Pollut. Res. Int. 2020, 27, 2273–2286. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and Human Health Risk Assessment of Antibiotic Residues in Large-Scale Drinking Water Sources in Chongqing Area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Dynamic Evolution of Antibiotic Resistance Genes in Plastisphere in the Vertical Profile of Urban Rivers. Water Res. 2024, 249, 120946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and Antibiotic Resistance Genes in Global Lakes: A Review and Meta-Analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and Antibiotic Resistant Genes (ARGs) in Groundwater: A Global Review on Dissemination, Sources, Interactions, Environmental and Human Health Risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Liu, K. Occurrence and Source Identification of Antibiotics and Antibiotic Resistance Genes in Groundwater Surrounding Urban Hospitals. J. Hazard. Mater. 2024, 465, 133368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A Systematic Review of Antibiotics and Antibiotic Resistance Genes in Estuarine and Coastal Environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar] [CrossRef]
- Shen, J. Land Use Conversion to Uplands Significantly Increased the Risk of Antibiotic Resistance Genes in Estuary Area. Environ. Int. 2024, 191, 108953. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Ding, J.; Du, S.; Zhu, D. Tire Particles and Its Leachates: Impact on Antibiotic Resistance Genes in Coastal Sediments. J. Hazard. Mater. 2024, 465, 133333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Wang, B.; Zhao, Q.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; Chen, Y.; et al. Antibiotics in Drinking Water in Shanghai and Their Contribution to Antibiotic Exposure of School Children. Environ. Sci. Technol. 2016, 50, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ho, K.W.K.; Ying, G.G.; Deng, W.J. Veterinary Antibiotics in Food, Drinking Water, and the Urine of Preschool Children in Hong Kong. Environ. Int. 2017, 108, 246–252. [Google Scholar] [CrossRef] [PubMed]
- He, L.-X.; He, L.-Y.; Gao, F.-Z.; Zhang, M.; Chen, J.; Jia, W.-L.; Ye, P.; Jia, Y.-W.; Hong, B.; Liu, S.-S.; et al. Mariculture Affects Antibiotic Resistome and Microbiome in the Coastal Environment. J. Hazard. Mater. 2023, 452, 131208. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef]
- Choi, S.; Sim, W.; Jang, D.; Yoon, Y.; Ryu, J.; Oh, J.; Woo, J.-S.; Kim, Y.M.; Lee, Y. Antibiotics in Coastal Aquaculture Waters: Occurrence and Elimination Efficiency in Oxidative Water Treatment Processes. J. Hazard. Mater. 2020, 396, 122585. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Yao, Z.; Li, M. A Review on the Alternatives to Antibiotics and the Treatment of Antibiotic Pollution: Current Development and Future Prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef] [PubMed]
- Chávez, A.M.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.L.; Álvarez, P.M.; Beltrán, F.J. Treatment of Highly Polluted Industrial Wastewater by Means of Sequential Aerobic Biological Oxidation-Ozone Based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Ma, D.; Wang, Y.; Gao, B.; Yue, Q.; Xu, X. The Application of UV/O3 Process on Ciprofloxacin Wastewater Containing High Salinity: Performance and Its Degradation Mechanism. Chemosphere 2021, 276, 130220. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J. Degradation and Mineralization of Ofloxacin by Ozonation and Peroxone (O3/H2O2) Process. Chemosphere 2021, 269, 128775. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, E.J.; Linden, K.G.; Canonica, S.; Von Gunten, U. Comparison of the Efficiency of OH Radical Formation during Ozonation and the Advanced Oxidation Processes O3/H2O2 and UV/H2O2. Water Res. 2006, 40, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Niu, Q.; Wu, S.; Lin, Y.; Biswas, J.K.; Yang, C. Hydroxyl Radicals in Ozone-Based Advanced Oxidation of Organic Contaminants: A Review. Environ. Chem. Lett. 2024, 22, 3059–3106. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Aly Hassan, A.; Ali, J.; Jung, J. A Critical Review of the Recent Developments in Micro–Nano Bubbles Applications for Domestic and Industrial Wastewater Treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, W.; Huang, X.; Wang, Y.; Li, H.; Zhang, Z. Studies on the ·OH Inactivation of Non-Native Aquatic Organisms and Potential Disinfection Byproduct Formation for Oceanic Environmental Safety. Plasma Chem. Plasma Process. 2018, 38, 1051–1062. [Google Scholar] [CrossRef]
- Bai, M.; Huang, X.; Zhong, Z.; Cao, M.; Gao, M. Comparison of OH and NaClO on Geosmin Degradation in the Process of Algae Colonies Inactivation at a Drinking Water Treatment Plant. Chem. Eng. J. 2020, 393, 123243. [Google Scholar] [CrossRef]
- Bai, M.; Bai, X.; Zhang, Z.; Bai, M.; Yang, B. Treatment of Red Tide in Ocean Using Non-Thermal Plasma Based Advanced Oxidation Technology*. Plasma Chem. Plasma Process. 2005, 25, 539–550. [Google Scholar] [CrossRef]
- GB 3097-1997; Sea Water Quality Standard. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 1997.
- GB 5749-2006; Sanitary Standards for Drinking Water Quality. National Health Commission of the People’s Republic of China: Beijing, China, 2006.
- Ochir, D.; Lee, Y.; Shin, J.; Kim, S.; Kwak, J.; Chon, K. Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters. Separations 2021, 8, 101. [Google Scholar] [CrossRef]
- Akatah, B.; Izinyon, C. Self-Purification Capacity of Mmubete Stream in Rivers State, Nigeria. IOSR J. Mech. Civil. Eng. 2023, 20, 37–45. [Google Scholar] [CrossRef]
- Elevli, S.; Uzgören, N.; Bingöl, D.; Elevli, B. Drinking Water Quality Control: Control Charts for Turbidity and pH. J. Water Sanit. Hyg. Dev. 2016, 6, 511–518. [Google Scholar] [CrossRef]
- Simpson, A.M.-A.; Mitch, W.A. Chlorine and Ozone Disinfection and Disinfection Byproducts in Postharvest Food Processing Facilities: A Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1825–1867. [Google Scholar] [CrossRef]
- Ben, W.; Qiang, Z.; Pan, X.; Nie, Y. Degradation of Veterinary Antibiotics by Ozone in Swine Wastewater Pretreated with Sequencing Batch Reactor. J. Environ. Eng. 2012, 138, 272–277. [Google Scholar] [CrossRef]
- Hermes, N.; Jewell, K.S.; Falås, P.; Lutze, H.V.; Wick, A.; Ternes, T.A. Ozonation of Sitagliptin: Removal Kinetics and Elucidation of Oxidative Transformation Products. Environ. Sci. Technol. 2020, 54, 10588–10598. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, S.; Li, F.; Sun, L.; Lu, H. Ozone Micronano-Bubble-Enhanced Selective Degradation of Oxytetracycline from Production Wastewater: The Overlooked Singlet Oxygen Oxidation. Environ. Sci. Technol. 2022, 57, acs.est.2c06008. [Google Scholar] [CrossRef]
- Li, B. Effects of Common Inorganic Anions on the Ozonation of Polychlorinated Diphenyl Sulfides on Silica Gel: Kinetics, Mechanisms, and Theoretical Calculations. Water Res. 2020, 186, 116358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Zhou, L.; Wang, G.; Feng, Y.; Wang, Z.; Yang, X. Aqueous Photodegradation of Antibiotic Florfenicol: Kinetics and Degradation Pathway Studies. Environ. Sci. Pollut. Res. Int. 2016, 23, 6982–6989. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sijak, S.; Zheng, M.; Tang, L.; Xu, G.; Wu, M. Aquatic Photolysis of Florfenicol and Thiamphenicol under Direct UV Irradiation, UV/H2O2 and UV/Fe(II) Processes. Chem. Eng. J. 2015, 260, 826–834. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, X.; Zhang, Y.; Yang, D.; Pan, L.; Fang, W.; Zhu, C.; Wang, F. Development of a Novel 2D Ni-MOF Derived NiO@C Nanosheet Arrays Modified Ti/TiO2NTs/PbO2 Electrode for Efficient Electrochemical Degradation of Salicylic Acid Wastewater. Sep. Purif. Technol. 2021, 263, 118368. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in Water Treatment the Generation, Basic Properties of Ozone and Its Practical Application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Won, J.S.; Kaewsuk, J.; Jo, J.H.; Lim, D.-H.; Seo, G.T. A Density Functional Theory Study on the Ozone Oxidation of Sulfonamide Antibiotics. J. Adavanced Oxid. Technol. 2015, 18, 31–38. [Google Scholar] [CrossRef][Green Version]
- Hossain, A.; Nishimura, Y.; Salma, U.; Tokumura, M.; Nishino, T.; Raknuzzaman, M.; Noro, K.; Watanabe, K.; Amagai, T.; Makino, M. Effects of Water Matrices on the Removal of Oxytetracycline Antibiotic and Total Organic Carbon (TOC) Using Four Different Oxidation Processes. Results Eng. 2024, 24, 103183. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Z.; Du, J.; Yin, R.; Zhou, X.; Jin, S.; Ren, N. Degradation of Sulfadiazine in Water by a UV/O3process: Performance and Degradation Pathway. RSC Adv. 2016, 6, 57138–57143. [Google Scholar] [CrossRef]
- Luo, L.; Zou, D.; Lu, D.; Yu, F.; Xin, B.; Ma, J. Study of Catalytic Ozonation for Tetracycline Hydrochloride Degradation in Water by Silicate Ore Supported Co3O4. RSC Adv. 2018, 8, 41109–41116. [Google Scholar] [CrossRef] [PubMed]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E.; Heidari, Z. Degradation of Sulfonamide Antibiotics Using Ozone-Based Advanced Oxidation Process: Experimental, Modeling, Transformation Mechanism and DFT Study. Sci. Total Environ. 2020, 734, 139446. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, C.-Y.; Liao, G.-Y. Degradation of Antibiotic Tetracycline by Ultrafine-Bubble Ozonation Process. J. Water Process Eng. 2020, 37, 101463. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Cass, G.R. Mathematical Modeling of Chemically Reactive Pollutants in Indoor Air. Environ. Sci. Technol. 1986, 20, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet Oxygen: Properties, Generation, Detection, and Environmental Applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zhang, Z.; Xue, X.; Yang, X.; Hua, L.; Fan, D. Killing Effects of Hydroxyl Radical on Algae and Bacteria in Ship’s Ballast Water and on Their Cell Morphology. Plasma Chem. Plasma Process. 2010, 30, 831–840. [Google Scholar] [CrossRef]
- Huang, X.; Quan, X.; Cheng, W.; Cheng, C.; Cheng, Z.; Yang, L.; Jiang, L. Enhancement of Ozone Mass Transfer by Stainless Steel Wire Mesh and Its Effect on Hydroxyl Radical Generation. Ozone-Sci. Eng. 2020, 42, 347–356. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of Photochemical Reaction Constants of Organic Micropollutants Required for UV Advanced Oxidation Processes in Water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Hickel, B.; Sehested, K. Reaction Of Hydroxyl Radicals with Ammonia In Liquid Water at Elevated-Temperatures. Radiat. Phys. Chem. 1992, 39, 355–357. [Google Scholar] [CrossRef]
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Mota, A.J.; Sánchez-Polo, M.; Leyva-Ramos, R. Effect of Radical Peroxide Promoters on the Photodegradation of Cytarabine Antineoplastic in Water. Chem. Eng. J. 2016, 284, 995–1002. [Google Scholar] [CrossRef]
- Von Gunten, U.; Oliveras, Y. Advanced Oxidation of Bromide-Containing Waters: Bromate Formation Mechanisms. Environ. Sci. Technol. 1998, 32, 63–70. [Google Scholar] [CrossRef]
- Lin, T.; Wu, S.; Chen, W. Formation Potentials of Bromate and Brominated Disinfection By-Products in Bromide-Containing Water by Ozonation. Environ. Sci. Pollut. Res. Int. 2014, 21, 13987–14003. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Canonica, S.; Von Gunten, U. Efficiency and Energy Requirements for the Transformation of Organic Micropollutants by Ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]




| Class | Antibiotics | Molecular Structure | Molecular Mass (g/mol) |
|---|---|---|---|
| Tetracyclines | Tetracycline (TC) | 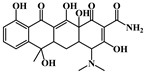 | 444.43 |
| Oxytetracycline (OTC) |  | 460.43 | |
| Chlorotetracycline (CTC) |  | 478.88 | |
| Sulfonamides | Sulfadiazine (SDZ) |  | 250.28 |
| Sulfamerazine (SMZ) | 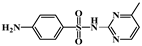 | 264.30 | |
| Sulfamethoxazole (SMX) |  | 253.23 | |
| Phenicols | Chloramphenicol (CHL) | 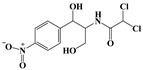 | 323.13 |
| Florfenicol (FLO) |  | 358.21 |
| Antibiotics | Precursor Ion | Transitions (m/z) | Fragmentor (V) | Collision Energy (V) |
|---|---|---|---|---|
| TC | [M + H]+ | 445→410 | 120 | 20 |
| OTC | [M + H]+ | 461→426 | 120 | 20 |
| CTC | [M + H]+ | 479→444 | 120 | 20 |
| SDZ | [M + H]+ | 251→92 | 100 | 30 |
| SMZ | [M + H]+ | 265→92 | 120 | 30 |
| SMX | [M + H]+ | 254→92 | 110 | 30 |
| CHL | [M − H]+ | 321→257 | 130 | 5 |
| FLO | [M − H]+ | 356→336 | 140 | 5 |
| Detected Items | Aquaculture Tank Effluent | Biological Filter Effluent | Effluent After OAHC | Limit Values |
|---|---|---|---|---|
| Temperature (°C) | 24.2 | 24.3 | 23.9 | — |
| Salinity (‰) | 34.8 | 35.1 | 34.9 | — |
| pH | 7.20 | 7.35 | 7.86 | 7.8~8.5 1 |
| Dissolved oxygen (mg/L) | 9.30 | 9.79 | 13.19 | >5 1 |
| Turbidity (NTU) | 1.44 | 0.62 | 0.27 | — |
| CODMn (mg/L) | 2.71 | 2.12 | 1.84 | ≤3 1 |
| Ammonia nitrogen (mg/L) | 1.20 | 0.43 | 0.44 | — |
| Nitrite nitrogen (mg/L) | 0.18 | 0.14 | 0.01 | — |
| Sulfide (mg/L) | 0.04 | 0.03 | 0.04 | ≤0.05 1 |
| Bromate (μg/L) | ND | — | ND | ≤10 2 |
| Trichloromethane (μg/L) | 0.12 | — | 0.16 | ≤60 2 |
| Bromodichloromethane (μg/L) | 0.01 | — | 0.03 | ≤60 2 |
| Dibromochloromethane (μg/L) | 0.01 | — | 0.13 | ≤100 2 |
| Tribromomethane (μg/L) | 0.03 | — | 4.87 | ≤100 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yang, D.; Song, L.; Jiang, Y. Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation. Water 2025, 17, 566. https://doi.org/10.3390/w17040566
Huang X, Yang D, Song L, Jiang Y. Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation. Water. 2025; 17(4):566. https://doi.org/10.3390/w17040566
Chicago/Turabian StyleHuang, Xiaodian, Dong Yang, Liang Song, and Yongcan Jiang. 2025. "Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation" Water 17, no. 4: 566. https://doi.org/10.3390/w17040566
APA StyleHuang, X., Yang, D., Song, L., & Jiang, Y. (2025). Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation. Water, 17(4), 566. https://doi.org/10.3390/w17040566






