Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design: Sampling and Mesocosm Setup
2.2. Nitrate Enrichment Experiment
2.3. Analytical Methods
2.4. Calculation of Benthic Fluxes (NO3−, NO2−, and N2)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Macrophyte Selection as a Key Factor in Optimizing Water Treatment Performance
3.2. Denitrification Capacity Along the NO3− Gradient
3.3. Biofilms as Hotspots of NO3− Removal via Denitrification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A New Wine in an Old Bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vigiak, O.; Udías, A.; Grizzetti, B.; Zanni, M.; Aloe, A.; Weiss, F.; Hristov, J.; Bisselink, B.; de Roo, A.; Pistocchi, A. Recent Regional Changes in Nutrient Fluxes of European Surface Waters. Sci. Total Environ. 2023, 858, 160063. [Google Scholar] [CrossRef] [PubMed]
- Souliotis, I.; Voulvoulis, N. Operationalising Nature-Based Solutions for the Design of Water Management Interventions. Nat. Based Solut. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Rizzo, A.; Sarti, C.; Nardini, A.; Conte, G.; Masi, F.; Pistocchi, A. Nature-Based Solutions for Nutrient Pollution Control in European Agricultural Regions: A Literature Review. Ecol. Eng. 2023, 186, 106772. [Google Scholar] [CrossRef]
- Mancuso, G.; Bencresciuto, G.F.; Lavrnić, S.; Toscano, A. Diffuse Water Pollution from Agriculture: A Review of Nature-Based Solutions for Nitrogen Removal and Recovery. Water 2021, 13, 1893. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Zhu, B.; Stefanakis, A.I.; Ajibade, F.O.; Dzakpasu, M.; Soana, E.; Wang, T.; Arif, M.; Muyembe, D.K.; Agboola, T.D. Advances in Ecotechnological Methods for Diffuse Nutrient Pollution Control: Wicked Issues in Agricultural and Urban Watersheds. Front. Environ. Sci. 2023, 11, 1199923. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z.; et al. Constructed Wetlands for Pollution Control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent Research Challenges in Constructed Wetlands for Wastewater Treatment: A Review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Lee, C.G.; Fletcher, T.D.; Sun, G. Nitrogen Removal in Constructed Wetland Systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Djodjic, F.; Geranmayeh, P.; Collentine, D.; Markensten, H.; Futter, M. Cost Effectiveness of Nutrient Retention in Constructed Wetlands at a Landscape Level. J. Environ. Manag. 2022, 324, 116325. [Google Scholar] [CrossRef]
- García-Herrero, L.; Lavrnić, S.; Guerrieri, V.; Toscano, A.; Milani, M.; Cirelli, G.L.; Vittuari, M. Cost-Benefit of Green Infrastructures for Water Management: A Sustainability Assessment of Full-Scale Constructed Wetlands in Northern and Southern Italy. Ecol. Eng. 2022, 185, 106797. [Google Scholar] [CrossRef]
- Nan, X.; Lavrnić, S.; Mancuso, G.; Toscano, A. Effects of Design and Operational Conditions on the Performance of Constructed Wetlands for Agricultural Pollution Control—Critical Review. Water Air Soil Pollut. 2023, 234, 434. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of Root Exudates on Denitrifier Gene Abundance, Community Structure and Activity in a Micro-Polluted Constructed Wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Wang, R.; Baldy, V. Purification Processes Involved in Sludge Treatment by a Vertical Flow Wetland System: Focus on the Role of the Substrate and Plants on N and P Removal. Bioresour. Technol. 2012, 105, 9–14. [Google Scholar] [CrossRef]
- Pierobon, E.; Castaldelli, G.; Mantovani, S.; Vincenzi, F.; Fano, E.A. Nitrogen Removal in Vegetated and Unvegetated Drainage Ditches Impacted by Diffuse and Point Sources of Pollution. Clean 2013, 41, 24–31. [Google Scholar] [CrossRef]
- Cui, Z.; Huang, J.; Gao, J.; Han, J. Characterizing the Impacts of Macrophyte-Dominated Ponds on Nitrogen Sources and Sinks by Coupling Multiscale Models. Sci. Total Environ. 2022, 811, 152208. [Google Scholar] [CrossRef]
- Piña-Ochoa, E.; Álvarez-Cobelas, M. Denitrification in Aquatic Environments: A Cross-System Analysis. Biogeochemistry 2006, 81, 111–130. [Google Scholar] [CrossRef]
- Deng, D.; He, G.; Ding, B.; Liu, W.; Yang, Z.; Ma, L. Denitrification Dominates Dissimilatory Nitrate Reduction across Global Natural Ecosystems. Glob. Change Biol. 2024, 30, e17256. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen Loading Affects Microbes, Nitrifiers and Denitrifiers Attached to Submerged Macrophyte in Constructed Wetlands. Sci. Total Environ. 2018, 622, 121–126. [Google Scholar] [CrossRef]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic Biofilms in Freshwater and Interactions with Macrophytes: Current Understanding and Future Directions. Aquat. Bot. 2022, 176, 103467. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Jiang, C. Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates. Water 2024, 16, 3706. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Chandra, H.; Kalra, S.J.S.; Mishra, P.; Khan, H.; Yadav, P. Plant–Microbe Interaction in Aquatic System and Their Role in the Management of Water Quality: A Review. Appl. Water Sci. 2017, 7, 1079–1090. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Hu, Y.; Ding, J.; Ma, Q.; Zong, K.; Yang, Z. Effect of Carbon Source Derived from Macrophytes on Microbial Denitrification in Constructed Wetlands: Role of Plant Species. Bioresour. Technol. Rep. 2019, 7, 100217. [Google Scholar] [CrossRef]
- Alldred, M.; Baines, S.B. Effects of Wetland Plants on Denitrification Rates: A Meta-analysis. Ecol. Appl. 2016, 26, 676–685. [Google Scholar] [CrossRef]
- Ruiz-Rueda, O.; Hallin, S.; Bañeras, L. Structure and Function of Denitrifying and Nitrifying Bacterial Communities in Relation to the Plant Species in a Constructed Wetland. FEMS Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef]
- Donato, M.; Johnson, O.; Steven, B.; Lawrence, B.A. Nitrogen Enrichment Stimulates Wetland Plant Responses Whereas Salt Amendments Alter Sediment Microbial Communities and Biogeochemical Responses. PLoS ONE 2020, 15, e0235225. [Google Scholar] [CrossRef]
- Tyler, H.L.; Moore, M.T.; Locke, M.A. Influence of Three Aquatic Macrophytes on Mitigation of Nitrogen Species from Agricultural Runoff. Water Air Soil Pollut. 2012, 223, 3227–3236. [Google Scholar] [CrossRef]
- Gebremariam, S.Y.; Beutel, M.W. Nitrate Removal and DO Levels in Batch Wetland Mesocosms: Cattail (Typha spp.) versus Bulrush (Scirpus spp.). Ecol. Eng. 2008, 34, 1–6. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Böhlke, J.K.; Tobias, C.R. Tracking the Fate of Nitrate through Pulse-flow Wetlands: A Mesocosm Scale 15N Enrichment Tracer Study. Ecol. Eng. 2017, 106, 597–608. [Google Scholar] [CrossRef]
- Vymazal, J. Plants Used in Constructed Wetlands with Horizontal Subsurface Flow: A Review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Retta, B.; Coppola, E.; Ciniglia, C.; Grilli, E. Constructed Wetlands for the Wastewater Treatment: A Review of Italian Case Studies. Appl. Sci. 2023, 13, 6211. [Google Scholar] [CrossRef]
- Costa, D.; Sutter, C.; Shepherd, A.; Jarvie, H.; Wilson, H.; Elliott, J.; Liu, J.; Macrae, M.L. Impact of Climate Change on Catchment Nutrient Dynamics: Insights from Around the World. Environ. Rev. 2022, 31, 4–25. [Google Scholar] [CrossRef]
- Castaldelli, G.; Aschonitis, V.; Vincenzi, F.; Fano, E.A.; Soana, E. The Effect of Water Velocity on Nitrate Removal in Vegetated Waterways. J. Environ. Manag. 2018, 215, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Soana, E.; Bartoli, M.; Milardi, M.; Fano, E.A.; Castaldelli, G. An Ounce of Prevention Is Worth a Pound of Cure: Managing Macrophytes for Nitrate Mitigation in Irrigated Agricultural Watersheds. Sci. Total Environ. 2019, 647, 301–312. [Google Scholar] [CrossRef]
- Soana, E.; Fano, E.A.; Castaldelli, G. Estimate of Gas Transfer Velocity in the Presence of Emergent Vegetation Using Argon as a Tracer: Implications for Whole-System Denitrification Measurements. Chemosphere 2018, 213, 526–532. [Google Scholar] [CrossRef]
- Soana, E.; Gavioli, A.; Tamburini, E.; Fano, E.A.; Castaldelli, G. To Mow or Not to Mow: Reed Biofilms as Denitrification Hotspots in Drainage Canals. Ecol. Eng. 2018, 113, 1–10. [Google Scholar] [CrossRef]
- Veraart, A.J.; Dimitrov, M.R.; Schrier-Uijl, A.P.; Smidt, H.; de Klein, J.J.M. Abundance, Activity and Community Structure of Denitrifiers in Drainage Ditches in Relation to Sediment Characteristics, Vegetation and Land-Use. Ecosystems 2017, 20, 928–943. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of Denitrification and Dissimilatory Nitrate Reduction to Ammonium. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; Wiley and Sons: New York, NY, USA, 1988; pp. 179–244. [Google Scholar]
- Owens, M.S.; Cornwell, J.C. The Benthic Exchange of O2, N2 and Dissolved Nutrients Using Small Core Incubations. J. Vis. Exp. 2016, 114, e54098. [Google Scholar] [CrossRef]
- Kana, T.M.; Darkangelo, C.; Hunt, M.D.; Oldham, J.B.; Bennett, G.E.; Cornwell, J.C. Membrane Inlet Mass Spectrometer for Rapid High-Precision Determination of N2, O2, and Ar in Environmental Water Samples. Anal. Chem. 1994, 66, 4166–4170. [Google Scholar] [CrossRef]
- Weiss, R.F. The Solubility of Nitrogen, Oxygen and Argon in Water and Seawater. Deep-Sea Res. Oceanogr. Abstr. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Armstrong, F.A.J.; Stearns, C.R.; Strickland, J.D.H. The Measurement of Upwelling and Subsequent Biological Process by Means of the Technicon Autoanalyzer® and Associated Equipment. Deep-Sea Res. Oceanogr. Abstr. 1967, 14, 381–389. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewaters, 8th ed.; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Bower, C.E.; Holm-Hansen, T. A Salicylate–Hypochlorite Method for Determining Ammonia in Seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Negi, D.; Verma, S.; Singh, S.; Daverey, A.; Lin, J.G. Nitrogen Removal via Anammox Process in Constructed Wetland—A Comprehensive Review. Chem. Eng. J. 2022, 437, 135434. [Google Scholar] [CrossRef]
- Shen, L.; Zheng, P.; Ma, S. Nitrogen Loss through Anaerobic Ammonium Oxidation in Agricultural Drainage Ditches. Biol. Fertil. Soils 2016, 52, 127–136. [Google Scholar] [CrossRef]
- Soana, E.; Gavioli, A.; Vincenzi, F.; Fano, E.A.; Castaldelli, G. Nitrate Availability Affects Denitrification in Phragmites australis Sediments. J. Environ. Qual. 2020, 49, 194–209. [Google Scholar] [CrossRef]
- Laursen, A.E.; Seitzinger, S.P. Measurement of Denitrification in Rivers: An Integrated, Whole Reach Approach. Hydrobiologia 2002, 485, 67–81. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Bírgand, F. Comparison of four nitrate removal kinetic models in two distinct wetland restoration mesocosm systems. Water 2017, 9, 517. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smitt, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R package version 1.10.2.090002; R Foundation for Statistical Computing: Vienna, Austria, 2024; Volume 34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Linear and Nonlinear Mixed Effects Models; New Prairie Press: Manhattan, KS, USA, 2016. [Google Scholar]
- Allen, C.R.; Burr, M.D.; Camper, A.K.; Moss, J.J.; Stein, O.R. Seasonality, C:N Ratio and Plant Species Influence on Denitrification and Plant Nitrogen Uptake in Treatment Wetlands. Ecol. Eng. 2023, 191, 106946. [Google Scholar] [CrossRef]
- Racchetti, E.; Longhi, D.; Ribaudo, C.; Soana, E.; Bartoli, M. Nitrogen Uptake and Coupled Nitrification–Denitrification in Riverine Sediments with Benthic Microalgae and Rooted Macrophytes. Aquat. Sci. 2017, 79, 487–505. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Nicolaisen, M.H.; Revsbech, N.P.; Lomstein, B.A. Competition between Ammonia-Oxidizing Bacteria and Benthic Microalgae. Appl. Environ. Microbiol. 2004, 70, 5528–5537. [Google Scholar] [CrossRef]
- Bastviken, S.K.; Eriksson, P.G.; Ekström, A.; Tonderski, K. Seasonal Denitrification Potential in Wetland Sediments with Organic Matter from Different Plant Species. Water Air Soil Pollut. 2007, 183, 25–35. [Google Scholar] [CrossRef]
- Toyama, T.; Nishimura, Y.; Ogata, Y.; Sei, K.; Mori, K.; Ike, M. Effects of Planting Phragmites australis on Nitrogen Removal, Microbial Nitrogen Cycling, and Abundance of Ammonia-Oxidizing and Denitrifying Microorganisms in Sediments. Environ. Technol. 2016, 37, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhu, J.N.; Liu, Q.F.; Liu, Y.; Liu, M.; Liu, L.; Zhang, Q. Comparison of the diversity of root-associated bacteria in Phragmites australis and Typha angustifolia L. in artificial wetlands. World J. Microbiol. Biotechnol. 2013, 29, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Roley, S.S.; Tank, J.L.; Grace, M.R.; Cook, P.L.M. The Influence of an Invasive Plant on Denitrification in an Urban Wetland. Freshw. Biol. 2018, 63, 353–365. [Google Scholar] [CrossRef]
- Payne, E.G.I.; Fletcher, T.D.; Russell, D.G.; Grace, M.R.; Cavagnaro, T.R.; Evrard, V.; Deletic, A.; Hatt, B.E.; Cook, P.L.M. Temporary Storage or Permanent Removal? The Division of Nitrogen between Biotic Assimilation and Denitrification in Stormwater Biofiltration Systems. PLoS ONE 2014, 9, e90890. [Google Scholar] [CrossRef]
- Speir, S.L.; Taylor, J.M.; Scott, J.T. Seasonal Differences in Relationships between Nitrate Concentration and Denitrification Rates in Ditch Sediments Vegetated with Rice Cutgrass. J. Environ. Qual. 2017, 46, 1500–1509. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, M.; He, D.; Zhu, J.; Yang, S.; Fang, F.; Yang, L. Submerged Macrophyte Promoted Nitrogen Removal Function of Biofilms in Constructed Wetland. Sci. Total Environ. 2024, 914, 169666. [Google Scholar] [CrossRef]
- Deng, H.; Li, Q.; Li, M.; Sun, L.; Li, B.; Wang, Y.; Wu, Q.L.; Zeng, J. Epiphytic Microorganisms of Submerged Macrophytes Effectively Contribute to Nitrogen Removal. Environ. Res. 2024, 242, 117754. [Google Scholar] [CrossRef]
- Toet, S.; Huibers, L.H.F.A.; Van Logtestijn, R.S.P.; Verhoeven, J.T.A. Denitrification in the Periphyton Associated with Plant Shoots and in the Sediment of a Wetland System Supplied with Sewage Treatment Plant Effluent. Hydrobiologia 2003, 501, 29–44. [Google Scholar] [CrossRef]
- Bourgues, S.; Hart, B.T.H. Nitrogen Removal Capacity of Wetlands: Sediment versus Epiphytic Biofilms. Water Sci. Technol. 2007, 55, 175–182. [Google Scholar] [CrossRef]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The Ecology and Biogeochemistry of Stream Biofilms. Nat. Rev. Microb. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Venterink, H.O.; Hummelink, E.; Van Den Hoorn, M.W. Denitrification Potential of a River Floodplain during Flooding with Nitrate-Rich Water: Grasslands versus Reedbeds. Biogeochemistry 2003, 65, 233–244. [Google Scholar] [CrossRef]
- Yamamoto, M.; Murai, H.; Takeda, A.; Okunishi, S.; Morisaki, H. Bacterial Flora of the Biofilm Formed on the Submerged Surface of the Reed Phragmites australis. Microbes Environ. 2005, 20, 14–24. [Google Scholar] [CrossRef][Green Version]
- Eriksson, P.G.; Weisner, S.E.B. Nitrogen Removal in a Wastewater Reservoir: The Importance of Denitrification by Epiphytic Biofilms on Submersed Vegetation. J. Environ. Qual. 1997, 26, 905–910. [Google Scholar] [CrossRef]
- Eriksson, P.G. Interaction Effects of Flow Velocity and Oxygen Metabolism on Nitrification and Denitrification in Biofilms on Submersed Macrophytes. Biogeochemistry 2001, 55, 29–44. [Google Scholar] [CrossRef]
- Bastviken, S.K.; Eriksson, P.G.; Martins, I.; Neto, J.M.; Leonardson, L.; Tonderski, K. Potential Nitrification and Denitrification on Different Surfaces in a Constructed Treatment Wetland. J. Environ. Qual. 2003, 32, 2414–2420. [Google Scholar] [CrossRef]
- Schaller, J.L.; Royer, T.V.; David, M.B.; Tank, J.L. Denitrification Associated with Plants and Sediments in an Agricultural Stream. J. N. Am. Benthol. Soc. 2004, 23, 667–676. [Google Scholar] [CrossRef]
- Adame, M.F.; Waltham, N.J.; Iram, N.; Farahani, B.S.; Salinas, C.; Burford, M.; Ronan, M. Denitrification within the Sediments and Epiphyton of Tropical Macrophyte Stands. Inland Waters 2021, 11, 257–266. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Zhang, W.; Xia, Y. Regulation of Denitrification/Ammonia Volatilization by Periphyton in Paddy Fields and Its Promise in Rice Yield Promotion. J. Sci. Food Agric. 2023, 103, 4119–4130. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
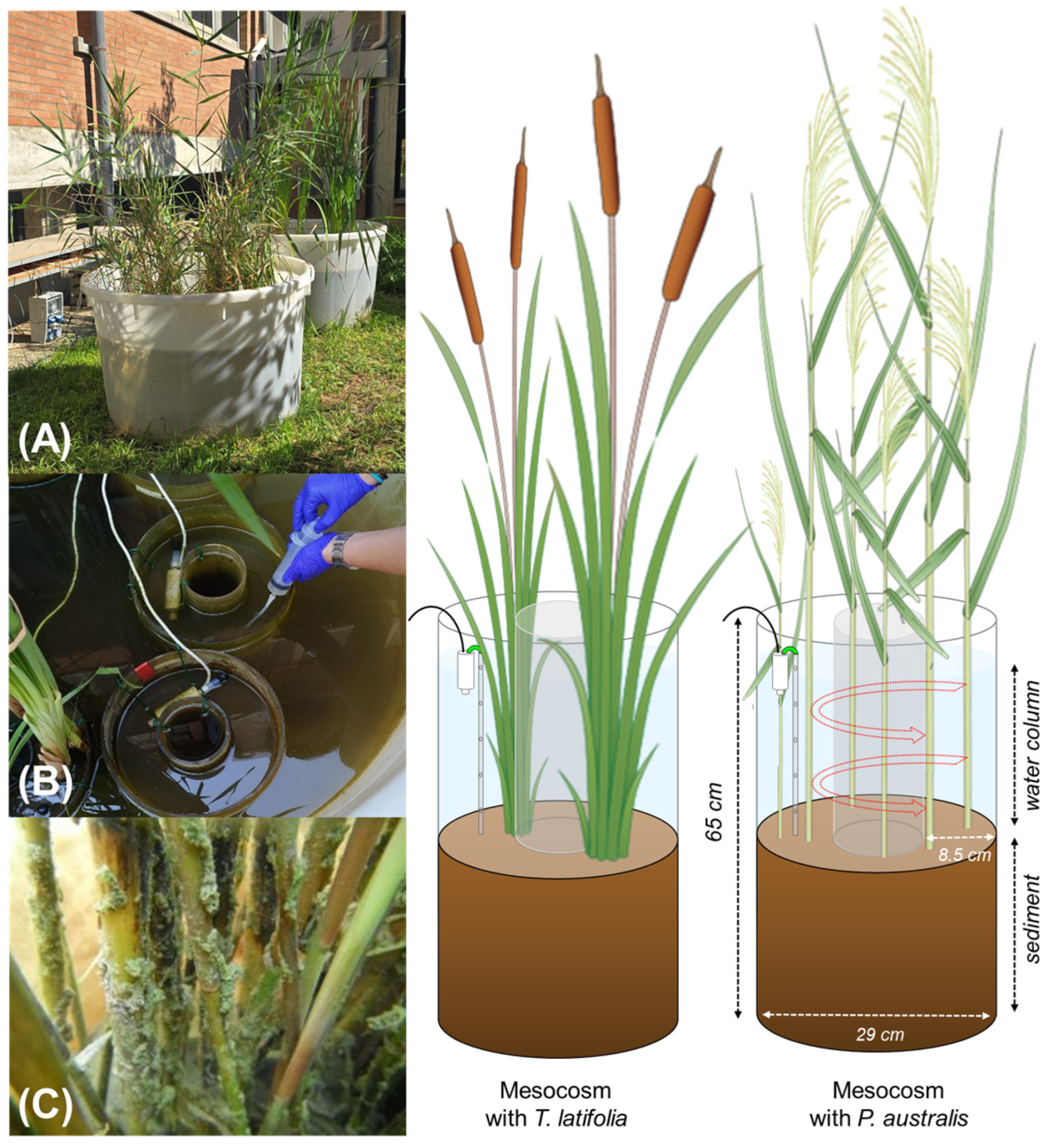
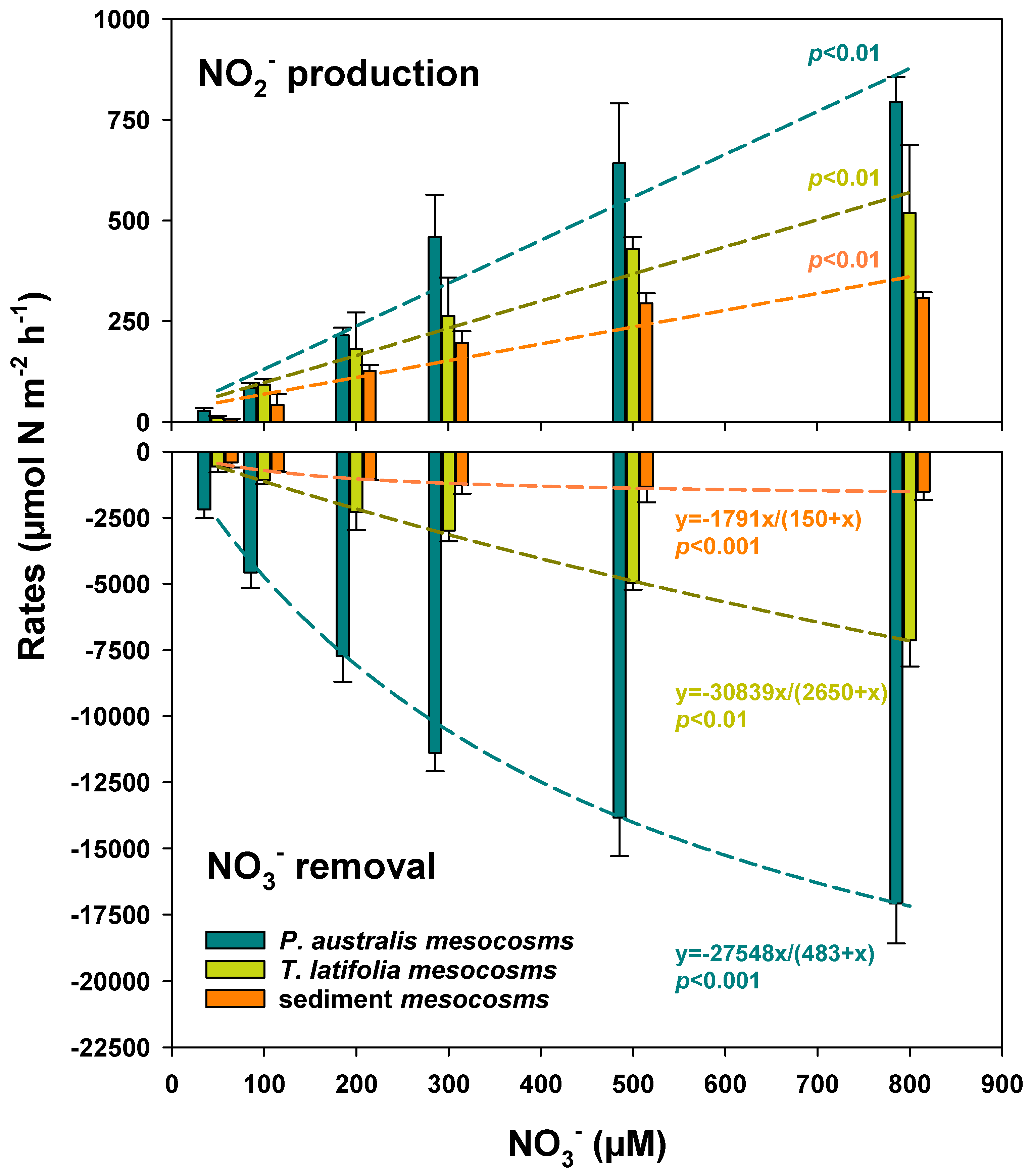
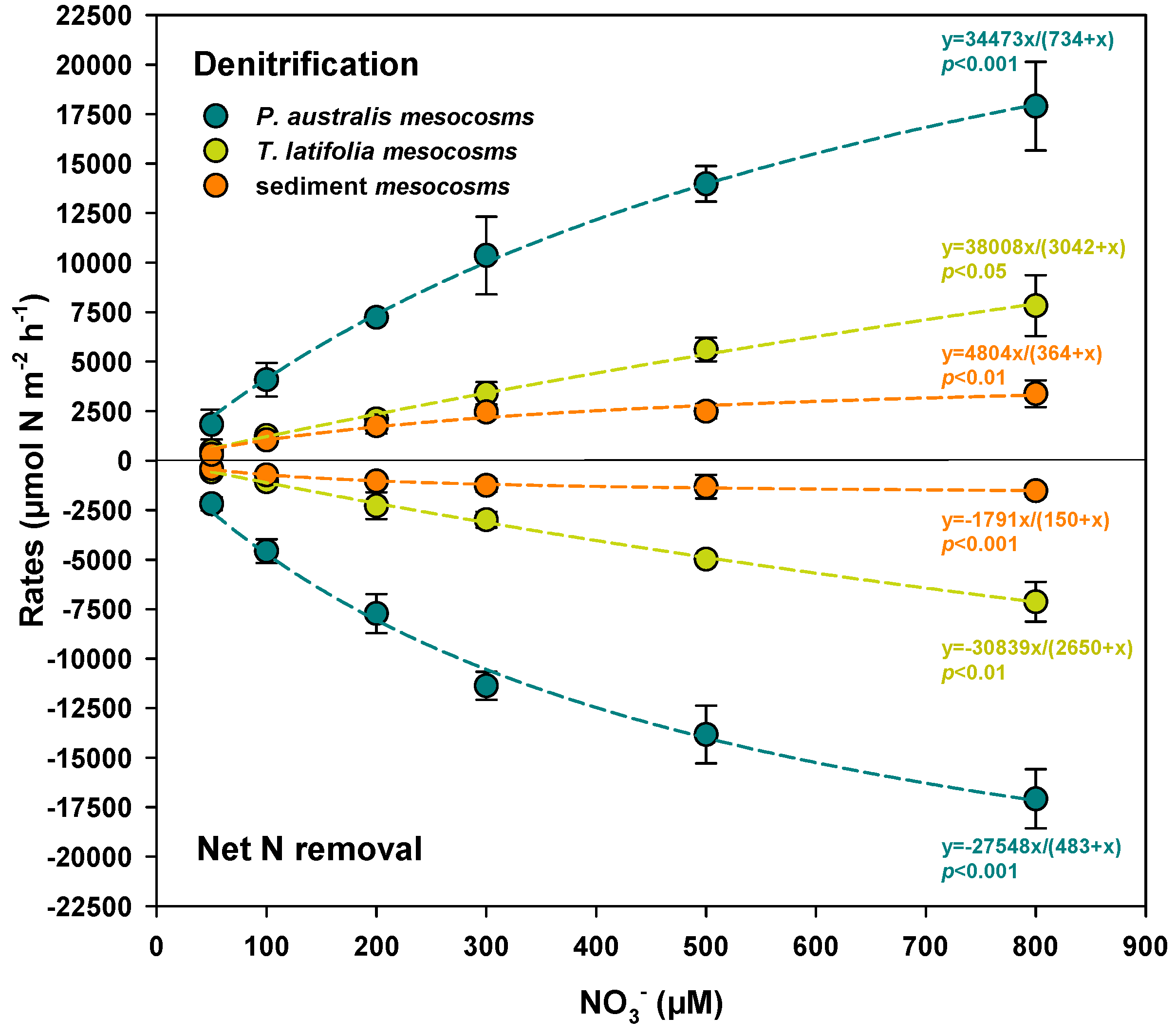
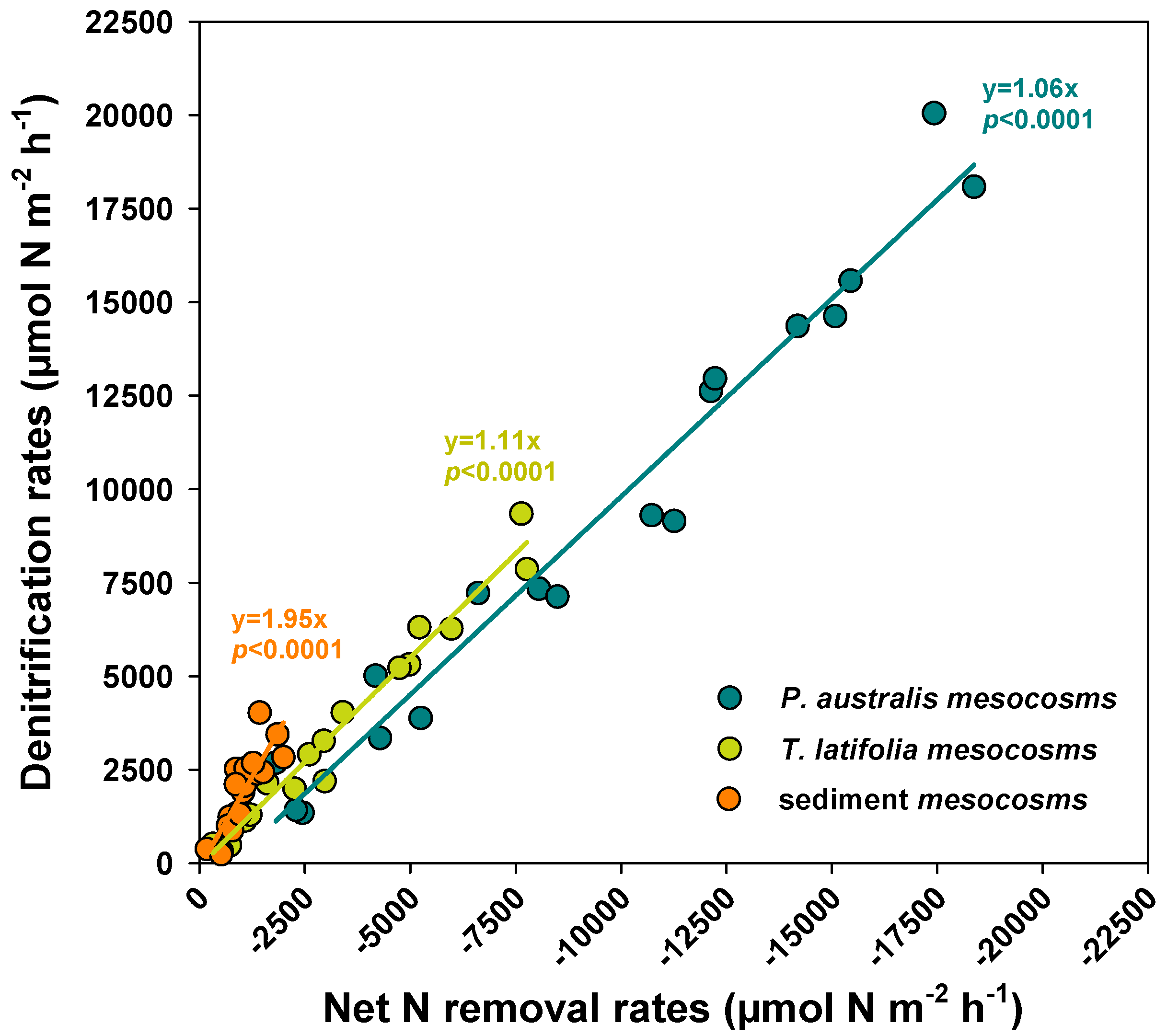
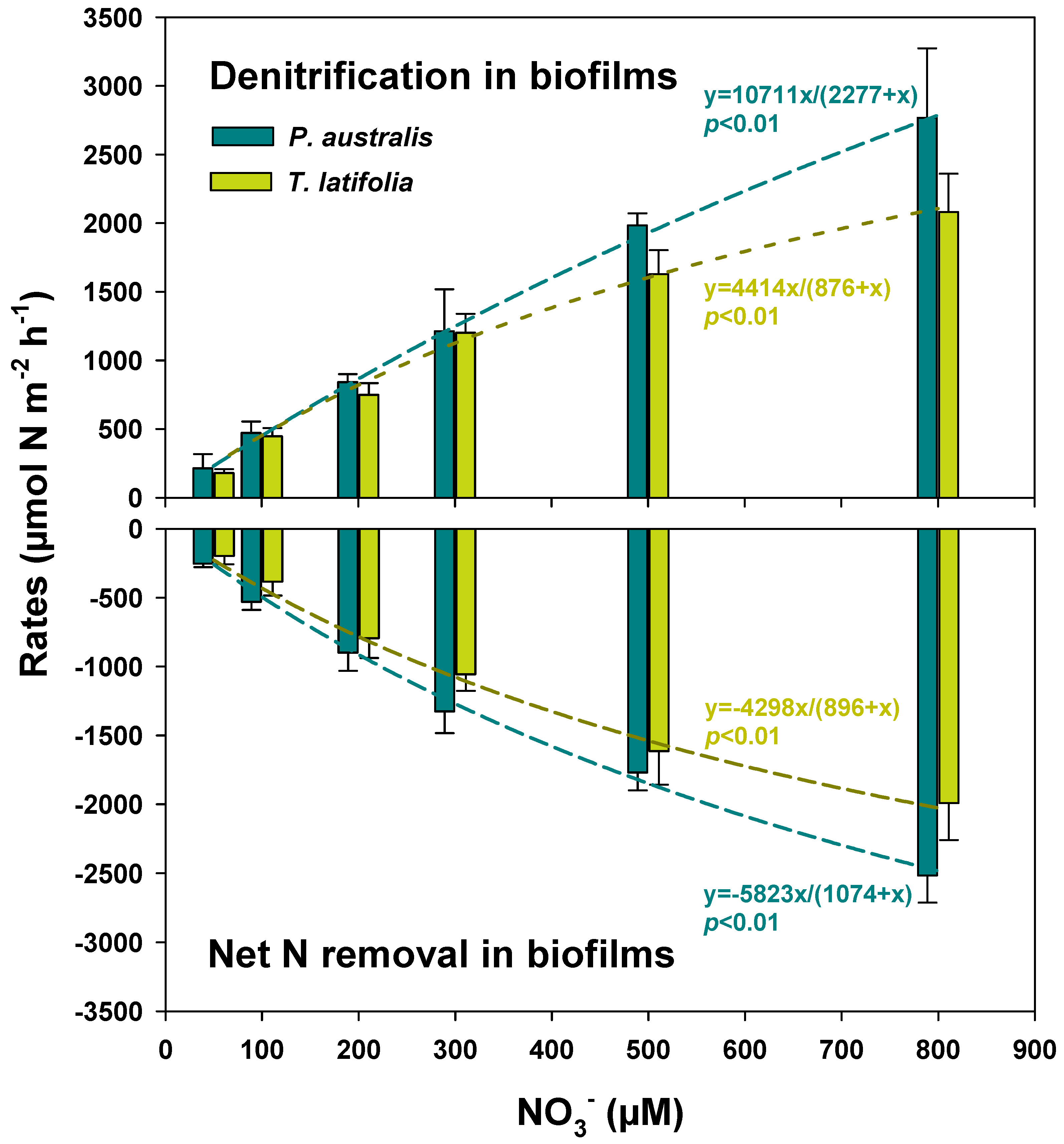
| Benthic Flux | Factor | Rate Expressed per m2 of Sediment | Rates Expressed per m2 of Surface Available for Biofilm Colonization | ||||
|---|---|---|---|---|---|---|---|
| df | F-Value | p-Value | df | F-Value | p-Value | ||
| NO3− removal | Plant type | 2 | 873.985 | <0.0001 | 1 | 0.0013 | 0.9715 |
| NO3− concentration | 5 | 212.769 | <0.0001 | 5 | 264.7168 | <0.0001 | |
| Plant type × NO3− concentration | 10 | 24.347 | <0.0001 | 5 | 5.9449 | 0.0013 | |
| NO2− production | Plant type | 2 | 45.305 | <0.0001 | 1 | 36.8892 | <0.0001 |
| NO3− concentration | 5 | 147.246 | <0.0001 | 5 | 50.2283 | <0.0001 | |
| Plant type × NO3− concentration | 10 | 2.831 | 0.0114 | 5 | 1.7726 | 0.1602 | |
| Net N removal | Plant type | 2 | 761.83 | <0.0001 | 1 | 0.7952 | 0.3822 |
| NO3− concentration | 5 | 162.002 | <0.0001 | 5 | 209.451 | <0.0001 | |
| Plant type × NO3− concentration | 10 | 21.677 | <0.0001 | 5 | 4.78 | 0.0042 | |
| Denitrification | Plant type | 2 | 435.672 | <0.0001 | 1 | 2.6417 | 0.1183 |
| NO3− concentration | 5 | 196.673 | <0.0001 | 5 | 173.3046 | <0.0001 | |
| Plant type × NO3− concentration | 10 | 12.117 | <0.0001 | 5 | 2.9863 | 0.0332 | |
| Treatment | Rate | R2 | Rmax | Km |
|---|---|---|---|---|
| µmol N m−2 h−1 | µM | |||
| P. australis mesocosms | Net N removal | 0.99 | −27,548 ± 2035 *** | 483 ± 69 *** |
| Denitrification | 0.99 | 34,473 ± 1826 *** | 734 ± 66 *** | |
| T. latifolia mesocosms | Net N removal | 0.99 | −30,839 ± 5079 ** | 2650 ± 535 ** |
| Denitrification | 0.99 | 38,008 ± 11,064 * | 3042 ± 1062 * | |
| Sediment mesocosms | Net N removal | 0.99 | 1791 ± 68 *** | 150 ± 17 ** |
| Denitrification | 0.96 | 4804 ± 742 ** | 364 ± 121 * |
| Macrophyte Species | T (°C) | NO3− Concentration (µM) | Denitrification Rate (µmol N m−2 h−1) | Method | Reference |
|---|---|---|---|---|---|
| Potamogeton pectinatus | 18 | 295 | 15−500 | Acetylene inhibition technique | Eriksson and Weisner, 1997 [70] |
| Potamogeton pectinatus | 20 | 715 | 3−15 | Isotope pairing technique | Eriksson, 2001 [71] |
| Myriophyllum spicatum | 20 | 1000 | 300−450 | Acetylene inhibition technique | Bastviken et al., 2003 [72] |
| Phragmites australis | 14−18 | 70 | 100−350 | Acetylene inhibition technique | Toet et al., 2003 [65] |
| Elodea nuttallii | 14−18 | 60 | 50−70 | Acetylene inhibition technique | Toet et al., 2003 [65] |
| Phragmites australis | 16 | 200 | 5−150 | Acetylene inhibition technique | Venterink et al., 2003 [68] |
| Potamogeton spp., Cladophora spp. | 14−28 | 50 | 2−200 | Acetylene inhibition technique | Schaller et al., 2004 [73] |
| Phragmites australis | 20 | 1000 | 100–480 | Acetylene inhibition technique | Yamamoto et al., 2005 [69] |
| Emergent macrophytes (stand of Phragmites australis and other species) | 20 | 35−1200 | 980−1860 | Acetylene inhibition technique | Bourgues and Hart, 2007 [66] |
| Actinoscirpus grossus, Nymphae spp. | 23−28 | <15 | 135−250 | Isotope pairing technique | Adame et al., 2021 [74] |
| Oryza sativa | 16 | 1100 | 25−325 | Net N2 flux measurement | Abulaiti et al., 2023 [75] |
| Vallisneria natans, Hydrilla verticillata | 24 | 50−80 | 5−170 | Isotope pairing technique | Deng et al., 2024 [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soana, E.; Vincenzi, F.; Gavioli, A.; Castaldelli, G. Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter? Water 2025, 17, 560. https://doi.org/10.3390/w17040560
Soana E, Vincenzi F, Gavioli A, Castaldelli G. Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter? Water. 2025; 17(4):560. https://doi.org/10.3390/w17040560
Chicago/Turabian StyleSoana, Elisa, Fabio Vincenzi, Anna Gavioli, and Giuseppe Castaldelli. 2025. "Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter?" Water 17, no. 4: 560. https://doi.org/10.3390/w17040560
APA StyleSoana, E., Vincenzi, F., Gavioli, A., & Castaldelli, G. (2025). Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter? Water, 17(4), 560. https://doi.org/10.3390/w17040560








