Environmental DNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in Dongshan Bay, China
Abstract
1. Introduction
2. Methods
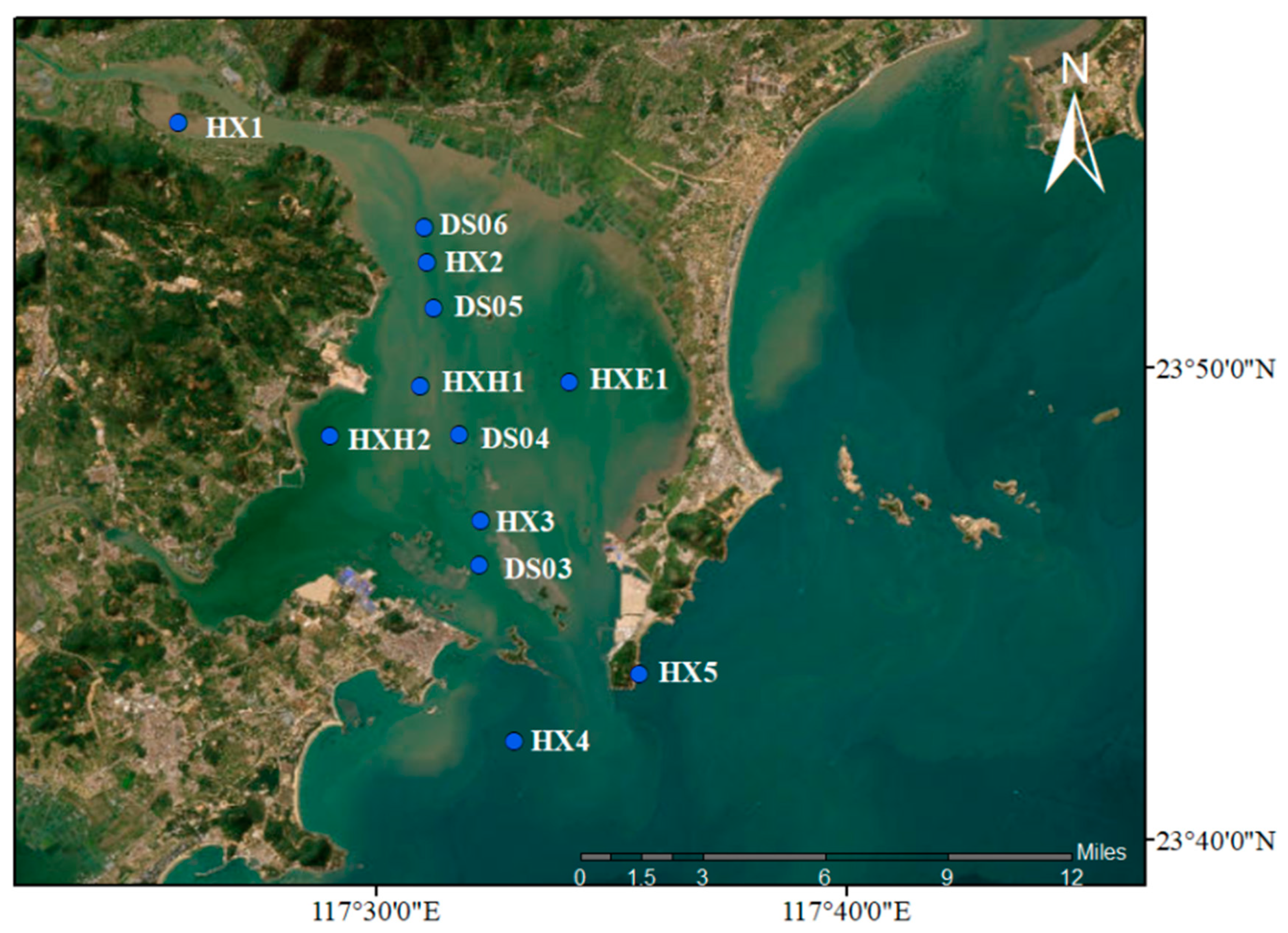
2.1. Field Site and Sample Collection
2.2. Measurement of Environmental Factors
2.3. PCR, Sequencing and Annotation
2.4. Data Analysis
3. Results
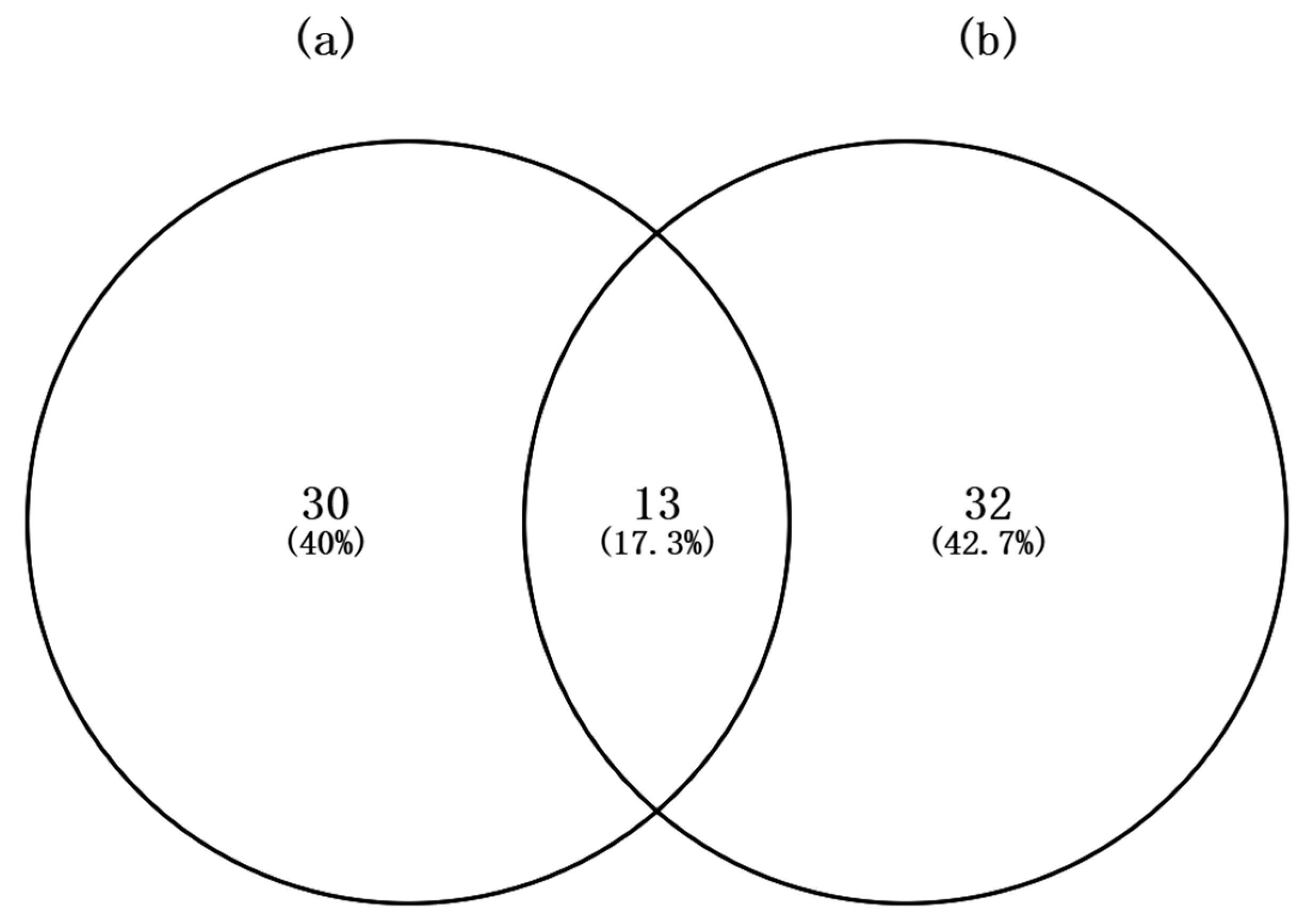
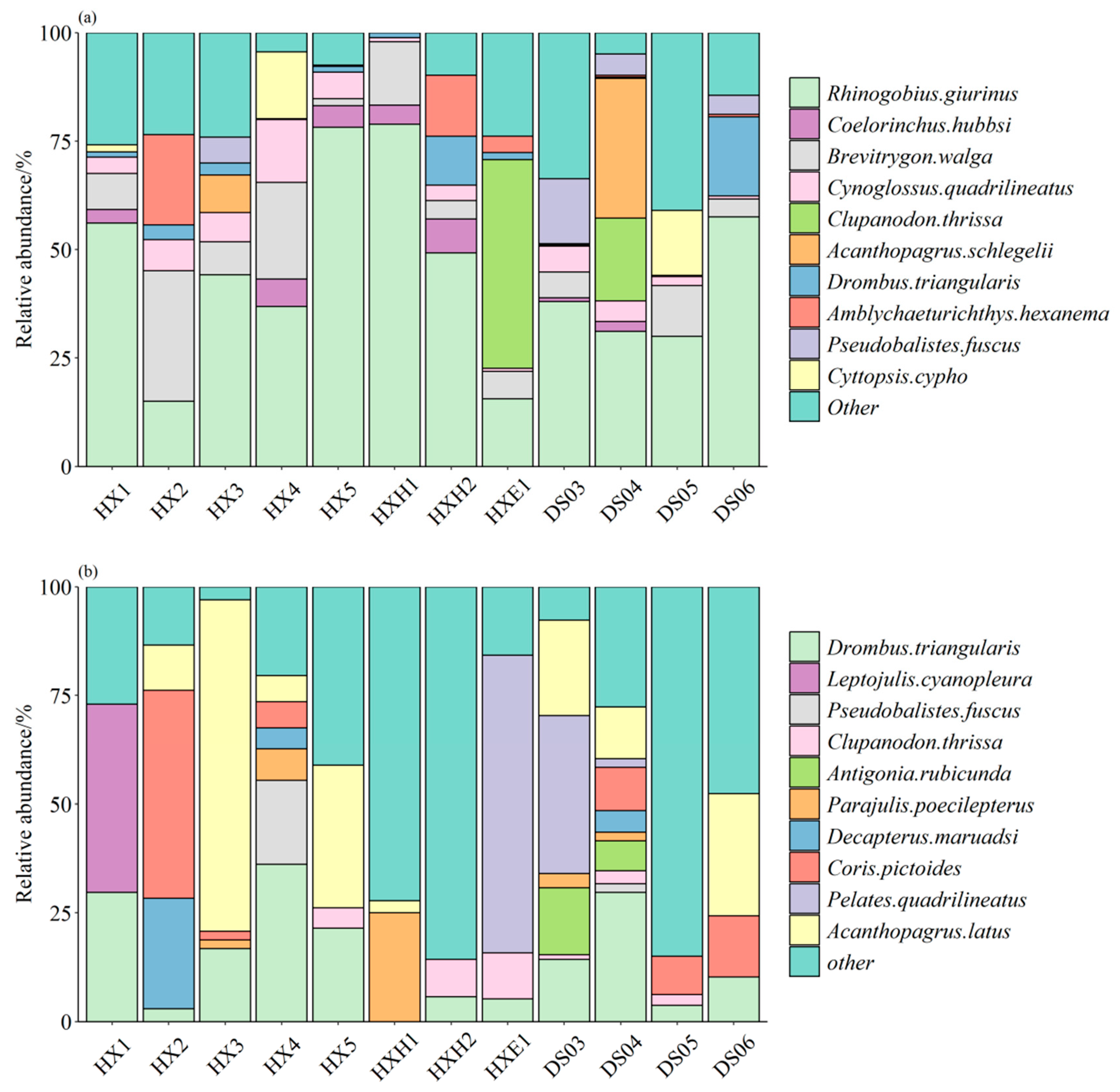
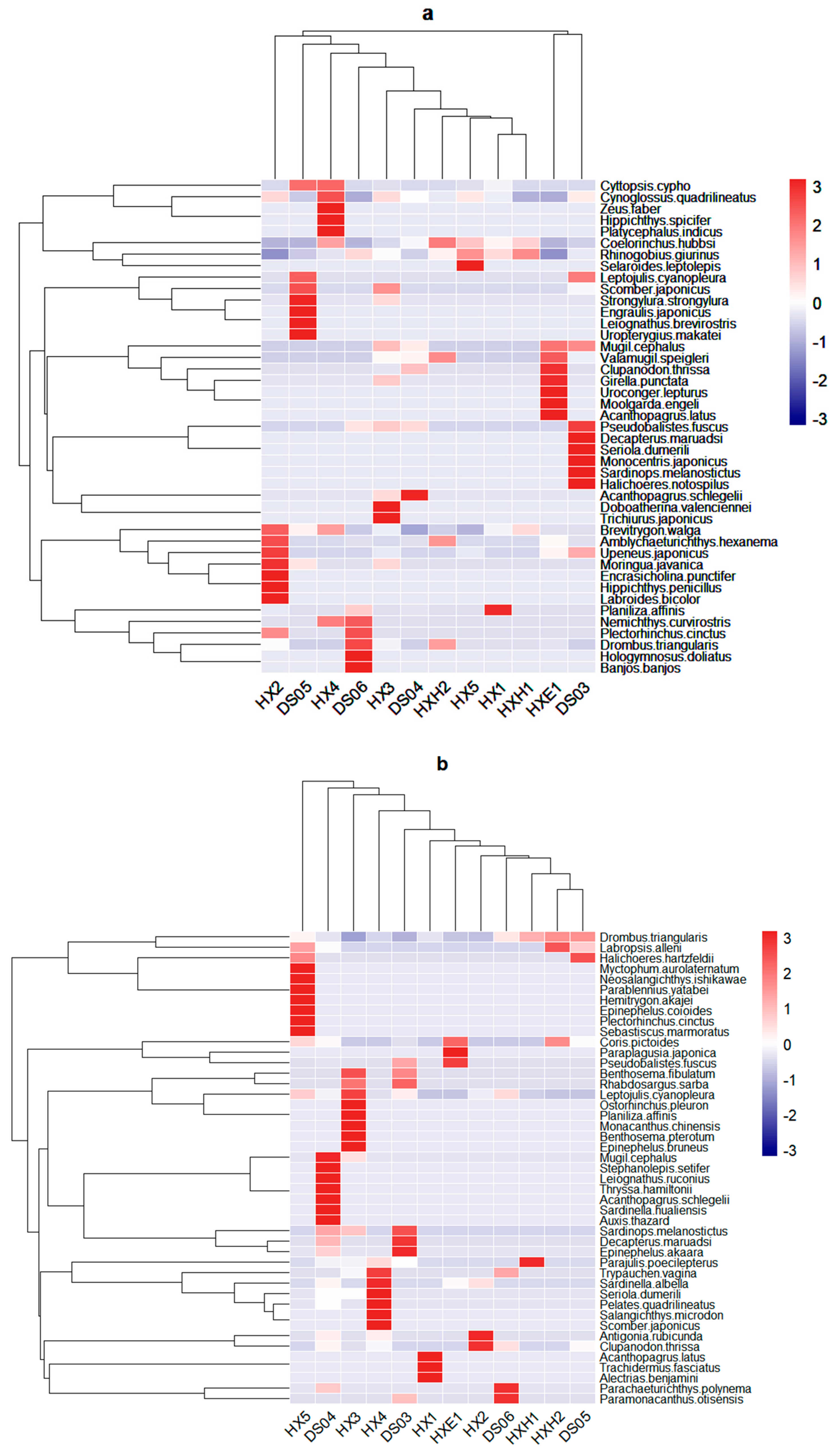
3.1. Species Composition
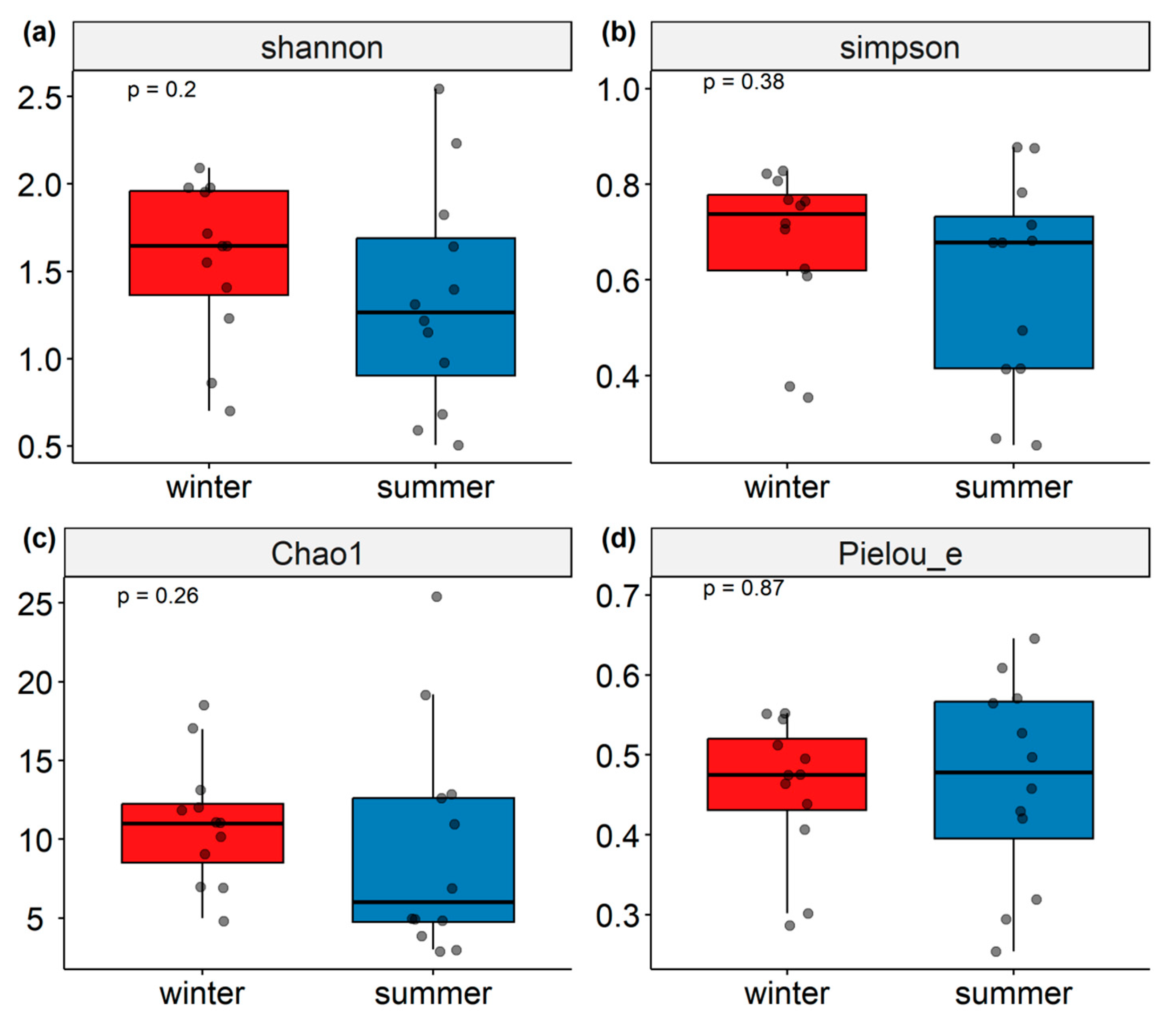
3.2. Alpha Diversity Analysis
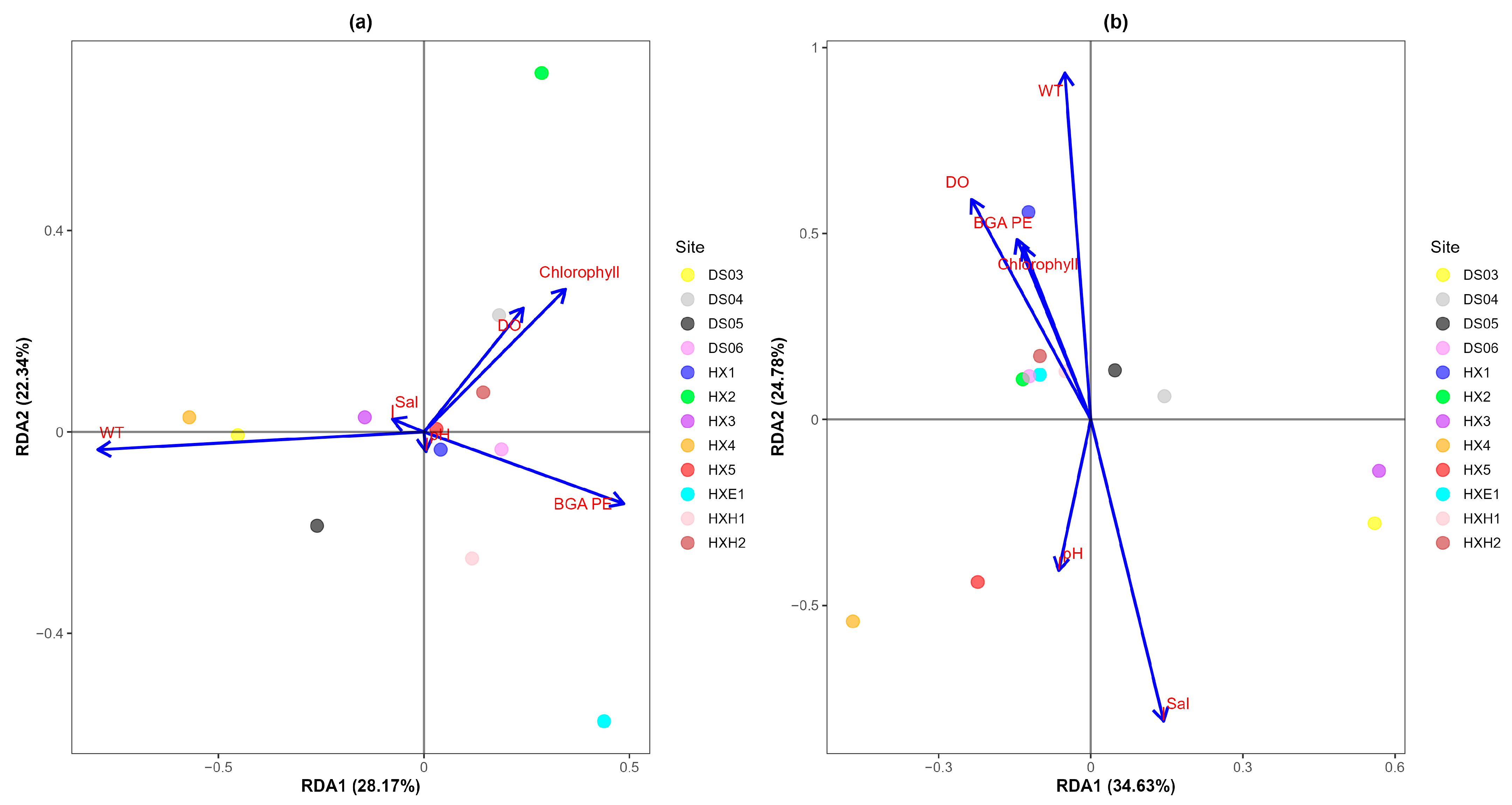
3.3. Correlation Analysis of Environmental Factors
4. Discussion
4.1. Fish Species Composition in Dongshan Bay During Winter and Summer
4.2. Alpha Diversity Analysis and Correlation with Environmental Factors
4.3. Impact of Thermal Discharge from Zhangzhou Nuclear Power Plant on Fish Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levêque, C.; Oberdorff, T.; PAUGy, D.; Stiassny, M.; Tedesco, P.A. Global diversity of fish (Pisces) in freshwater. In Freshwater Animal Diversity Assessment; Springer: Dordrecht, The Netherland, 2008; pp. 545–567. [Google Scholar]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. BioScience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Yamamoto, S.; Minami, K.; Fukaya, K.; Takahashi, K.; Sawada, H.; Murakami, H.; Tsuji, S.; Hashizume, H.; Kubonaga, S.; Horiuchi, T. Environmental DNA as a ‘snapshot’of fish distribution: A case study of Japanese jack mackerel in Maizuru Bay, Sea of Japan. PLoS ONE 2016, 11, e0149786. [Google Scholar]
- Evans, N.T.; Lamberti, G.A. Freshwater fisheries assessment using environmental DNA: A primer on the method, its potential, and shortcomings as a conservation tool. Fish. Res. 2018, 197, 60–66. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Hajibabaei, M.; Rieseberg, L.H. Environmental DNA. Mol. Ecol. 2012, 21, 1789. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.F.; Willerslev, E. Environmental DNA–An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Andruszkiewicz, E.A.; Yamahara, K.M.; Closek, C.J.; Boehm, A.B. Quantitative PCR assays to detect whales, rockfish, and common murre environmental DNA in marine water samples of the Northeastern Pacific. PLoS ONE 2020, 15, e0242689. [Google Scholar] [CrossRef]
- West, K.M.; Stat, M.; Harvey, E.S.; Skepper, C.L.; DiBattista, J.D.; Richards, Z.T.; Travers, M.J.; Newman, S.J.; Bunce, M. eDNA metabarcoding survey reveals fine-scale coral reef community variation across a remote, tropical island ecosystem. Mol. Ecol. 2020, 29, 1069–1086. [Google Scholar] [CrossRef]
- Shaw, J.L.; Clarke, L.J.; Wedderburn, S.D.; Barnes, T.C.; Weyrich, L.S.; Cooper, A. Comparison of environmental DNA metabarcoding and conventional fish survey methods in a river system. Biol. Conserv. 2016, 197, 131–138. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Cilleros, K.; Valentini, A.; Allard, L.; Dejean, T.; Etienne, R.; Grenouillet, G.; Iribar, A.; Taberlet, P.; Vigouroux, R.; Brosse, S. Unlocking biodiversity and conservation studies in high-diversity environments using environmental DNA (eDNA): A test with Guianese freshwater fishes. Mol. Ecol. Resour. 2019, 19, 27–46. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Møller, P.R.; Rasmussen, M.; Willerslev, E. Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLoS ONE 2012, 7, e41732. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, E.E.; Nielsen, I.B.; Carl, H.; Krag, M.A.; Knudsen, S.W.; Xing, Y.; Holm-Hansen, T.H.; Møller, P.R.; Thomsen, P.F. Seawater environmental DNA reflects seasonality of a coastal fish community. Mar. Biol. 2017, 164, 128. [Google Scholar] [CrossRef]
- Stat, M.; Huggett, M.J.; Bernasconi, R.; DiBattista, J.D.; Berry, T.E.; Newman, S.J.; Harvey, E.S.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef]
- Chen, B.; Ji, W.; Zhou, K.; He, Q.; Fu, T. Nutrient and eutrophication characteristics of the Dongshan Bay, South China. Chin. J. Oceanol. Limnol. 2014, 32, 886–898. [Google Scholar] [CrossRef]
- Xu, W.; Gong, L.; Yang, S.; Gao, Y.; Ma, X.; Xu, L.; Chen, H.; Luo, Z. Spatiotemporal dynamics of Vibrio communities and abundance in Dongshan Bay, South of China. Front. Microbiol. 2020, 11, 575287. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, Q.; Jiang, R.; Li, W.; Sun, X.; Lin, H.; Jiang, S.; Huang, H. Microplastic pollution and ecological risk assessment in an estuarine environment: The Dongshan Bay of China. Chemosphere 2021, 262, 127876. [Google Scholar] [CrossRef]
- Deiner, K.; Walser, J.-C.; Mächler, E.; Altermatt, F. Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol. Conserv. 2015, 183, 53–63. [Google Scholar] [CrossRef]
- He, W.; Wang, L.; Ou, D.; Li, W.; Huang, H.; Ou, R.; Qiu, J.; Cai, L.; Lin, L.; Zhang, Y. Fish Diversity Monitoring Using Environmental DNA Techniques in the Clarion–Clipperton Zone of the Pacific Ocean. Water 2023, 15, 2123. [Google Scholar] [CrossRef]
- Miya, M.; Gotoh, R.O.; Sado, T. MiFish metabarcoding: A high-throughput approach for simultaneous detection of multiple fish species from environmental DNA and other samples. Fish. Sci. 2020, 86, 939–970. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Yoshizawa, S.; Iwasaki, W.; Li, Y.; Xian, W.; Zhang, H. Seasonal variation and assessment of fish resources in the Yangtze Estuary based on environmental DNA. Water 2020, 12, 2874. [Google Scholar] [CrossRef]
- Yamamoto, S.; Masuda, R.; Sato, Y.; Sado, T.; Araki, H.; Kondoh, M.; Minamoto, T.; Miya, M. Environmental DNA metabarcoding reveals local fish communities in a species-rich coastal sea. Sci. Rep. 2017, 7, 40368. [Google Scholar] [CrossRef] [PubMed]
- Huang-Rong, W. Survey on status of fishery resources of Dongshan Bay in Fujian. J. Fish. Res. 2016, 38, 112. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Bolker, B. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008; Volume 396. [Google Scholar]
- Oksanen, J.; Minchin, P.R. Instability of ordination results under changes in input data order: Explanations and remedies. J. Veg. Sci. 1997, 8, 447–454. [Google Scholar] [CrossRef]
- Beamish, R.; Leask, K.; Ivanov, O.; Balanov, A.; Orlov, A.; Sinclair, B. The ecology, distribution, and abundance of midwater fishes of the Subarctic Pacific gyres. Prog. Oceanogr. 1999, 43, 399–442. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamamoto, S.; Sato, Y.; Sado, T.; Minamoto, T.; Miya, M. Comparing local-and regional-scale estimations of the diversity of stream fish using eDNA metabarcoding and conventional observation methods. Freshw. Biol. 2018, 63, 569–580. [Google Scholar] [CrossRef]
- Jing, Z.; Pu-Qing, S.; Zhi-Hui, Z.; Long-Shan, L. Species composition and abundance temporal-spatial distribution of fish egg, larvae and juveniles in Dongshan Bay of Fujian. J. Fish. Res. 2013, 35, 1. [Google Scholar]
- Sun-Zhong, Y.; Dong-Lian, L.; Jian-Di, C.; Yong, L.; Chao, M.; Fang, Y.; Shu-Yue, G.; Chang-Chun, S. Characteristics of community structure and biomass distribution of fishery resources in Dongshan Bay. J. Fish. Res. 2018, 40, 358. [Google Scholar]
- Chen, X.; Ji, P.; Wu, Y.; Zhao, Y.; Zeng, L. Coupling simulation of overland flooding and underground network drainage in a coastal nuclear power plant. Nucl. Eng. Des. 2017, 325, 129–134. [Google Scholar] [CrossRef]
- Ding, X.; Tian, W.; Zhai, A.; Kang, B.; Zhu, Q. Research on Water Intake, Usage and Drainage Impact Demonstration for Coastal Nuclear Power Plants. IOP Conf. Ser. Earth Environ. Sci. 2019, 223, 012004. [Google Scholar] [CrossRef]
- Liu, N.; Huang, Z.; Fang, Y.; Dong, Z. Impacts of Thermal Drainage on Bacterial Diversity and Community Construction in Tianwan Nuclear Power Plant. Microb. Ecol. 2023, 86, 2981–2992. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Caselle, J.E.; Gaymer, C.F.; Palma, A.T.; Petit, I.; Varas, E.; Muñoz Wilson, A.; Sala, E. Marine biodiversity in Juan Fernández and Desventuradas Islands, Chile: Global endemism hotspots. PLoS ONE 2016, 11, e0145059. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Plante, C.; Lalonde, S. Fish production correlated with primary productivity, not the morphoedaphic index. Can. J. Fish. Aquat. Sci. 1990, 47, 1929–1936. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yokoduka, T. Radioactive contamination of fishes in lake and streams impacted by the Fukushima nuclear power plant accident. Sci. Total Environ. 2014, 482, 184–192. [Google Scholar] [CrossRef]
- Rotherham, D.; Chapman, M.; Underwood, A.; Gray, C.; Johnson, D. Untangling spatial and temporal variation in abundances of estuarine fish sampled with multi-mesh gillnets. Mar. Ecol. Prog. Ser. 2011, 435, 183–195. [Google Scholar] [CrossRef][Green Version]
- Larson, J.H.; Trebitz, A.S.; Steinman, A.D.; Wiley, M.J.; Mazur, M.C.; Pebbles, V.; Braun, H.A.; Seelbach, P.W. Great Lakes rivermouth ecosystems: Scientific synthesis and management implications. J. Great Lakes Res. 2013, 39, 513–524. [Google Scholar] [CrossRef]
- Wang, S.; Yan, Z.; Hänfling, B.; Zheng, X.; Wang, P.; Fan, J.; Li, J. Methodology of fish eDNA and its applications in ecology and environment. Sci. Total Environ. 2021, 755, 142622. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fleming, L.E.; Squicciarini, D.; Backer, L.C.; Clark, R.; Abraham, W.; Benson, J.; Cheng, Y.S.; Johnson, D.; Pierce, R. Literature review of Florida red tide: Implications for human health effects. Harmful Algae 2004, 3, 99–115. [Google Scholar] [CrossRef]
- Agersborg, H. The influence of temperature on fish. Ecology 1930, 11, 136–144. [Google Scholar] [CrossRef]
- Mallya, Y.J. The Effects of Dissolved Oxygen on Fish Growth in Aquaculture. The United Nations University Fisheries Training Programme, Final Project. 2007. Available online: https://www.grocentre.is/static/gro/publication/58/document/yovita07prf.pdf (accessed on 11 November 2024).
- Zhang, H.; Yoshizawa, S.; Iwasaki, W.; Xian, W. Seasonal fish assemblage structure using environmental DNA in the Yangtze Estuary and its adjacent waters. Front. Mar. Sci. 2019, 6, 515. [Google Scholar] [CrossRef]
- Osborne, L.L.; Wiley, M.J. Influence of tributary spatial position on the structure of warmwater fish communities. Can. J. Fish. Aquat. Sci. 1992, 49, 671–681. [Google Scholar] [CrossRef]
- Eaton, J.G.; Scheller, R.M. Effects of climate warming on fish thermal habitat in streams of the United States. Limnol. Oceanogr. 1996, 41, 1109–1115. [Google Scholar] [CrossRef]
- Neuman, E. Thermal discharge and fish fauna in Sweden. Water Sci. Technol. 1983, 15, 67–87. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Sastry, J.; Swamy, G.N. Implication of Thermal Discharges into the Sea—A Review. 1991. Available online: http://drs.nio.org/drs/handle/2264/3339 (accessed on 22 November 2024).
- Tang, Y. Politics of the risk management for marine invasive alien species in China: Constructing a cooperation system. Appl. Mech. Mater. 2013, 295, 520–527. [Google Scholar] [CrossRef]
- Du, Y.; Meng, F.; Fu, W.; Wang, Z. Distribution, speciation and bioaccumulation of Hg and As in mariculture sediments from Dongshan Bay, China. Soil Sediment Contam. Int. J. 2016, 25, 489–504. [Google Scholar] [CrossRef]
| Order | Family | Species | Season | |
|---|---|---|---|---|
| Winter | Summer | |||
| Mugiliformes | Mugilidae | Planiliza affinis (Günther, 1861) | * | * |
| Valamugil speigleri (Bleeker, 1858) | * | |||
| Moolgarda engeli (Bleeker, 1858) | * | |||
| Mugil cephalus (Linnaeus, 1758) | * | * | ||
| Clupeiformes | Clupeidae | Sardinops melanostictus (Jenyns, 1842) | * | * |
| Clupanodon thrissa (Linnaeus, 1758) | * | * | ||
| Sardinella albella (Valenciennes, 1847) | * | |||
| Sardinella hualiensis (Chu & Tsai, 1958) | * | |||
| Engraulidae | Encrasicholina punctifer (Fowler, 1938) | * | ||
| Engraulis japonicus (Temminck & Schlegel, 1846) | * | |||
| Thryssa hamiltonii (Gray, 1835) | * | |||
| Centrarchiformes | Girellidae | Girella punctata (Gray, 1835) | * | |
| Terapontidae | Pelates quadrilineatus (Bloch, 1790) | * | ||
| Chaetodontiformes | Leiognathidae | Leiognathus brevirostris (Valenciennes, 1835) | * | |
| Leiognathus ruconius (Hamilton, 1822) | ||||
| Acropomatiformes | Banjosidae | Banjos banjos (Richardson, 1846) | * | |
| Anguilliformes | Congridae | Uroconger lepturus (Richardson, 1845) | * | |
| Moringuidae | Moringua javanica (Kaup, 1856) | * | ||
| Muraenidae | Uropterygius makatei (Bleeker, 1864) | * | ||
| Nemichthyidae | Nemichthys curvirostris (Strömman, 1896) | * | ||
| Atheriniformes | Atherinidae | Hypoatherina valenciennei (Bleeker, 1854) | * | |
| Beloniformes | Belonidae | Strongylura strongylura (van Hasselt, 1823) | * | |
| Blenniiformes | Blenniidae | Parablennius yatabei (Jordan & Snyder, 1900) | * | |
| Caproiformes | Caproidae | Antigonia rubicunda (Ogilby, 1910) | * | |
| Carangiformes | Carangidae | Decapterus maruadsi (Temminck & Schlegel, 1843) | * | * |
| Selaroides leptolepis (Cuvier, 1833) | * | |||
| Seriola dumerili (Risso, 1810) | * | * | ||
| Gadiformes | Macrouridae | Coelorinchus hubbsi (Matsubara, 1936) | * | |
| Gobiiformes | Gobiidae | Amblychaeturichthys hexanema (Bleeker, 1853) | * | |
| Drombus triangularis (Weber, 1909) | * | * | ||
| Parachaeturichthys polynema (Bleeker, 1853) | * | |||
| Rhinogobius giurinus (Rutter, 1897) | * | |||
| Trypauchen vagina (Bloch & Schneider, 1801) | * | |||
| Kurtiformes | Apogonidae | Ostorhinchus pleuron (Fraser, 2005) | * | |
| Labriformes | Labridae | Cheilinus oxycephalus (Bleeker, 1853) | * | |
| Halichoeres notospilus (Günther, 1864) | * | |||
| Halichoeres hartzfeldii (Bleeker, 1852) | * | |||
| Hologymnosus doliatus (Lacepède, 1801) | * | |||
| Labroides bicolor (Fowler & Bean, 1928) | * | |||
| Labroides alleni (Randall, 1981) | * | |||
| Leptojulis cyanopleura (Bleeker, 1853) | * | * | ||
| Parajulis poecilepterus (Temminck & Schlegel, 1845) | * | |||
| Coris pictoides (Randall & Kuiter, 1982) | * | |||
| Lutjaniformes | Haemulidae | Plectorhinchus cinctus (Temminck & Schlegel, 1843) | * | * |
| Perciformes | Platycephalidae | Platycephalus indicus (Linnaeus, 1758) | * | |
| Cottidae | Trachidermus fasciatus (Heckel, 1837) | * | ||
| Sebastidae | Sebastiscus marmoratus (Cuvier, 1829) | * | ||
| Serranidae | Epinephelus akaara (Temminck & Schlegel, 1842) | * | ||
| Epinephelus bruneus (Bloch, 1793) | * | |||
| Epinephelus coioides (Hamilton, 1822) | * | |||
| Stichaeidae | Alectrias benjamini (Jordan & Snyder, 1902) | * | ||
| Pleuronectiformes | Cynoglossidae | Cynoglossidae quadrilineatus (Bleeker, 1851) | * | |
| Paraplagusia japonica (Temminck & Schlegel, 1846) | * | |||
| Scombriformes | Scombridae | Scomber japonicus (Houttuyn, 1782) | * | * |
| Auxis thazard (Lacepède, 1800) | * | |||
| Trichiuridae | Trichiurus japonicus (Temminck & Schlegel, 1844) | * | ||
| Spariformes | Sparidae | Acanthopagrus latus (Houttuyn, 1782) | * | * |
| Acanthopagrus schlegelii (Bleeker, 1854) | * | * | ||
| Rhabdosargus sarba (Forsskål, 1775) | * | |||
| Mulliformes | Mullidae | Upeneus japonicus (Houttuyn, 1782) | * | |
| Myctophiformes | Myctophidae | Benthosema fibulatum (Gilbert & Cramer, 1897) | * | |
| Benthosema pterotum (Alcock, 1890) | * | |||
| Myctophum aurolaternatum (Garman, 1899) | * | |||
| Syngnathiformes | Syngnathidae | Hippichthys penicillus (Cantor, 1849) | * | |
| Hippichthys spicifer (Rüppell, 1838) | * | |||
| Tetraodontiformes | Balistidae | Pseudobalistes fuscus (Bloch & Schneider, 1801) | * | * |
| Monacanthidae | Monacanthus chinensis (Osbeck, 1765) | * | ||
| Paramonacanthus otisensis (Whitley, 1931) | * | |||
| Stephanolepis setifer (Bennett, 1831) | * | |||
| Trachichthyiformes | Monocentridae | Monocentris japonicus (Houttuyn, 1782) | * | |
| Zeiformes | Parazenidae | Cyttopsis cypho (Fowler, 1934) | * | |
| Zeidae | Zeus faber (Linnaeus, 1758) | * | ||
| Myliobatiformes | Dasyatidae | Brevitrygon walga (Müller & Henle, 1841) | * | |
| Hemitrygon akajei (Müller & Henle, 1841) | * | |||
| Osmeriformes | Salangidae | Neosalangichthys ishikawae (Wakiya & Takahashi, 1913) | * | |
| Salangichthys microdon (Bleeker, 1860) | * | |||
| Site | Shannon Index | Simpson Index | Chao1 Index | Pielou_e Index | goods_coverage Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | S | W | S | W | S | W | S | W | S | |
| HX3 | 1.979 | 1.151 | 0.768 | 0.414 | 18.333 | 19.200 | 0.475 | 0.295 | 0.998 | 0.931 |
| DS03 | 2.093 | 1.823 | 0.807 | 0.783 | 17.000 | 12.500 | 0.512 | 0.527 | 1.000 | 0.967 |
| DS04 | 1.645 | 2.545 | 0.756 | 0.878 | 11.000 | 25.500 | 0.476 | 0.571 | 0.999 | 0.931 |
| DS05 | 1.953 | 0.591 | 0.828 | 0.268 | 12.000 | 5.000 | 0.545 | 0.254 | 0.998 | 0.988 |
| HXH2 | 1.550 | 0.506 | 0.706 | 0.255 | 7.000 | 3.000 | 0.552 | 0.319 | 1.000 | 1.000 |
| HXE1 | 1.716 | 0.977 | 0.719 | 0.494 | 13.000 | 5.000 | 0.464 | 0.421 | 1.000 | 0.982 |
| DS06 | 1.408 | 1.396 | 0.623 | 0.682 | 11.000 | 7.000 | 0.407 | 0.497 | 1.000 | 1.000 |
| HX1 | 1.232 | 1.218 | 0.608 | 0.678 | 7.000 | 4.000 | 0.439 | 0.609 | 1.000 | 1.000 |
| HX2 | 1.978 | 1.311 | 0.822 | 0.678 | 12.000 | 5.000 | 0.553 | 0.565 | 1.000 | 1.000 |
| HX5 | 0.861 | 1.643 | 0.377 | 0.715 | 9.000 | 13.000 | 0.287 | 0.458 | 0.994 | 0.972 |
| HXH1 | 0.701 | 0.681 | 0.354 | 0.415 | 5.000 | 3.000 | 0.302 | 0.430 | 1.000 | 0.972 |
| HX4 | 1.646 | 2.234 | 0.765 | 0.876 | 10.000 | 11.000 | 0.495 | 0.646 | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; He, W.; Wang, L.; Ou, D.; Qiu, J.; Li, W.; Huang, H. Environmental DNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in Dongshan Bay, China. Water 2025, 17, 452. https://doi.org/10.3390/w17030452
Zhang Y, He W, Wang L, Ou D, Qiu J, Li W, Huang H. Environmental DNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in Dongshan Bay, China. Water. 2025; 17(3):452. https://doi.org/10.3390/w17030452
Chicago/Turabian StyleZhang, Yanxu, Weiyi He, Lei Wang, Danyun Ou, Jinli Qiu, Weiwen Li, and Hao Huang. 2025. "Environmental DNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in Dongshan Bay, China" Water 17, no. 3: 452. https://doi.org/10.3390/w17030452
APA StyleZhang, Y., He, W., Wang, L., Ou, D., Qiu, J., Li, W., & Huang, H. (2025). Environmental DNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in Dongshan Bay, China. Water, 17(3), 452. https://doi.org/10.3390/w17030452









