Migration and Transformation of Nitrogen in Clay-Rich Soil Under Shallow Groundwater Depth: In Situ Experiment and Numerical Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment and Measurements
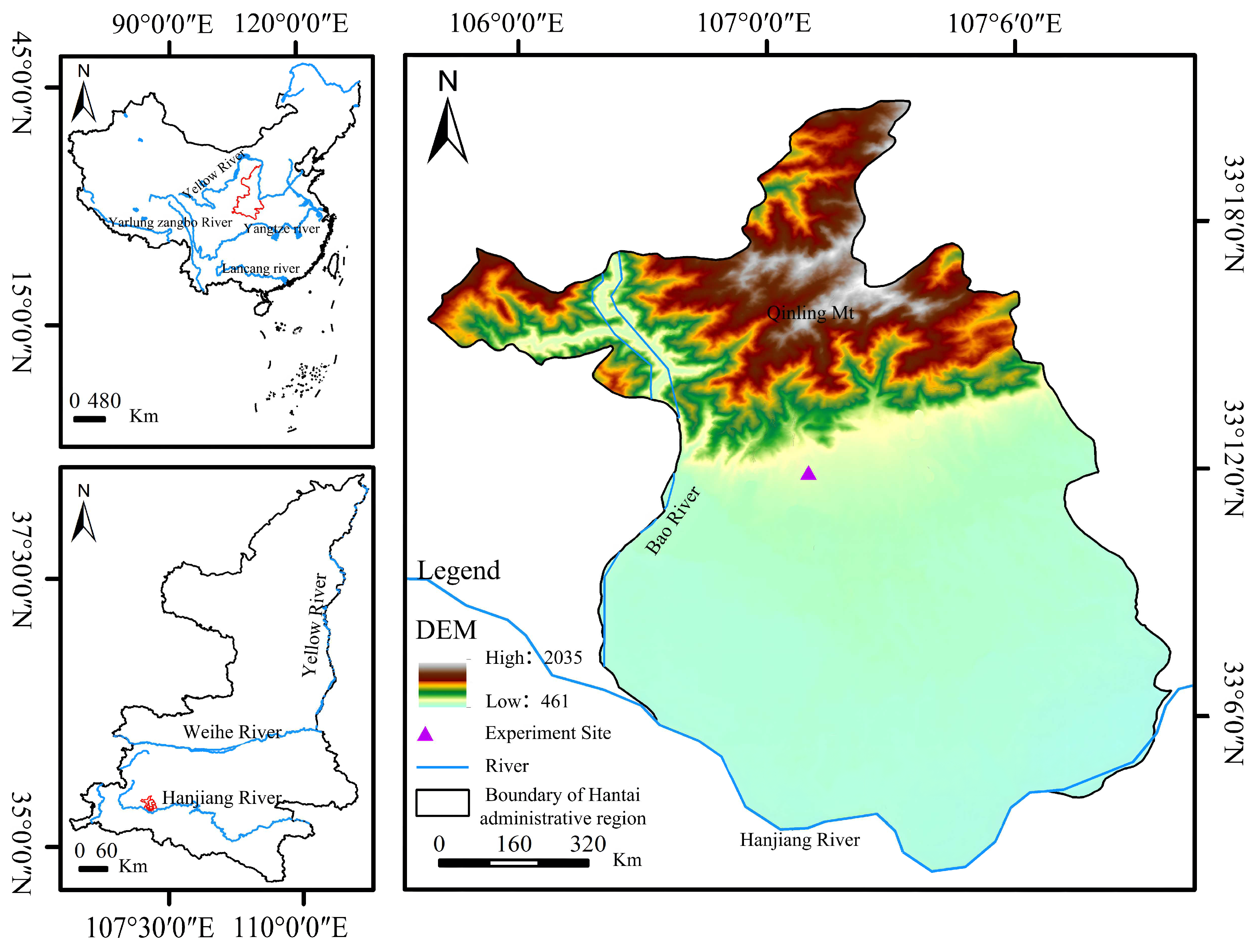
2.1.1. Site Description
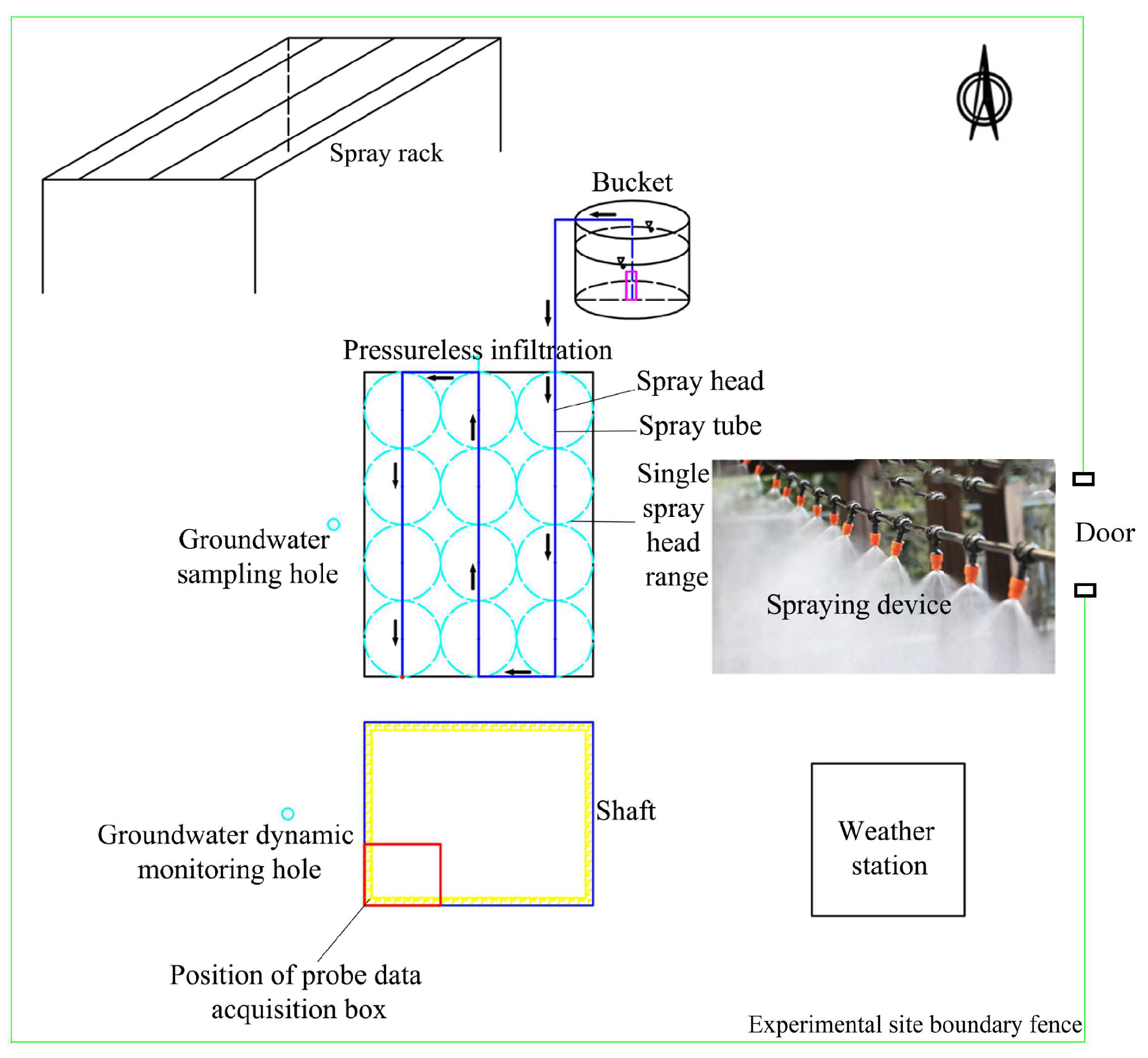
2.1.2. Instrument Set-Up
2.1.3. Sample Collection and Analysis
2.2. Numerical Model
2.2.1. Numerical Description
2.2.2. Initial and Boundary Conditions
2.2.3. Hydraulic and Solute Transport Parameters
2.3. Model Evaluation
2.4. Nitrogen Balance Calculation
3. Results
3.1. Impact of Rainfall and Groundwater on Nitrogen Migration
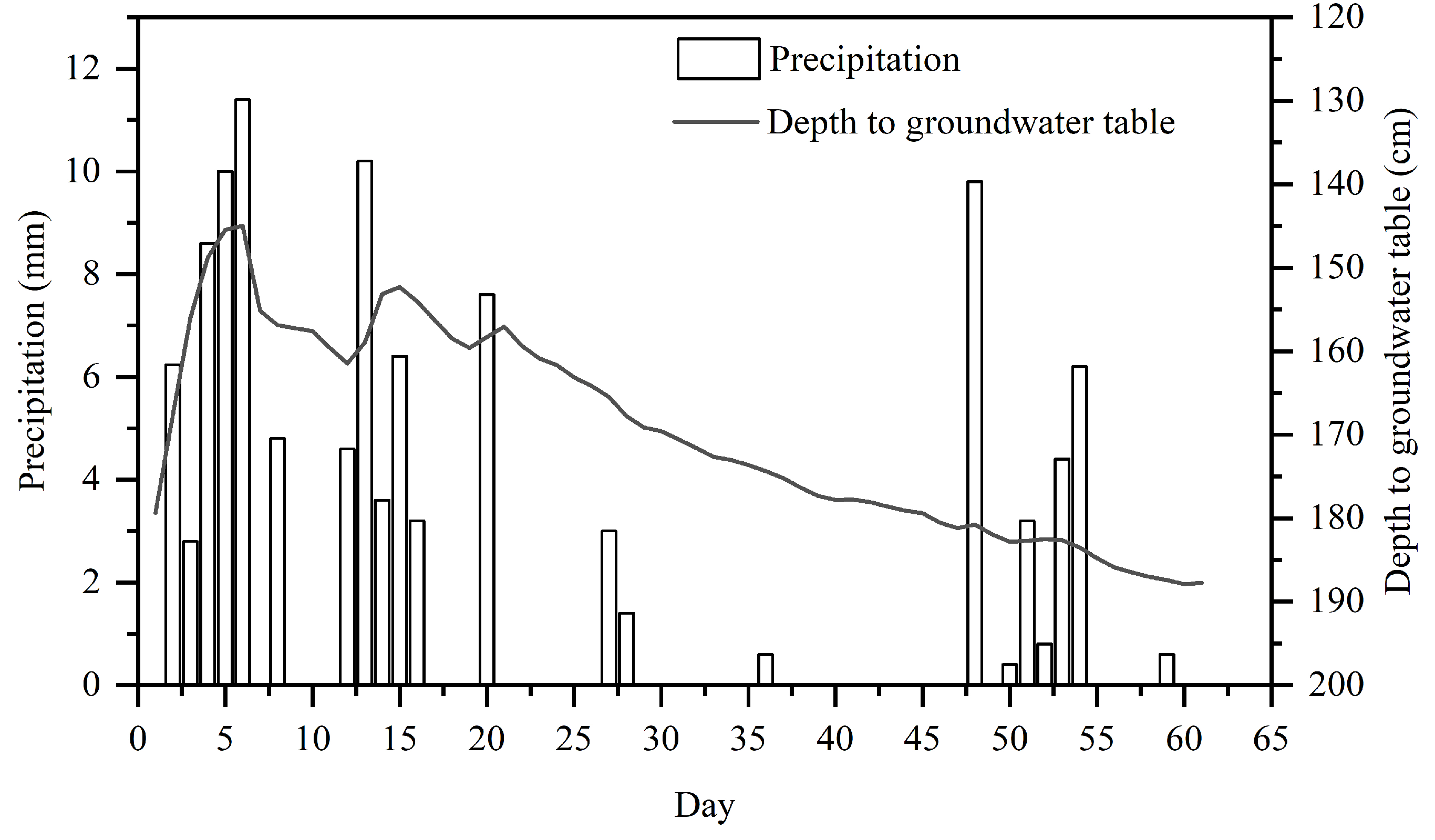
3.1.1. Variation in Rainfall and Groundwater Depth
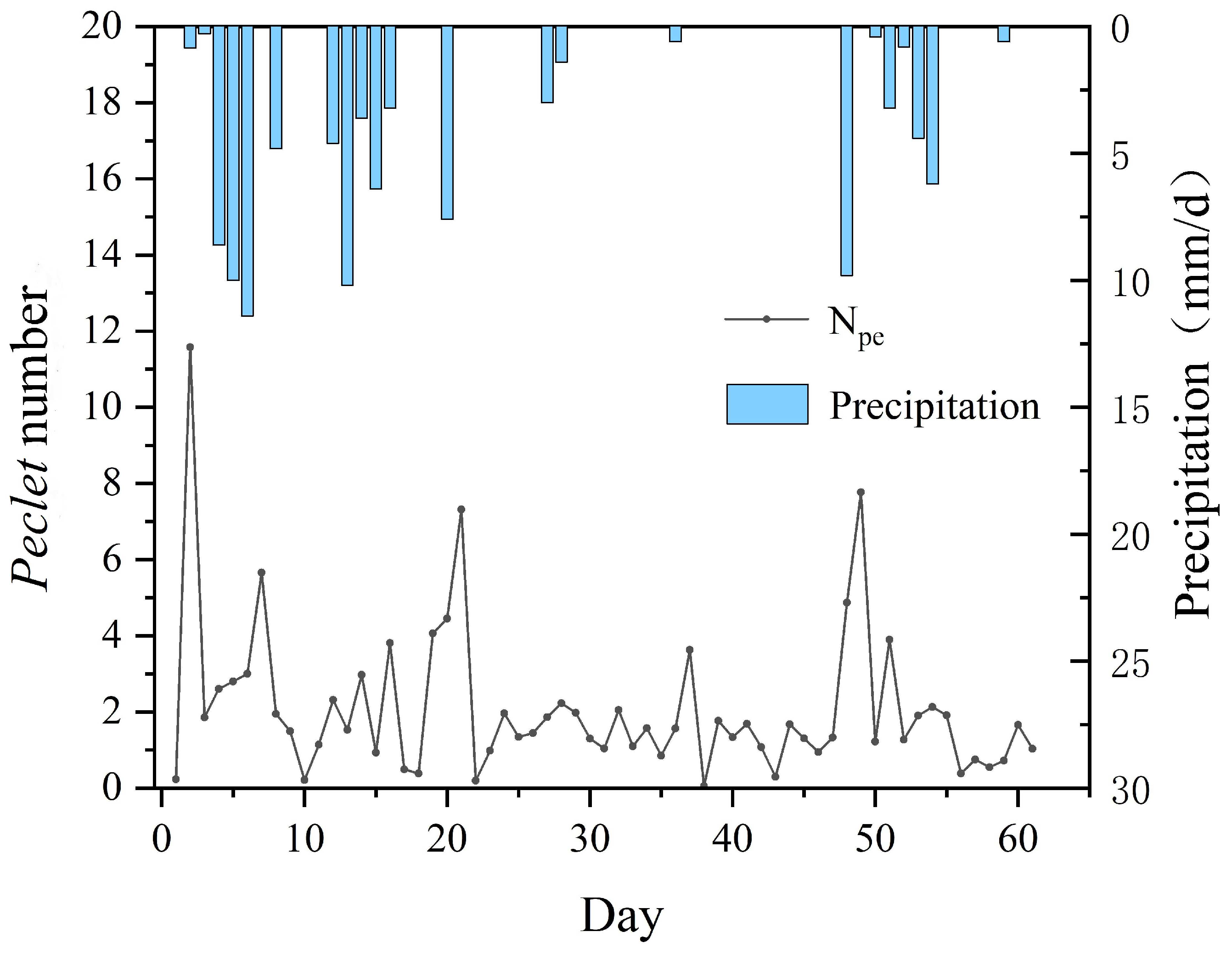
3.1.2. The Effect of Precipitation on the Nitrogen Migration
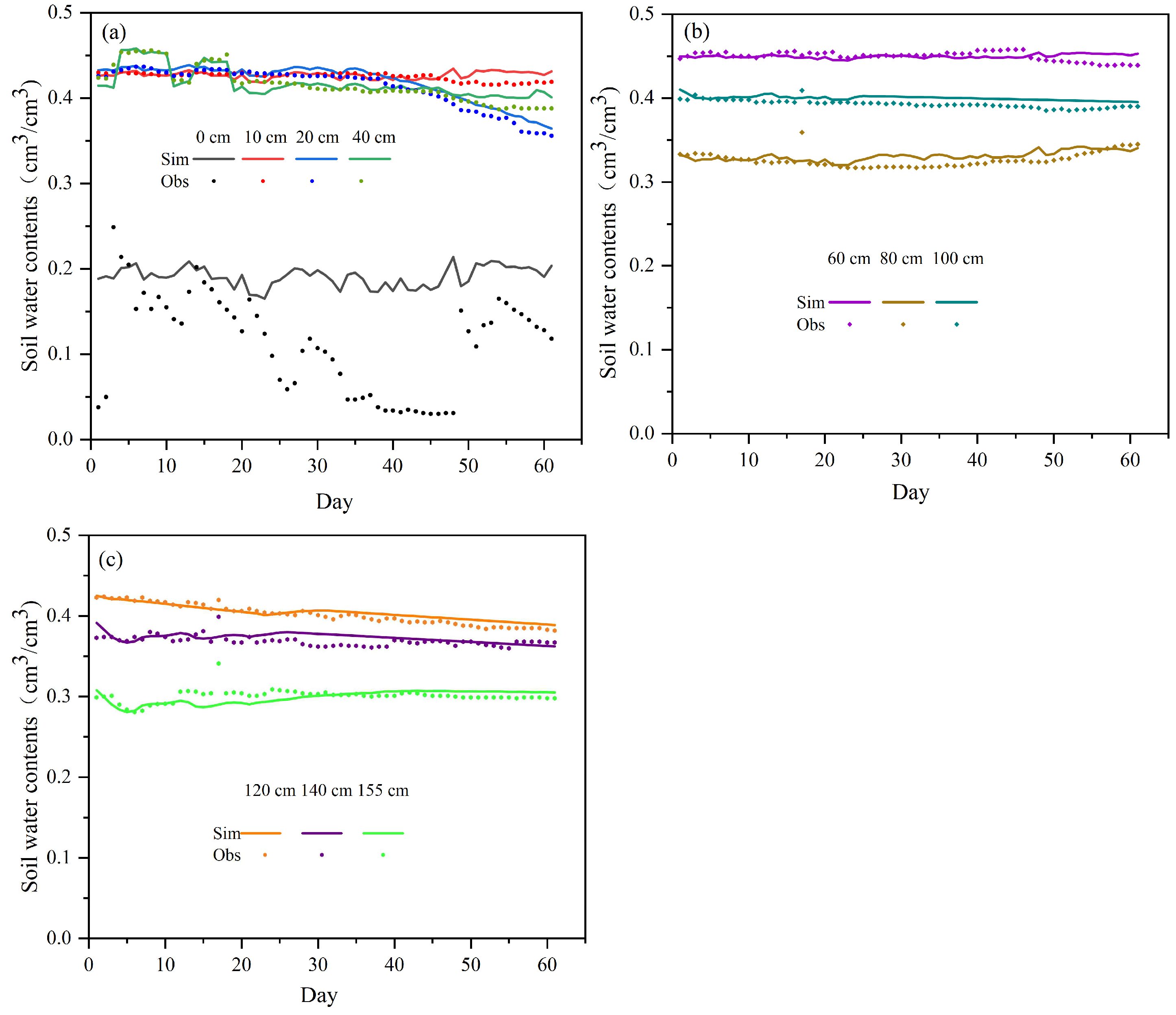
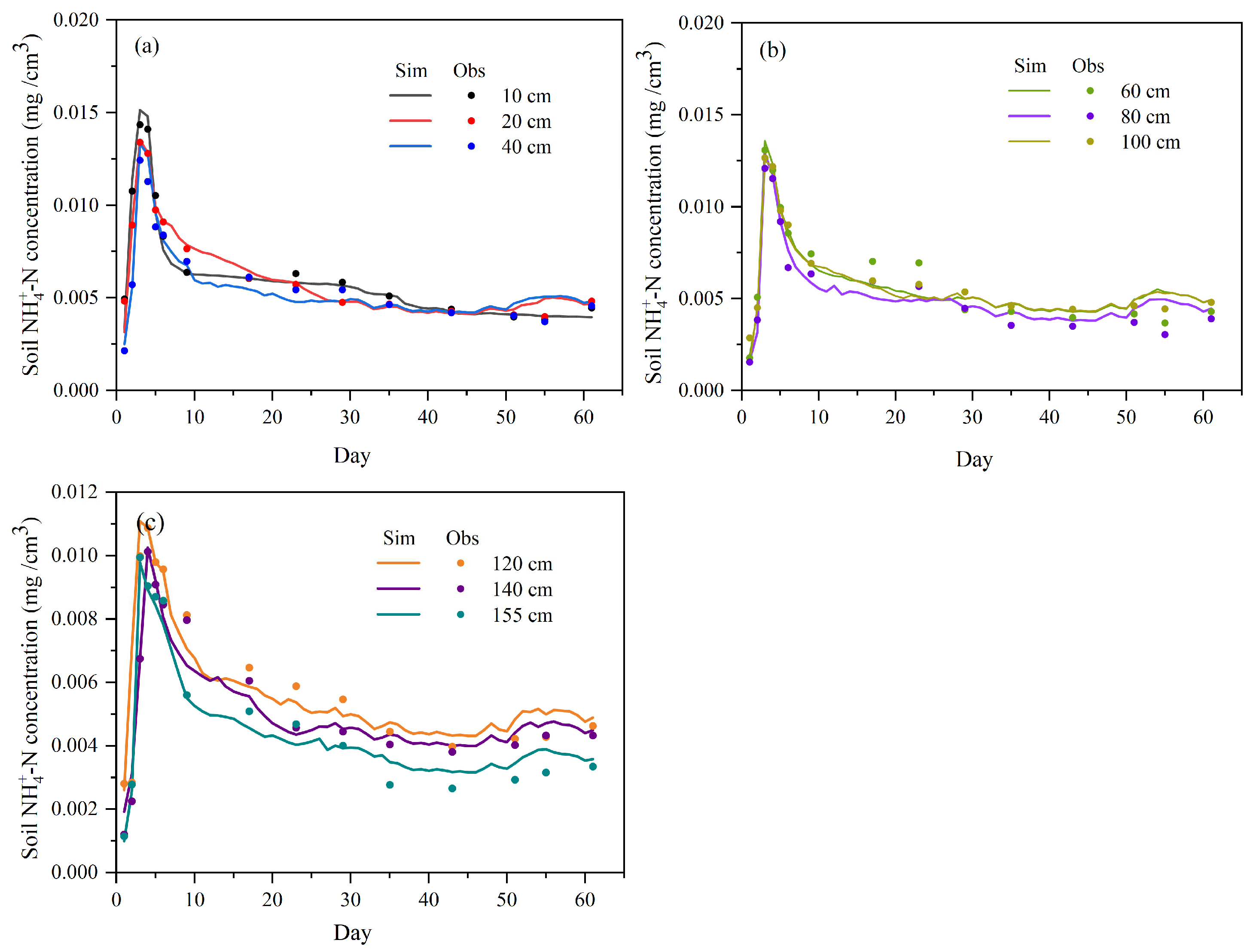
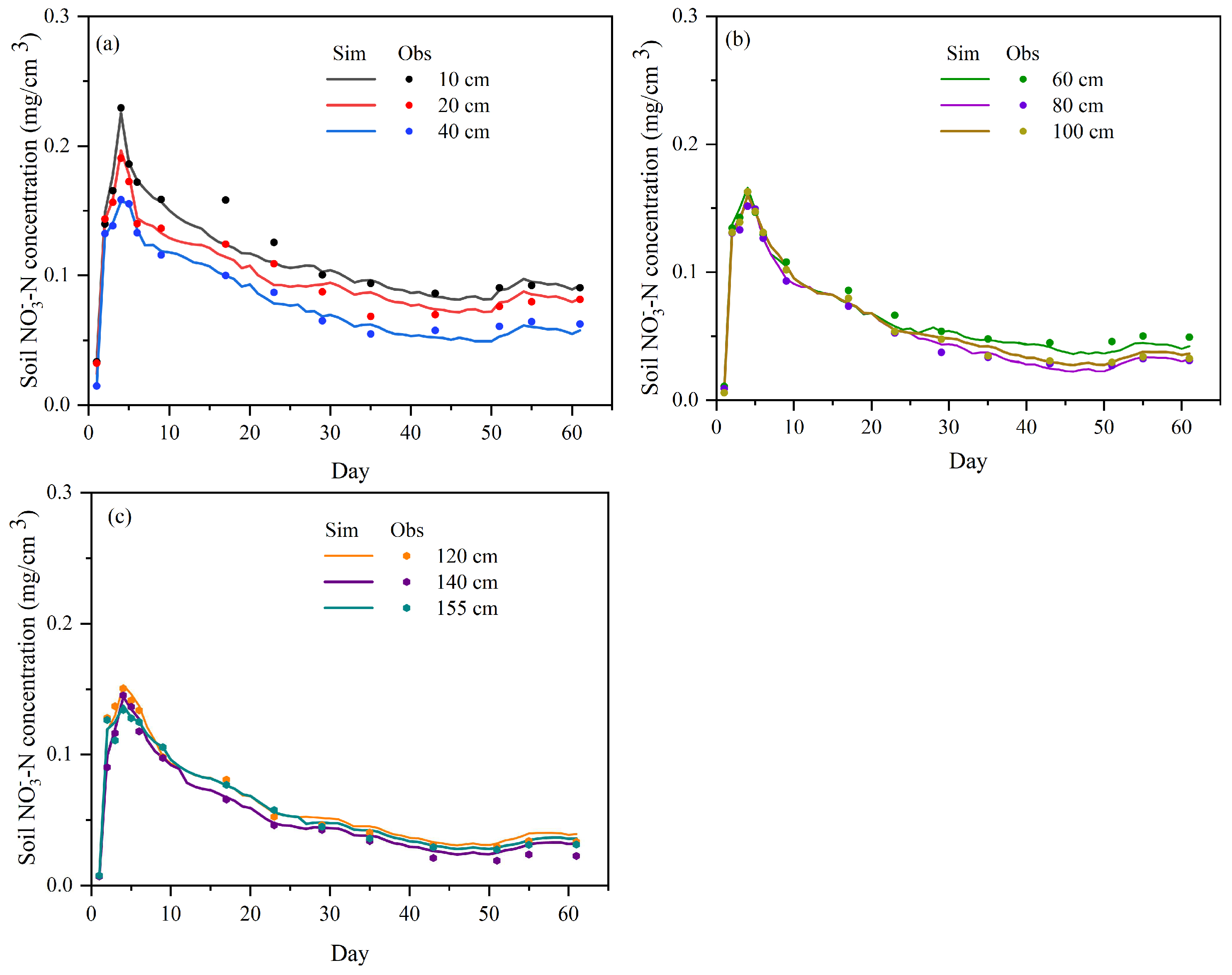
3.2. Model Verification of Soil Water Content and Nitrogen
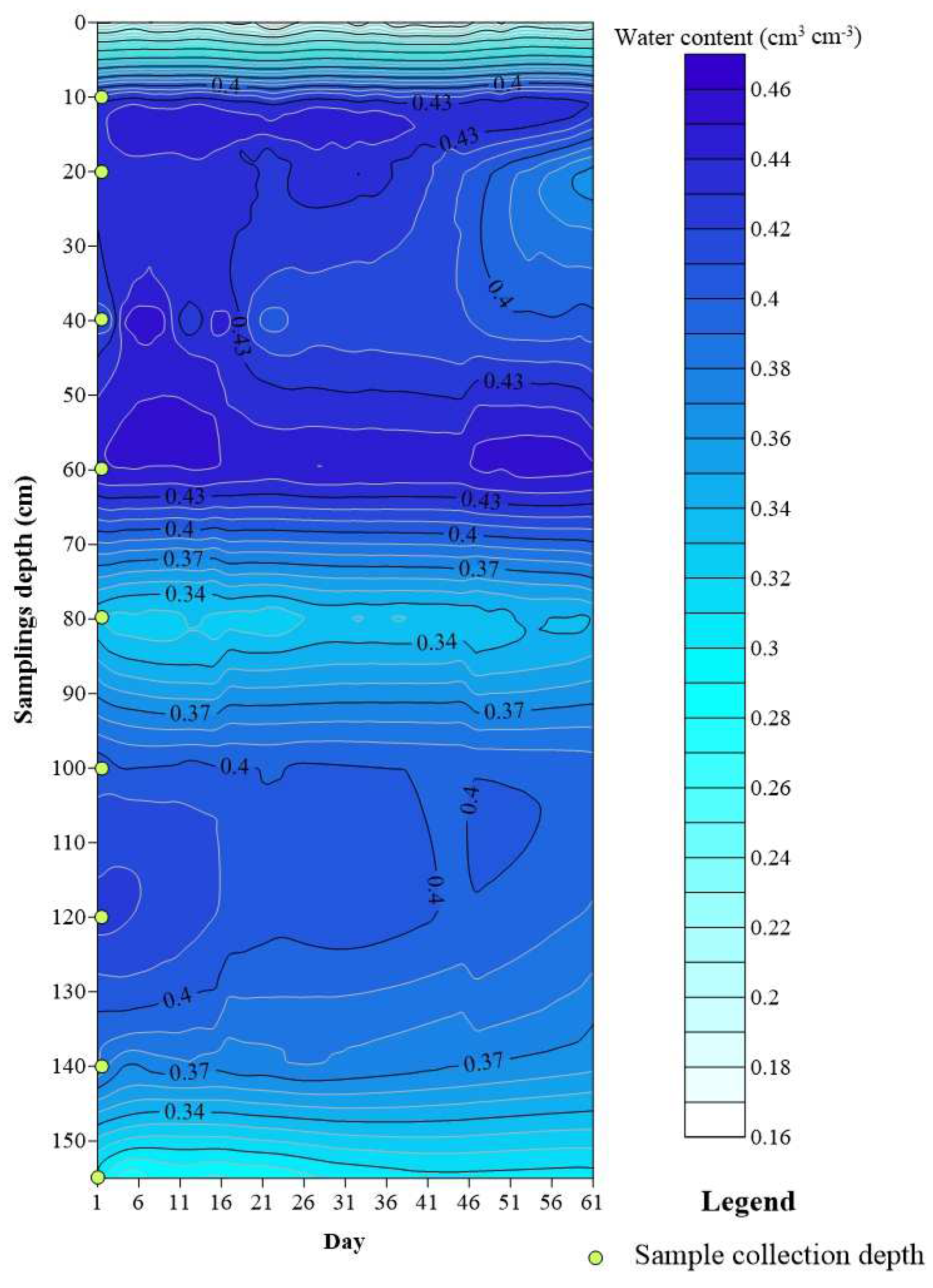
3.3. Temporal and Spatial Variation in Soil Water Content
3.4. Distribution of Nitrogen
3.5. Mass Balance of Water and Nitrogen
3.5.1. Water Balance
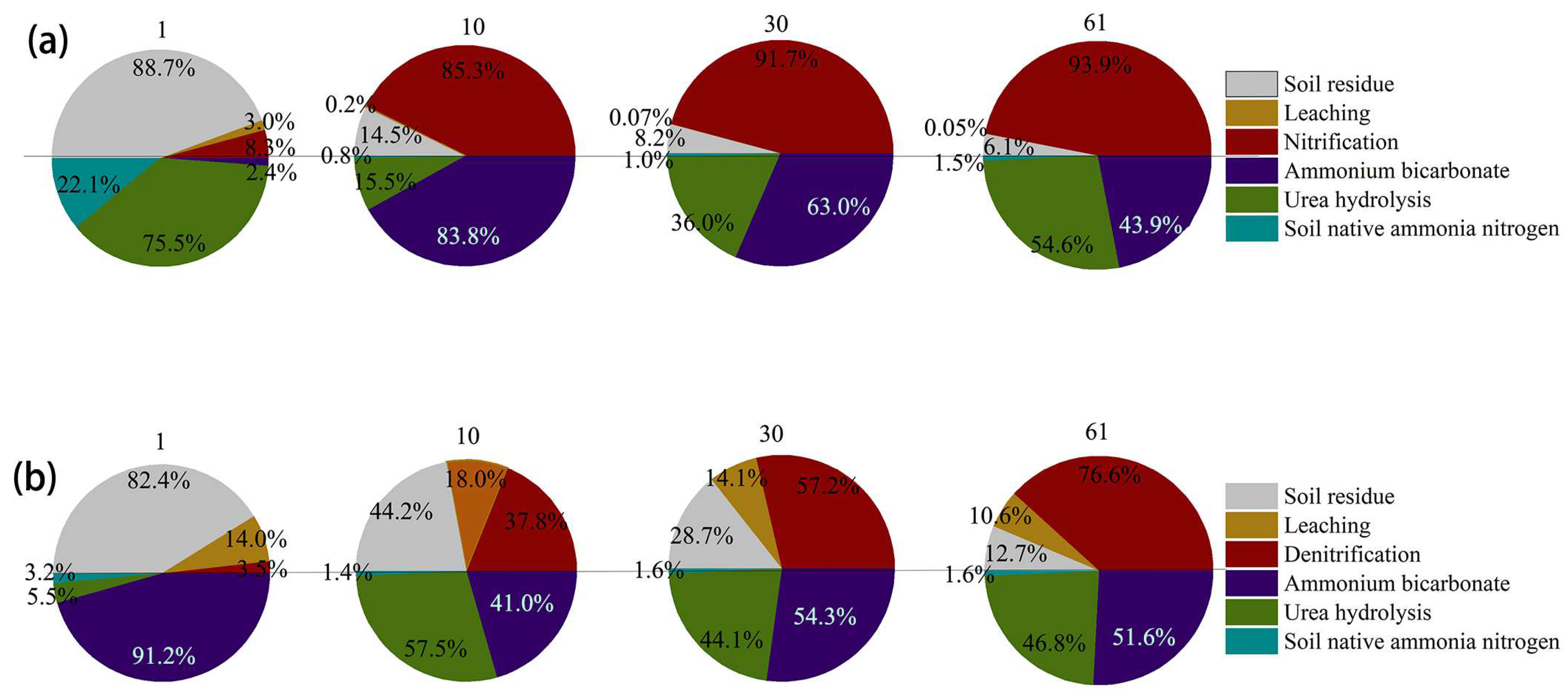
3.5.2. Nitrogen Balance
4. Discussion
4.1. Analysis of Distribution Characteristics of NH4+-N and NO3−-N Pollutants
4.2. The Influence of Shallow Groundwater Table on Nitrogen Migration
4.3. Control of the Effect of Clay-Unsaturated Zone on the Migration and Transformation of Nitrogen Pollutants
5. Conclusions
- (1)
- Peak concentrations of NH4+-N occurred earlier (on the third day) than NO3−-N (on the fourth day);
- (2)
- By the end of this experiment, the concentrations of both ammonia and -N had not returned to background concentrations;
- (3)
- The depth of the groundwater table fluctuated between 145.5 and 187.9 cm below ground level. Consequently, NH4+-N and NO3−-N were able to migrate downward and then enter the groundwater convected by precipitation recharge;
- (4)
- The pattern of nitrogen migration is dominated by convection during precipitation, while convection and dispersion act together during dry periods, according to the Peclet number;
- (5)
- The leakage loss of NO3−-N in soil accounts for 14% to 18%. The nitrification reaction of NH4+-N and NO3−-N is rapid over time, with increases from 8.3% to 85.3% and from 3.5% to 76.6%, respectively.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castaldelli, G.; Colombani, N.; Tamburini, E.; Vincenzi, F.; Mastrocicco, M. Soil type and microclimatic conditions as drivers of urea transformation kinetics in maize plots. Catena 2018, 166, 200–208. [Google Scholar] [CrossRef]
- Li, Y.; Šimůnek, J.; Zhang, Z.; Jing, L.; Ni, L. Evaluation of nitrogen balance in a direct-seeded-rice field experiment using Hydrus-1D. Agric. Water Manag. 2015, 148, 213–222. [Google Scholar] [CrossRef]
- Weitzman, J.N.; Brooks, J.R.; Compton, J.E.; Faulkner, B.R.; Mayer, P.M.; Peachey, R.E.; Rugh, W.D.; Coulombe, R.A.; Hatteberg, B.; Hutchins, S.R. Deep soil nitrogen storage slows nitrate leaching through the vadose zone. Agric. Ecosyst. Environ. 2022, 332, 107949. [Google Scholar] [CrossRef]
- Ascott, M.J.; Gooddy, D.C.; Wang, L.; Stuart, M.E.; Lewis, M.A.; Ward, R.S.; Binley, A.M. Global patterns of nitrate storage in the vadose zone. Nat. Commun. 2017, 8, 1416. [Google Scholar] [CrossRef]
- Xin, J.; Liu, Y.; Chen, F.; Duan, Y.; Wei, G.; Zheng, X.; Li, M. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019, 165, 114977. [Google Scholar] [CrossRef]
- Liang, X.; Tian, G.; Li, H.; Chen, X.; Zhu, S. Characteristics of nitrogen and phosphorus runoff loss in paddy field under natural rainfall conditions. J. Soil Water Conserv. 2005, 1, 59–63, (In Chinese with English abstract). [Google Scholar]
- Mo, X.; Peng, H.; Xin, J.; Wang, S. Analysis of urea nitrogen leaching under high-intensity rainfall using HYDRUS-1D. J. Environ. Manag. 2022, 312, 114900. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Wang, J.; Hu, X.; Wang, L.; Miao, Z. Study on the law of nitrogen transfer and conversion and use of fertilizer nitrogen in paddy fields under water-saving irrigation mode. Water 2019, 11, 218. [Google Scholar] [CrossRef]
- Yang, R.; Tong, J.; Hu, B.X.; Li, J.; Wei, W. Simulating water and nitrogen loss from an irrigated paddy field under continuously flooded condition with Hydrus-1D model. Environ. Sci. Pollut. Res. 2017, 24, 15089–15106. [Google Scholar] [CrossRef]
- Zhou, J.B.; Xi, J.G.; Chen, Z.J.; Li, S.X. Leaching and transformation of nitrogen fertilizers in soil after application of N with Irrigation: A Soil Column Method. Pedosphere 2006, 16, 245–252. [Google Scholar] [CrossRef]
- Bollmann, A.; Conrad, R. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob. Change Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Mekala, C.; Nambi, I.M. Understanding the hydrologic control of N cycle: Effect of water filled pore space on heterotrophic nitrification, denitrification and dissimilatory nitrate reduction to ammonium mechanisms in unsaturated soils. J. Contam. Hydrol. 2017, 202, 11–22. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, Y. Experimental study on purification of reclaimed water by river sediment under different pH conditions. J. Ecol. Rural Environ. 2022, 38, 1181–1187. [Google Scholar]
- Yu, S.; Ehrenfeld, J.G. The effects of changes in soil moisture on nitrogen cycling in acid wetland types of the New Jersey Pinelands (USA). Soil Biol. Biochem. 2009, 41, 2394–2405. [Google Scholar] [CrossRef]
- Brar, H.S.; Bhullar, M.S. Nutrient uptake by direct seeded rice and associated weeds as influenced by sowing date, variety and weed control. Indian J. Agric. Res. 2013, 47, 353–358. [Google Scholar]
- Gu, Y.; Sun, D.B.; Wang, Q.S. Studies on groundwater nitrate nitrogen distribution and it’s affecting factors in Chao lake watershed. J. Agric. Sci. Technol. 2011, 13, 68–74. [Google Scholar]
- Jiao, P.J.; Wang, S.L.; Xu, D.; Wang, Y.Z. Effect of crop vegetation type on nitrogen and phosphorus runoff losses from farmland in one rainstorm event. Shuili Xuebao/J. Hydraul. Eng. 2009, 40, 296–302. [Google Scholar]
- Peng, S.; He, Y.; Yang, S.; Xu, J. Effect of controlled irrigation and drainage on nitrogen leaching losses from paddy fields. Paddy Water Environ. 2015, 13, 303–312. [Google Scholar] [CrossRef]
- Tafteh, A.; Sepaskhah, A.R. Application of HYDRUS-1D model for simulating water and nitrate leaching from continuous and alternate furrow irrigated rapeseed and maize fields. Agric. Water Manag. 2012, 113, 19–29. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Gu, W.; Liu, H. Field analysis of water and nitrogen fate in lowland paddy fields under different water managements using HYDRUS-1D. Agric. Water Manag. 2015, 150, 67–80. [Google Scholar] [CrossRef]
- Alhaj Hamoud, Y.; Guo, X.; Wang, Z.; Shaghaleh, H.; Chen, S.; Hassan, A.; Bakour, A. Effects of irrigation regime and soil clay content and their interaction on the biological yield, nitrogen uptake and nitrogen-use efficiency of rice grown in southern China. Agric. Water Manag. 2019, 213, 934–946. [Google Scholar] [CrossRef]
- Jarvis, N.; Etana, A.; Stagnitti, F. Water repellency, near-saturated infiltration and preferential solute transport in a macroporous clay soil. Geoderma 2008, 143, 223–230. [Google Scholar] [CrossRef]
- Karup, D.; Moldrup, P.; Paradelo, M.; Katuwal, S.; Norgaard, T.; Greve, M.H.; de Jonge, L.W. Water and solute transport in agricultural soils predicted by volumetric clay and silt contents. J. Contam. Hydrol. 2016, 192, 194–202. [Google Scholar] [CrossRef]
- Tian, L.Y.; Wang, S.Q.; Wei, S.C.; Liu, B.X.; Liu, B.B.; Hu, C.S. Effect of the thickness of clay layer in the layered vadose zone on nitrate nitrogen migration. Trans. Chin. Soc. Agric. Eng. 2020, 36, 55–62. [Google Scholar]
- Dong, P.; Li, Y.; Sun, Y.; Xie, Z.H.; Liu, Y.C. Experimental study on the filtration capability of clayey soils in the vadose zone prevention nitrogen from polluting groundwater. Geoscience 2016, 30, 688–694. [Google Scholar]
- Liu, X.; Zuo, R.; Meng, L.; Li, P.; Li, Z.P.; He, Z.K.; Li, Q.; Wang, J.S. Study on the variation law of nitrate pollution during the rise of groundwater level. China Environ. Sci. 2021, 41, 232–238. [Google Scholar]
- Pu, F.; Huang, J.T.; Song, G.; Wang, J.W.; Li, Z.Z.; Tian, H.; Zhang, F.; Sun, F.Q. Movement and transformation of nitrogen in clay unsaturated zone under shallow groundwater table: In-situ experiment. China Environ. Sci. 2022, 42, 2707–2713. [Google Scholar]
- Antonopoulos, V.Z. Modelling of water and nitrogen balances in the ponded water and soil profile of rice fields in Northern Greece. Agric. Water Manag. 2010, 98, 321–330. [Google Scholar] [CrossRef]
- Chowdary, V.M.; Rao, N.H.; Sarma, P.B.S. A coupled soil water and nitrogen balance model for flooded rice fields in India. Agric. Ecosyst. Environ. 2004, 103, 425–441. [Google Scholar] [CrossRef]
- Dash, D.; Kumar, S.; Mallika, C.; Kamachi Mudali, U. Temperature Dependency Studies on Volumetric Change and Structural Interaction in Aqueous Rare Earth Nitrate Solution. J. Solut. Chem. 2015, 44, 1812–1832. [Google Scholar] [CrossRef]
- Jeon, J.H.; Yoon, C.G.; Ham, J.H.; Jung, K.W. Model development for nutrient loading estimates from paddy rice fields in Korea. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2004, 39, 845–860. [Google Scholar] [CrossRef]
- Jyotiprava Dash, C.; Sarangi, A.; Singh, D.K.; Singh, A.K.; Adhikary, P.P. Prediction of root zone water and nitrogen balance in an irrigated rice field using a simulation model. Paddy Water Environ. 2015, 13, 281–290. [Google Scholar] [CrossRef]
- Li, Y.; Šimůnek, J.; Jing, L.; Zhang, Z.; Ni, L. Evaluation of water movement and water losses in a direct-seeded-rice field experiment using Hydrus-1D. Agric. Water Manag. 2014, 142, 38–46. [Google Scholar] [CrossRef]
- Patil, M.D.; Das, B.S. Assessing the effect of puddling on preferential flow processes through under bund area of lowland rice field. Soil Tillage Res. 2013, 134, 61–71. [Google Scholar] [CrossRef]
- Phogat, V.; Yadav, A.K.; Malik, R.S.; Kumar, S.; Cox, J. Simulation of salt and water movement and estimation of water productivity of rice crop irrigated with saline water. Paddy Water Environ. 2010, 8, 333–346. [Google Scholar] [CrossRef]
- Sutanto, S.J.; Wenninger, J.; Coenders-Gerrits, A.M.J.; Uhlenbrook, S. Partitioning of evaporation into transpiration, soil evaporation and interception: A comparison between isotope measurements and a HYDRUS-1D model. Hydrol. Earth Syst. Sci. 2012, 16, 2605–2616. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Liu, H. Simulating soil water regime in lowland paddy fields under different water managements using HYDRUS-1D. Agric. Water Manag. 2014, 132, 69–78. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, K.K.; Hyun, Y.; Clement, T.P.; Hamilton, D. Nitrogen transformation and transport modeling in groundwater aquifers. Ecol. Model. 2006, 192, 143–159. [Google Scholar] [CrossRef]
- Gao, T.Z.; Fu, H.Y. Migration and transformation regularity of nitrogen in vadose zone in Hebei plain. J. Saf. Environ. 2015, 15, 217–221. [Google Scholar]
- Jiang, G.H.; Wang, W.K. Numerical simulation and prediction of N transportation and transformation in unsaturated zone in Guanzhong Basin. J. Northwest Univ. 2007, 95, 825–829. [Google Scholar]
- HJ/T166-2004; The Technical Specification for Soil Environmental Monitoring. China Environmental Science Press: Beijing, China, 2004.
- HJ634-2012; Soil-Determination of Ammonium, Nitrite and Nitrate by Extraction with Potassium Chloride Solution-Spectrophotometric Methods. China Environmental Science Press: Beijing, China, 2012.
- Yang, Y.R.; Tang, Z.H. Numerical simulation study on nitrate-nitrogen transportation and transformation in soil. Yangtze River 2019, 50, 53–57, (In Chinese with English abstract). [Google Scholar]
- Pfannkuch, H. Contribution à l’étude des déplacements de fluides miscibles dans un milieu poreux [Contribution to the study of the displacement of miscible fluids in a porous medium]. Rev. Inst. Fr. Pétr. 1963, 2, 215–270. [Google Scholar]
- Saiki, M.; Nguyen, T.P.M.; Shindo, J.; Nishida, K. Correction to: Nitrogen balance in paddy fields under flowing-irrigation condition. Nutr. Cycl. Agroecosyst. 2020, 117, 411. [Google Scholar] [CrossRef]
- Feng, S.Y.; Zheng, Y.Q. Transformation and Loss of Nitrogen in Farmland and Its Impact on Aquatic Environment. Agric. Environ. Prot. 1996, 6, 277–279. [Google Scholar]
- Yang, Q.H.; Zhang, H.T.; Hu, W.B.; Wang, H.J. Study on the Impact of Groundwater Level Changes on Nitrogen Export in the Middle Reaches of the Yangtze River. J. Hydrogeol. 2013, 34, 1–6. [Google Scholar]
- Ma, Z.; Lian, X.; Jiang, Y.; Meng, F.; Xi, B.; Yang, Y.; Yuan, Z.; Xu, X. Nitrogen transport and transformation in the saturated-unsaturated zone under recharge, runoff, and discharge conditions. Environ. Sci. Pollut. Res. 2016, 23, 8741–8748. [Google Scholar] [CrossRef]
- Sugita, F.; Nakane, K. Combined effects of rainfall patterns and porous media properties on nitrate leaching. Vadose Zone J. 2007, 6, 548–553. [Google Scholar] [CrossRef]
- Gu, C.H.; William, J.R. Combined effects of short term rainfall patterns and soil texture on soil nitrogen cycling-A modeling analysis. J. Contam. Hydrol. 2010, 112, 141–154. [Google Scholar] [CrossRef]
- Lazaratou, C.V.; Vayenas, D.V.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Liu, J. Study on Nitrogen Leaching Regulations on Three Textures of Soil; Beijing Forestry University: Beijing, China, 2010. [Google Scholar]
- Horn, R.; Taubner, H.; Wuttke, M.; Baumgartl, T. Soil physical properties related to soil structure. Soil Tillage Res. 1994, 30, 187–216. [Google Scholar] [CrossRef]
- Jarvis, N.J. A review of non-equilibrium water flow and solute transport in soil macropores: Principles, controlling factors and consequences for water quality. Eur. J. Soil Sci. 2020, 71, 279–302. [Google Scholar] [CrossRef]
- Bronswijk, J.J.B.; Hamminga, W.; Oostindie, K. Field-scale solute transport in a heavy clay soil. Water Resour. Res. 1995, 31, 517–526. [Google Scholar] [CrossRef]
- Kurtzman, D.; Scanlon, B.R. Groundwater Recharge through Vertisols: Irrigated Cropland vs. Natural Land, Israel. Vadose Zone J. 2011, 10, 662–674. [Google Scholar] [CrossRef]
- Sahile Gutema, S. Effects of Capillary Rise Saturation on Properties of Sub Grade Soil. Am. J. Constr. Build. Mater. 2021, 5, 32–49. [Google Scholar] [CrossRef]
- Ben Neriah, A.; Assouline, S.; Shavit, U.; Weisbrod, N. Impact of ambient conditions on evaporation from porous media. Water Resour. Res. 2014, 50, 6696–6712. [Google Scholar] [CrossRef]
| Depth (cm) | Soil Particle Size Distribution | Texture | Bulk Density (g/cm3) | ||
|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | |||
| 0–10 | 2.2 | 68.1 | 29.7 | silt clay | 1.50 |
| 10–20 | 1.7 | 61.7 | 36.6 | silt clay | 1.65 |
| 20–40 | 1.7 | 56.6 | 41.7 | silt clay | 1.60 |
| 40–60 | 0.0 | 58.7 | 41.3 | silt clay | 1.76 |
| 60–80 | 0.0 | 55.2 | 44.8 | silt clay | 1.68 |
| 80–100 | 0.0 | 56.6 | 43.4 | silt clay | 1.63 |
| 100–120 | 0.0 | 54.2 | 45.8 | silt clay | 1.70 |
| 120–140 | 0.9 | 60.2 | 38.9 | silt clay | 1.50 |
| 140–155 | 1.5 | 59.1 | 39.4 | silt clay | 1.35 |
| 155–175 | 2.3 | 57.4 | 40.3 | silt clay | 1.35 |
| Solute Migration State | |
|---|---|
| Absolute dominance of dispersion | |
| Interaction of convective and dispersion | |
| Convection dominant | |
| Absolute convection dominance |
| Depth (cm) | RMSE | R2 | ||||
|---|---|---|---|---|---|---|
| Soil Water Content | NH4+-N | NO3−-N | Soil Water Content | NH4+-N | NO3−-N | |
| 0–10 | 0.096 | 0.711 | ||||
| 10–20 | 0.007 | 0.0006 | 0.0107 | 0.887 | 0.9708 | 0.9543 |
| 20–40 | 0.008 | 0.0005 | 0.0080 | 0.945 | 0.9715 | 0.9676 |
| 40–60 | 0.009 | 0.0007 | 0.0045 | 0.863 | 0.9548 | 0.9895 |
| 60–80 | 0.007 | 0.0009 | 0.0054 | 0.855 | 0.9244 | 0.9981 |
| 80–100 | 0.009 | 0.0008 | 0.0038 | 0.897 | 0.9407 | 0.9957 |
| 100–120 | 0.008 | 0.0005 | 0.0028 | 0.911 | 0.9706 | 0.9968 |
| 120–140 | 0.005 | 0.0012 | 0.0048 | 0.917 | 0.9026 | 0.9895 |
| 140–155 | 0.009 | 0.0005 | 0.0053 | 0.900 | 0.9424 | 0.9888 |
| 155–410 | 0.010 | 0.0005 | 0.0048 | 0.856 | 0.9697 | 0.9887 |
| Depth (cm) | θr (cm3·cm−3) | θs (cm3·cm−3) | α (cm−1) | n [-] | Ks (cm·min−1) | L [-] | DL (cm) | Kd | μ’w,1 (d−1) | μw,2 (d−1) | μ’w,2 (d−1) | μ’s,2 (d−1) | μw,3 (d−1) | μs,3 (d−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 0.03 | 0.30 | 0.0079 | 1.531 | 10.0 | 0.5 | 7 | 0.50 | 0.35 | 0.04 | 0.070 | 0.070 | 0.001 | 0.001 |
| 10–20 | 0.03 | 0.46 | 0.0050 | 1.100 | 10.3 | 0.5 | 14 | 0.50 | 0.32 | 0.02 | 0.062 | 0.062 | 0.001 | 0.001 |
| 20–40 | 0.03 | 0.47 | 0.0060 | 1.100 | 11.8 | 0.5 | 10 | 0.50 | 0.18 | 0.02 | 0.060 | 0.060 | 0.001 | 0.001 |
| 40–60 | 0.03 | 0.50 | 0.0050 | 1.200 | 10.8 | 0.5 | 12 | 0.50 | 0.17 | 0.02 | 0.060 | 0.060 | 0.002 | 0.002 |
| 60–80 | 0.03 | 0.50 | 0.0050 | 1.100 | 12.0 | 0.5 | 12 | 0.50 | 0.17 | 0.01 | 0.042 | 0.042 | 0.002 | 0.002 |
| 80–100 | 0.03 | 0.45 | 0.0110 | 1.300 | 11.5 | 0.5 | 10 | 0.15 | 0.17 | 0.01 | 0.038 | 0.038 | 0.002 | 0.002 |
| 100–120 | 0.03 | 0.50 | 0.0100 | 1.300 | 10.0 | 0.5 | 10 | 0.10 | 0.17 | 0.01 | 0.038 | 0.038 | 0.002 | 0.002 |
| 120–140 | 0.03 | 0.47 | 0.0080 | 1.300 | 10.0 | 0.5 | 10 | 0.10 | 0.17 | 0.01 | 0.038 | 0.038 | 0.002 | 0.002 |
| 140–155 | 0.03 | 0.45 | 0.0070 | 1.600 | 10.0 | 0.5 | 11 | 0.10 | 0.17 | 0.01 | 0.038 | 0.038 | 0.002 | 0.002 |
| 155–410 | 0.03 | 0.33 | 0.0060 | 2.600 | 10.0 | 0.5 | 8 | 0.10 | 0.17 | 0.01 | 0.038 | 0.038 | 0.002 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Lv, Q.; Yang, Z.; Pu, F.; Song, G.; Wang, J.; Li, Z.; Fang, T.; Huang, T.; Zhang, F.; et al. Migration and Transformation of Nitrogen in Clay-Rich Soil Under Shallow Groundwater Depth: In Situ Experiment and Numerical Simulation. Water 2025, 17, 427. https://doi.org/10.3390/w17030427
Huang J, Lv Q, Yang Z, Pu F, Song G, Wang J, Li Z, Fang T, Huang T, Zhang F, et al. Migration and Transformation of Nitrogen in Clay-Rich Soil Under Shallow Groundwater Depth: In Situ Experiment and Numerical Simulation. Water. 2025; 17(3):427. https://doi.org/10.3390/w17030427
Chicago/Turabian StyleHuang, Jinting, Qiu Lv, Zhan Yang, Fang Pu, Ge Song, Jiawei Wang, Zongze Li, Tuo Fang, Tian Huang, Fang Zhang, and et al. 2025. "Migration and Transformation of Nitrogen in Clay-Rich Soil Under Shallow Groundwater Depth: In Situ Experiment and Numerical Simulation" Water 17, no. 3: 427. https://doi.org/10.3390/w17030427
APA StyleHuang, J., Lv, Q., Yang, Z., Pu, F., Song, G., Wang, J., Li, Z., Fang, T., Huang, T., Zhang, F., & Sun, F. (2025). Migration and Transformation of Nitrogen in Clay-Rich Soil Under Shallow Groundwater Depth: In Situ Experiment and Numerical Simulation. Water, 17(3), 427. https://doi.org/10.3390/w17030427






