Acidic Wastewater from Electrode Foil Manufacturing: Treatment Advances and Future Pathways
Abstract
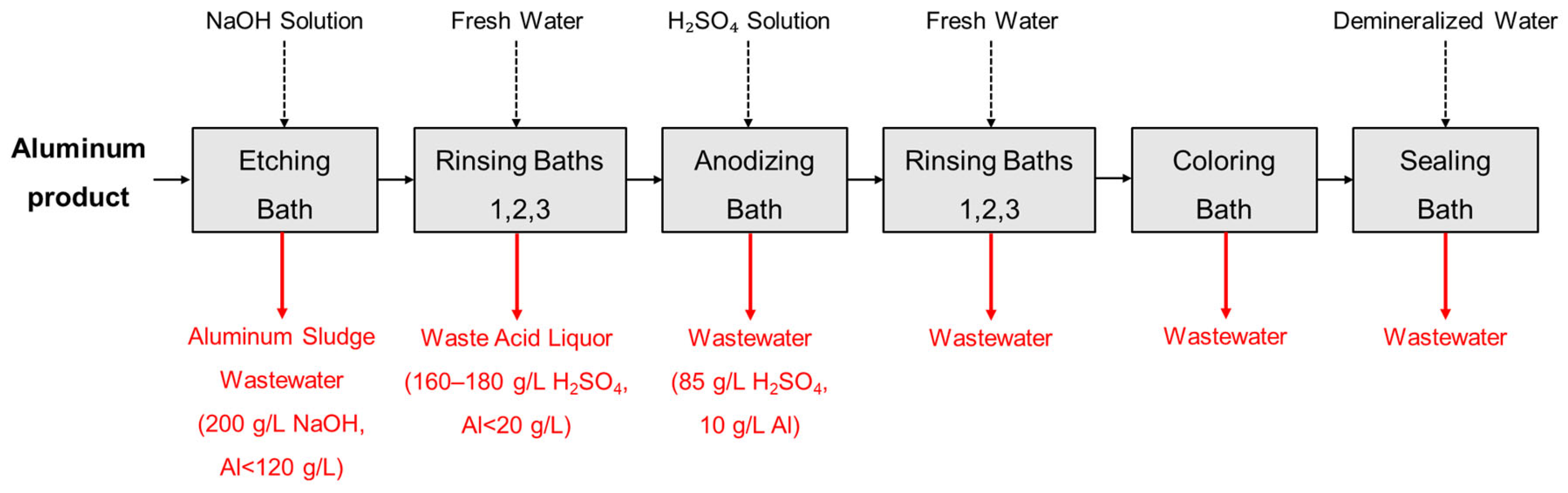
1. Introduction
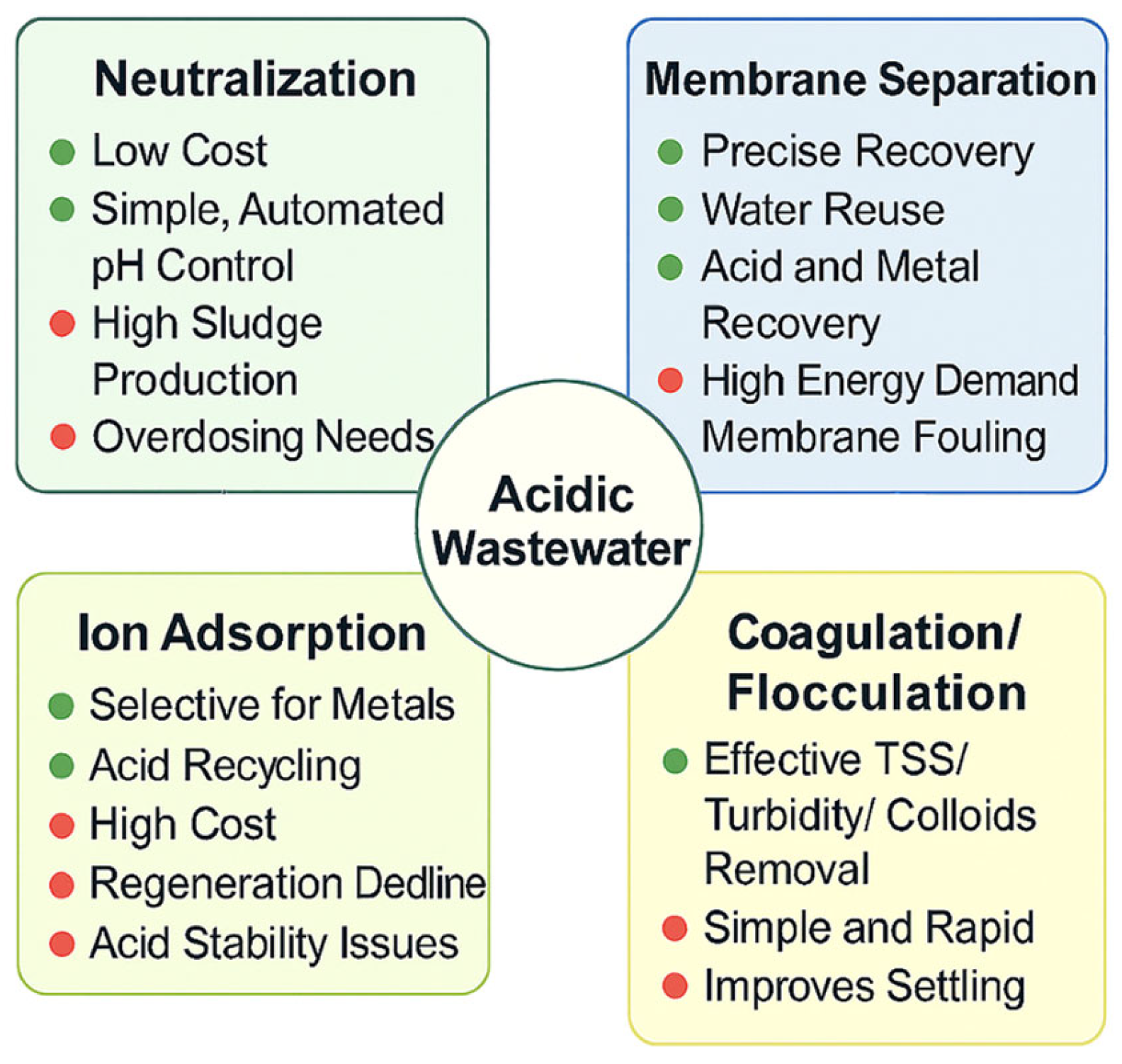
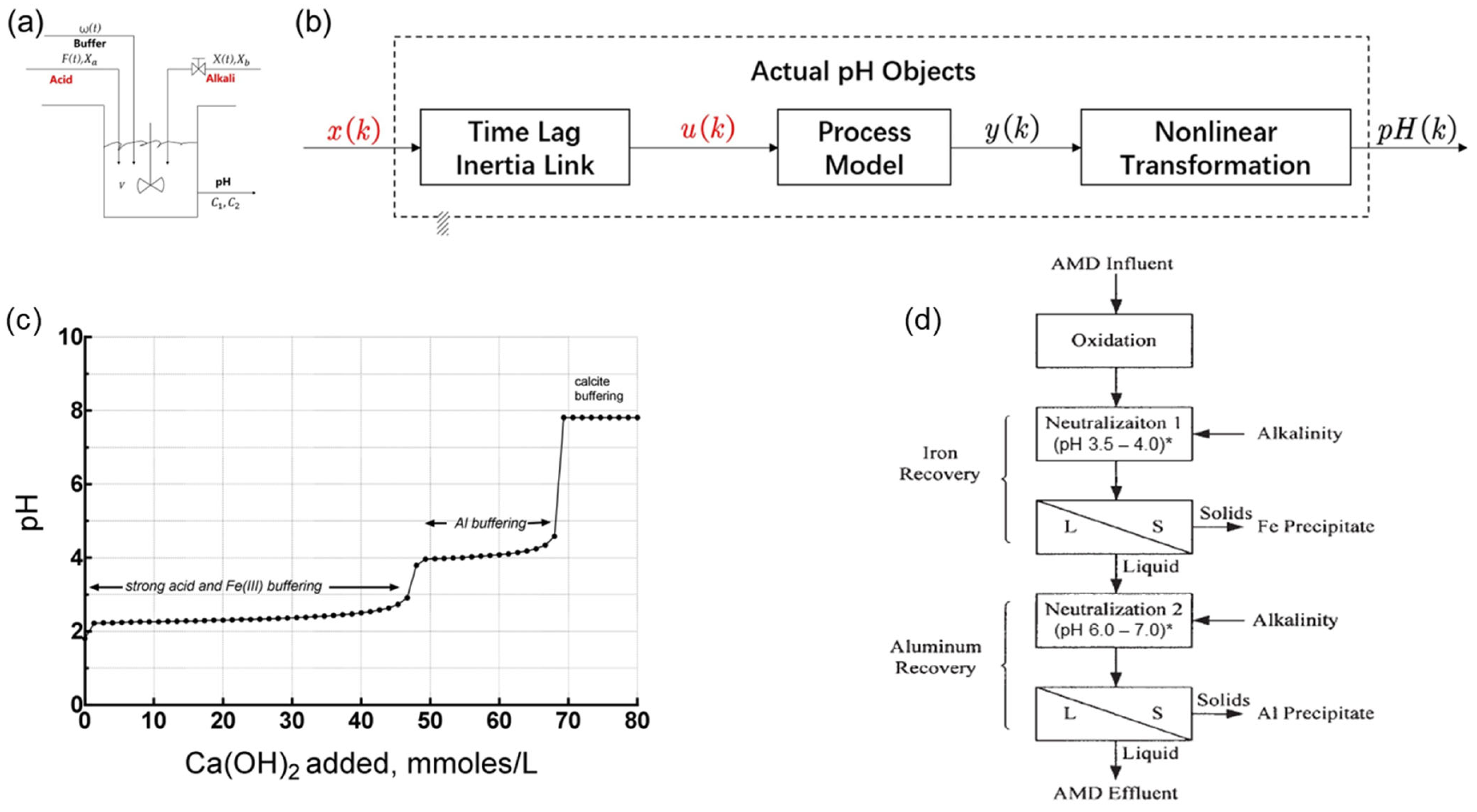
2. Advances and Applications of Neutralization Technology
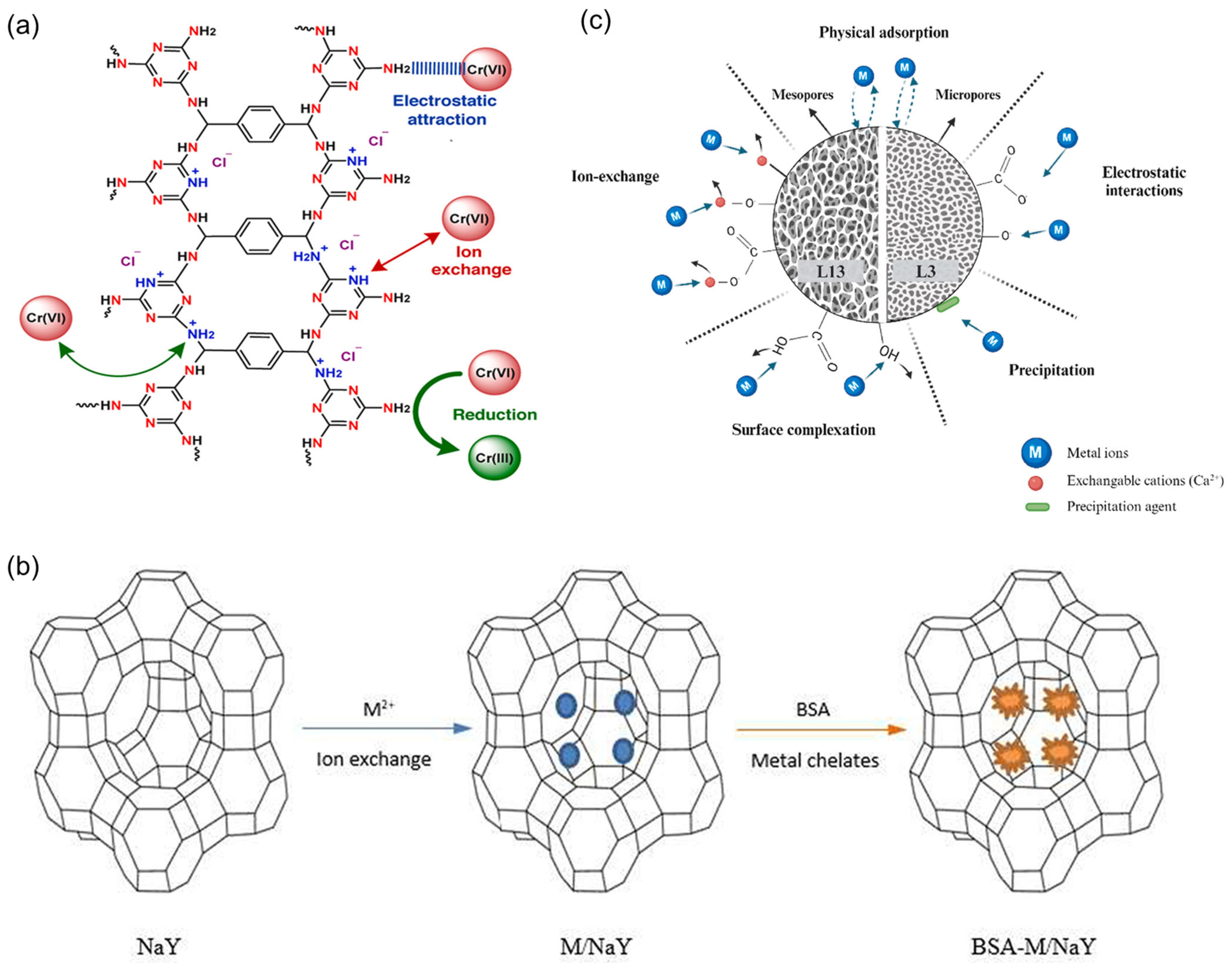
3. Recent Progress in Ionic Adsorption for Wastewater Treatment
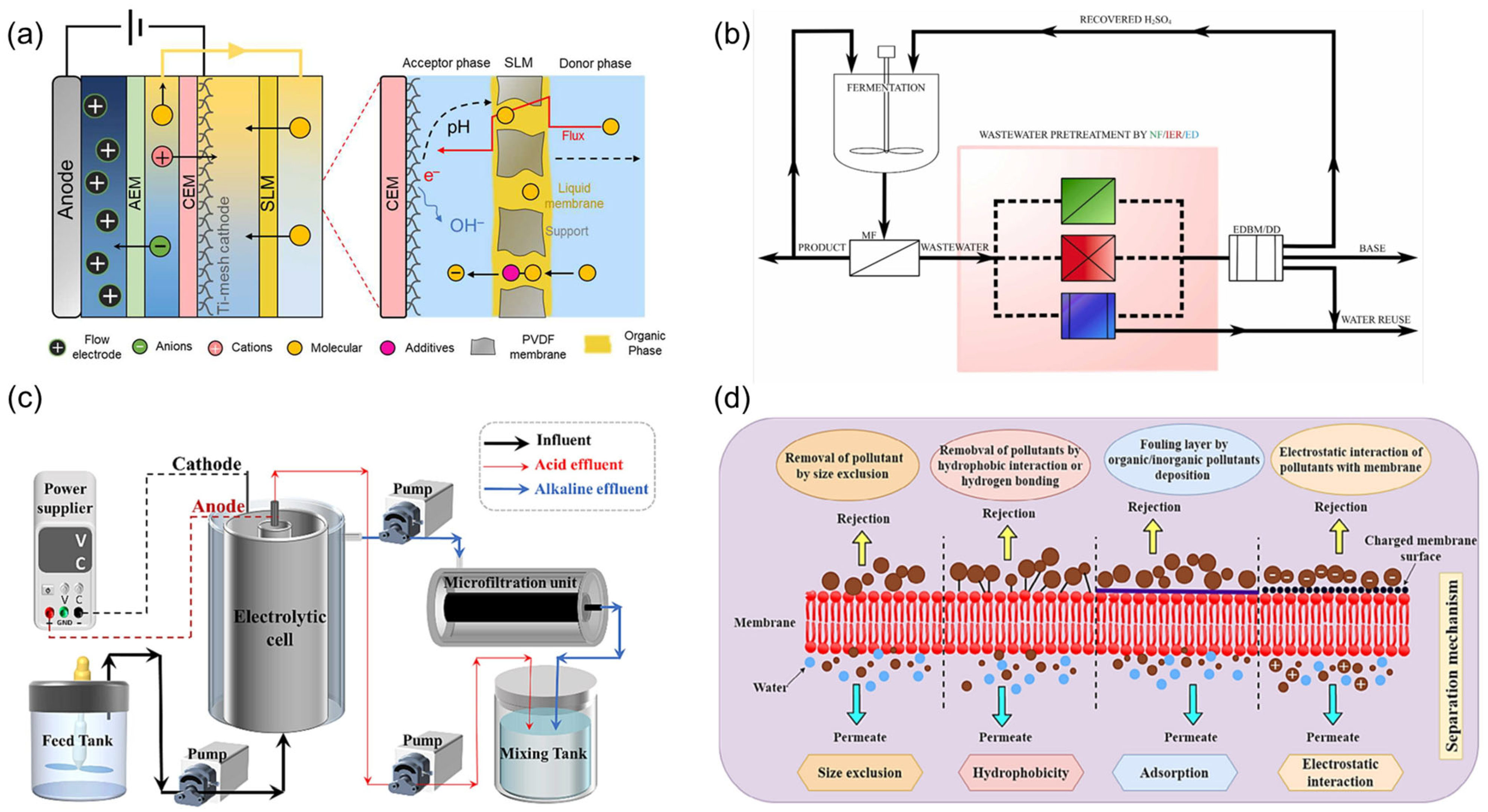
4. Membrane Separation Technology
4.1. Principles and Classification of Membrane Separation Technology
4.2. Typical Application Cases
- Case Study 1: NF-RO Combined Process
- Case Study 2: ED-UF Process for Synergistic Recovery of Hydrochloric Acid and Aluminum Resources
- Case Study 3: Membrane Distillation–Crystallization (MDCr) Treatment of Concentrated Sulfanilic Acid Wastewater
4.3. Advantages and Limitations of Membrane Separation Technology
5. Coagulation/Flocculation
6. Integrated and Multi-Stage Treatment Processes
7. Future Outlook
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuzer, B.; Aydin, M.I.; Yildiz, H.; Hasançebi, B.; Selcuk, H.; Kadmi, Y. Optimal performance of electrodialysis process for the recovery of acid wastes in wastewater: Practicing circular economy in aluminum finishing industry. Chem. Eng. J. 2022, 434, 134755. [Google Scholar] [CrossRef]
- Silva, G.O.R.e.; Carpanez, T.G.; Dos Santos, C.R.; Casella, G.S.; Moreira, V.R.; de Paula, E.C.; Amaral, M.C.S. Biohydrogen production from wastewater: Production technologies, environmental and economic aspects. J. Environ. Chem. Eng. 2024, 12, 114104. [Google Scholar] [CrossRef]
- Lagos, A.S.; Landázuri, A.C. Impact of Water Circularity on Climate Change: Removal of Fats, Oils and Grease (FOG) from Water Using Green and Simple Extraction Methods. Sustainability 2023, 15, 4176. [Google Scholar] [CrossRef]
- Charnnok, B.; Laosiripojana, N. Integrative process for rubberwood waste digestibility improvement and levulinic acid production by hydrothermal pretreatment with acid wastewater conversion process. Bioresour. Technol. 2022, 360, 127522. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Liu, T.-J.; Kang, L.-L.; Wang, Y.-T.; Li, J.-G.; Wang, F.-P.; Yu, Q.; Wang, X.-M.; Liu, H.; Guo, H.-W.; et al. A review of metallurgical slag for efficient wastewater treatment: Pretreatment, performance and mechanism. J. Clean Prod. 2022, 380, 135076. [Google Scholar] [CrossRef]
- Farzaneh, H.; Saththasivam, J.; McKay, G.; Parthasarathy, P. Adsorbent Minimization for Removal of Ibuprofen from Water in a Two-Stage Batch Process. Processes 2022, 10, 453. [Google Scholar] [CrossRef]
- Shakeri, H.; Motiee, H.; McBean, E. Forecasting impacts of climate change on changes of municipal wastewater production in wastewater reuse projects. J. Clean Prod. 2021, 329, 129790. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies. Water 2023, 15, 1195. [Google Scholar] [CrossRef]
- Barba-Lobo, A.; García-González, B.; Guerrero, J.L.; Bolívar, J.P. Sedimentary environmental quality of a biosphere reserve estuary in southwestern Iberian Peninsula. Mar. Pollut. Bull. 2024, 201, 116225. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A review of acid mine drainage: Formation mechanism, treatment technology, typical engineering cases and resource utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- GB 8978-1996; Integrated Wastewater Discharge Standard. State Environmental Protection Administration: Beijing, China, 1996.
- Ouadah, M.; Chemlal, R.; Mameri, N. Improvement of ultrafiltration of olive mill wastewater via ultrasound coupling. Int. J. Environ. Sci. Technol. 2023, 21, 3103–3114. [Google Scholar] [CrossRef]
- Raj, V.; Chauhan, M.S.; Pal, S.L. Potential of sugarcane bagasse in remediation of heavy metals: A review. Chemosphere 2022, 307, 135825. [Google Scholar] [CrossRef] [PubMed]
- Juve, J.-M.A.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Du, G.; Ho, H.-J.; Iizuka, A. A critical review of current treatment methods of acid mine drainage with an assessment of associated CO2 emissions toward carbon neutrality. J. Water Process Eng. 2025, 77, 108347. [Google Scholar] [CrossRef]
- Nguegang, B.; Ambushe, A.A. Sustainable acid mine drainage treatment: A comprehensive review of passive, combined, and emerging technologies. Environ. Eng. Res. 2025, 30, 156–186. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xu, J.; Fu, J.; Zheng, G.; Zhou, L. Modified chemical mineralization-alkali neutralization technology: Mineralization behavior at high iron concentrations and its application in sulfur acid spent pickling solution. Water Res. 2022, 218, 118513. [Google Scholar] [CrossRef]
- Amudha, V.; Judes, J. Persuade of solar driven photo-Fenton process for the effective sludge reduction through chemical deflocculation. Desalination Water Treat. 2022, 246, 258–264. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, Z.; Tang, Y.; Yang, W.; Chen, B.; Jiang, J.; Lin, Y. Chemical leaching−Flexible precipitation for sustainable recovery of fluoride from aluminum electrolysis multisource hazardous waste. Chem. Eng. J. 2024, 500, 157138. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, R.; Hu, M. Alkaline chemical neutralization to treat acid mine drainage with high concentrations of iron and manganese. Water 2024, 16, 821. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Yang, M.; Lu, C.; Quan, X.; Cao, D. Mechanism of acid mine drainage remediation with steel slag: A review. Acs Omega 2021, 6, 30205–30213. [Google Scholar] [CrossRef]
- Yang, L.; Tang, Y.; Cao, D.; Yang, M. Remediation of acid mine drainage (AMD) using steel slag: Mechanism of the alkalinity decayed process. Int. J. Environ. Res. Public Health 2023, 20, 2805. [Google Scholar] [CrossRef]
- Oladimeji, T.; Oyedemi, M.; Emetere, M.; Agboola, O.; Adeoye, J.; Odunlami, O. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Z.; Luan, H.; Liu, Z.; Xu, B.; Wang, Z.; He, W. An Improved Method of Model-Free Adaptive Predictive Control: A Case of pH Neutralization in WWTP. Processes 2023, 11, 1448. [Google Scholar] [CrossRef]
- Moyo, A.; Parbhakar-Fox, A.; Meffre, S.; Cooke, D.R. Alkaline industrial wastes–Characteristics, environmental risks, and potential for mine waste management. Environ. Pollut. 2023, 323, 121292. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, H.; Zhu, X.; Sun, Z. Review on electrochemical processes for the treatment of heavy metal complexes in wastewater: Performance, mechanism, application and improvement. Int. J. Electrochem. Sci. 2025, 20, 100971. [Google Scholar] [CrossRef]
- Kirk Nordstrom, D. Geochemical Modeling of Iron and Aluminum Precipitation during Mixing and Neutralization of Acid Mine Drainage. Minerals 2020, 10, 547. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C., Jr.; Buzby, K.M. Recovery of iron and aluminum from acid mine drainage by selective precipitation. Environ. Eng. Sci. 2005, 22, 745–755. [Google Scholar] [CrossRef]
- Zhou, S.; Mei, Y.; Yang, W.; Jiang, C.; Guo, H.; Feng, S.-P.; Tang, C.Y. Energy harvesting from acid mine drainage using a highly proton/ion-selective thin polyamide film. Water Res. 2024, 255, 121530. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Xue, C.; Lin, Y.; Lee, J.-F.; Yi, X.; Dang, Z. Efficient removal of heavy metals from acid mine drainage by ε-MnO2 adsorption. J. Clean Prod. 2024, 452, 141936. [Google Scholar] [CrossRef]
- Hwang, S.M.; Yeo, Y.H.; Park, W.H. Facile preparation of tannin-coated waste silk fabric as an effective heavy metal adsorbent. J. Environ. Chem. Eng. 2022, 10, 108233. [Google Scholar] [CrossRef]
- Wang, R.-D.; Zhang, W.-Q.; Lv, H.-B.; Chen, Y.-T.; Wang, L.; Zhou, S.-H.; Du, L.; Zhao, Q.-H. Sulfate-functionalized Fe-based MOF for removal of Pb(Ⅱ) and NO3- in industrial wastewater. J. Environ. Chem. Eng. 2024, 12, 112167. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of heavy metals: Mechanisms, kinetics, and applications of various adsorbents in wastewater remediation—A review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Xue, M.; Lv, R.; Fan, C.; Li, A. Adsorption mechanism of Ca2+, Mg2+, Fe3+, and Al3+ ions in phosphoric acid–nitric acid solution on 001× 7 and S957 resins. RSC Adv. 2024, 14, 7234–7240. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal–organic framework-based materials for adsorption and detection of uranium (VI) from aqueous solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef]
- Huang, W.-H.; Wu, R.-M.; Chang, J.-S.; Juang, S.-Y.; Lee, D.-J. Manganese ferrite modified agricultural waste-derived biochars for copper ions adsorption. Bioresour. Technol. 2023, 367, 128303. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Sen, T.K. Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review. Molecules 2023, 28, 5575. [Google Scholar] [CrossRef]
- Furtado, L.M.; Fuentes, D.P.; Ando, R.A.; Oliveira, P.V.; Siqueira Petri, D.F. Carboxymethyl cellulose/sugarcane bagasse/polydopamine adsorbents for efficient removal of Pb2+ ions from synthetic and undergraduate laboratory wastes. J. Clean Prod. 2022, 380, 134969. [Google Scholar] [CrossRef]
- Qing, M.; Liu, W.; Liu, L.; Huang, S.; He, Z.; Yin, Y.; Xiang, J. Effective fixation of Cu(II) and Cr(III) in solution by food waste biochar–Innovative and valuable treatment method for municipal solid waste. Fuel 2024, 361, 130679. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-D.; Wei, W.-M.; Li, H.; Shen, T.-Z.; Wang, L.; Zhou, S.-H.; Zhang, W.-Q.; Du, L.; Zhao, Q.-H. Metal-Organic framework derived vulcanized functional materials for ultra-efficient treatment of Hg(Ⅱ) ions in water. J. Mol. Liq. 2024, 401, 124609. [Google Scholar] [CrossRef]
- Gonsalves, O.S.; Nemade, P.R. Ultrafast adsorption of hexavalent chromium from aqueous effluents using covalent triazine frameworks. Chemosphere 2024, 351, 141246. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Sankaran, R.; Show, P.L.; Ooi, C.W.; Liu, B.-L.; Chai, W.S.; Chang, Y.-K. Removal of protein wastes by cylinder-shaped NaY zeolite adsorbents decorated with heavy metal wastes. Int. J. Biol. Macromol. 2021, 185, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.K.; Senthil Kumar, P.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef]
- Raji, Z.; Ebtehaj, I.; Bonakdari, H.; Khalloufi, S. Artificial intelligence-driven assessment of critical inputs for lead adsorption by agro-food wastes in wastewater treatment. Chemosphere 2024, 368, 143801. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Xiang, D.; Chen, Y.; Wang, S.; Zhu, R.; Zhang, D.; Peng, Z.; Fu, L. The design of high-efficient MOFs for selective Ag(I) capture: DFT calculations and practical applications. J. Hazard. Mater. 2024, 476, 135204. [Google Scholar] [CrossRef]
- de Castro-Alves, L.; Yánez-Vilar, S.; González-Goméz, M.A.; Garcia-Acevedo, P.; Arnosa-Prieto, Á.; Pineiro-Redondo, Y.; Rivas, J. Understanding adsorption mechanisms and metal ion selectivity of superparamagnetic beads with mesoporous CMK-3 carbon and commercial activated carbon. Microporous Mesoporous Mater. 2024, 374, 113159. [Google Scholar] [CrossRef]
- Alsehli, B.R. Toward sustainable environmental cleanup: Metal–organic frameworks in adsorption-A review. Desalination Water Treat. 2023, 316, 44–70. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of copper ions from wastewater: A review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Devi, N.; Siwal, S.S.; Alsanie, W.F.; Thakur, M.K.; Thakur, V.K. Metal–organic framework-based materials for wastewater treatment: Superior adsorbent materials for the removal of hazardous pollutants. ACS Omega 2023, 8, 9004–9030. [Google Scholar] [CrossRef] [PubMed]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, J.; Quan, W.; Chen, Q.; Long, X.; Wang, A. Advances in the adsorption of heavy metal ions in water by UiO-66 composites. Front. Chem. 2023, 11, 1211989. [Google Scholar] [CrossRef]
- Gebreslassie, G.; Desta, H.G.; Dong, Y.; Zheng, X.; Zhao, M.; Lin, B. Advanced membrane-based high-value metal recovery from wastewater. Water Res. 2024, 265, 122122. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qin, M. The Application of Cation Exchange Membranes in Electrochemical Systems for Ammonia Recovery from Wastewater. Membranes 2021, 11, 494. [Google Scholar] [CrossRef]
- Abuhantash, F.; Hegab, H.M.; Aljundi, I.H.; Hasan, S.W. Synergistic design of polylactic acid/functionalized multi-walled carbon nanotubes composite membrane for enhanced oil-water separation. J. Environ. Chem. Eng. 2023, 11, 111566. [Google Scholar] [CrossRef]
- Sanchis-Perucho, P.; Aguado, D.; Ferrer, J.; Seco, A.; Robles, Á. A comprehensive review of the direct membrane filtration of municipal wastewater. Environ. Technol. Innov. 2024, 35, 103732. [Google Scholar] [CrossRef]
- Alterkaoui, A.; Eskikaya, O.; Keskinler, B.; Dizge, N.; Balakrishnan, D.; Hiremath, P.; Naik, N. Caustic recovery from caustic-containing polyethylene terephthalate (PET) washing wastewater generated during the recycling of plastic bottles. Sci. Rep. 2025, 15, 2916. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, H.; Liu, C.; Wang, M.; Li, W.; Zhang, B.; Shen, Y.; Shi, W. Microfiltration pretreatment of polymer-flooding produced wastewater before desalination: Role of Ca2+ and Mg2+ in membrane fouling. Desalination 2022, 539, 115934. [Google Scholar] [CrossRef]
- Sayegh, A.; Shylaja Prakash, N.; Pedersen, T.H.; Horn, H.; Saravia, F. Treatment of hydrothermal liquefaction wastewater with ultrafiltration and air stripping for oil and particle removal and ammonia recovery. J. Water Process Eng. 2021, 44, 102427. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Teow, Y.H.; Mohammad, A.W.; Hilal, N. Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review. J. Water Process Eng. 2022, 45, 102478. [Google Scholar] [CrossRef]
- Li, Z.; Zhen, H.; Jia, Y.; Xiao, W.; Li, X.; Wu, X.; Li, T.; He, G.; Jiang, X. Polyelectrolyte fabricated nanofiltration membrane with heterogeneously charged channels for high efficient cephalexin wastewater treatment. J. Hazard. Mater. 2024, 480, 136356. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Sanders, K.T.; Childress, A.E. Reclaiming wastewater with increasing salinity for potable water reuse: Water recovery and energy consumption during reverse osmosis desalination. Desalination 2021, 520, 115316. [Google Scholar] [CrossRef]
- Ngo, M.T.T.; Diep, B.Q.; Sano, H.; Nishimura, Y.; Boivin, S.; Kodamatani, H.; Takeuchi, H.; Sakti, S.C.W.; Fujioka, T. Membrane distillation for achieving high water recovery for potable water reuse. Chemosphere 2022, 288, 132610. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Peng, S.; Zhou, Z.; Xu, L.; Wang, Y.; Zhu, J.; Zhang, P.; Chen, Z.; Lei, Z.; Wu, D. Selective ammonia recovery from wastewater by SDS-AC based microfiltration membrane flow electrode capacitor deionization. Sep. Purif. Technol. 2025, 359, 130555. [Google Scholar] [CrossRef]
- Wang, D.; Li, T.; Yan, C.; Zhou, Y.; Zhou, L. A novel bio-flocculation combined with electrodialysis process: Efficient removal of pollutants and sustainable resource recovery from swine wastewater. Sep. Purif. Technol. 2023, 304, 122330. [Google Scholar] [CrossRef]
- GB/T 50050-2017; Code for Design of Industrial Recirculating Cooling Water Treatment. China Planning Press: Beijing, China, 2017.
- Ricci, B.C.; Ferreira, C.D.; Aguiar, A.O.; Amaral, M.C. Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent. Sep. Purif. Technol. 2015, 154, 11–21. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Ahmad, N.N.R.; Teow, Y.H. Role of Nanofiltration Process for Sustainability in Industries: Reuse, Recycle, and Resource Recovery. In Nanofiltration for Sustainability; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–13. [Google Scholar]
- Zhang, X.; Li, C.; Wang, X.; Wang, Y.; Xu, T. Recovery of hydrochloric acid from simulated chemosynthesis aluminum foils wastewater: An integration of diffusion dialysis and conventional electrodialysis. J. Membr. Sci. 2012, 409, 257–263. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal recovery from wastewater using electrodialysis separation. Metals 2023, 14, 38. [Google Scholar] [CrossRef]
- Creusen, R.; van Medevoort, J.; Roelands, M.; van Duivenbode, A.v.R.; Hanemaaijer, J.H.; van Leerdam, R. Integrated membrane distillation–crystallization: Process design and cost estimations for seawater treatment and fluxes of single salt solutions. Desalination 2013, 323, 8–16. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Xu, Z.; Bai, A.-P.; Resina-Gallego, M.; Ji, Z.-G. Separation and recycling of concentrated heavy metal wastewater by tube membrane distillation integrated with crystallization. Membranes 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Li, T.; Peng, S.; Wu, D. Simultaneous desalination and molecular resource recovery from wastewater using an electrical separation system integrated with a supporting liquid membrane. Water Res. 2023, 246, 120706. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, G.; Liu, R.; Ji, Q.; Liu, H. Integration of concentration and electro-driven membrane system for effective water-saved acid recycling performance. Resour. Conserv. Recycl. 2023, 191, 106885. [Google Scholar] [CrossRef]
- Qiu, Y.; Ren, L.-F.; Xia, L.; Zhong, C.; Shao, J.; Zhao, Y.; Van der Bruggen, B. Recovery of Fluoride-Rich and Silica-Rich Wastewaters as Valuable Resources: A Resource Capture Ultrafiltration–Bipolar Membrane Electrodialysis-Based Closed-Loop Process. Environ. Sci. Technol. 2022, 56, 16221–16229. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Saurina, J.; Granados, M.; Cortina, J.L. Integration of Nanofiltration and Reverse Osmosis Technologies in Polyphenols Recovery Schemes from Winery and Olive Mill Wastes by Aqueous-Based Processing. Membranes 2022, 12, 339. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Q.; Huang, X.; Yan, Z.; Lin, X.; Ye, W.; Arcadio, S.; Luis, P.; Bi, J.; Van der Bruggen, B.; et al. Integrated loose nanofiltration-electrodialysis process for sustainable resource extraction from high-salinity textile wastewater. J. Hazard. Mater. 2021, 419, 126505. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Chi, M.; Eygen, G.V.; Guan, K.; Matsuyama, H. Comprehensive review of nanofiltration membranes for efficient resource recovery from textile wastewater. Chem. Eng. J. 2025, 506, 160132. [Google Scholar] [CrossRef]
- Knežević, K.; Saracevic, E.; Krampe, J.; Kreuzinger, N. Comparison of ion removal from waste fermentation effluent by nanofiltration, electrodialysis and ion exchange for a subsequent sulfuric acid recovery. J. Environ. Chem. Eng. 2022, 10, 108423. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Z.; Yuan, W.; Song, W.; Zhou, Y.; Lei, Y.; Jiang, B. High-flux electrochemical phosphorus recovery in an undivided electrolytic cell coupled with microfiltration with low energy consumption. Chem. Eng. J. 2024, 484, 149801. [Google Scholar] [CrossRef]
- Shen, Y.; Badireddy, A.R. Synergistic effect of alternating current-based electric and acoustic fields on flux recovery in crossflow microfiltration of synthetic wastewater. Sep. Purif. Technol. 2023, 306, 122534. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.; Priya, A.; Hawash, H.B.; Yap, P.S. Membrane technology for energy saving: Principles, techniques, applications, challenges, and prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Chen, T.; Bi, J.; Ji, Z.; Yuan, J.; Zhao, Y. Application of bipolar membrane electrodialysis for simultaneous recovery of high-value acid/alkali from saline wastewater: An in-depth review. Water Res. 2022, 226, 119274. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Teixeira, A.R.; Jorge, N.; Peres, J.A. Industrial wastewater treatment by coagulation–flocculation and advanced oxidation processes: A review. Water 2025, 17, 1934. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Asheghmoalla, M.; Mehrvar, M. Integrated and hybrid processes for the treatment of actual wastewaters containing micropollutants: A review on recent advances. Processes 2024, 12, 339. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Safitri, H.; Khairurrijal, K.; Taher, T.; Arham, L.O.; Jasipto, A.; Danasla, M.A.; Fadhilah, R.; Army, E.K.; Hakim, H.Z. Recent advances in acid mine drainage treatment through hybrid technology: Comprehensive review of scientific literature. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100945. [Google Scholar]
- Wang, Y.; Cao, J.; Biswas, A.; Fang, W.; Chen, L. Acid mine wastewater treatment: A scientometrics review. J. Water Process Eng. 2024, 57, 104713. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation technologies for acid mine drainage: Recent trends and future perspectives. Chemosphere 2023, 311, 137089. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Sun, J.; He, Y.; Wu, B.; Ge, L.; Pan, J. Ultrathin zwitterionic COF membranes from colloidal 2D-COF towards precise molecular sieving. Water Res. 2025, 274, 123073. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Tao, H.; Meng, J.; Yang, J.; Shuai, Q.; Asakura, Y.; Huang, L.; Yamauchi, Y. Covalent Organic Framework Nanoarchitectonics: Recent Advances for Precious Metal Recovery. Adv. Mater. 2024, 36, e2405399. [Google Scholar] [CrossRef]
- Xia, K.; Qin, Y.; Ni, C.; Liu, C.; Yan, H.; Zou, J.; Luo, S. Hydrazide-functionalized COFs exhibit ultra-high palladium adsorption capacity and excellent selectivity. Chem. Eng. J. 2024, 494, 153027. [Google Scholar] [CrossRef]
- Yan, H.; Peng, K.; Yan, J.; Jiang, C.; Wang, Y.; Feng, H.; Yang, Z.; Wu, L.; Xu, T. Bipolar membrane-assisted reverse electrodialysis for high power density energy conversion via acid-base neutralization. J. Membr. Sci. 2022, 647, 120288. [Google Scholar] [CrossRef]
- Ruan, H.; Guo, L.; Ding, N.; Cui, H.; Lu, Y.; Qiu, Y.; Yao, Y.; Liao, J.; Shen, J. Enhanced recovery of p-Aminophenol from high-salt wastewater via optimized bipolar membrane electrodialysis in a Water-Ethanol system. Sep. Purif. Technol. 2025, 360, 131038. [Google Scholar] [CrossRef]
- Hussain, A.; Yan, H.; Ul Afsar, N.; Wang, H.; Yan, J.; Jiang, C.; Wang, Y.; Xu, T. Acid recovery from molybdenum metallurgical wastewater via selective electrodialysis and nanofiltration. Sep. Purif. Technol. 2022, 295, 121318. [Google Scholar] [CrossRef]
- Sathvik, S.; Kumar, R.; Ulloa, N.; Shakor, P.; Ujwal, M.S.; Onyelowe, K.; Kumar, G.S.; Christo, M.S. Modelling the mechanical properties of concrete produced with polycarbonate waste ash by machine learning. Sci. Rep. 2024, 14, 11552. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiao, F.; Liu, W.; Wang, D.; Chen, W.; Qin, W. Selective preparation of lithium carbonate from overhaul slag by high temperature sulfuric acid roasting–Water leaching. J. Environ. Manag. 2024, 359, 120963. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Safaeipour, N.; Othman, R.S.; Otadi, M.; Sheibani, R.; Kargaran, F.; Van Le, Q.; Khonakdar, H.A.; Li, C. Agricultural waste-derived (nano)materials for water and wastewater treatment: Current challenges and future perspectives. J. Clean Prod. 2023, 421, 138524. [Google Scholar] [CrossRef]
- Ullah, H.I.; Dickson, R.; Mancini, E.; Malanca, A.A.; Pinelo, M.; Mansouri, S.S. An integrated sustainable biorefinery concept towards achieving zero-waste production. J. Clean Prod. 2022, 336, 130317. [Google Scholar] [CrossRef]
- Wang, N.; Li, T.; Zhang, M.; Sun, H.; Zhu, Z.; Li, J.; Liang, W. Excellent acid resistance and MXene enhanced photothermal conversion of bilayered porous solar evaporator fabricated by palygorskite and pectin. Desalination 2024, 591, 118053. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, L.; Liu, J.; Wang, D.; Chai, J.; Wu, M.; Tang, L. Recent progress of photoelectrocatalysis systems for wastewater treatment. J. Water Process Eng. 2023, 53, 103609. [Google Scholar] [CrossRef]
- Li, F.; Su, Z.; Wang, G.-m. An effective integrated control with intelligent optimization for wastewater treatment process. J. Ind. Inf. Integr. 2021, 24, 100237. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Kong, S.; Yan, H.; Li, C.; Wang, H. Green treatment of turbid traditional Chinese medicine (TCM) wastewater: Optimization of UF-MD coupling process. Desalination Water Treat. 2024, 320, 100810. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, J.; Hu, X.; Zhu, F.; Peng, X. Reductive Removal and Recovery of As(V) and As(III) from Strongly Acidic Wastewater by a UV/Formic Acid Process. Environ. Sci. Technol. 2022, 56, 9732–9743. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Zhang, W.; Zhou, A.; Xu, Q.; He, Z.; Yang, C.; Wang, A. Short-chain fatty acid production from waste activated sludge and in situ use in wastewater treatment plants with life cycle assessment. Resour. Conserv. Recycl. 2023, 198, 107186. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.S.; Sim, S.J. Enhancement of microalgal biomass productivity through mixotrophic culture process utilizing waste soy sauce and industrial flue gas. Bioresour. Technol. 2023, 373, 128719. [Google Scholar] [CrossRef]
- Mora-León, A.G.; Castro-Jiménez, C.C.; Saldarriaga-Molina, J.C.; García A, E.F.; Correa-Ochoa, M.A. Aluminium recovered coagulant from water treatment sludge as an alternative for improving the primary treatment of domestic wastewater. J. Clean Prod. 2022, 346, 131229. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Wang, A.; Yue, B.; Lin, T.; Ding, M. Microalgae treatment of food processing wastewater for simultaneous biomass resource recycling and water reuse. J. Environ. Manag. 2024, 369, 122394. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, C.; Xu, X.; Li, Z.; Wang, H.; Zhao, Y.; Zhao, Y. An ultrastable cationic covalent organic framework for selective capture of silver from aqueous solution. J. Mol. Struct. 2023, 1292, 136173. [Google Scholar] [CrossRef]
| Item/Process | Influent Characteristics | Operating Setpoints | Key Performance Indicators | Remarks/Notes |
|---|---|---|---|---|
| Two-stage neutralization + flocculation | pH 2.5; Al3+ = 500 mg/L; H2SO4 system | Stage 1: add lime milk to pH 4.5 (CaSO4 precipitation); Stage 2: add NaOH to pH 7.0 | Final pH to 6.8; decrease Al3+ to 0.8 mg/L; SO42− removal > 90%; sludge = 0.15 kg m−3 | Effluent meets GB 8978-1996 [11]; cost ≈ 0.3–0.7 USD/t; 1.2–1.5 times overdosing and sludge handling |
| General operation for acidic multi-metal wastewater | High-acid mixed Al/Cu system | Target pH 5–9 (2-stage typical); controlled mixing and residence time | Metal removal > 86–99% (analogous AMD study); lime cheaper but produces more sludge | Solid–liquid separation via settling + filter press/centrifuge; high ionic strength may need coagulation aid |
| Adsorbent Type | Target Metal(s) | Max Adsorption Capacity (mg Metal/g) | Approximate Influent pH or pH Range | Acid/High Ionic Strength Tolerance | Regeneration/Stability (Cycles, Residual Capacity) | Approximate Cost | Reference |
|---|---|---|---|---|---|---|---|
| Strong-acid cation exchange resin (e.g., sulfonated styrene-divinylbenzene) | Cu2+, Pb2+, Al3+ | ~70–300 | ~pH 1–4 | Good at low pH for some systems; competing H+ reduces efficiency | ~10–20 cycles, ~80–90% remaining capacity (literature-dependent) | Moderate (USD tens-hundreds per kg resin) | Qasem et al. [21] |
| Modified activated carbon (e.g., Fe3O4@SiO2-NH2) | Al3+, Cu2+ | ~50–100 | ~pH 2–6 | Moderate; magnetic recovery improves practicality | Example: ~12% capacity loss after ~10 cycles reported | Moderate/high | Liu et al. [52] |
| Metal–organic framework (MOF) (e.g., phospho-MIL-101(Al), functionalized MOFs) | Al3+, Pb2+, Cd2+ | ~100–500 | ~pH 2–6 | High ionic strength resistance reported | >90% regeneration efficiency in some studies | High (USD hundreds per kg or more) | Zadehahmadi et al. [54] |
| Covalent triazine framework (CTF) or ion-imprinted polymer (IIP) (emerging) | Cr(VI), Cu2+ etc. | ~70–250 | ~pH 1–5 | Designed for acid resistance; early stage | Limited cycle data; some high reusability claimed | Lower cost target (commercial data limited) | Lei et al. [55] |
| Adsorbent/System | Influent Characteristics | Operating Setpoints | Key Performance Indicators | Remarks/Notes |
|---|---|---|---|---|
| Strong-acid cation exchange resin (H+-selective) | Acidic wastewater with Al3+ ≈ 1500 mg L−1 | pH ≈ 1.5 (H+ exchange dominant region) | 85% sulfuric acid recovery; >99% Al3+ removal | Industrial-scale application; resin swelling in strong acid noted |
| Fe3O4@SiO2-NH2-modified activated carbon + magnetic separation + electrolytic regeneration | Mixed Al3+/Cu2+ acidic wastewater | Batch mode; magnetic solid recovery; electro-regeneration | Al3+ uptake: 95 mg/g; Cu2+ uptake: 28 mg/g; recovered metal purity > 99%; hazardous waste reduction ≈ 90% | 12% capacity loss after about 10 cycles |
| Phospho-functionalized MIL-101(Al) MOF | Al3+ in acidic chloride medium (50 g/L Cl−) | pH ≈ 2–3; acidic high-salinity environment | Al3+ capacity 195 mg/g maintained; selectivity coefficient (Al3+/Fe3+) = 16.3; regeneration efficiency > 93% | High acid/salt tolerance; higher sorbent cost (~200 USD/kg synthesis) |
| Process/Membrane Type | Influent/Pretreatment | Operating Setpoints | Key Performance Indicators | Remarks/Notes |
|---|---|---|---|---|
| MF pretreatment (before NF–RO system) | Suspended solids are about 500 mg/L | PP/PVDF MF, 0.2 µm, 0.3 MPa, flux ≈ 80 L/m2/h | Decrease SS to <10 mg L−1; fouling cycle extended to 30 days | Pre-NF pH adjusted 1.8 to 2.5 to avoid Al colloids |
| NF (acid-resistant, within NF–RO train) | H2SO4–Al3+ wastewater (pH ≈ 2.5) | MWCO ≈ 200 Da; 1.5 MPa; 25 °C | SO42− retention > 95%; Al3+ rejection 98%; permeate Al3+ < 20 mg L−1; concentrate 30% of feed; add H2SO4 to 18% | Concentrate reused internally; supports closed acid loop |
| RO (polishing after NF) | NF permeate | SW30HR polyamide; about 4 MPa | Permeate conductivity < 100 µS cm−1; overall Al3+ total removal > 99.5%; effluent Al3+ < 0.5 mg L−1 | Water treatment cost decreased from 2 to 1 USD/t; Decrease sludge to 70% |
| UF–ED combined (HCl acid regeneration + Al recovery) | 8% HCl; Al3+ ≈ 800 mg/L; COD ≈ 200 mg/L | UF: Al2O3–ZrO2 ceramic, 50 nm, 0.4 MPa, pH 1.5; ED: 200 pairs of AMV/CMV membranes, 50 mA cm−2, 120 V | UF retains gel Al(OH)3 (decrease Al3+ to 80 mg L−1; flux ≈ 60 L m−2 h−1); ED regenerates 12% HCl with 75% acid recovery; Al < 50 mg L−1 in dilution | Al recovery 92%; energy decrease from 30 to 12 kWh/t; >98% γ-Al2O3 product |
| VMD + Al2(SO4)3 crystallization | 20% H2SO4; Fe3+ ≈ 200 mg/L; Cu2+ ≈ 50 mg/L | PTFE 0.22 µm; 60 °C; −90 kPa; followed by 1:1.2 mol Al(OH)3 reaction | Water vapor recovery 80%; H2SO4 concentrated to 40% (90% acid recovery); Al2(SO4)3 crystal purity > 98% | Sludge reduction 95% |
| UF (general acid-resistant ceramic) | Colloidal Al(OH)3 removal stage | α-Al2O3/ZrO2 UF; pH 1–14; ≤80 °C | Flux 60–80 L/m2/h; Al rejection > 85% | Long-term acid stability verified (>2 years) |
| Technology | Advantages | Disadvantages | Current Challenges | Future Perspectives | Representative References |
|---|---|---|---|---|---|
| Neutralization (alkali addition + precipitation) | Rapid pH control and bulk removal of dissolved metals; low reagent cost (lime, carbide slag); simple and mature process for large-scale operation. | High sludge yield and disposal cost; residual metals and hardness in effluent; reagent overdosing (1.2–1.5 × theoretical). | Optimizing pH setpoints for multi-metal systems; sludge dewatering and valorization; maintaining efficiency at high sulfate/chloride load. | AI-based dosing and process control; resource recovery from Al(OH)3 to α-Al2O3; integration with electrochemical or membrane polishing. | Zhao et al. [20]; Qasem et al. [21]; Du et al. [15] |
| Ionic Adsorption/Ion Exchange | High selectivity for target ions (Al3+, Cu2+, Zn2+); lower sludge generation; feasible for acid/metal resource recovery. | Reduced capacity at very low pH (pH < 3); high cost of advanced adsorbents; chemical consumption during regeneration. | Development of acid-resistant resins/MOFs/CTFs; improved regeneration efficiency and mechanical stability; scaled up under high-salinity feeds. | Design of low-cost, recyclable, acid-resistant materials; electro-desorption/supercritical CO2 regeneration; hybrid adsorption–membrane or adsorption–electrochemical systems. | Qasem et al. [21]; Kaur et al. [53]; Zadehahmadi et al. [54] |
| Membrane Separation | High-precision separation of acid, metal, and water; enables water reuse and resource recovery; modular and automatable. | Fouling and scaling by colloids/metal hydroxides; concentrate management issue; high pressure with high energy cost. | Ensuring long-term acid resistance (pH 1–3); flux decline and membrane degradation; energy optimization and concentrate disposal. | Development of fluoropolymer/ceramic anti-fouling membranes; hybrid UF–NF–RO or UF–ED–BMED trains for acid + metal recovery; renewable energy-coupled low-carbon systems. | Ahmad et al. [63],; Wei et al. [65]; Chen et al. [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Wang, L.; Qin, B.; Meng, F.; He, Y.; Wang, X.; Bai, J.; Zhang, J.; Wang, Y. Acidic Wastewater from Electrode Foil Manufacturing: Treatment Advances and Future Pathways. Water 2025, 17, 3325. https://doi.org/10.3390/w17223325
Wu G, Wang L, Qin B, Meng F, He Y, Wang X, Bai J, Zhang J, Wang Y. Acidic Wastewater from Electrode Foil Manufacturing: Treatment Advances and Future Pathways. Water. 2025; 17(22):3325. https://doi.org/10.3390/w17223325
Chicago/Turabian StyleWu, Guodong, Lu Wang, Bing Qin, Fanbin Meng, Yonghu He, Xin Wang, Jing Bai, Jingpeng Zhang, and Yuanhao Wang. 2025. "Acidic Wastewater from Electrode Foil Manufacturing: Treatment Advances and Future Pathways" Water 17, no. 22: 3325. https://doi.org/10.3390/w17223325
APA StyleWu, G., Wang, L., Qin, B., Meng, F., He, Y., Wang, X., Bai, J., Zhang, J., & Wang, Y. (2025). Acidic Wastewater from Electrode Foil Manufacturing: Treatment Advances and Future Pathways. Water, 17(22), 3325. https://doi.org/10.3390/w17223325





