Abstract
Acid mine drainage (AMD), characterized by low pH and high sulfate concentrations, poses severe environmental risks. Sulfate-reducing bacteria (SRB) are promising for AMD bioremediation, but their activity is often inhibited in such extreme conditions. This study proposed two strategies—SRB pre-cultivation and SRB-enhanced sediment amendment—to address this limitation, and systematically examined the effects of sulfate concentration, pH, inoculum size, and carbon source on sulfate removal. Results showed that pre-cultivation significantly improved SRB’s acid tolerance, expanding the effective AMD treatment pH range from 6.8–8.8 to 4.8–8.8. At pH 7.8, pre-cultivated SRB achieved 50% removal of 11,760 mg/L sulfate within 24 h and complete removal within 150 h. The SRB-enhanced sediment system further enabled efficient and stable remediation of real AMD (sulfate removal > 97%, Fe/Mn co-removal > 90%). This work provides a practical solution to overcome SRB inhibition in harsh AMD environments and contributes to the development of low-cost, sustainable AMD bioremediation technologies.
1. Introduction
Acid mine drainage (AMD) is widely recognized as a critical global environmental challenge, with urgent remediation demands [1,2]. AMD primarily originates from mining activities: when sulfide minerals, such as pyrite, are exposed to air and water, a series of microbially mediated reactions occur [3,4,5]. These reactions ultimately generate acidic effluents with pH < 4, high concentrations of SO42− (usually ranging from 1000–10,000 mg/L), and elevated levels of a mixture of major cations, oxyhydroxide-forming metals, and potentially toxic metalloids—such as iron ions (100–2000 mg/L), manganese (Mn) up to 250 mg/L, and copper (Cu) up to 2670 mg/L [3,4,6,7]. Once AMD released into surrounding aquatic or soil environments, it not only causes acidification and disrupts the balance of aquatic ecosystems, but the potentially toxic metalloids may bioaccumulate through food chains, posing serious risks to plants, animals, and human health [5,8]. In addition, high sulfate concentrations may induce soil salinization and alkalization, further exacerbating regional ecological degradation [2,9].
Current strategies for AMD remediation can be broadly categorized into physicochemical and biological approaches. Physicochemical methods are capable of achieving rapid contaminant removal in the short term; however, they generally suffer from high operational costs, excessive reagent consumption, secondary pollution risks, and difficulties in maintaining long-term stability [10,11,12]. In contrast, biological remediation has attracted increasing attention due to its low cost, environmental compatibility, absence of secondary pollution, and potential for effective removal of sulfate ions and immobilization of heavy metalloids in AMD [4,13]. Among these, sulfate-reducing bacteria (SRB)-mediated bioremediation has emerged as a particularly promising strategy, owing to its unique metabolic mechanisms and broad application potential [14,15,16,17].
SRB are functional microbial consortia capable of using sulfate as a terminal electron acceptor under anaerobic conditions, obtaining energy by oxidizing organic substrates such as lactate, ethanol, or acetate, and reducing sulfate to sulfide [18]. In AMD remediation, the sulfide generated by SRB metabolism reacts with dissolved heavy metal ions to form sparingly soluble sulfide precipitates (e.g., FeS, CuS, ZnS), while simultaneously consuming protons (H+) and thereby increasing the pH of the wastewater [2,13,14]. This coupled process achieves three concurrent goals, sulfate removal, heavy metal immobilization, and acidity neutralization, fundamentally mitigating the environmental hazards of AMD and aligning with the concept of green and sustainable remediation [4,19]. Studies showed that SRB-based bioremadiation achieved up to 93.97% of sulfate reduction, with metal recovery rates of 95% for nickel, 98% for iron and copper, and 99% for zinc under optimized conditions [13]. Despite these advantages and significant progress at the theoretical and laboratory scales, the practical application of SRB-based remediation of AMD under complex environmental conditions still faces critical challenges. The pH of AMD effluents is often below 4, where extreme acidity, high sulfate and metal concentrations strongly inhibit microbial growth and metabolism [6,7,11,20,21]. As a result, sulfate reduction rates decline markedly and metal immobilization becomes unstable, greatly hindering the large-scale implementation of SRB-based technologies [16,19,22]. To overcome the inhibition of SRB activity in extreme AMD environments, various strategies have been explored. These include the cultivation of acidophilic or acid-tolerant strains from environmental samples [8,23,24,25]; strain screening and acclimation or the development of off-line systems to avoid direct contact between bacteria and acidic wastewater; as well as cell immobilization and the application of functional additives to enhance the environmental adaptability of SRB [26]; and supply of various substrate as carbon source to achieve long-term reaction [27,28,29]. With these strategies, certain progress has been achieved; however, several unresolved issues still hinder their practical application in real AMD remediation scenarios. For instance, acid-tolerant/acidophilic strains (e.g., Desulfosporosinus acidophilus and Desulfosporosinus acididurans) [30], often exhibit incomplete substrate oxidation metabolism, like acetic acid, which are toxic to microbes, reduce available electrons for sulfate reduction, and increase the COD of effluent [31]. Another critical challenge is the lack of organic carbon sources in AMD, as SRB require organic matter as electron donors for their metabolic activities.
To address the aforementioned limitations, we propose the following research objectives: (i) Develop a pre-cultivation strategy, in which SRB are first enriched under favorable conditions to achieve sufficient biomass before being inoculated into AMD systems. This pre-adaptation step is designed to enhances the strains’ tolerance to extreme AMD environments and accelerates the sulfate reduction kinetics; (ii) systematically investigate key operational parameters-including sulfate concentration, pH, inoculum size, and carbon source-to elucidate their effects on remediation performance; (iii) Establish an SRB-enhanced sediment remediation approach by constructing an integrated SRB-in situ sediment system and applied to real AMD treatment. Sediment from contaminated sites is inherently rich in metabolic products of indigenous microorganisms [32], which can be further metabolized by native microbes to serve as bioavailable carbon sources for SRB-addressing the critical issue of organic carbon deficiency in AMD. Collectively, this study clarifies the role of pre-cultivation in expanding the effective application scope of SRB, verifies the practical feasibility of the bioaugmented sediment remediation system, and provides both theoretical and technical support for the optimization and large-scale implementation of AMD bioremediation technologies.
2. Materials and Methods
2.1. Bacterial Strains and Culture
The SRB strain Desulfovibrio vulgaris was obtained from the laboratory culture collection. D. vulgaris was cultivated and enriched in Postgate’s medium, which contained (g/L): NH4Cl 1.0, MgSO4·7H2O 2.0, Na2SO4 1.0, K2HPO4 0.5, CaCl2 0.86, yeast extract 1.0, and sodium lactate (70%) 3.33 mL/L. The medium was adjusted to pH 7.8 and deoxygenated by purging with high-purity nitrogen gas for 30 min. After autoclaving (121 °C, 20 min), sterile-filtered (0.22 μm) solutions of FeSO4·7H2O (0.5 g/L), sodium thioglycolate (0.1 g/L), and sodium L-ascorbate (0.1 g/L) were added aseptically.
2.2. Sediment
Fresh sediment was collected from the same 0–10 cm depth layer at three replicate points (10 m apart) in the discharge channel and then thoroughly mixed in a sterile anaerobic chamber (O2 < 0.1%) to ensure initial homogeneity, the homogenized sediment was immediately divided into 50 g sterile centrifuge tubes and sealed with oxygen-impermeable caps to maintain anaerobic conditions. The tubes were transported to the laboratory under cold-chain conditions and placed in a −80 °C freezer via a pre-cooled (−20 °C) gradient freezing box until further use. Microbial community diversity in the sediment was analyzed by 16S rRNA gene amplicon sequencing, conducted by Shanghai Sangon Biotech (Shanghai, China).
2.3. AMD Wastewater
Two types of AMD wastewater were used in this study: simulated AMD and real AMD. The simulated wastewater contained (g/L): NH4Cl 1.0, MgSO4·7H2O 2.0, Na2SO4 1.0, K2HPO4 0.5, CaCl2 0.86, yeast extract 1.0, sodium lactate (70%) 3.33 mL/L, and FeSO4·7H2O 0.5; the pH was adjusted to 7.8. Real AMD wastewater was collected from effluent discharge points and boreholes at the Liugou and Miaogou abandoned coal mines in the Shandi River Basin, Yangquan. After sampling, the wastewater was transported under low-temperature conditions to the laboratory for water quality analysis (Table S1). For remediation experiments, additional carbon sources were supplemented with yeast extract (1.0 g/L) and sodium lactate (70%, 3.33 mL/L).
2.4. Determination of Sulfate Reduction Performance
The sulfate content was quantified using the barium sulfate turbidimetric method [27]. In acidic solution, barium chromate reacts with sulfate for 5 min to form barium sulfate precipitate while releasing chromate ions. After neutralization, both the excess barium chromate and the generated barium sulfate remain as precipitates and are removed by filtration. Under alkaline conditions, chromate ions exhibit a yellow color, and their absorbance was measured at 420 nm. The sulfate concentration was then calculated based on a standard calibration curve.
2.5. Study on Sulfate Reduction Performance of SRB
The preserved SRB strain was inoculated into 50 mL of Postgate’s liquid medium at 4% (v/v) and incubated anaerobically at 35 °C. The optical density of the culture was periodically measured at 600 nm (OD600) using a UV spectrophotometer (Metash, Shanghai, China). Once the culture reached the stationary growth phase, 2 mL of the suspension was transferred into fresh medium for the next round of cultivation. During the cultivation process, the sulfate concentration in the medium was gradually increased, enabling the strain to adapt to the target sulfate concentration through stepwise acclimation. All experiments were performed in triplicate and incubated in a 35 °C constant-temperature chamber.
To ensure an adequate SRB population in the treatment system, the bacteria were pre-cultivated by transferring cultures grown to the stationary phase at specific sulfate concentrations. The cultures were centrifuged, the supernatant discarded, and the cell pellets resuspended in AMD wastewater to be treated. Single-factor experiments were conducted to investigate the effects of key environmental parameters, including pH, initial sulfate concentration, carbon source content, and inoculum size, on sulfate removal by SRB, with the aim of optimizing the treatment process. The experimental parameters were set as follows:
- Effect of pH: The initial pH of the medium was adjusted to 2.8, 3.8, 4.8, 5.8, 6.8, 7.8, and 8.8.
- Effect of sulfate concentration: Initial sulfate concentrations were set at 840, 1680, 3504, 8400, and 11,760 mg/L.
- Effect of inoculum size: Acclimated SRB cultures were centrifuged and resuspended into fresh AMD systems at volumes of 10, 50, and 75 mL.
- Effect of carbon source: Using sodium lactate in Postgate’s medium as the baseline, the amount of carbon source added was adjusted to 5.5, 16.5, and 38.85 mg/L.
Control group: Experimental matrix (e.g., AMD or culture medium) without SRB or sediment. Conditions (temperature: 35 °C, initial pH, anaerobic control) were identical to the experimental group, serving to eliminate background interference from reagents, matrix, and environmental factors.
- Prior to inoculation with SRB, both the growth medium and real AMD samples were purged with high-purity nitrogen for 30 min to remove dissolved oxygen. The DO concentration was measured and maintained at <0.5 mg/L, the Eh value was stably maintained at −150 to −300 mV.
2.6. Evaluation of Sediment-Mediated AMD Remediation Efficiency
Prior to use, visible debris was manually removed. The sediment was then homogenized thoroughly using a sterile stainless-steel sieve (2 mm mesh) to ensure uniformity.
Ten grams of sediment from the effluent discharge point of a representative AMD were mixed with 40 mL of sterile water to prepare a bacterial suspension. Ten milliliters of this suspension were transferred into an anaerobic bottle containing 40 mL of medium and incubated until the culture became turbid, with an OD600 exceeding 2. The resulting bacterial suspension was then introduced into the AMD wastewater at a 1:5 (v/v) ratio, incubated at 35 °C under anaerobic conditions, and samples were periodically collected every 24 h (first 8 days) or 48 h to monitor sulfate reduction. Remediation experiments were conducted using (i) SRB alone, (ii) sediment alone, and (iii) a combined SRB–sediment system for both simulated AMD and real wastewater from the representative mine.
2.7. Determination of Total Fe and Mn
The concentrations of total Fe and Mn in the solution were measured using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, 7300 V, PerkinElmer, Shelton, CT, USA). The samples were first filtered through a 0.22 μm polyethersulfone filter, and then the concentrations of Fe and Mn in the resulting filtrate (2 mL) were determined by ICP-OES.
2.8. Statistical Analysis
All experiments were performed in triplicate to ensure reproducibility. The mean value () of the three parallel measurements was calculated as the final result of each sample, following the formula: , where x1, x2, and x3 represent the individual measurements. Standard deviation (SD) was used to evaluate the precision of the method, calculated using the formula: , where n = 3 (number of replicates) and xi is the i-th measurement. The relative standard deviation (RSD) was derived as
, with a threshold of ≤3% for acceptable precision. Statistical significance was confirmed with one-way ANOVA followed by Tukey’s test (p < 0.05). Error bars in figures correspond to the standard deviation of triplicate measurements, providing a visual representation of data variability.
3. Results and Discussion
3.1. Characterization of Sediment
Based on species abundance, Desulfofarcimen was identified as the most dominant genus in the sediment (Figure 1b). Under anaerobic conditions, it can reduce sulfate to sulfide, which subsequently reacts with dissolved heavy metal ions to form insoluble precipitates, playing a crucial role in sulfur cycling and heavy metal immobilization in the AMD sediment. Another abundant genus, Aneurinibacillus, contains species with strong environmental adaptability; some can form spores to withstand harsh conditions, while others participate in the decomposition and metabolism of organic matter, thereby promoting carbon cycling and transformation within the sediment. The presence of genera such as Pseudomonas and Clostridium indicates that the sediment possesses the capacity to degrade organic pollutants [33,34,35], contributing to the breakdown of residual organic contaminants in the sediment. The metabolic products of these microorganisms may serve as nutrients for other microbial species. In addition to the dominant genera mentioned above, others such as Ciceribacter, Clostridium, and Sedimentibacter were also detected, reflecting relatively high microbial genus-level diversity and suggesting a complex food web and ecological functionality within the sediment ecosystem. Many species, including Desulfovibrio, Desulfosporosinus, Desulfitobacterium, and Acidibacillus, exhibit typical adaptations to acidic environments [19,36,37]. Furthermore, the diversity of microbial taxa in the sediment implies comprehensive functional roles in elemental cycling of carbon, nitrogen, sulfur, and iron. For example, Desulfofarcimen participates in sulfur cycling, Aneurinibacillus may contribute to carbon cycling, Rhizobium is involved in nitrogen cycling, and Acidibacillus can oxidize ferrous iron, collectively maintaining the material balance of the sediment ecosystem [38,39,40].
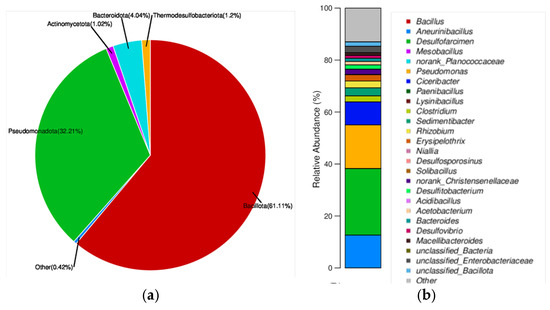
Figure 1.
Microbial species diversity in the sediment. (a) Microbial community composition at the phylum level. Different colors represent different taxa, and the area of each pie segment corresponds to the relative abundance of the respective taxon. (b) Microbial community composition at the genus level. The y-axis represents the relative abundance of each genus. Colors correspond to the names of the taxa at this taxonomic level, and the width of each color block indicates the relative abundance of the respective genus. Species with a relative abundance below 1% in the sample are grouped as “others,” while the remaining taxa are considered dominant and analyzed accordingly.
Notably, AMD treatment systems without sediment or organic matter-transforming microbes (e.g., pure SRB) require exogenous carbon supplementation like to sustain sulfate reduction and microbial metabolism, boosting reaction rates [13,27,29]. Unlike these approaches, the functional genera (e.g., Aneurinibacillus, Pseudomonas, Clostridium) decompose endogenous organics into a “self-sustaining carbon cycle,” reducing external carbon reliance. This microbe-driven carbon transformation cuts operational costs and enhances harsh AMD adaptability—An advantage pure SRB or exogenous carbon-supplemented reactors typically lack.
3.2. Growth Characteristics of SRB
The strain is slightly curved, Gram-negative, rod-shaped, with dimensions of approximately 0.5 μm × 1.5 μm. On lactate–sulfate agar medium containing ferrous salts, it forms entirely black, circular colonies (Figure 2). The bacterium is strictly anaerobic and chemoorganoheterotrophic, deriving energy via anaerobic respiration by reducing sulfur or other reducible sulfides to H2S. This metabolic activity enables efficient reduction of sulfate ions in AMD. The optimal growth temperature ranges from 25 to 35 °C, and the optimal pH is 7.0–7.5 [14,19].
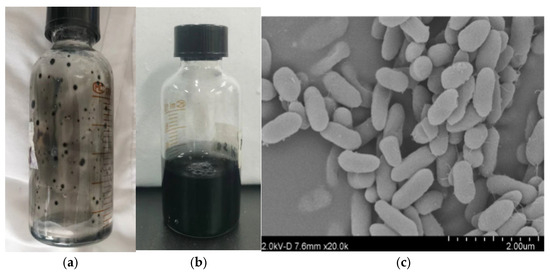
Figure 2.
Characteristics of SRB. (a) Formation of single black colonies on the inner wall of solid medium. (b) Activation of the strain in liquid medium, resulting in the formation of black FeS precipitates. (c) Scanning electron microscopy (SEM) image of the strain.
The strain was inoculated into SRB medium without Fe2+. As shown in the growth curve (Figure 3), bacterial growth was slow during the first 18 h, corresponding to the lag phase. Between 18 and 48 h, the optical density at 600 nm increased rapidly to 1.54, indicating exponential cell proliferation and entry into the logarithmic growth phase. Around 48 h, cell density reached its maximum. Subsequently, due to nutrient depletion and environmental limitations, the cell density gradually declined, indicating the onset of the stationary and death phases. In the experimental group, the initial pH was 7.4. During the first 20 h, pH increased, followed by a decrease, reaching a minimum at 36 h, then rising again to a peak of 7.5 before gradually stabilizing around 7.2. The overall trend of pH, first rising, then decreasing, and subsequently increasing, correlates with bacterial growth characteristics. During the initial growth phase, bacteria reduce sulfate to H2S, decreasing H+ concentration and increasing alkalinity, which leads to a rise in pH [41]. Upon entering the exponential phase, accumulation of metabolic products, including organic acids, caused a slight pH decrease. During the stationary phase, pH gradually increased and stabilized. The increase in pH during sulfate reduction by SRB is mainly attributed to the formation of HCO3− from the oxidation of organic substrates [2,15,42].
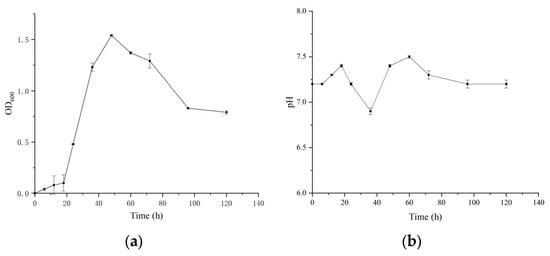
Figure 3.
Growth characteristics of Desulfovibrio vulgaris at 35 °C and pH 7.5. (a) Changes in bacterial cell density during cultivation. (b) Changes in pH during cultivation.
3.3. Sulfate Reduction Performance Testing
To investigate the factors influencing sulfate reduction by SRB, the effects of different sulfate concentrations, pH, inoculum size, and carbon source on SRB reduction performance were systematically examined.
3.3.1. Effect of Sulfate Concentration
To evaluate the sulfate reduction efficiency of SRB under varying sulfate concentrations, experiments were conducted using simulated AMD at pH 7.8 and 35 °C, with initial sulfate concentrations ranging from 840 to 11,760 mg/L. The results demonstrated that pre-cultivated SRB exhibited strong sulfate reduction performance across the entire tested range (Figure 4a). Sulfate concentrations decreased continuously over time, showing a rapid decline during the initial phase, followed by a slower reduction and eventual stabilization. At equilibrium, sulfate concentrations were all below the discharge limit of 250 mg/L, and no inhibition of reduction due to high sulfate levels was observed. In the lowest concentration group (840 mg/L), significant black precipitate (presumably ferrous sulfide) appeared within 12 h, indicating that SRB cells rapidly initiated the reduction process, with complete sulfate removal achieved within 24 h. This rapid response is likely due to the absence of significant inhibitory effects of low sulfate on SRB metabolism, allowing the population to quickly reach the logarithmic growth phase and rapidly activate intracellular sulfate-reducing enzymes, such as adenosine-5′-phosphosulfate reductase.
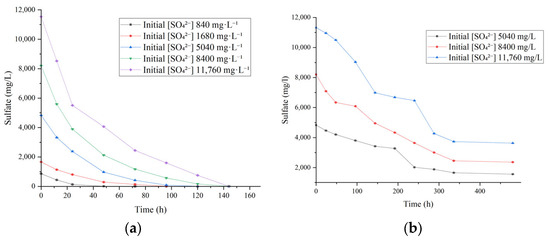
Figure 4.
Sulfate reduction by SRB at 35 °C under different initial sulfate concentrations (initial pH 7.8). (a) Pre-cultivated SRB, (b) SRB without pre-cultivation.
In higher concentration groups (1680–11,760 mg/L), sulfate reduction rates during the first 24 h were comparable (average rate 25.3–28.7 mg L−1 h−1), with sulfate concentrations decreasing to less than 50% of their initial values. Complete removal times increased with initial sulfate concentration, occurring at 90 h, 100 h, 120 h, and 220 h, respectively, but the final removal efficiency exceeded 97% in all cases. These results indicate that SRB’s overall reduction capacity during the early phase was not significantly affected by elevated sulfate levels. After 24 h, SRB activity under high sulfate stress slightly decreased but still maintained metabolic function, ultimately achieving complete sulfate removal.
Notably, pre-cultivated SRB exhibited substantial reduction capability even at 11,760 mg/L sulfate, a concentration far exceeding the sulfate levels typically observed in coal mine AMD (3000–8000 mg/L) [43], demonstrating strong high-sulfate tolerance and adaptability. This tolerance may be attributed to the regulation of intracellular and extracellular osmotic pressure to maintain membrane stability [2,44], as well as an efficient electron transfer system that accelerates sulfate conversion to sulfide, alleviating intracellular sulfate-induced toxicity. By contrast, non-pre-cultivated SRB exhibited very slow reduction rates when treating 5040–11,760 mg/L sulfate, with no effective removal observed even after 480 h (Figure 4b). This suggests that SRB populations without pre-cultivation struggle to rapidly adapt to high-sulfate environments and activate effective reduction pathways. The likely reason is that only a small fraction of the original population is tolerant to high sulfate, limiting the overall reduction capacity.
During sulfate removal, the removal of Fe and Mn ions in the system was also monitored (Figure 5). The results indicate that SRB can achieve effective co-removal of Fe and Mn during sulfate reduction. The removal of these metals lagged behind sulfate reduction. Biogenic sulfides produced by SRB metabolism provide the necessary conditions for Fe and Mn precipitation, highlighting the potential of biogeochemical synergy in heavy metal remediation. The removal of metal ions involves multiple mechanisms. In addition to biological reduction and immobilization by SRB, the extracellular polymeric substances (EPS) of the cells and the associated organic functional groups play important roles in metal immobilization [18,45]. SRB EPS contains large amounts of non-polar and acidic amino acids, carrying abundant negative charges that enhance electron transfer [46] and effectively promote interactions with metal cations [44,47]. Moreover, EPS can encapsulate the surfaces of metal sulfides, reducing their toxicity to the cells and thereby improving the overall treatment efficiency of the SRB system [48].
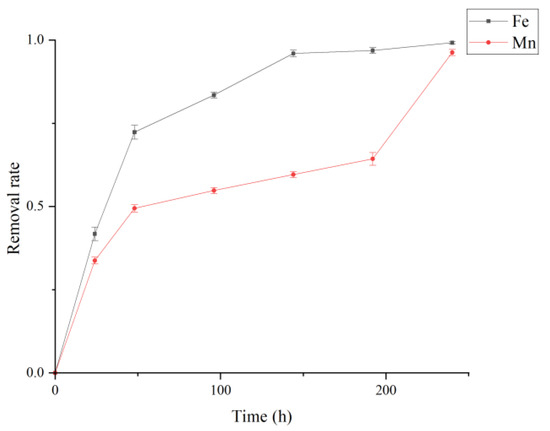
Figure 5.
Removal and immobilization of Fe and Mn in the cultivation system at 35 °C and pH 7.8. Concentration of Fe is 474 mg/L and Mn is 54 mg/L.
3.3.2. Effect of pH Conditions
This study systematically investigated the effect of pH on the sulfate reduction performance of SRB. In Figure 6a, without pre-cultivation, effective sulfate reduction was observed only within a pH range of 6.8–8.8, while negligible reduction occurred at pH below 5.8. Under pH 7.8, complete sulfate removal required 220 h. After pre-cultivation, SRB was inoculated into systems with different initial pH values (2.8–8.8), and the results are shown in Figure 6b. At an initial pH of 7.8, SRB exhibited the highest sulfate reduction efficiency, with sulfate concentration decreasing from 11,760 mg/L to below 4000 mg/L within 48 h, corresponding to a removal rate exceeding 66%. Complete removal was achieved at 160 h. Under mildly acidic or alkaline conditions (pH 6.8 and 8.8), SRB growth and reduction activity were slightly reduced but still maintained high metabolic capability, with complete sulfate removal requiring 220 h and 260 h, respectively. When the initial pH was further decreased to 5.8 and 4.8, bacterial growth slowed lightly, and the reduction process proceeded at a slower rate. At pH 3.8 and 2.8, SRB growth was almost completely inhibited, and after 500 h, sulfate concentrations decreased only to 7500 mg/L and 8000 mg/L, indicating that the reduction process was severely suppressed.
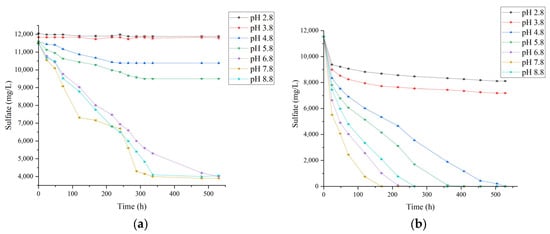
Figure 6.
Sulfate reduction by SRB at 35 °C under different initial pH conditions (initial sulfate concentration 11,760 mg/L). (a) SRB without pre-cultivation; (b) Pre-cultivated SRB.
These results indicate that SRB exhibit high sulfate reduction efficiency within a pH range of 6.8–7.8, whereas growth and metabolic activity are significantly inhibited at lower pH values (<5.8), consistent with previous studies [14,44]. “SRB pre-cultivation + sediment enhancement” strategy expands the effective pH range to 4.8–8.8, whereas most pure SRB systems reported in literature only operate stably at pH 6.0–9.0 [13,19]. This addresses the acid tolerance limitation of prior approaches. Changes in pH can disrupt intracellular homeostasis, as free acids or bases in the environment can cross the cell membrane and dissociate within the cytoplasm, interfering with proton gradients and electrolyte balance. If the pH gradient between the extracellular and intracellular environment is too large, most of the energy generated from redox processes is consumed to maintain pH homeostasis rather than supporting cell growth, which is highly detrimental to SRB growth and metabolism [49]. Moreover, pH affects the speciation of substances in solution [45,50,51]. For example, under acidic conditions, the proportion of undissociated H2S increases, allowing it to penetrate cell membranes, disrupt intracellular pH homeostasis, and inhibit cytochrome activity, ultimately hindering SRB energy metabolism and reducing their activity [41].
3.3.3. Effect of Inoculum Size
This study further investigated the effect of inoculation volume on SRB-mediated reduction of high-concentration sulfate (11,760 mg/L) (Figure 7). Under constant cultivation conditions (35 °C, pH 7.8), three inoculation volumes-15 mL, 50 mL, and 75 mL-were tested. The results showed that the highest inoculation volume (75 mL) exhibited a faster sulfate reduction rate compared to 50 mL and 15 mL. Complete sulfate removal was achieved within 240 h in all groups, indicating that SRB possess strong self-proliferation and adaptability at this concentration, maintaining normal metabolic activity. The system provided sufficient nutrients to sustain proper microbial growth and activity.
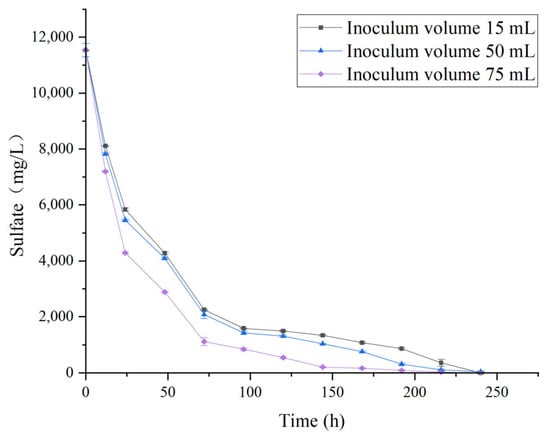
Figure 7.
Sulfate reduction by SRB at 35 °C and pH 7.8 under different inoculation volumes (initial sulfate concentration 11,760 mg/L).
3.3.4. Effect of Carbon Source Conditions
This study systematically evaluated the effect of carbon source availability on SRB-mediated reduction of high-concentration sulfate (11,760 mg/L) (Figure 8). As the sulfate concentration was seven times that of the standard Postgate medium, the influence of carbon source concentration on sulfate reduction was investigated. Sodium lactate, used in this study, is widely recognized as one of the preferred electron donors for SRB. Organic acid salts such as lactate can help maintain a near-neutral pH, which is favorable for SRB metabolism, whereas sugar-based carbon sources tend to acidify the medium and inhibit SRB activity [19].
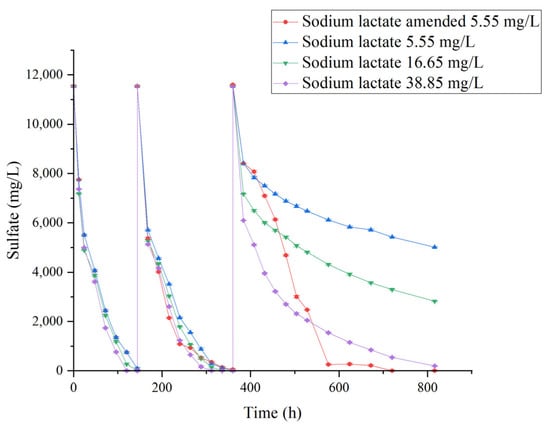
Figure 8.
Sulfate reduction by SRB at 35 °C and pH 7.8 under different carbon source conditions (initial sulfate concentration 11,760 mg/L). After complete removal, sulfate was replenished to the initial concentration. “Sodium lactate amended” indicates that sodium lactate was added to 5.55 mg/L after the second cycle.
Within a short-term period (≤150 h), SRB exhibited similar sulfate reduction efficiencies across all carbon source groups, achieving complete sulfate removal during the first cycle. When sulfate was replenished to the initial concentration for a second cycle, no significant differences were observed among the groups. However, during the third reduction cycle, the carbon source concentration had a pronounced effect on SRB performance, with higher carbon availability enhancing reduction efficiency (Figure 8). At the end of the third cycle, sulfate removal rates were 90%, 70%, and 50% for the high-, medium-, and low-carbon source groups, respectively. In contrast, after completing the first two cycles, replenishing the 1× carbon source group to the initial concentration of 5.5 mg/L restored SRB reduction capacity, enabling sulfate to be lowered to 250 mg/L within 200 h. These results indicate that sufficient carbon supply is a critical factor for maintaining high metabolic activity. In lactate-containing environments, microbial proliferation and sulfate reduction proceeded most rapidly, while a near-neutral pH was maintained, favoring SRB metabolism. Although sugar-based carbon sources can initially promote bacterial growth, they tend to acidify the medium, inhibiting SRB activity [16].
3.4. Treatment of Real AMD Wastewater by SRB
Based on the investigation of reaction-influencing factors, the sulfate reduction performance of SRB was further evaluated using three actual AMD samples (labeled L, 20, and 6-B), as shown in Figure 9. After 25 h of treatment, all three samples exhibited sulfate removal rates exceeding 50%, demonstrating strong initial reduction activity. As the reaction progressed, SRB ultimately achieved complete sulfate removal in all samples. The sample with the lowest initial concentration (20) reached full reduction within 70 h, while samples L and 6-B required 120 h and 145 h, respectively. These results indicate that SRB can efficiently and completely remove sulfate from actual AMD of varying concentrations, and the duration of the reduction process is positively correlated with the initial sulfate load. The lower the initial sulfate concentration, the shorter the time required for SRB to achieve complete reduction [14].
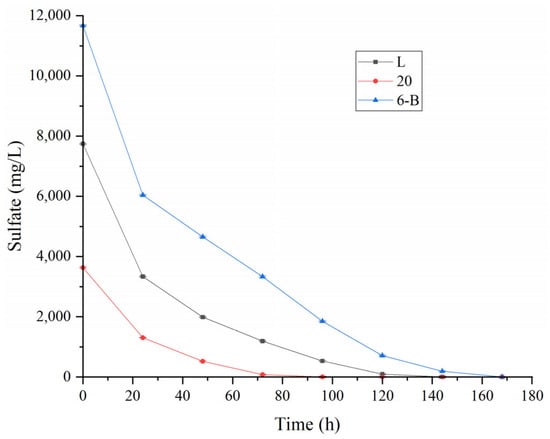
Figure 9.
Sulfate removal by SRB from actual AMD samples L, 20, and 6-B.
3.5. SRB Combined with Sediment for Treatment of Actual AMD
Considering the adaptability of laboratory strains under extreme conditions in actual AMD—polluted environments, this study investigated the sulfate-reducing performance of a combined system of lab-cultured SRB and in situ sediment. Sediment from the Miaogou outflow site was mixed with the laboratory SRB at a 1:1 ratio, and different treatment groups were applied to actual AMD with an initial sulfate concentration of 5500 mg/L. The results indicated that pure SRB, sediment alone, and the SRB—sediment combination all exhibited some sulfate-reducing capacity, but significant differences were observed among the groups (Figure 10). Both the pure SRB group and the SRB—sediment reinforced group achieved complete sulfate removal within 150 h, with higher removal rates and final removal efficiencies than the sediment-only group, which reached a maximum removal of 63% without further progress.
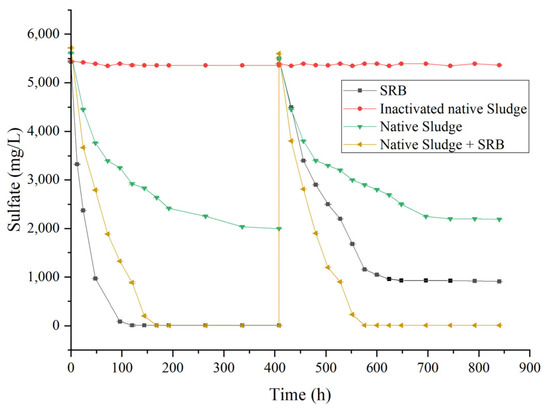
Figure 10.
Sulfate reduction efficiency of different microbial treatment groups under the cultivation conditions of 35 °C, pH 7.8, and an initial sulfate concentration of 5500 mg/L. Once the sulfate concentration in each group stabilized, it was replenished to the initial level. Abbreviations: SRB = pure sulfate-reducing bacteria; Native sludge = sediment alone; Native sludge + SRB = SRB-sediment combination; Inactivated native sludge = high-temperature sterilized control group.
The pure SRB and native microbial community demonstrated efficient sulfate reduction, and in the SRB-augmented sediment group, the treatment performance remained reproducible over two consecutive cycles when sulfate was replenished to the initial concentration, indicating stable and sustained activity. In contrast, the pure SRB group showed high removal rates in the first cycle, which declined in the second cycle, with a maximum removal of only 80%, likely due to carbon source depletion. The sustained and efficient reduction in the bio-reinforced system is likely attributable to functional complementarity and synergy between the laboratory strain and the native microbial community: while the laboratory SRB exhibit high metabolic activity, their adaptability and stability under actual environmental conditions may be limited, whereas the indigenous microbes possess strong resilience to environmental stress [15]. The combined system retains the ecological stability of native microbes while introducing the metabolic advantages of highly active strains, enabling rapid and continuous sulfate reduction. Furthermore, sediment contains diverse organic compounds [32] and associated heterotrophic microorganisms such as Pseudomonas and Clostridium, which can convert organic matter into organic acids, supplying carbon sources for functional microbes and sustaining system operation.
Under conditions of abundant influent, sufficient carbon supply can be maintained. No significant sulfate removal was observed in the high-temperature-sterilized control, indicating that abiotic processes (e.g., adsorption, precipitation) contributed negligibly to sulfate removal, and the reduction was primarily driven by microbial activity. The “functional strain–native community” strategy has considerable potential in many bioremediation applications, as exogenous high-efficiency strains can enhance the activity of typically low-activity native microbes in complex polluted environments [2,44].
3.6. Techno-Economical Perspective
Compared to conventional AMD remediation technologies, “SRB pre-cultivation + sediment enhancement” strategy has notable cost advantages—relying on endogenous organic carbon from on-site sediment and small doses of low-cost sodium lactate to avoid expensive exogenous carbon sources; pre-cultivation uses simple anaerobic reactors with on-site sediment, eliminating transportation costs for external amendments; and it simultaneously achieves sulfate removal (>97%, Figure 10), metal immobilization (Fe/Mn > 90%, Figure 5), skipping separate post-treatment processes. This confirms the strategy’s techno-economical competitiveness for practical use.
4. Conclusions
This study developed and validated two strategies—“SRB pre-cultivation” and “sediment enhancement”—to tackle the central challenge of inhibited SRB activity in actual acid mine drainage (AMD) environments. Key findings and implications are summarized below:
Firstly, SRB pre-cultivation substantially improves acid tolerance and high-sulfate adaptability, thereby enhancing sulfate reduction performance. Under controlled conditions, pre-cultivated SRB extended the effective AMD treatment pH range from 6.8–8.8 (for non-pre-cultivated SRB) to 4.8–8.8. It achieved 50% removal of 11,760 mg/L sulfate within 24 h and complete removal within 150 h at pH 7.8.
Secondly, SRB-reinforced sediment boosts system stability and the capacity for multi-pollutant removal. Relying on indigenous microbes in the sediment to convert endogenous organic matter into carbon sources, this synergistic system reduces dependence on exogenous carbon. It achieves simultaneous sulfate removal (>97%) and Fe/Mn coprecipitation (>90%) in real acid mine drainage, surpassing the performance of pure SRB systems.
From a practical standpoint, the combined strategy demonstrates significant techno-economic competitiveness, serving as a low-cost, field-applicable solution for AMD bioremediation. However, limitations persist: the system is inhibited at pH < 4.8, and pre-cultivation involves an 18–48 h lag phase. Future research will concentrate on screening acid-resistant SRB strains to broaden pH adaptability and on developing slow-release carbon carriers to extend long-term operation.
Overall, this study confirms the efficacy of the SRB-reinforced sediment remediation approach, providing theoretical and technical support for optimizing AMD bioremediation and its industrial application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17223308/s1, Table S1. Genes with significant differential expression in the presence of pyrite.
Author Contributions
Conceptualization, L.Z. and B.L.; methodology, L.Z. and C.Y.; validation, L.Z., H.Z. and W.L.; formal analysis, H.Z., Y.W. and C.Y.; investigation, W.L.; resources, H.H., C.C. and C.Y.; data curation, W.L., H.H. and C.C.; writing—original draft preparation, L.Z. and B.L.; writing—review and editing, B.L., T.Q., X.L., H.Z. and Y.W.; visualization, T.Q., X.L. and Y.W.; supervision, T.Q. and B.L.; project administration, T.Q. and X.L.; funding acquisition, Y.W., H.H. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by National Key R&D Program of China (No. 2023YFC3710005), Shanxi Center of Technology Innovation for Mining Groundwater Pollution Prevention and Remediation in Karst Area, the Fundamental Research Program of Shanxi Province (No. 20210302123119), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0816) and Horizontal Scientific Research Funds of Taiyuan University of Technology (RH2500002125).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Lei Zhang was employed by the company Shanxi Low-carbon Environmental Protection Industry Group Co., Ltd.; Author Yue Wang was employed by the company Shanxi Transition Comprehensive Reform Demonstration Zone Construction Development Co., Ltd., Author Wenjun Li was employed by the company Shanxi Transportation Holding Ecological Environment Co., Ltd.; Author Hucheng Huang and Cong Cao were employed by the company Shanxi Institute of Geological Survey Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmad, M.; Ling, J.; Yin, J.; Chen, L.; Yang, Q.; Zhou, W.; Zhang, Y.; Huang, X.; Khan, I.; Dong, J. Evaluation of the Different Nutritional and Environmental Parameters on Microbial Pyrene Degradation by Mangrove Culturable Bacteria. Int. J. Mol. Sci. 2023, 24, 8282. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Rodrigues, V.G.S. A Systematic Literature Review of Treatment Approaches with Sulfate-Reducing Bacteria for Acid Mine Drainage. Water Air Soil Pollut. 2025, 236, 271. [Google Scholar] [CrossRef]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Isa, Y.M. Bioremediation of acid mine drainage—Review. Alex. Eng. J. 2023, 65, 1047–1075. [Google Scholar] [CrossRef]
- Anekwe, I.M.; Isa, Y.M. Wastewater and Bioventing Treatment Systems for Acid Mine Drainage–Contaminated Soil. Soil Sediment Contam. Int. J. 2021, 30, 518–531. [Google Scholar] [CrossRef]
- Sulonen, M.L.K.; Baeza, J.A.; Gabriel, D.; Guisasola, A. Optimisation of the operational parameters for a comprehensive bioelectrochemical treatment of acid mine drainage. J. Hazard. Mater. 2021, 409, 124944. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Wei, Y.; Lin, W.; Yi, X.; Lu, G.; Dang, Z. Cosolubilization synergism occurrence in codesorption of PAH mixtures during surfactant-enhanced remediation of contaminated soil. Chemosphere 2016, 144, 583–590. [Google Scholar] [CrossRef]
- Aghaei, E.; Wang, Z.; Tadesse, B.; Tabelin, C.B.; Quadir, Z.; Alorro, R.D. Performance Evaluation of Fe-Al Bimetallic Particles for the Removal of Potentially Toxic Elements from Combined Acid Mine Drainage-Effluents from Refractory Gold Ore Processing. Minerals 2021, 11, 590. [Google Scholar] [CrossRef]
- Ryu, S.; Naidu, G.; Hasan Johir, M.A.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid mine drainage treatment by integrated submerged membrane distillation–sorption system. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef]
- Patel, A.; Enman, J.; Gulkova, A.; Guntoro, P.I.; Dutkiewicz, A.; Ghorbani, Y.; Rova, U.; Christakopoulos, P.; Matsakas, L. Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. Chemosphere 2021, 263, 128306. [Google Scholar] [CrossRef] [PubMed]
- Mafane, D.; Ngulube, T.; Mphahlele-Makgwane, M.M. Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions. Sustainability 2025, 17, 3567. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Z.; Chen, Y.; Tang, J.; Zeng, L.; Peng, C.; Chen, L.; Wang, J. Unlocking soil revival: The role of sulfate-reducing bacteria in mitigating heavy metal contamination. Environ. Geochem. Health 2024, 46, 417. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Verma, A.K. Bioaugmentation: An approach to biological treatment of pollutants. Biodegradation 2024, 35, 117–135. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Liu, T.; Feng, C. Effects of adding different carbon sources on the microbial behavior of sulfate-reducing bacteria in sulfate-containing wastewater. J. Clean. Prod. 2023, 392, 136332. [Google Scholar] [CrossRef]
- Kuang, X.; Peng, L.; Chen, S.; Peng, C.; Song, H. Immobilization of metal(loid)s from acid mine drainage by biological soil crusts through biomineralization. J. Hazard. Mater. 2023, 443, 130314. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Kovářová, A.; Dordevic, D.; Gaine, J.; Kollar, P.; Vítězová, M.; Rittmann, S.K.-M.R. Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application. Processes 2021, 9, 1843. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Gao, Z.; Di, J.; Wang, D.; Guo, X.; Hu, Z.; Gao, X.; Wang, Y. Study on Growth Influencing Factors and Desulfurization Performance of Sulfate Reducing Bacteria Based on the Response Surface Methodology. ACS Omega 2023, 8, 4046–4059. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Wang, R.; Yang, L.; Cao, Y.; Wang, H. A novel approach for treating acid mine drainage by forming schwertmannite driven by a combination of biooxidation and electroreduction before lime neutralization. Water Res. 2022, 221, 118748. [Google Scholar] [CrossRef]
- Brar, K.K.; Etteieb, S.; Magdouli, S.; Calugaru, L.; Brar, S.K. Novel approach for the management of acid mine drainage (AMD) for the recovery of heavy metals along with lipid production by Chlorella vulgaris. J. Environ. Manag. 2022, 308, 114507. [Google Scholar] [CrossRef]
- Bouchez, M.; Blanchet, D.; Vandecasteele, J.P. Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: Inhibition phenomena and cometabolism. Appl. Microbiol. Biotechnol. 1995, 43, 156–164. [Google Scholar] [CrossRef]
- González, D.; Liu, Y.; Villa Gomez, D.; Southam, G.; Hedrich, S.; Galleguillos, P.; Colipai, C.; Nancucheo, I. Performance of a sulfidogenic bioreactor inoculated with indigenous acidic communities for treating an extremely acidic mine water. Miner. Eng. 2019, 131, 370–375. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, H.L.; Nguyen, M.H.; Nguyen, T.K.N.; Dinh, H.T. Sulfate Reduction for Bioremediation of AMD Facilitated by an Indigenous Acidand Metal-Tolerant Sulfate-Reducer. J. Microbiol. Biotechnol. 2020, 30, 1005–1012. [Google Scholar] [CrossRef]
- de Matos, L.P.; Costa, P.F.; Moreira, M.; Gomes, P.C.S.; de Queiroz Silva, S.; Gurgel, L.V.A.; Teixeira, M.C. Simultaneous removal of sulfate and arsenic using immobilized non-traditional SRB mixed culture and alternative low-cost carbon sources. Chem. Eng. J. 2018, 334, 1630–1641. [Google Scholar] [CrossRef]
- Wu, Z.; Firmin, K.A.; Cheng, M.; Wu, H.; Si, Y. Biochar enhanced Cd and Pb immobilization by sulfate-reducing bacterium isolated from acid mine drainage environment. J. Clean. Prod. 2022, 366, 132823. [Google Scholar] [CrossRef]
- Li, J.; Tabassum, S. Synergism of hydrolytic acidification and sulfate reducing bacteria for acid production and desulfurization in the anaerobic baffled reactor: High sulfate sewage wastewater treatment. Chem. Eng. J. 2022, 444, 136611. [Google Scholar] [CrossRef]
- Mukwevho, M.J.; Maharajh, D.; Chirwa, E.M.N. Evaluating the Effect of pH, Temperature, and Hydraulic Retention Time on Biological Sulphate Reduction Using Response Surface Methodology. Water 2020, 12, 2662. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, Q.; Chen, Y.; An, H.; Zhang, L.; Wu, Z.; Xiao, E. Nitrate reduction pathway of iron sulphides based MFC-CWs purifying low C/N wastewater: Competitive mechanism to inorganic and organic electrons. Chem. Eng. J. 2024, 479, 147379. [Google Scholar] [CrossRef]
- Alazard, D.; Joseph, M.; Battaglia-Brunet, F.; Cayol, J.-L.; Ollivier, B. Desulfosporosinus acidiphilus sp. nov.: A moderately acidophilic sulfate-reducing bacterium isolated from acid mining drainage sediments. Extremophiles 2010, 14, 305–312. [Google Scholar] [CrossRef]
- Colipai, C.; Southam, G.; Oyarzún, P.; González, D.; Díaz, V.; Contreras, B.; Nancucheo, I. Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor. Minerals 2018, 8, 35. [Google Scholar] [CrossRef]
- She, Z.; Wang, J.; He, C.; Jiang, Z.; Pan, X.; Wang, M.; Ma, D.; Shi, Q.; Yue, Z. Molecular insights into the impacts of acid mine drainage on dissolved organic matter dynamics in pit lakes. Sci. Total Environ. 2023, 888, 164097. [Google Scholar] [CrossRef]
- Li, Y.P.; Tian, Y.X.; Hao, Z.D.; Ma, Y.L. Transcriptome analysis of the degradation characteristics of fluoranthene by Pseudomonas aeruginosa DN1. Microbiol. Bull. 2020, 47, 54–65. [Google Scholar]
- Chen, Z.; Hu, H.; Xu, P.; Tang, H. Soil bioremediation by Pseudomonas brassicacearum MPDS and its enzyme involved in degrading PAHs. Sci. Total Environ. 2022, 813, 152522. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Z.; Yang, C.; Ma, C.; Tao, F.; Xu, P. Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresour. Technol. 2011, 102, 4111–4116. [Google Scholar] [CrossRef]
- Holanda, R.; Hedrich, S.; Ňancucheo, I.; Oliveira, G.; Grail, B.M.; Johnson, D.B. Isolation and characterisation of mineral-oxidising “Acidibacillus” spp. from mine sites and geothermal environments in different global locations. Res. Microbiol. 2016, 167, 613–623. [Google Scholar] [CrossRef]
- Bourguignon, N.; Irazusta, V.; Isaac, P.; Estévez, C.; Maizel, D.; Ferrero, M.A. Identification of proteins induced by polycyclic aromatic hydrocarbon and proposal of the phenanthrene catabolic pathway in Amycolatopsis tucumanensis DSM 45259. Ecotoxicol. Environ. Saf. 2019, 175, 19–28. [Google Scholar] [CrossRef]
- Pernicova, I.; Novackova, I.; Sedlacek, P.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers-1. Isolation and Characterization of the Bacterium. Polymers 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-García, A.; Torres, M.J.; Salas, A.; Bedmar, E.J.; Girard, L.; Delgado, M.J. Rhizobium etli Produces Nitrous Oxide by Coupling the Assimilatory and Denitrification Pathways. Front. Microbiol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Kang, J.-X.; Wang, Y.-Y. The role of Acidithiobacillus ferrooxidans in Fe(II) oxidation of pyrite in bioleaching processes. J. Chem. Technol. Biotechnol. 2022, 97, 2013–2023. [Google Scholar] [CrossRef]
- Janyasuthiwong, S.; Rene Eldon, R.; Esposito, G.; Lens Piet, N.L. Effect of pH on the Performance of Sulfate and Thiosulfate-Fed Sulfate Reducing Inverse Fluidized Bed Reactors. J. Environ. Eng. 2016, 142, C4015012. [Google Scholar] [CrossRef]
- Hao, T.-W.; Xiang, P.-Y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.-K.; van Loosdrecht, M.C.M.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Lam, P.K.-S. (Eds.) Global Contamination Trends of Persistent Organic Chemicals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Y.; Zhang, Z.; Tang, Y.; Su, P.; Lin, Z. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 2022, 376, 134109. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Chen, Y. Advances in heavy metal removal by sulfate-reducing bacteria. Water Sci. Technol. 2020, 81, 1797–1827. [Google Scholar] [CrossRef]
- Zhuang, Z.; Xia, X.; Yang, G.; Zhuang, L. The Role of Exopolysaccharides in Direct Interspecies Electron Transfer. Front. Microbiol. 2022, 13, 927246. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Li, M.-M.; Chen, T.-H.; Zhou, Y.-F.; Yue, Z.-B. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria. Bioresour. Technol. 2014, 163, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Piva, A.; Fortin, D. Enrichment of sulfate-reducing bacteria and resulting mineral formation in media mimicking pore water metal ion concentrations and pH conditions of acidic pit lakes. FEMS Microbiol. Ecol. 2012, 79, 69–84. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Mu, S.; Zhang, D.; Pan, X.; Lee, D.-J.; Chang, J.-S. Removal of antimony (Sb(V)) from Sb mine drainage: Biological sulfate reduction and sulfide oxidation—Precipitation. Bioresour. Technol. 2013, 146, 799–802. [Google Scholar] [CrossRef]
- Basu, A.; Apte, S.K.; Phale, P.S. Preferential utilization of aromatic compounds over glucose by Pseudomonas putida CSV86. Appl. Environ. Microbiol. 2006, 72, 2226–2230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).