Adsorption Ability of Soft Magnetic FeCo Alloys for Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Materials Preparation Methods
2.3. Material Characterization and Analysis
2.4. Adsorption of FeCo on PE-MPs
3. Results and Discussion
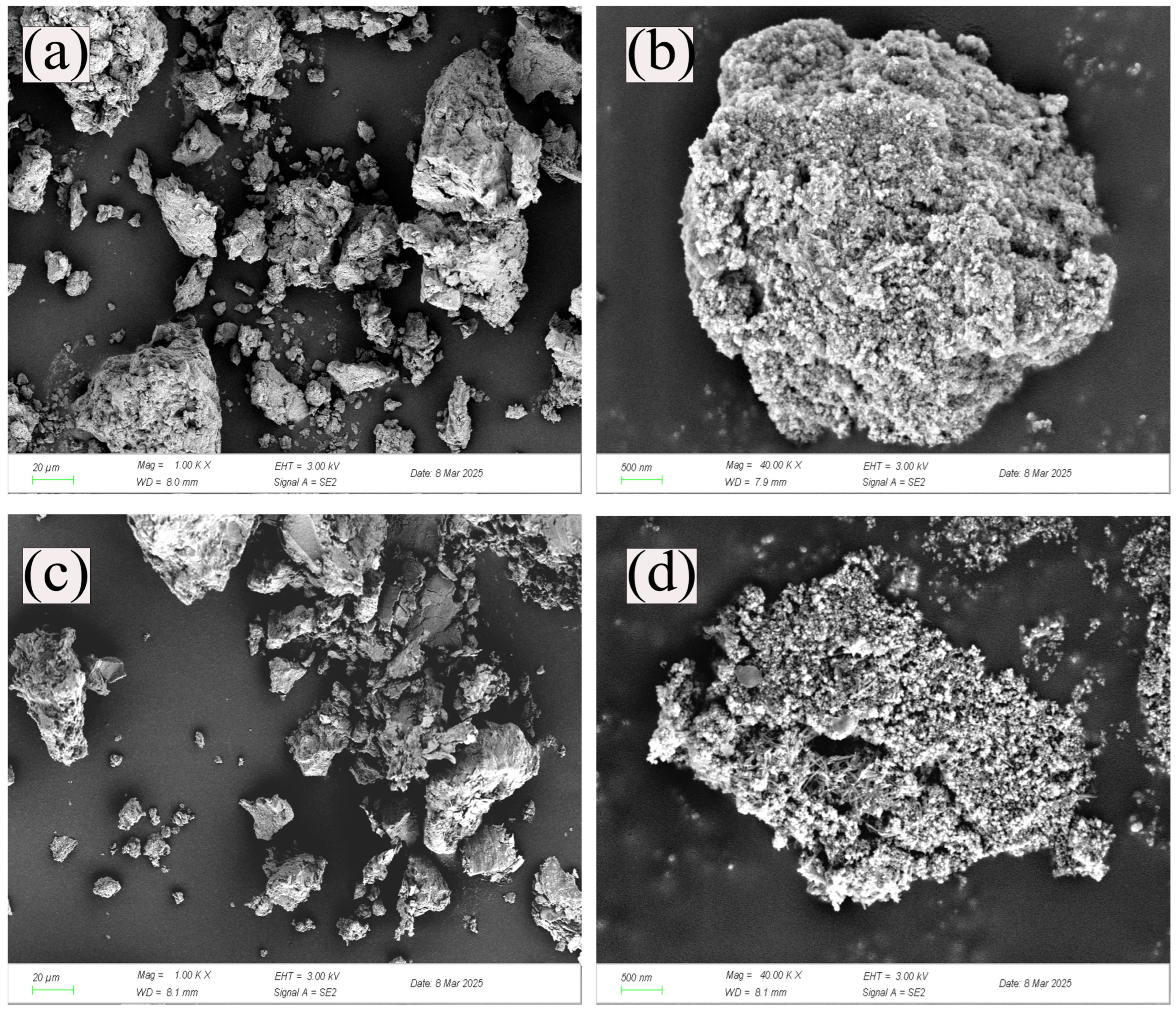
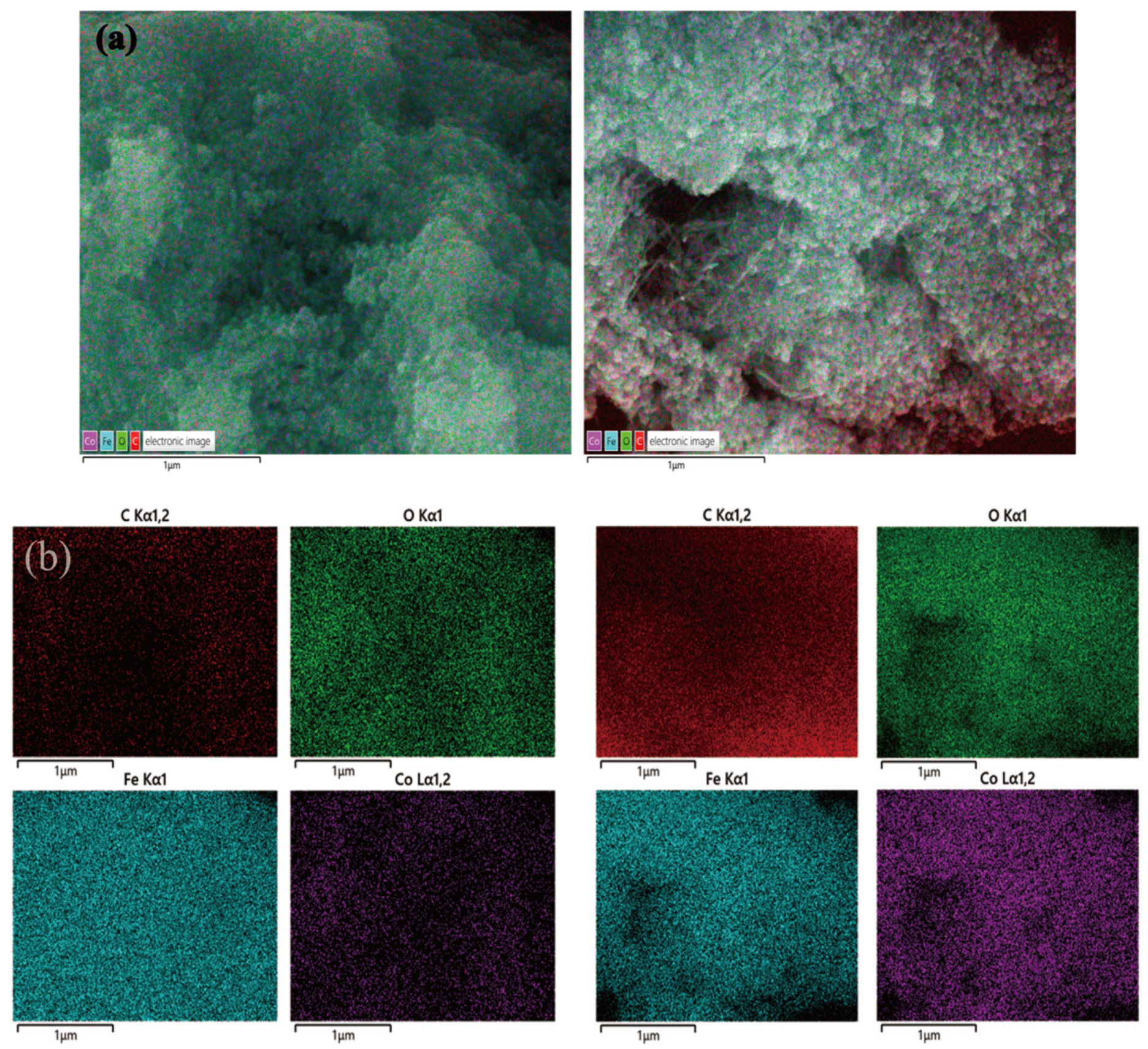
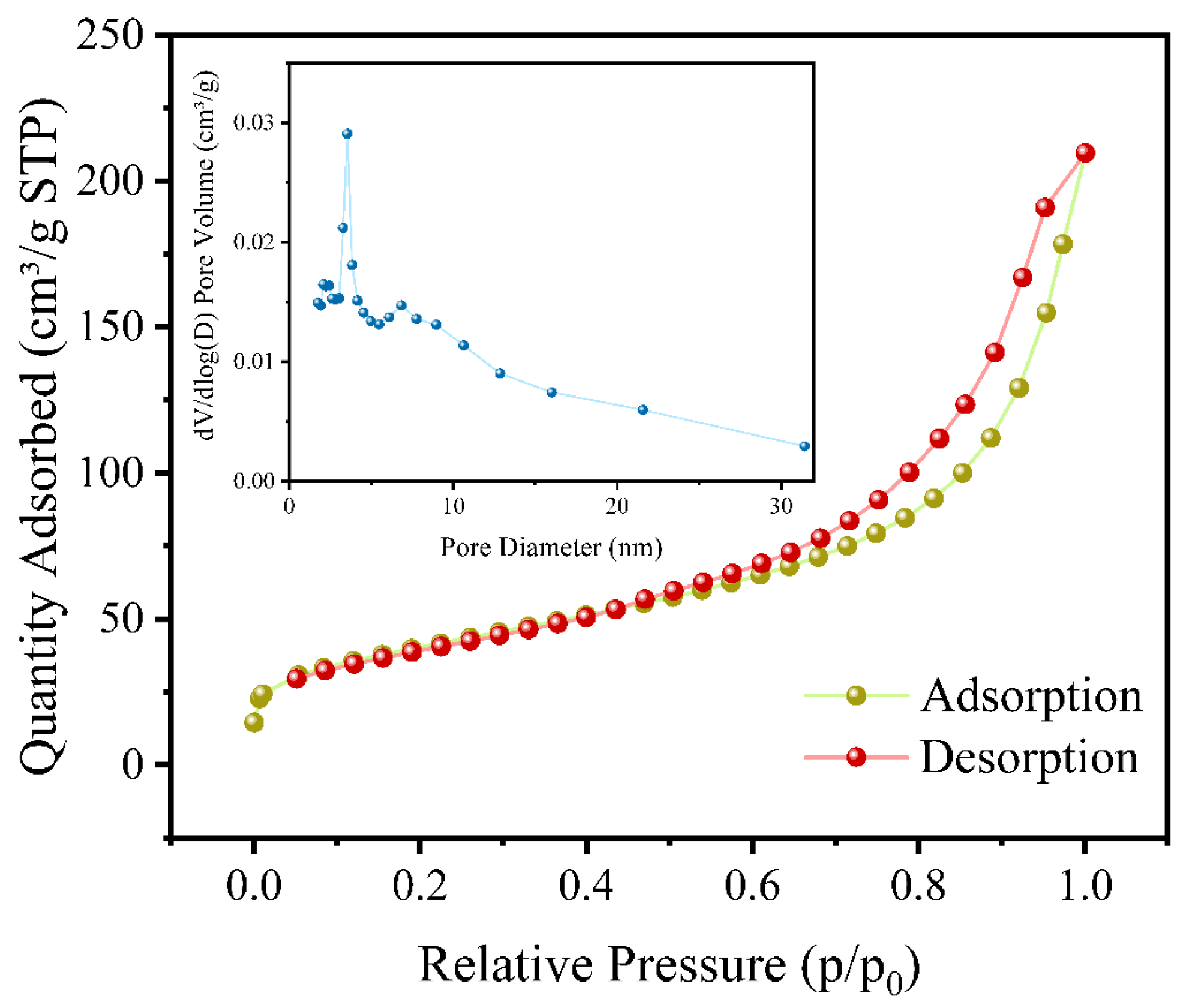
3.1. Characterization Analysis of Adsorption Materials
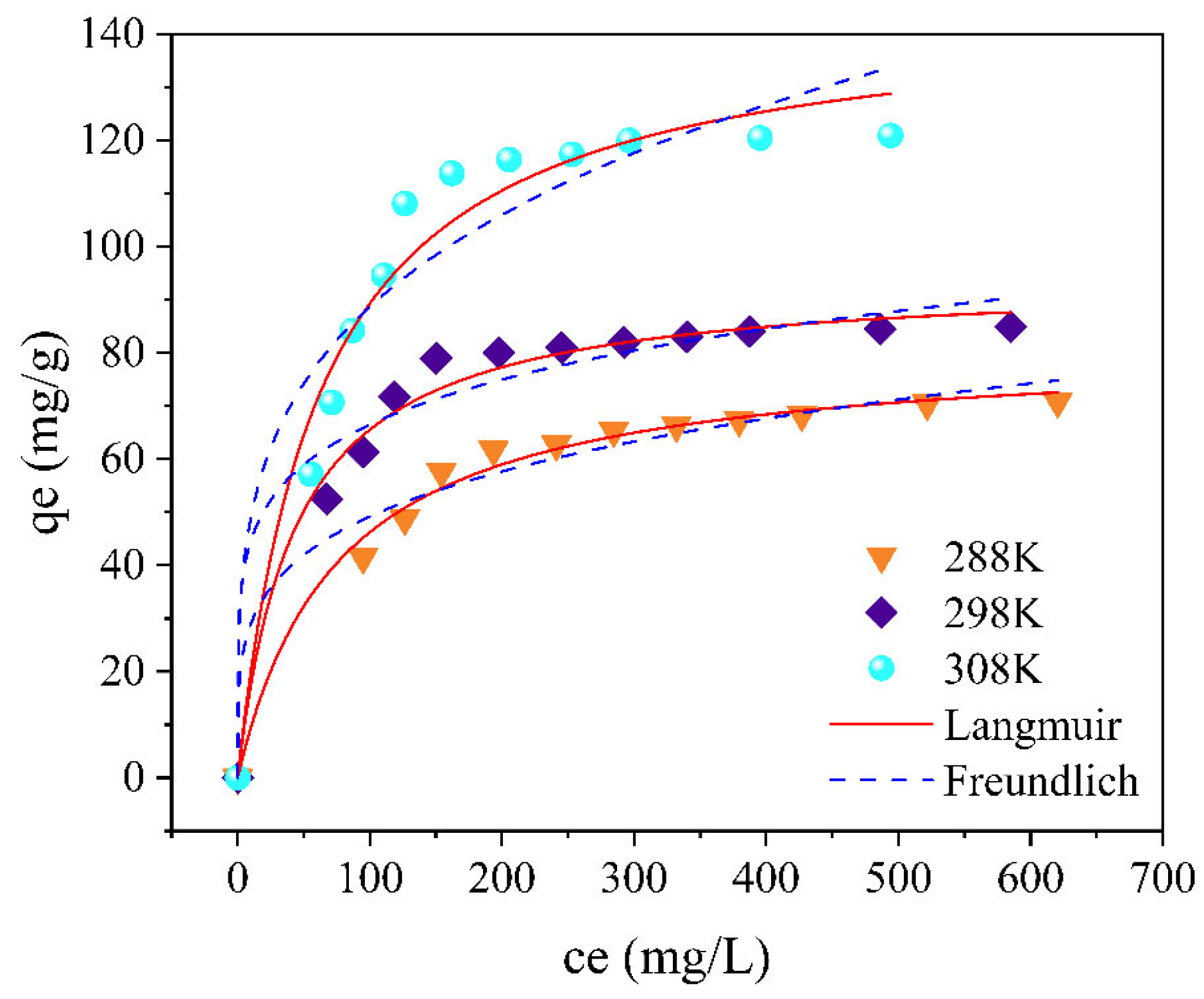
3.2. Isothermal Adsorption Experiment
3.3. Adsorption Kinetics Experiment
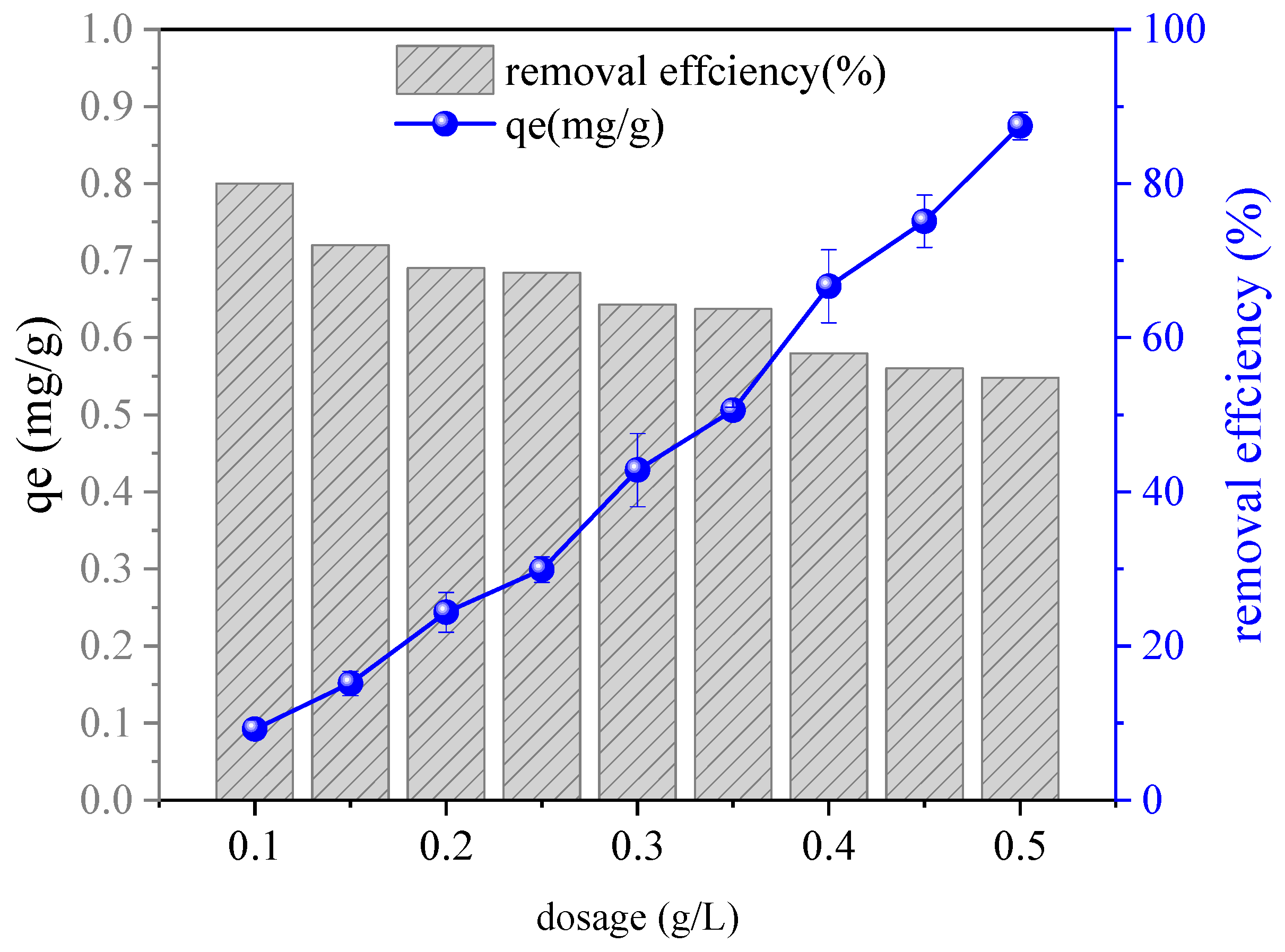
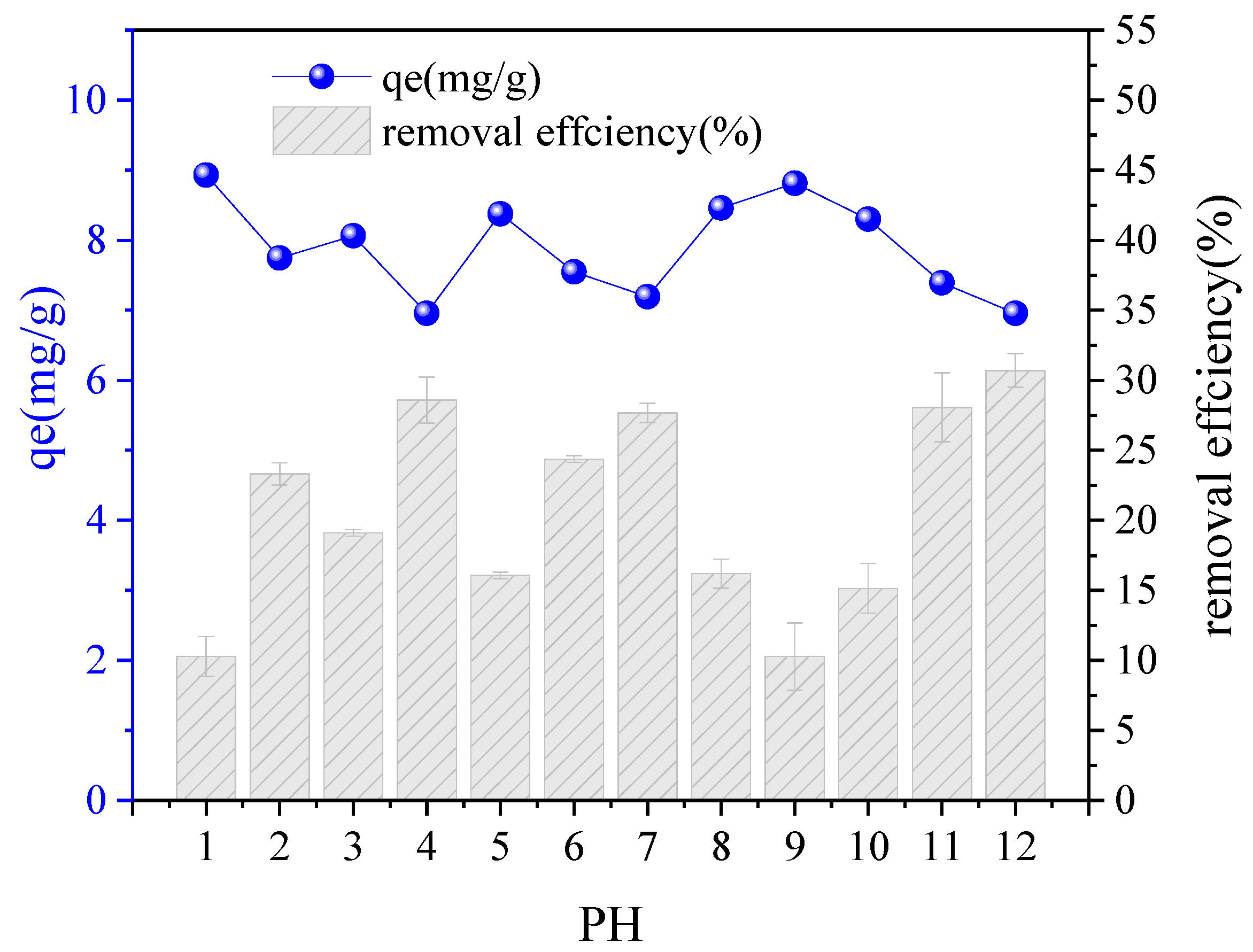
3.4. Study on the Adsorption Behaviors Observed Under Different Driving Factors
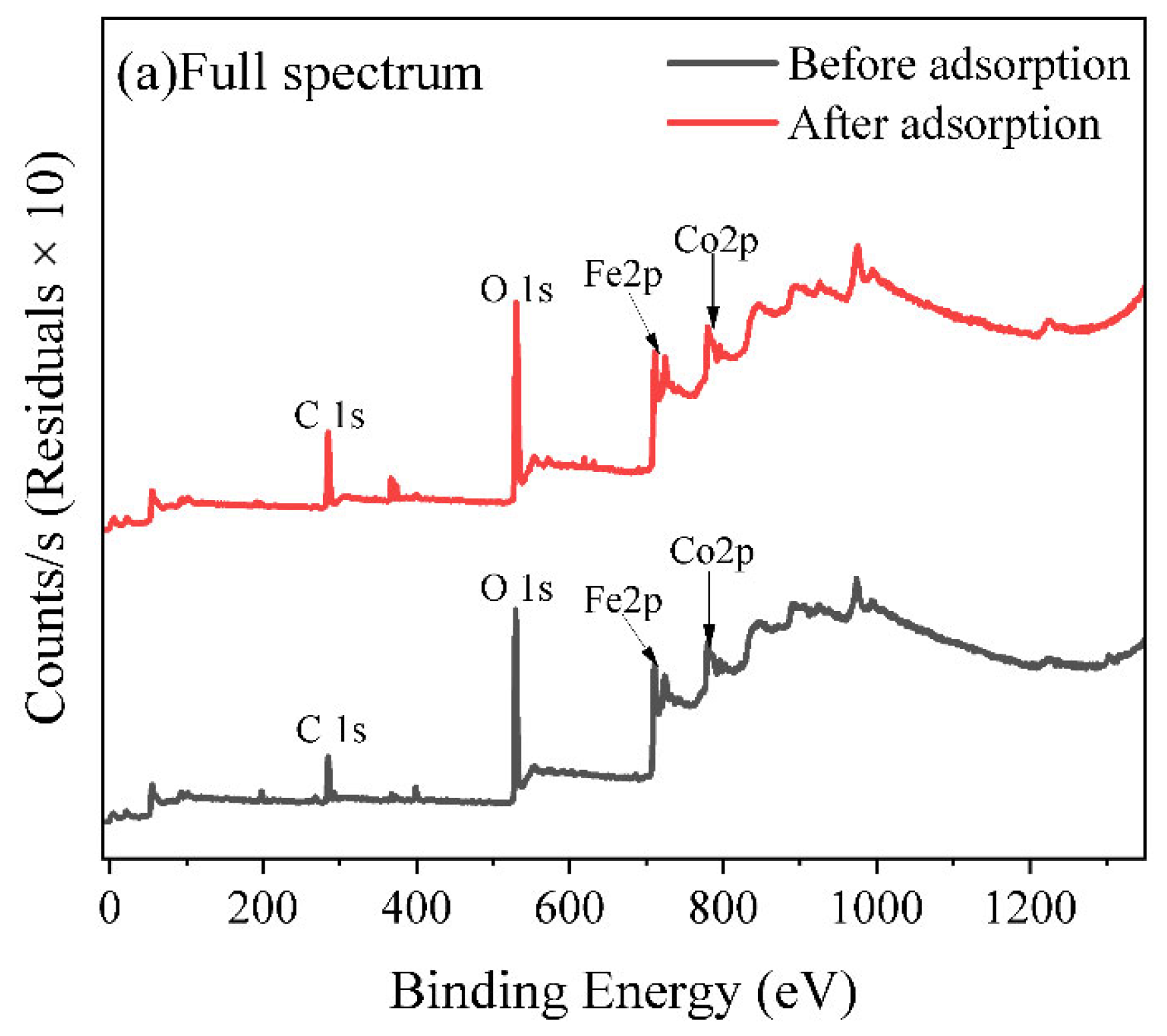
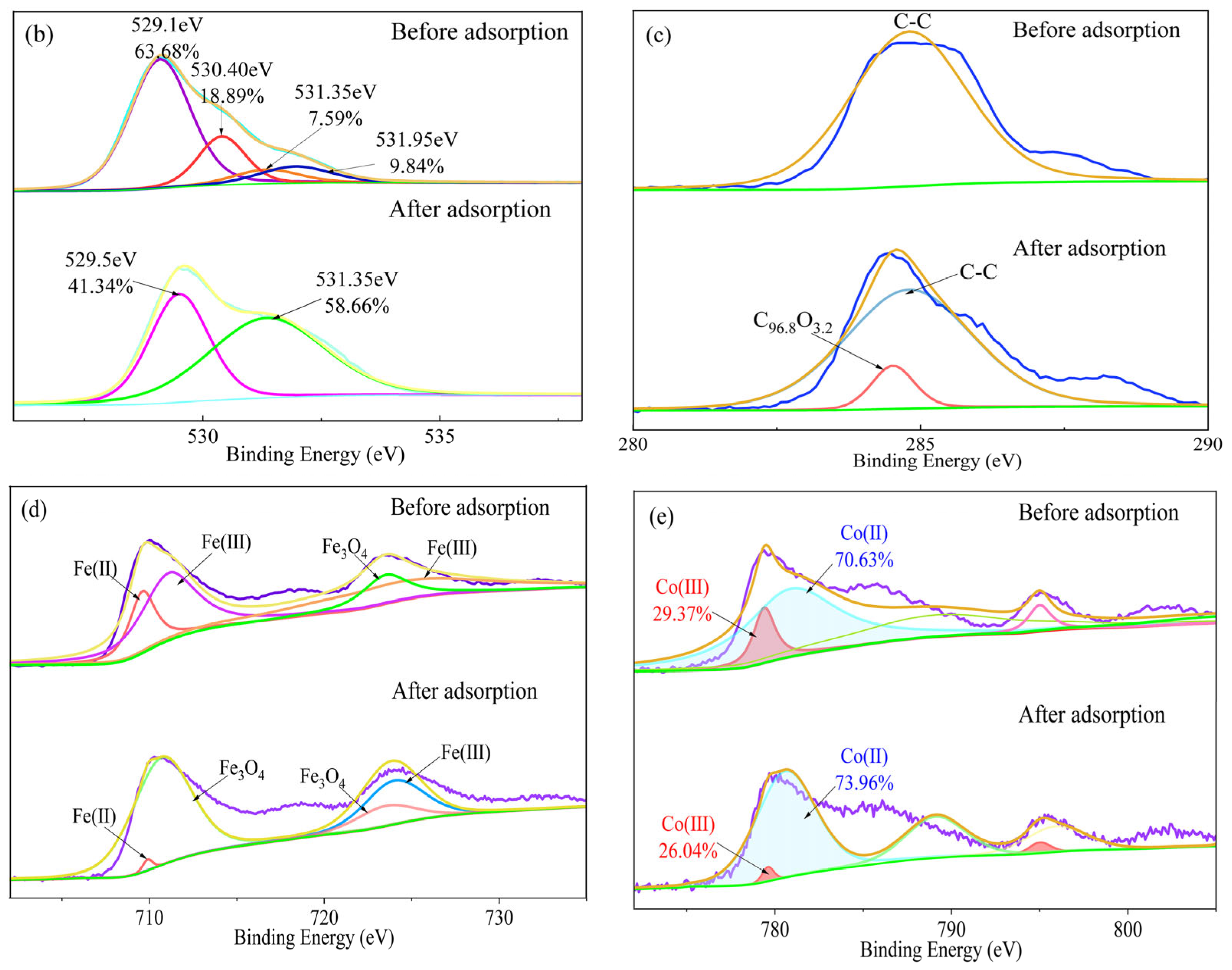
3.5. Adsorption Mechanism Analysis
4. Discussion
4.1. The Impact of Material Site Substitution Behaviors on Adsorption Performance
4.2. Adsorption Kinetics and Thermodynamic Processes
4.3. Study on the Adsorption Mechanism and an Analysis of the Driving Factors
5. Conclusions
- (1)
- The present study employs the coprecipitation method to integrate cobalt and iron into porous, high-surface-area CMC precursors at a Co:Fe ratio of 7:3, thereby constructing high-quality soft magnetic FeCo materials with iron–cobalt alloys. SEM and XRD characterizations reveal that the prepared FeCo sample exhibits the characteristic peaks of soft magnetic iron–cobalt alloy materials and a typical solid solution structure. Its abundant pore structure is conducive to the surface and pore adsorption of polyethylene microplastics.
- (2)
- The quasisecond-order kinetic fitting model can partially describe the adsorption kinetic process of FeCo on PE-MPs. The adsorption behavior of PS-MPs on the adsorbent conforms to the Freundlich isotherm model. The adsorption of FeCo on PE-MPS is a chemical adsorption process, with a maximum adsorption capacity of 120.92 mg/g for FeCo on PE-MPs at 45 °C. The influence of the pH on the adsorption of FeCo is relatively small.
- (3)
- The adsorption process of FeCo on PE-MPs involves the formation of hydrogen bonds, the coordination complexation of metal with hydroxyl groups, and hydrophobic interactions. The site substitution of cobalt for iron changes the structure and electronic properties of the material, enhancing the adsorption effect of the material on PE-MPs, and the formation of a solid alloy solution stabilizes the material structure. In summary, the process of preparing FeCo materials is simple and cost-effective and is capable of achieving efficient PE-MP adsorption; thus, the toxicity of PE-MPs in water can be effectively controlled, and these materials have good application prospects in water treatment and pollution remediation scenarios.
6. Environmental Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.-H.; Maheshwaran, S.; Park, Y.-K.; Ong, H.C. Iron-based electrode material composites for electrochemical sensor application in the environment: A review. Sci. Total Environ. 2024, 953, 176128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dhiware, P.; Laddha, H.; Janu, V.C.; Gupta, R. Harnessing magnetically separable iron based adsorbents for enhanced uranium adsorption. Coord. Chem. Rev. 2024, 508, 215766. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Y.; Li, H.; Wu, R.; Ju, J.; Liu, S.; Yang, Y.; Wang, B. Adsorption of heavy metal ions by iron tailings: Behavior, mechanism, evaluation and new perspectives. J. Clean. Prod. 2022, 344, 131065. [Google Scholar] [CrossRef]
- Lei, Q.; Chen, W.; Zhou, K.; Yang, Q.; Zhou, H.; Zhang, M.; Peng, Y.; He, D. Modification and preparation of iron salt-silica aerogel composite material for effective arsenic adsorption in wastewater: Mechanisms and application potential. J. Water Process Eng. 2025, 79, 108899. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus immobilization in water and sediment using iron-based materials: A review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, Q.; Qiu, Y.; Chen, Y.; Lin, Z.; Yang, X. Iron-based metal–organic frameworks for rapid and effective phosphorus removal from eutrophic lake water. Appl. Organomet. Chem. 2024, 38, e7606. [Google Scholar] [CrossRef]
- Wan, J.; Gao, R.; Feng, X. Insights into the relationship between Nitrilotri (methylphosphonic acid) (NTMP) adsorption and physicochemical properties of iron oxides. Process Saf. Environ. Prot. 2024, 192, 750–759. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Qian, C.; Ye, Z.; Yu, F.; Yao, Y. Synergistic adsorption and degradation of CBZ by an adenine-anchored porous iron-based catalyst. Sep. Purif. Technol. 2025, 376, 133949. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, M.; Li, J.; Zheng, Z. Influence of moderate pre-oxidation treatment on the physical, chemical and phosphate adsorption properties of iron-containing activated carbon. J. Environ. Sci. 2014, 26, 519–528. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.-H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing microplastics from aquatic environments: A critical review. Environ. Sci. Ecotechnol. 2023, 13, 100222. [Google Scholar] [CrossRef]
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2020, 19, 1229–1274. [Google Scholar] [CrossRef]
- Sun, B.; Xu, F.; Liu, F.; Wang, H.; Ding, C.; Cheng, Y.; Tang, Z.; Zhang, J.; Shen, W.; Zhu, S. Study on adsorption characteristics and regeneration effect of iron—Based alumina composites. E3S Web Conf. 2019, 136, 06030. [Google Scholar] [CrossRef]
- Feng, X.; Gu, X.; Xuan, G.; Wu, H.; Li, S. Efficient microplastic removal in aquatic environments using iron–nitrogen co-doped layered biocarbon materials. Chem. Eng. J. 2025, 506, 160095. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Zhou, X.; Yang, Y. Oxygen-vacancy-boosted visible light driven photocatalytic oxidative dehydrogenation of saturated N-heterocycles over Nb2O5 nanorods. Appl. Catal. B Environ. 2022, 316, 121622. [Google Scholar] [CrossRef]
- Siu, B.; Chowdhury, A.R.; Yan, Z.; Humphrey, S.M.; Hutter, T. Selective adsorption of volatile organic compounds in metal-organic frameworks (MOFs). Coord. Chem. Rev. 2023, 485, 215119. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Sun, C.; Li, H.; Sun, J.; Liu, X. Adsorption performance and kinetic study of hierarchical porous Fe-based MOFs for toluene removal. Sci. Total Environ. 2021, 793, 148622. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Wang, Y.; Zhao, W.; Gu, J. Study on the Adsorption Properties of Iron Tailings for GO. Coatings 2021, 11, 768. [Google Scholar] [CrossRef]
- Dong, W.; Liu, R.; Geng, L.; Xie, Z.; Wu, Y.; Sun, W. Inquire into Co-doped pyrite crystal structure modifications and implications for flotation behavior: A DFT study. Appl. Surf. Sci. 2024, 653, 159404. [Google Scholar] [CrossRef]
- Han, Y.; Li, Z.; Zhang, M.; Han, F.; Liu, Z.; Zhou, W. Isomorphic substitution of goethite by cobalt: Effects on the pathway and performance of peroxymonosulfate activation. Chem. Eng. J. 2022, 450, 138460. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yang, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Lu, X.; Xue, H.; Gong, H.; Bai, M.; Tang, D.; Ma, R.; Sasaki, T. 2D Layered Double Hydroxide Nanosheets and Their Derivatives Toward Efficient Oxygen Evolution Reaction. Nano-Micro Lett. 2020, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, H.; Peng, C.; Bu, L.; Chiang, C.; Tian, K.; Zhao, Y.; Zhao, J.; Lin, Y.-G.; Lee, J.-M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 21, 2002426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, X.; Liu, M.; Zhao, H.; Wang, Z.; Ni, R.; Wang, Y.; Yang, J.; Gao, F.; Li, Y.; et al. Composition-engineered FeCo nanoalloys with lattice expansion and optimized electron structure boosting electrocatalytic Nitrate reduction. Appl. Catal. B-Environ. Energy 2024, 355, 124205. [Google Scholar] [CrossRef]
- Gouamid, M.; Ouahrani, M.R.; Bensaci, M.B. Adsorption Equilibrium, Kinetics and Thermodynamics of Methylene Blue from Aqueous Solutions using Date Palm Leaves. Energy Procedia 2013, 36, 898–907. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Hou, W. Pseudo-Second-Order Kinetic Equation for Describing the Effect of Sorbent and Sorbate Concentrations. Langmuir 2024, 40, 3559–3568. [Google Scholar] [CrossRef]
- Henninger, M.; Postweiler, P.; Engelpracht, M.; Galvanin, F.; Bardow, A. Model-based design of experiments for adsorption isotherms. Adsorption 2025, 31, 103. [Google Scholar] [CrossRef]
- Chung, H.K.; Kim, W.H.; Park, J.; Cho, J.; Jeong, T.Y.; Park, P.J. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Lu, Z.; Ying, L.; Wang, X.; Zeng, Y.; Gao, Y.; Chen, Q.; Liu, P. Crystal Plane-Orientation Dependent Phase Evolution from Precursor to Porous Intermediate Phase in the Vapor Phase Dealloying of a Co-Zn Alloy. Acta Mater. 2022, 245, 118617. [Google Scholar] [CrossRef]
- Zhang, F.; Song, H. Effect of atomic size mismatch and chemical complexity on the local lattice distortion of BCC solid solution alloys. Mater. Today Commun. 2022, 33, 104367. [Google Scholar] [CrossRef]
- Santos, P.J.; Gabrys, P.A.; Zornberg, L.Z.; Lee, M.S.; Macfarlane, R.J. Macroscopic materials assembled from nanoparticle superlattices. Nature 2021, 591, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Bahuleyan, B. Homonuclear Bimetallic Fe (II) or Co (II) Complexes for Selective Oligomerization Process. Yanbu J. Eng. Sci. 2025, 22, 14–19. [Google Scholar] [CrossRef]
- Monson, P.A. Understanding adsorption/desorption hysteresis for fluids in mesoporous materials using simple molecular models and classical density functional theory. Micropor. Mesopor. Mat. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Stasiak, O.; Kordek-Khalil, K. Polymer-based porous carbon doped with iron nanoparticles for enhanced organic compounds removal. Adsorption 2024, 30, 279–291. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Dimitris, S. Argyropoulos. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]
- Ji, C.; Wu, D.; Lu, J.; Shan, C.; Ren, Y.; Li, T.; Lv, L.; Pan, B.; Zhang, W. Temperature regulated adsorption and desorption of heavy metals to A-MIL-121: Mechanisms and the role of exchangeable protons. Water Res. 2021, 189, 116599. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.S.; Bollinger, J.C.; Chao, H. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Wang, J.; Chen, T.; Ding, X.; Chen, H. Insight into surface hydroxyl groups for environmental purification: Characterizations, applications and advances. Surf. Interfaces 2021, 25, 101272. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Zhang, J. Insights into adsorption rate constants and rate laws of preset and arbitrary orders. Sep. Purif. Technol. 2021, 255, 117713. [Google Scholar] [CrossRef]
- Jayabrata, M.; Kumar, R.S. Competitive Removal of Cu(II) and Cd(II) from Water Using a Biocomposite Hydrogel. J. Phys. Chem. B 2017, 121, 10988–11001. [Google Scholar]
- Zhang, J.; Zhan, S.; Zhong, L.-B.; Wang, X.; Qiu, Z.; Zheng, Y.-M. Adsorption of typical natural organic matter on microplastics in aqueous solution: Kinetics, isotherm, influence factors and mechanism. J. Hazard. Mater. 2023, 443, 130130. [Google Scholar] [CrossRef]
- Xiong, W.; Qiu, X.; Zhong, R.; Yang, D. Characterization of the adsorption properties of a phosphorylated kraft lignin-based polymer at the solid/liquid interface by the QCM-D approach. Holzforschung 2016, 70, 937–945. [Google Scholar] [CrossRef]
- Tian, H.; Cui, K.; Sun, S.; Li, H.; Chen, X. Construction of acidic microenvironments to overcome the pH dependence of iron-based catalysts and application to the degradation of micropollutants by AOPs. Chem. Eng. J. 2024, 488, 150934. [Google Scholar] [CrossRef]
- Xie, K.; Xie, J.; Zhao, Z.; Meng, X.; Chen, B. Synthesis of iron-modified montmorillonite/Al2O3 composite adsorbents and their phosphorus adsorption performance study. Sci. Rep. 2025, 15, 34873. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Li, S.; Feng, L.; Wang, Z.; Wang, D.; Wanga, Q.; Wang, H. Corals-inspired magnetic absorbents for fast and efficient removal of microplastics in various water sources. RSC Adv. 2024, 14, 11908–11913. [Google Scholar] [CrossRef]
- Cao, Y.; Sathish, C.I.; Li, Z.; Ahmed, M.I. Plastics adsorption and removal by 2D ultrathin iron oxide nanodiscs: From micro to nano. Chem. Eng. J. 2024, 497, 154610. [Google Scholar] [CrossRef]
- Xia, H.; Duan, N.; Song, B.; Li, Y.; Xu, H.; Geng, Y.; Wang, X. Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration. Nanomaterials 2025, 15, 915. [Google Scholar] [CrossRef] [PubMed]
- Mcafee, L. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry; Part B: Application in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed.; Kazuo, N., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2016; p. 1122. [Google Scholar]
- Sheng, X.; Jiang, X.; Zhao, H.; Wan, D.; Liu, Y.; Ngwenya, C.A.; Du, L. FTIR study of hydrogen bonding interaction between fluorinated alcohol and unsaturated esters. Spectrochim Acta A 2018, 198, 239–247. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.; Liu, X.; Jiang, Q. Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates. Nat. Commun. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ratheesh, A.; Sreelekshmy, B.R.; Namitha, S.; Sasidharan, S.; Nair, K.S.; George, S.; Shibli, S.M.A. Regulation of extracellular electron transfer by sustained existence of Fe2+/Fe3+ redox couples on iron oxide-functionalized woody biochar anode surfaces in bioelectrochemical systems. Surf. Interfaces 2024, 54, 105114. [Google Scholar] [CrossRef]
- Vien, T.K.; Dormann, J.L.; Gall, H.L. Crystal Field Splitting in Octahedral and Tetrahedral Symmetry for Fe3+ Ions in Y3Fe5012. Phys. Status Solidi B 2010, 71, 731–739. [Google Scholar] [CrossRef]
- Wang, Y.; WANG, L.; Sun, C. The 2p3/2 binding energy shift of Fe surface and Fe nanoparticles. Chem. Phys. Lett. 2009, 480, 243–246. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Fernández, A.; Espinós, J.P.; González-Elipe, A.R. The state of the oxygen at the surface of polycrystalline cobalt oxide. J. Electron Spectrosc. 1995, 71, 61–71. [Google Scholar] [CrossRef]
- Loi, F.; Pozzo, M.; Sbuelz, L.; Bignardi, L.; Lacovig, P.; Tosi, E.; Lizzit, S.; Kartouzian, A.; Heiz, U.; Larciprete, R.; et al. Breakdown of the correlation between oxidation states and core electron binding energies at the sub-nanoscale. Appl. Surf. Sci. 2023, 619, 156755. [Google Scholar] [CrossRef]
- Che, X.; Wu, Q.; Hu, S.; Wang, G.; Pang, H.; Sun, W.; Ma, H.; Wang, X.; Tan, L.; Yang, G. Directed synthesis of an unusual uniform trimetallic hydrogen evolution catalyst by a predesigned cobalt-bipy modified bivanadyl capped polymolybdate. J. Solid State Chem. 2022, 314, 123403. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, J.; Liu, J.; Ye, Q.; Gao, X.; Lu, J.; Cheng, Y. Modulating electronic structure of cobalt phosphide porous nanofiber by ruthenium and nickel dual doping for highly-efficiency overall water splitting at high current density. Appl. Catal. B Environ. 2021, 298, 120488. [Google Scholar] [CrossRef]
- Li, X.; Xiao, L.; Zhou, L.; Xu, Q.; Weng, J.; Xu, J.; Liu, B. Adaptive Bifunctional Electrocatalyst of Amorphous CoFe Oxide @ 2D Black Phosphorus for Overall Water Splitting. Chem 2020, 59, 21106–21113. [Google Scholar]
- Karimi, S.; Yaraki, M.T.; Karri, R.R. A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renew. Sust. Energ. Rev. 2019, 107, 535–553. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, X.; Huang, Y.; Keller, A.A.; Lan, J. Competitive removal of Pb2+ and malachite green from water by magnetic phosphate nanocomposites. Water Res. 2019, 150, 442–451. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Design 2023, 230, 111952. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, H.; Ye, J. Constructing and controlling of highly dispersed metallic sites for catalysis. Nano. Today 2018, 19, 108–125. [Google Scholar] [CrossRef]





| Element | Wt% | Wt% Sigma | At% | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| C | 11.63 | 55.19 | 0.21 | 0.11 | 31.49 | 70.48 |
| O | 12.22 | 25.28 | 0.09 | 0.10 | 24.84 | 24.23 |
| Fe | 53.73 | 13.84 | 0.20 | 0.07 | 31.29 | 3.80 |
| Co | 22.42 | 5.70 | 0.17 | 0.06 | 12.38 | 1.48 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | ||
| Parameter Sequence Number | Thermophysical | Parameter Value |
|---|---|---|
| 1 | BET surface area (m2g−1) | 142.8302 m2g−1 |
| 2 | Micropore volume (cm3g−1) | 0.004586 cm3g−1 |
| 3 | Micropore area (m2g−1) | 10.5186 m2g−1 |
| 4 | Average pore size (nm) | 7.7283 nm |
| Isotherm Model | T (K) | |||
|---|---|---|---|---|
| 288 | 298 | 308 | ||
| Qm | 84.96 | 97.47 | 163.40 | |
| Langmuir | KL | 0.011 | 0.019 | 0.011 |
| R2 | 0.942 | 0.922 | 0.920 | |
| KF | 17.07 | 29.93 | 27.15 | |
| Freundlich | n | 4.35 | 5.77 | 3.89 |
| R2 | 0.969 | 0.956 | 0.911 | |
| T (K) | lnK | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J·mol−1·K−1) |
|---|---|---|---|---|
| 288.15 | −1.60 | 3.83 | 33.21 | 101.63 |
| 298.15 | −1.26 | 3.11 | ||
| 308.15 | −0.70 | 1.79 |
| Material | Pollutants | Adsorption Capacity | Adsorption Efficiency | Removal Method | References |
|---|---|---|---|---|---|
| Fe3O4@PDA | PS | 4.0–10 mg/L Not specified | 98.5% | Magnetic removal | [49] |
| 2D ultrathin magnetic NDs | PS PMMA | 187.7 mg/g 188.4 mg/g | 90% | Magnetic removal | [50] |
| MFe@BC | PHBV | 31.96 mg/g | 98.53% | Filtration | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, L.; Wang, X.; Xia, P.; Zhang, Z.; Huang, X.; Guo, X. Adsorption Ability of Soft Magnetic FeCo Alloys for Microplastics. Water 2025, 17, 3305. https://doi.org/10.3390/w17223305
Zhang S, Zhang L, Wang X, Xia P, Zhang Z, Huang X, Guo X. Adsorption Ability of Soft Magnetic FeCo Alloys for Microplastics. Water. 2025; 17(22):3305. https://doi.org/10.3390/w17223305
Chicago/Turabian StyleZhang, Shenghao, Lan Zhang, Xingfu Wang, Pinhua Xia, Zhenming Zhang, Xianfei Huang, and Xuetao Guo. 2025. "Adsorption Ability of Soft Magnetic FeCo Alloys for Microplastics" Water 17, no. 22: 3305. https://doi.org/10.3390/w17223305
APA StyleZhang, S., Zhang, L., Wang, X., Xia, P., Zhang, Z., Huang, X., & Guo, X. (2025). Adsorption Ability of Soft Magnetic FeCo Alloys for Microplastics. Water, 17(22), 3305. https://doi.org/10.3390/w17223305







