L-Lysine-Modified Lignin for Polishing Alkaline Road-Marking Wash Water: High Uptake of Cationic Dyes with Acid-Enabled Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods
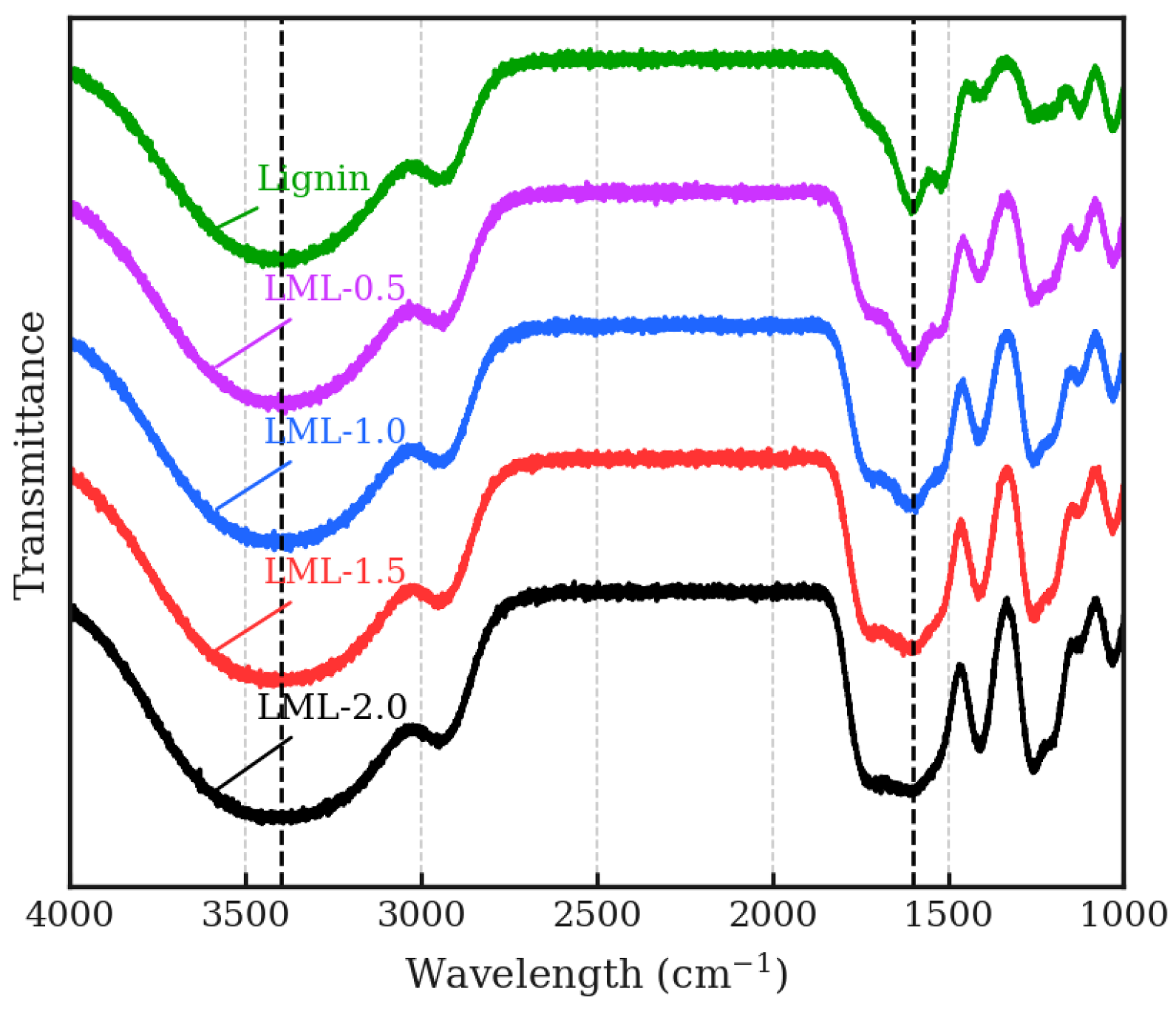
2.2.1. Preparation of L-Lysine-Modified Lignin
2.2.2. Adsorption Experiments
2.2.3. Regeneration Experiment
3. Results and Discussion
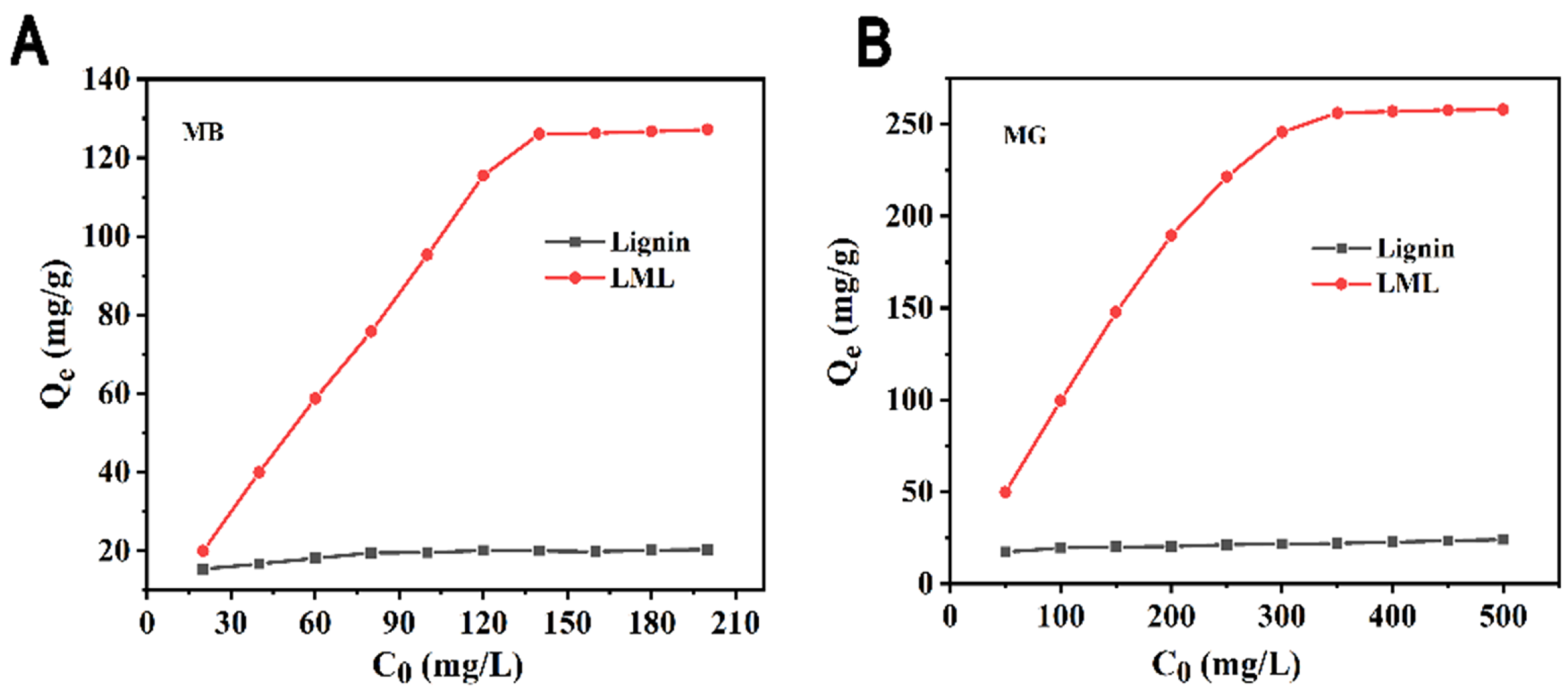
3.1. Effect of Modification on Adsorption
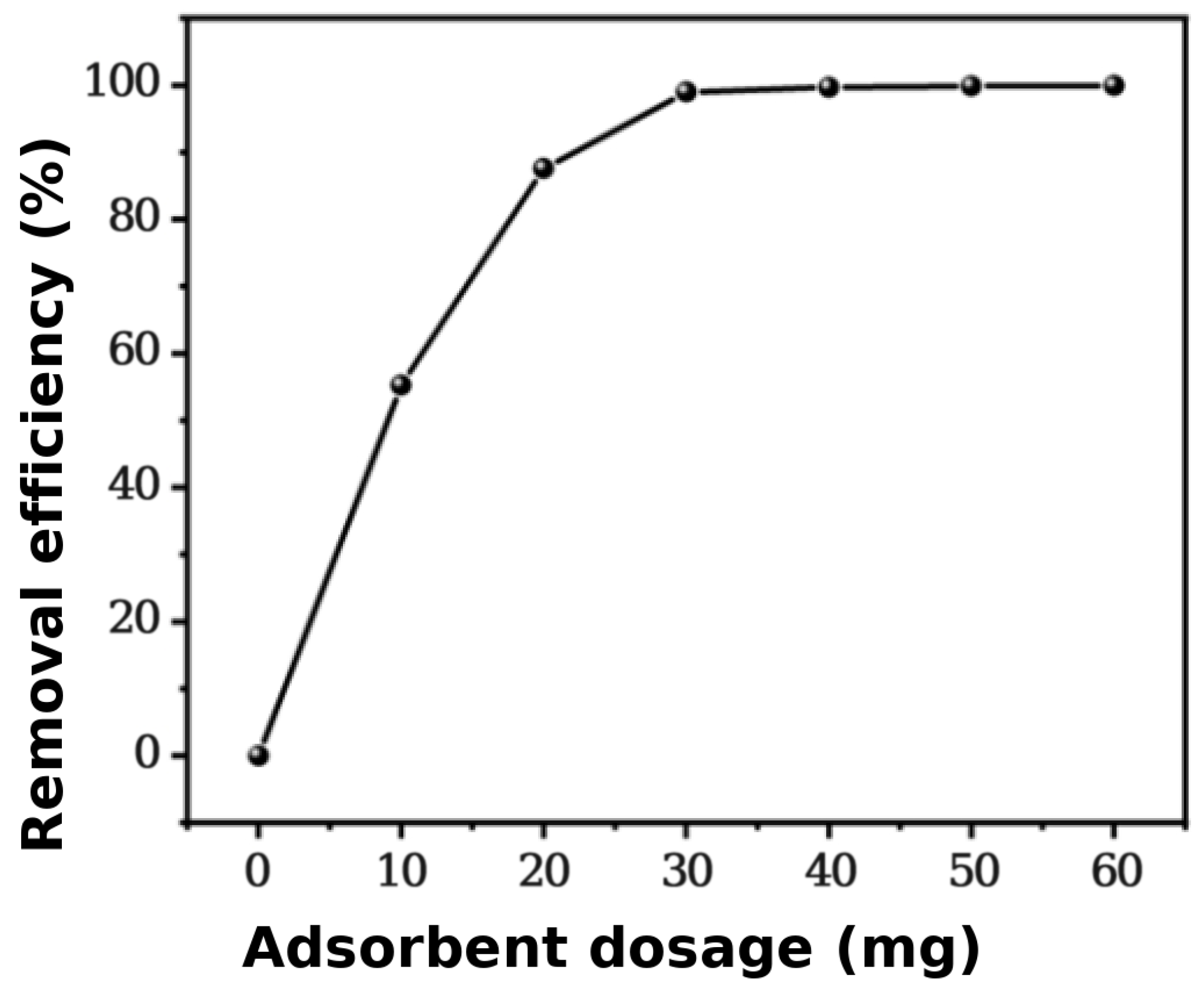
3.2. Effect of Adsorbent Dosage
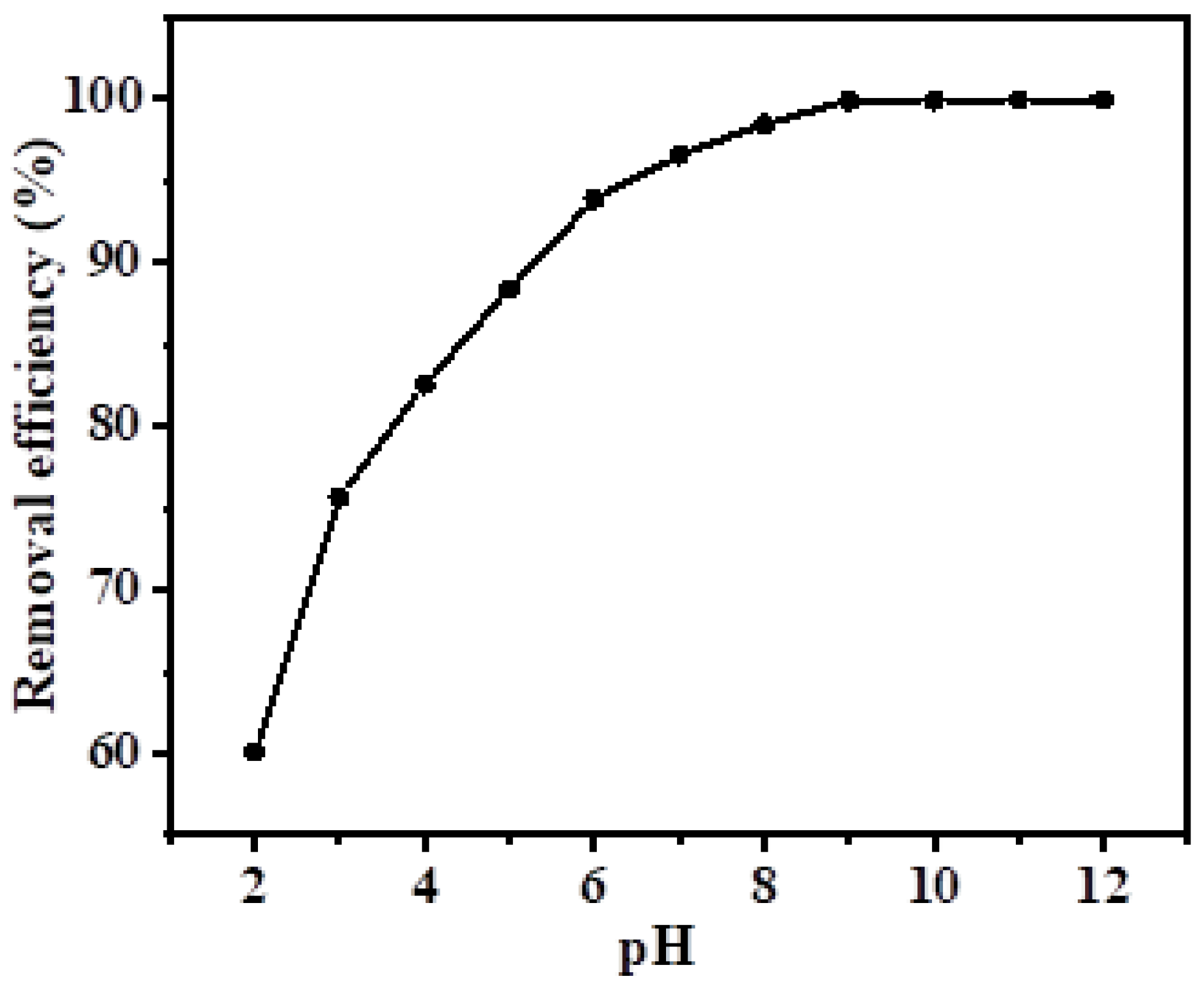
3.3. Effect of pH
3.4. Isotherm Adsorption
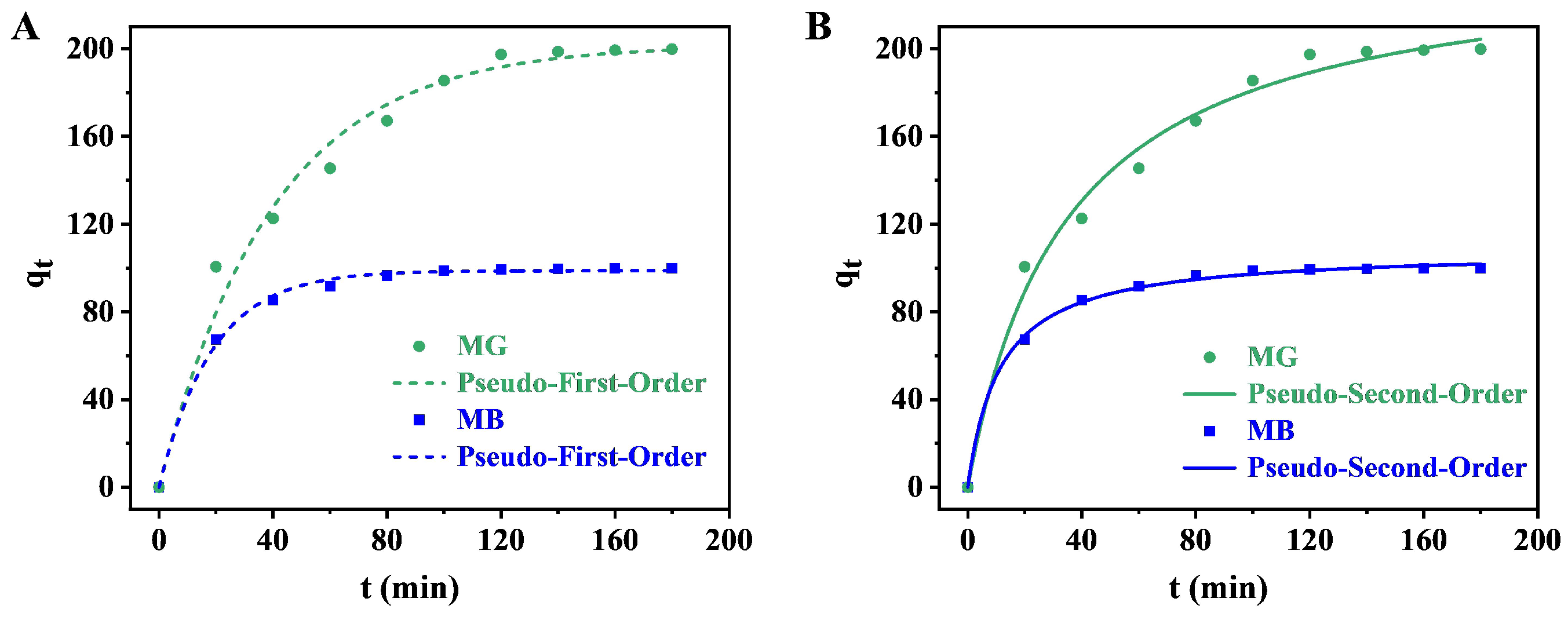
3.5. Adsorption Kinetics
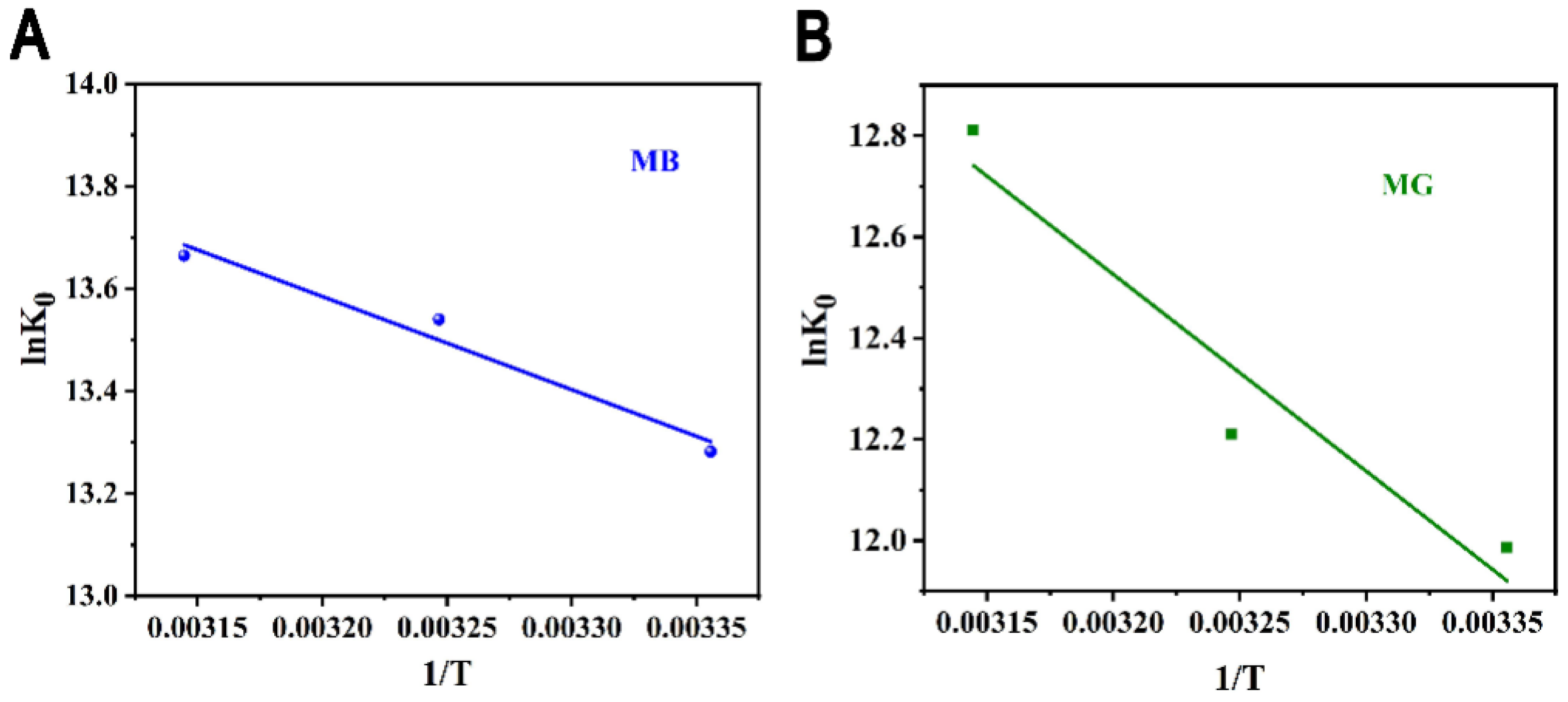
3.6. Thermodynamics of Adsorption
3.7. Reusability of LML
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Cui, K.; Wu, W. Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process. Water 2025, 17, 1322. [Google Scholar] [CrossRef]
- Chen, A.; Liang, H.; Chen, T.; Yang, W.; Ding, C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J. Soils Sediments 2016, 16, 1352–1359. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, B.; Husnain, N.; Wang, Q. Overview of Agricultural Machinery Automation Technology for Sustainable Agriculture. Agronomy 2025, 15, 1471. [Google Scholar] [CrossRef]
- Abdualrahman, M.A.Y.; Ma, H.; Zhou, C.; Yagoub, A.E.A.; Hu, J.; Yang, X. Thermal and single frequency counter-current ultrasound pretreatments of sodium caseinate: Enzymolysis kinetics and thermodynamics, amino acids composition, molecular weight distribution and antioxidant peptides. J. Sci. Food Agric. 2016, 96, 4861–4873. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xie, H.; Zhang, L.; Wang, X.; Zhong, Y.; Shen, Y.; Wang, H.; Hao, C. High-performance polyethylenimine-functionalized lignin/silica porous composite microsphere for the removal of hexavalent chromium, phosphate and Congo red from aqueous solutions. Ind. Crops Prod. 2023, 194, 116289. [Google Scholar] [CrossRef]
- Azam, S.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Huang, X.-W.; Zou, X.-B.; Shi, J.-Y.; Li, Z.-H.; Zhao, J.-W. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; Zhang, T.; Li, P.; Qiu, F. Conversion of agricultural waste biomass resource into high-added-value composite and its potential for boosting synergistic removal of ammonia nitrogen in practical water. Food Bioprod. Process. 2025, 150, 240–251. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a lignosulfonate–lysine hydrogel for the adsorption of heavy metal ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- Fan, X.; Peng, L.; Wang, X.; Han, S.; Yang, L.; Wang, H.; Hao, C. Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Ind. Crops Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using magnetic multiwalled carbon nanotubes as modified QuEChERS adsorbent for simultaneous determination of multiple mycotoxins in grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Morgan, H.M.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal behavior and kinetic study of the effects of zinc-modified biochar catalyst on lignin and low-density polyethylene (LDPE) co-pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
- Song, C.; Gao, C.; Fatehi, P.; Wang, S.; Jiang, C.; Kong, F. Influence of structure and functional group of modified kraft lignin on adsorption behavior of dye. Int. J. Biol. Macromol. 2023, 240, 124368. [Google Scholar] [CrossRef]
- Zahoor; Madadi, M.; Nazar, M.; Shah, S.W.A.; Li, N.; Imtiaz, M.; Zhong, Z.; Zhu, D. Green alkaline fractionation of sugarcane bagasse at cold temperature improves digestibility and delignification without the washing processes and release of hazardous waste. Ind. Crops Prod. 2023, 200, 116815. [Google Scholar] [CrossRef]
- Hai-Jew, S. Online Survey Design and Data Analytics: Emerging Research and Opportunities; IGI Global: Hershey, PA, USA, 2019. [Google Scholar]
- Jiang, C.; Wang, X.; Qin, D.; Da, W.; Hou, B.; Hao, C.; Wu, J. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019, 369, 50–61. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Meng, X.; Scheidemantle, B.; Li, M.; Wang, Y.Y.; Zhao, X.; Toro-González, M.; Singh, P.; Pu, Y.; Wyman, C.E.; Ozcan, S.; et al. Synthesis, characterization, and utilization of a lignin-based adsorbent for effective removal of azo dye from aqueous solution. ACS Omega 2020, 5, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, W. Sponge effect of aerated concrete on phosphorus adsorption-desorption from agricultural drainage water in rainfall. Soil Water Res. 2020, 15, 220–227. [Google Scholar] [CrossRef]
- Lee, S.L.; Park, J.H.; Kim, S.H.; Kang, S.W.; Cho, J.S.; Jeon, J.R.; Lee, Y.B.; Seo, D.C. Sorption behavior of malachite green onto pristine lignin to evaluate the possibility as a dye adsorbent by lignin. Appl. Biol. Chem. 2019, 62, 37. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T. Equilibrium, kinetics, and thermodynamic studies of malachite green adsorption onto fig (Ficus cartia) leaves. J. Anal. Methods Chem. 2020, 2020, 7384675. [Google Scholar] [CrossRef]
- Abewaa, M.; Mengistu, A.; Takele, T.; Fito, J.; Nkambule, T. Adsorptive removal of malachite green dye from aqueous solution using Rumex abyssinicus derived activated carbon. Sci. Rep. 2023, 13, 14701. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Li, P.; Liu, M.; Xiao, G.M.; Xiao, Z.Q.; Mao, J.W.; Gai, X.K. Removal of methylene blue by porous biochar obtained by KOH activation from bamboo biochar. Bioresour. Bioprocess. 2023, 10, 51. [Google Scholar] [CrossRef]
- Ngapa, Y.D.; Gago, J. Optimizing of Competitive Adsorption Methylene Blue and Methyl Orange using Natural Zeolite from Ende-Flores. JKPK (J. Kim. Pendidik. Kim.) 2021, 6, 39–48. [Google Scholar] [CrossRef]
- Babić, D.; Burghardt, T.E.; Babić, D. Application and characteristics of waterborne road marking paint. Int. J. Traffic Transp. Eng. 2015, 5, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Wu, P.; Yagoub, A.E.G.A.; Chen, L.; Taiye, M.A.; Zhou, C. Pretreatment of sugarcane bagasse with deep eutectic solvents affect the structure and morphology of lignin. Ind. Crops Prod. 2021, 173, 114108. [Google Scholar] [CrossRef]
- Shi, N.; Li, S.; He, L.; Feng, Y.; Saeed, M.; Ma, Y.; Ni, Z.; Zhu, D.; Chen, H. High-throughput screening and identification of lignin peroxidase based on spore surface display of Bacillus subtilis. J. Sci. Food Agric. 2025, 105, 2179–2189. [Google Scholar] [CrossRef]
- Lin, H.; Duan, Y.; Man, Z.; Zareef, M.; Wang, Z.; Chen, Q. Quantitation of volatile aldehydes using chemoselective response dyes combined with multivariable data analysis. Food Chem. 2021, 353, 129485. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable preparation of FeOOH/CuO@WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Boateng, I.D.; Soetanto, D.A.; Yang, X.M.; Zhou, C.; Saalia, F.K.; Li, F. Effect of pulsed-vacuum, hot-air, infrared, and freeze-drying on drying kinetics, energy efficiency, and physicochemical properties of Ginkgo biloba L. seed. J. Food Process Eng. 2021, 44, e13655. [Google Scholar] [CrossRef]
- Xu, L.; Sethupathy, S.; Liang, Z.; Zhuang, Z.; Zhang, Y.; Sun, J.; Zhu, D. NAD+ regeneration-coupled enzymatic bioconversion of lignin-derived vanillin into vanillic acid: A cleaner production approach. Ind. Crops Prod. 2024, 222, 119921. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Yang, Z.; Peng, F.; Yang, L. Renewable and functional composite film from epoxidized Eucommia ulmoides gum and industrial lignin. Ind. Crops Prod. 2023, 194, 116381. [Google Scholar] [CrossRef]
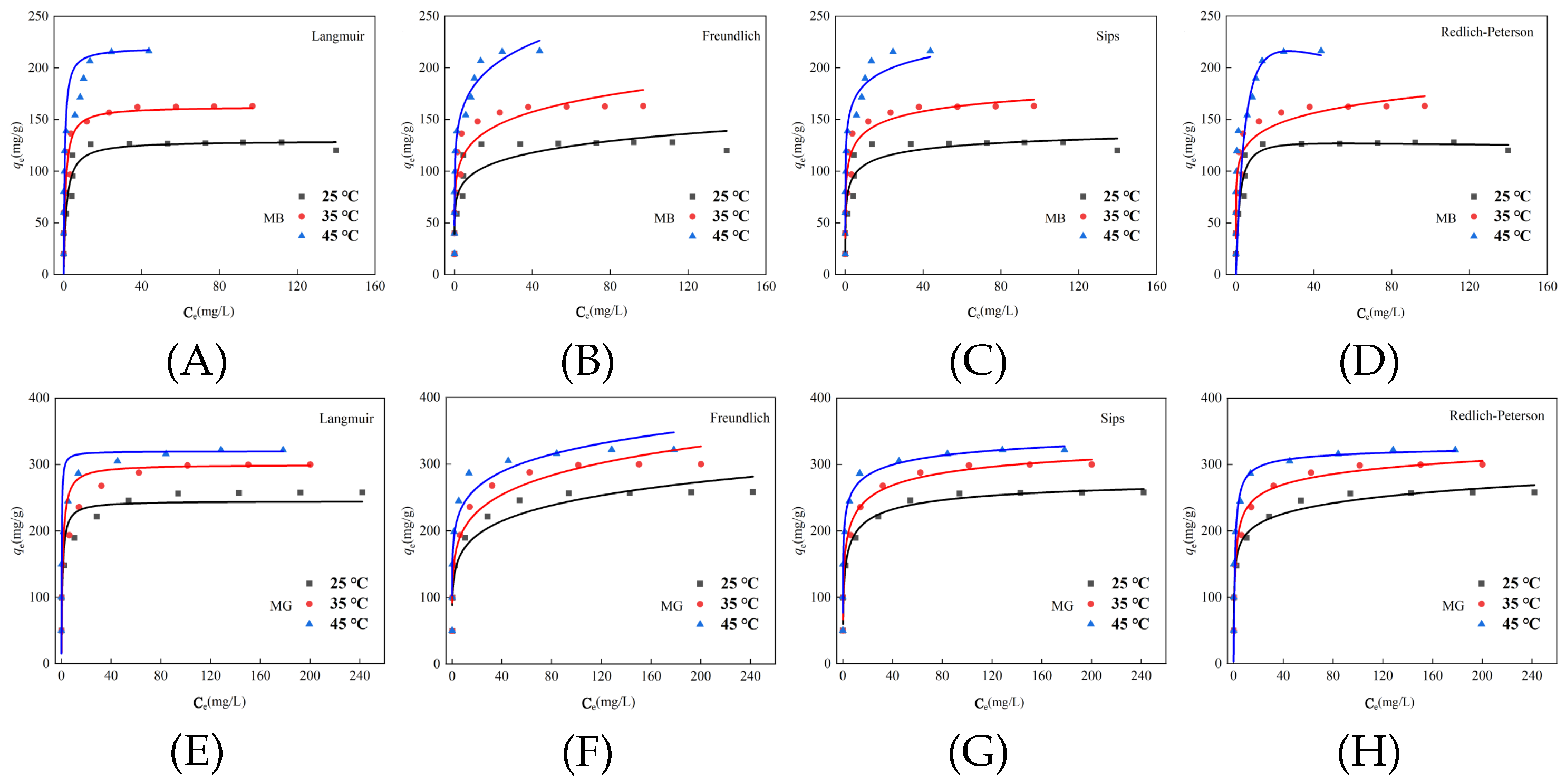

| Model | Parameters | 25 °C | 35 °C | 45 °C |
|---|---|---|---|---|
| Langmuir | (L mg−1) | 0.69 | 1.04 | 1.82 |
| (mg g−1) | 129.4 | 162.6 | 220.0 | |
| 0.84 | 0.80 | 0.70 | ||
| Freundlich | ((mg g−1)/(mg L−1)n) | 72.43 | 92.74 | 130.5 |
| n | 1.3 | 1.4 | 1.4 | |
| 0.84 | 0.90 | 0.95 | ||
| Sips | (mg g−1) | 154.3 | 218.7 | 320.0 |
| 0.94 | 0.82 | 0.78 | ||
| s | 0.37 | 0.31 | 0.24 | |
| 0.90 | 0.93 | 0.93 | ||
| Redlich–Peterson | 71.28 | 78.96 | 50.00 | |
| 0.48 | 0.89 | 0.10 | ||
| 1.03 | 0.89 | 1.19 | ||
| 0.84 | 0.93 | 0.35 |
| Model | Parameters | 25 °C | 35 °C | 45 °C |
|---|---|---|---|---|
| Langmuir | (L mg−1) | 1.16 | 0.95 | 4.84 |
| (mg g−1) | 244.9 | 300.0 | 320.0 | |
| 0.90 | 0.84 | 0.76 | ||
| Freundlich | ((mg g−1)/(mg L−1)n) | 123.3 | 142.9 | 182.4 |
| n | 1.5 | 1.6 | 1.2 | |
| 0.93 | 0.95 | 0.94 | ||
| Sips | (mg g−1) | 292.9 | 369.2 | 386.8 |
| 0.70 | 0.65 | 1.07 | ||
| s | 0.46 | 0.38 | 0.32 | |
| 0.99 | 0.99 | 0.98 | ||
| Redlich–Peterson | 774.2 | 300 | 320 | |
| 4.81 | 1.38 | 1.08 | ||
| 0.91 | 0.07 | 0.02 | ||
| 0.99 | 0.90 | 0.72 |
| Dye | Kinetic Model | Kinetic Parameter |
|---|---|---|
| Methylene blue | Pseudo-first-order | = 98.80 mg g−1 = 0.0536 min−1 R2 = 0.9710 |
| Pseudo-second-order | = 108.17 mg g−1 = 8.1949 g mg−1 min−1 R2 = 0.9811 | |
| Malachite green | Pseudo-first-order | = 201.64 mg g−1 = 0.0251 min−1 R2 = 0.9290 |
| Pseudo-second-order | = 243.53 mg g−1 = 1.1885 g mg−1 min−1 R2 = 0.9589 |
| Dye | T (K) | ln(K0) | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) | R2 |
|---|---|---|---|---|---|---|
| 298 | 13.28 | −32.91 | ||||
| Methylene blue | 308 | 13.54 | −34.67 | 15.13 | 161.4 | 0.9683 |
| 318 | 13.66 | −36.13 | ||||
| 298 | 11.99 | −29.70 | ||||
| Malachite green | 308 | 12.21 | −31.27 | 32.31 | 207.5 | 0.9252 |
| 318 | 12.81 | −33.87 |
| Adsorbent | Equilibrium Time | Adsorption Capacity | Refs |
|---|---|---|---|
| Pristine lignin | ≈30 min | MG 31.2 mg g−1 | [24] |
| Activated carbon from fig leaves | 200 min | MG 51.79 mg g−1 | [25] |
| Activated carbon from Rumex abyssinicus stems | 40 min | MG 98.43 mg g−1 | [26] |
| KOH-activated bamboo biochar (KBBC-900) | 20 min | MB 67.71 ± 0.19 mg g−1 | [27] |
| Natural zeolite | 60 min | MB 21.19 mg g−1 | [28] |
| LML | 120 min | MG 244.9 mg g−1 MB 129.4 mg g−1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Jing, P. L-Lysine-Modified Lignin for Polishing Alkaline Road-Marking Wash Water: High Uptake of Cationic Dyes with Acid-Enabled Regeneration. Water 2025, 17, 3234. https://doi.org/10.3390/w17223234
Xiong Z, Jing P. L-Lysine-Modified Lignin for Polishing Alkaline Road-Marking Wash Water: High Uptake of Cationic Dyes with Acid-Enabled Regeneration. Water. 2025; 17(22):3234. https://doi.org/10.3390/w17223234
Chicago/Turabian StyleXiong, Zeyu, and Peng Jing. 2025. "L-Lysine-Modified Lignin for Polishing Alkaline Road-Marking Wash Water: High Uptake of Cationic Dyes with Acid-Enabled Regeneration" Water 17, no. 22: 3234. https://doi.org/10.3390/w17223234
APA StyleXiong, Z., & Jing, P. (2025). L-Lysine-Modified Lignin for Polishing Alkaline Road-Marking Wash Water: High Uptake of Cationic Dyes with Acid-Enabled Regeneration. Water, 17(22), 3234. https://doi.org/10.3390/w17223234






