SARS-CoV-2, Noroviruses, Adenoviruses, and Antibiotic-Resistant Coliforms Within Chilean Rural Wastewater Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Monitoring
2.2. Analytical Methods and Instrumentation
2.2.1. Wastewater Viral Detection
2.2.2. Propidium Monoazide (PMA)
2.2.3. Detection of Antibiotic-Resistant Coliform Bacteria
3. Results and Discussion
3.1. Seasonal Behavior of Viruses in Rural WWTPs
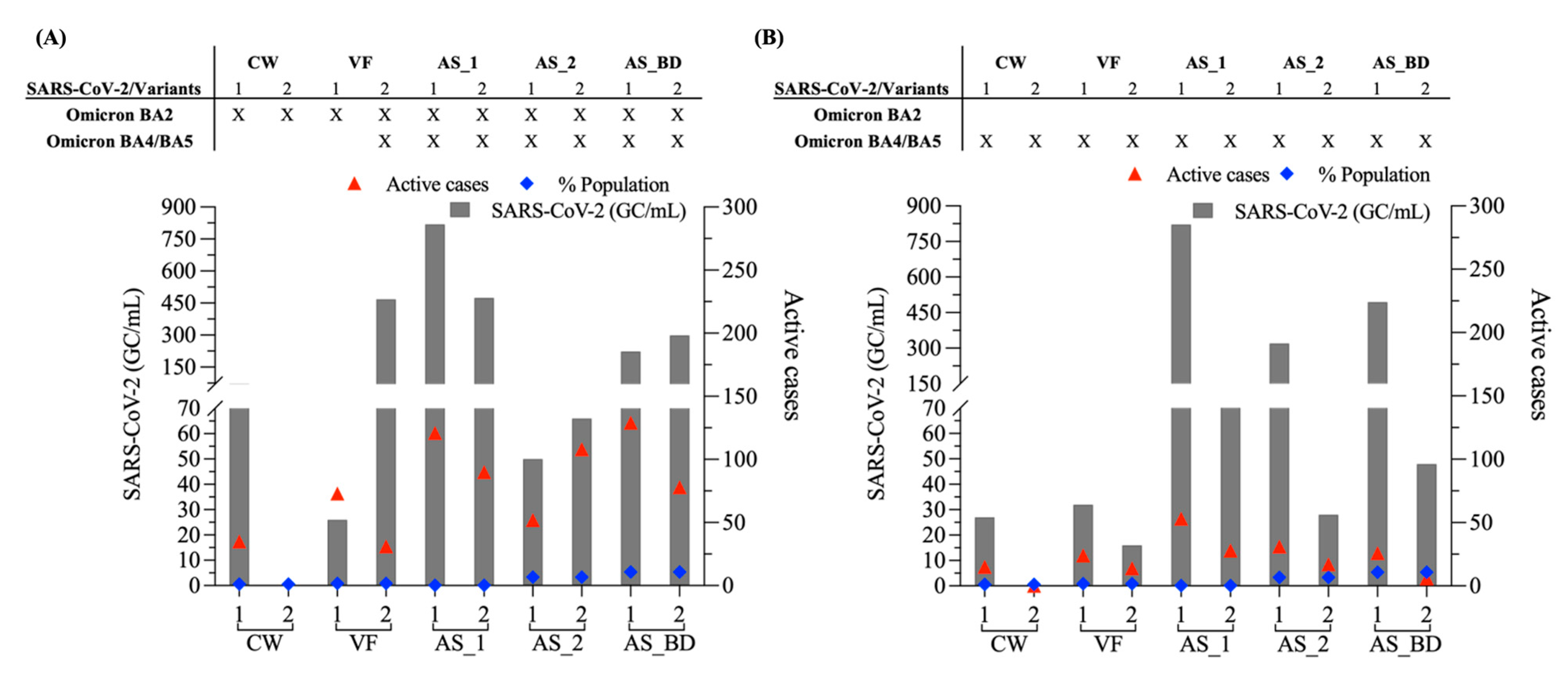
3.1.1. SARS-CoV-2
3.1.2. Norovirus GI and GII
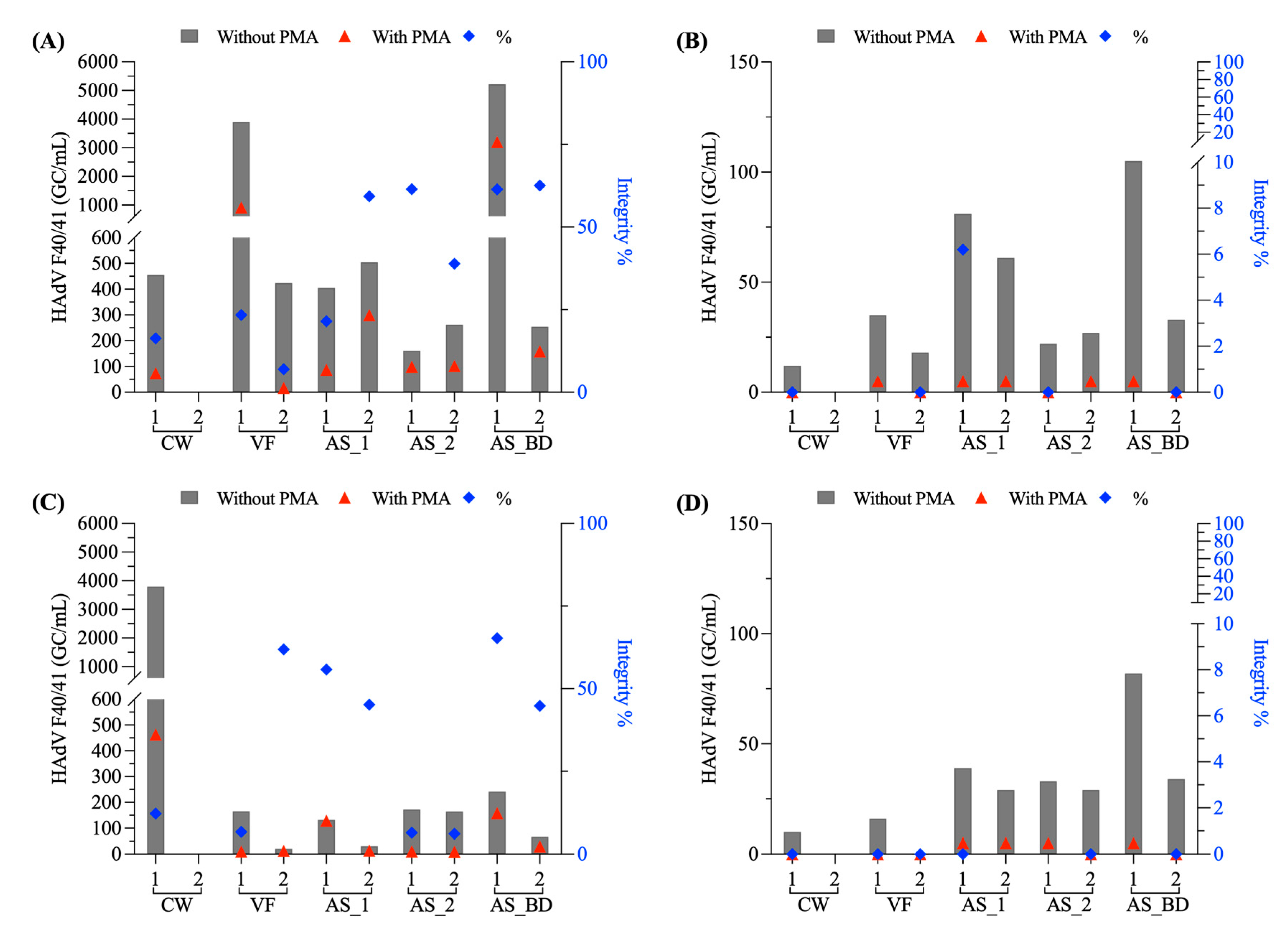
3.1.3. Adenovirus F40/41
3.1.4. Total and Fecal Coliforms in Rural WWTPs
3.1.5. Antibiotic-Resistant Total and Fecal Coliforms in Rural WWTPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Oh, B.S.; Yoon, S.; Shon, H.; Lee, S.; Hong, S. Wastewater Reclamation. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012; pp. 11873–11891. [Google Scholar]
- UNESCO; UN-Water. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UNESCO: Paris, France, 2023; Available online: https://www.unwater.org/publications/un-world-water-development-report-2023 (accessed on 25 July 2025).
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and particle associations in wastewater: Significance and implications for Treatmentand disinfection processe. Adv. Appl. Microbiol. 2016, 91, 83–119. [Google Scholar]
- Ahmed, W.; Bivins, A.; Bertsch, P.; Bibby, K.; Gyawali, P.; Sherchan, S.; Simpson, S.; Thomas, K.; Verhagen, R.; Kitajima, M.; et al. Intraday variability of indicator and pathogenic viruses in 1-h to 24-h composite wastewater samples: Implications for wastewater-based epidemiology. Environ. Res. 2021, 193, 110531. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Garrido, A.; Limaico, M.; Villamar-Ayala, C. Influence of wastewater Treatment technologies on virus removal under a bibliometric-statistical analysis. J. Water Process Eng. 2022, 47, 102642. [Google Scholar] [CrossRef]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, H.; Greenwood, A. Characterization of Bacterial Communities in Wastewater with Enhanced Taxonomic Resolution by Full-Length 16S RRNA Sequencing. Sci. Rep. 2019, 9, 9673. [Google Scholar] [CrossRef]
- Bohra, D.; Modasiya, V.; Bahura, C. The distribution of coliform bacteria in wastewater. Microbiol. Res. 2012, 3, e2. [Google Scholar] [CrossRef][Green Version]
- Ampuero, M.; Valenzuela, S.; Valiente-Echeverría, F.; Soto-Rifo, R.; Barriga, G.P.; Chnaiderman, J.; Gaggero, A. SARS-CoV-2 Detection in Sewage in Santiago, Chile-preliminary Results. MedRxiv 2020. [Google Scholar] [CrossRef]
- D’Aoust, P.M.; Towhid, S.T.; Mercier, É.; Hegazy, N.; Tian, X.; Bhatnagar, K.; Delatolla, R. COVID-19 wastewater surveillance in rural communities: Comparison of lagoon and pumping station samples. Sci. Total Environ. 2021, 801, 149618. [Google Scholar] [CrossRef]
- Plaza-Garrido, A.; Ampuero, M.; Gaggero, A.; Villamar-Ayala, C. Norovirus, Hepatitis A, and SARS-CoV-2 surveillance within Chilean rural wastewater treatment plants based on different biological treatment typologies. Sci. Total Environ. 2023, 863, 160685. [Google Scholar] [CrossRef]
- Kallem, P.; Hegab, H.; Alsafar, H.; Hasan, S.; Banat, F. SARS-CoV-2 detection and inactivation in water and wastewater: Review on analytical methods, limitations, and future research recommendations. Emerg. Microbes Infect. 2023, 12, 2222850. [Google Scholar] [CrossRef]
- Ekundayo, T.; Igere, B.; Oluwafemi, Y.; Iwu, C.; Olaniyi, O. Human norovirus contamination in water sources: A systematic review and meta-analysis. Environ. Pollut. 2021, 291, 118164. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, V.; van Zyl, W.; Taylor, M.; Mans, J. Quantification and potential viability of human noroviruses in final effluent from wastewater Treatment Works in Pretoria, South Africa. Food Environ. Virol. 2024, 16, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Reyne, M.; Allen, D.; Levickas, A.; Allingham, P.; Lock, J.; Fitzgerald, A.; McSparron, C.; Nejad, B.; McKinley, J.; Lee, A.; et al. Detection of human adenovirus F41 in wastewater and its relationship to clinical cases of acute hepatitis of unknown aetiology. Sci. Total Environ. 2023, 857, 159579. [Google Scholar] [CrossRef]
- Takuissu, G.R.; Kenmoe, S.; Ebogo-Belobo, T.; Kengne-Ndé, C.; Mbaga, D.D.; Bowo-Ngandji, A. Exploring adenovirus in water environments: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2023, 34, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jin, Y.; Sims, T.; Kneiel, K. Survival of human adenovirus 41 in land-Applied Manure and biosolids. Food Environ. Virol. 2009, 1, 148–154. [Google Scholar] [CrossRef]
- Rachmadi, A.T.; Kitajima, M.; Kato, T.; Okabe, S.; Sano, D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: A critical review. Environ. Sci. Technol. 2020, 105, 456–469. [Google Scholar] [CrossRef]
- Metcalf and Eddy, Inc.; Asano, T.; Burton, F.L.; Leverenz, H.; Tsuchihashi, R.; Tchobanoglous, G. Water Reuse United States of America; McGraw-Hill Professional Publishing: New York, NY, USA, 2007; pp. 6–15. [Google Scholar]
- Environmental Protection Agency (EPA). Technology Information Brochure Wastewater Disinfection with Chlorine; EPA 832-F-99-062; Environmental Protection Agency (EPA): Washington, DC, USA, 1999; 9p.
- Le Roux, J.; Plewa, M.J.; Wagner, E.D.; Nihemaiti, M.; Dad, A.; Croué, J.P. Chloramination of wastewater effluent: Toxicity and formation of disinfection byproducts. J. Environ. Sci. 2017, 58, 135–145. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A review on occurrence and spread of antibiotic resistance in wastewaters and in wastewater Treatment plants: Mechanisms and perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Salazar, D.; Ginn, O.; Brown, J.; Soria, F.; Garvizu, C. Assessment of antibiotic resistant coliforms from bioaerosol samples collected above a sewage-polluted river in La Paz-Bolivia. Int. J. Hyg. Environ. Health 2020, 228, 113494. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar]
- Łuczkiewicz, A.; Jankowska, K.; Fudala-Ksiazek, S.; Olańczuk-Neyman, K. Antimicrobial Resistance of Fecal Indicators in Municipal Wastewater Treatment Plant. Water Res. 2010, 44, 5089–5097. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Korzeniewska, A.; Harnisz, M. Antibiotic Resistant Escherichia Coli in Hospital and Municipal Sewage and Their Emission to the Environment. Ecotoxicol. Environ. Saf. 2013, 91, 96–102. [Google Scholar] [CrossRef]
- Grehs, B.; Lopes, A.; Moreira, N.; Fernandes, T.; Linton, M.; Silva, A.; Manaia, C.; Carissimi, E.; Nunes, O. Removal of microorganisms and antibiotic resistance genes from treated urban wastewater: A comparison between aluminium sulphate and tannin coagulants. Water Res. 2019, 166, 115056. [Google Scholar] [CrossRef]
- Villamar, C.; Vera-Puerto, I.; Rivera, D.; De la Hoz, F. Reuse and recycling of livestock and municipal wastewater in Chilean Agriculture: A preliminary Assessment. Water 2018, 10, 817. [Google Scholar] [CrossRef]
- Vera-Puerto, I.; Valdes, H.; Bueno, M.; Correa, C.; Olave, J.; Carrasco-Banavides, M.; Schiappacasse, F.; Arias, C. Reclamation of treated wastewater for irrigation in Chile: Perspectives of the current state and challenges. Water 2022, 14, 627. [Google Scholar] [CrossRef]
- Fumian, T.M.; Leite, J.P.G.; Castello, A.A.; Gaggero, A.; de Caillou, M.S.; Miagostovich, M.P. Detection of rotavirus a in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. J. Virol. Methods 2010, 170, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Levican, J.; Levican, A.; Ampuero, M.; Gaggero, A. JC polyomavirus circulation in one-year surveillance in wastewater in Santiago, Chile. Infect. Genet. Evol. 2019, 71, 151–158. [Google Scholar] [CrossRef]
- World Health Organization. Data COVID-19 Global Circulation. 2024. Available online: https://data.who.int/dashboards/covid19/circulation?n=o (accessed on 25 July 2025).
- Chielan Institute of Public Health. Deteccion de Casos Infectados Con Nuevo Linaje (BA.2) de la Variante Omicron. Available online: https://www.ispch.gob.cl/noticia/deteccion-de-casos-infectados-con-nuevo-sublinaje-ba-2-de-la-variante-omicron/ (accessed on 25 July 2025).
- Wilhelm, A.; Scotch, J.; Mainert-Berning, C.; Bastian, D.; Ciesek, S.; Teichgräber, B.; Wintgens, T.; Weber, F.; Widera, M. Early detection of SARS-CoV-2 Omicron BA.4/5 German wastewater. Viruses 2022, 14, 1876. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hossen, F.; Rahman, A.; Fahmida, K.; Nayeem, H.; Haque, A.; Sosa-Hernandez, J.; Oyervides-Muñoz, M.; Parra-Saldivar, R.; Ahmed, T.; et al. An opinion wastewater-based epidemiological monitoring (WBEM) with clinical diagnostics test (CDT) for detecting high-prevalence areas of community COVID-19 infections. Curr. Opin. Environ. Sci. Health 2022, 31, 100396. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Rahman, A.; Jakariya, M.N.; Hossen, F.; Kumar, S.; Salim, M.; Tasneem, A.; Haque, A.; Sera, F.; Kabir, I.; et al. A 30-day follow-up study on the prevalence of SARS-CoV-2 genetic markers in wastewater from the residence of COVID-19 patient and comparison with clinical positivity. Sci. Total Environ. 2022, 858, 159350. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- Balboa, S.; Mauricio-Iglesias, M.; Rodriguez, S.; Martínez-Lamas, L.; Vasallo, F.; Regueirom, B.; Lema, J. The fate of SARS-CoV-2 in WWTPs points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021, 772, 145268. [Google Scholar] [CrossRef] [PubMed]
- Shing, R.; Bhunia, P.; Dash, R. A mechanistic review on vermifiltration of wastewater: Design, operation, and performance. J. Environ. Manag. 2017, 197, 656–672. [Google Scholar] [CrossRef]
- Ghosh, D.; Gopal, B. Effect of hydraulic retention time on the treatment of secondary effluent in a subsurface flow constructed wetland. Ecol. Eng. 2010, 36, 1044–1051. [Google Scholar] [CrossRef]
- Conceicao, K.; Villamar-Ayala, C.; Plaza-Garrido, A.; Tolero-Neira, C. Seasonal behavior of pharmaceuticals and personal care products within Chilean Rural WWTPs under COVID-19 pandemic conditions. J. Environ. Chem. Eng. 2023, 11, 110984. [Google Scholar] [CrossRef]
- Ye, Y.; Ellenberg, R.; Graham, K.; Wigginton, K. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef]
- Cutrupi, F.; Madonna, M.; Postinghel, M.; Foladori, P. SARS-CoV-2 removal in municipal wastewater treatment plants: Focus on conventional activated sludge, membrane bioreactor and anaerobic digestion. Sci. Total Environ. 2024, 96, 167434. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, A.; Calderwood, L.; Wikswo, M.; Barclay, L.; Mattison, C.; Balachandran, N.; Vinjé, J.; Hall, A.; Mirza, S. Spatiotemporal trends in norovirus outbreaks in the United States, 2009–2019. Clin. Infect. Dis. 2022, 76, 667–673. [Google Scholar] [CrossRef]
- Tan, M.; Tian, Y.; Zhang, D.; Wang, D.; Gao, Z. Aerosol transmission of norovirus. Viruses 2024, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Ginn, O.; Rocha-Melogno, L.; Bivins, A.; Lowry, S.; Cadelino, M.; Nichols, D.; Tripathi, S.; Soria, F.; Andrade, M.; Bergin, M.; et al. Detection and quantification of enteric pathogens in aerosols near open wastewater canals in cities with poor sanitation. Environ. Sci. Technol. 2021, 55, 14758–14771. [Google Scholar] [CrossRef]
- Uhrbrand, K.; Schultz, A.; Madsen, A. Exposure to airborne noroviruses and other bioaerosol components at a wastewater Treatment plant in Denmark. Food Environ. Virol. 2011, 3, 130–137. [Google Scholar] [CrossRef]
- Bi, X.; Liu, D.; Wang, L.; Huo, Y.; Fu, M. Inactivation and genome damage of rotavirus and a human norovirus surrogate by monochloramine treatment and sequential application with UV. Water Res. 2022, 226, 119309. [Google Scholar] [CrossRef]
- Eggers, M.; Suchomel, M. In-vivo efficacy of alcohol-based hand rubs against noroviruses: A novel standardized European test method simulating practical conditions. J. Food Microbiol. 2023, 340, 109058. [Google Scholar] [CrossRef]
- Kennedy, L.; Costantini, V.; Huynh, K.; Loeb, S.; Jennings, W. Persistence of human norovirus (GII) in surface water: Decay rate constants and inactivation mechanisms. Environ. Sci. Technol. 2023, 57, 3671–3679. [Google Scholar] [CrossRef]
- Yu, Z.; Shao, Q.; Liu, Z.; Chen, J.; Xu, Z.; Hu, B.; Cheng, D. A novel strategy for norovirus removal from wastewater based on bacterial-viral interactions. Environ. Technol. Innov. 2024, 35, 103643. [Google Scholar] [CrossRef]
- Armanious, A.; Aeppli, M.; Jack, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at solid-water interfaces: A systematic assessment of interactions driving adsorption. Environ. Sci. Technol. 2015, 50, 732–743. [Google Scholar] [CrossRef]
- Al-Hazmi, H.; Shokrani, H.; Shokrani, A.; Jabbour, K.; Abida, O.; Mousavi, S.; Habibzadeh, S.; Sonawane, S.; Saeb, M.; Bonilla-Petriciolet, A.; et al. Recent advances in aqueous virus removal technologies. Chemosphere 2022, 305, 135441. [Google Scholar] [CrossRef]
- Grand, R. Pathogenicity and virulence of human adenovirus F41: Possible links to severe hepatitis in children. Virulence 2023, 14, 2242544. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.; Ahmed, N.; Badr, K.; Elmahdy, M. Detection and quantification of adenovirus, polyomavirus, and papillomavirus in urban sewage. J. Water Health 2024, 22, 401–413. [Google Scholar] [CrossRef]
- Maniah, K.; Nour, I.; Hanif, A.; Yassin, M.; Alkathiri, A.; Al-Ashkar, I.; Eifan, S. Molecular identification of human adenovirus isolated from different wastewater treatment plants in Riyadh, Saudi Arabia: Surveillance and Meteorological impacts. Water 2023, 15, 1367. [Google Scholar] [CrossRef]
- Sidhu, J.; Toze, S. Human pathogens and their indicators in biosolids: A literature review. Environ. Int. 2009, 35, 187–201. [Google Scholar] [CrossRef]
- Morató, J.; Codony, F.; Sanchez, O.; Pérez, L.; García, J.; Mas, J. Key design factors affecting microbial community composition and pathogenic organism removal in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2014, 481, 81–89. [Google Scholar] [CrossRef]
- Zurita, F.; Carreón-Álvarez, A. Performance of three pilot-scale hybrid constructed wetlands for total coliforms and Escherichia coli removal from primary effluent—A 2-year study in a subtropical climate. J. Water Health 2015, 13, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.; Mota e Silva, J.; Fia, R.; Coutinho de Oliveira, L.; Campos, C. Coliform removal in a constructed wetland system used in post-swine effluent treatment. Rev. Ambiente Água Interdiscip. J. Appl. Sci. 2019, 14, e2402. [Google Scholar] [CrossRef]
- Ministerio Secretaría General de la Presidencia. Decreto 90. Establece Norma de Emisión Para la Regulación de Contaminantes Asociados a Las Descargas de Residuos Líquidos a Aguas Marinas Y Continentales Superficiales; Diario Oficial de la República de Chile: Santiago, Chile, 2000.
- WHO. Guidelines for the Safe Use of Wastewater, Excreta, and Greywater; WHO: Geneva, Switzerland, 2006; Volume IV. [Google Scholar]
- Arora, S.; Rajpal, A.; Kazmi, A. Antimicrobial activity of bacterial community for removal of pathogens during vermifiltration. J. Environ. Eng. 2016, 142, 04016012. [Google Scholar] [CrossRef]
- Chilean Institure of Public Health. Informe Vigilancia de Consumo de Antimicrobianos en Chile; Chilean Institure of Public Health: Santiago, Chile, 2024. [Google Scholar]
- Pérez-López, M.; Miranda-Falcón, M.; Correa-Ramírez, M.; Loredo-Treviño, A. Effect of two types of wastewater Treatment plants on antibiotic resistance of fecal coliform. Water 2024, 16, 2364. [Google Scholar] [CrossRef]
- Wen, L.; Cui, Y.; Huang, L.; Wei, C.; Wang, G.; Zhang, J.; Jiang, Y.; Wei, Y.; Shen, P. Changes of composition and antibiotic resistance of fecal coliform bacteria in municipal wastewater Treatment plant. J. Environ. Sci. 2024, 146, 241–250. [Google Scholar] [CrossRef]
- Kali, M.; Kontogiannatos, D.; Koi, A.; Angeli, M.; Noutsopoulos, C.; Georgakopoulos, D.; Malamis, S.; Mammais, D. Removal of antibiotic resistant bacteria and genes by conventional and nature-based municipal wastewater Treatment systems. Environ. Process. 2025, 12, 38. [Google Scholar] [CrossRef]
- Ghonimy, M.; Alharbi, A.; Saad, S.; Hussein, N. Improving wastewater quality using ultrafiltration technology for sustainable irrigation reuse. Water 2025, 17, 870. [Google Scholar] [CrossRef]
- Azuma, T.; Usui, M.; Hayashi, T. Inactivation of antibiotic-resistant bacteria in wastewater by ozone-based advanced water Treatment processes. Antibiotics 2022, 11, 210. [Google Scholar] [CrossRef] [PubMed]
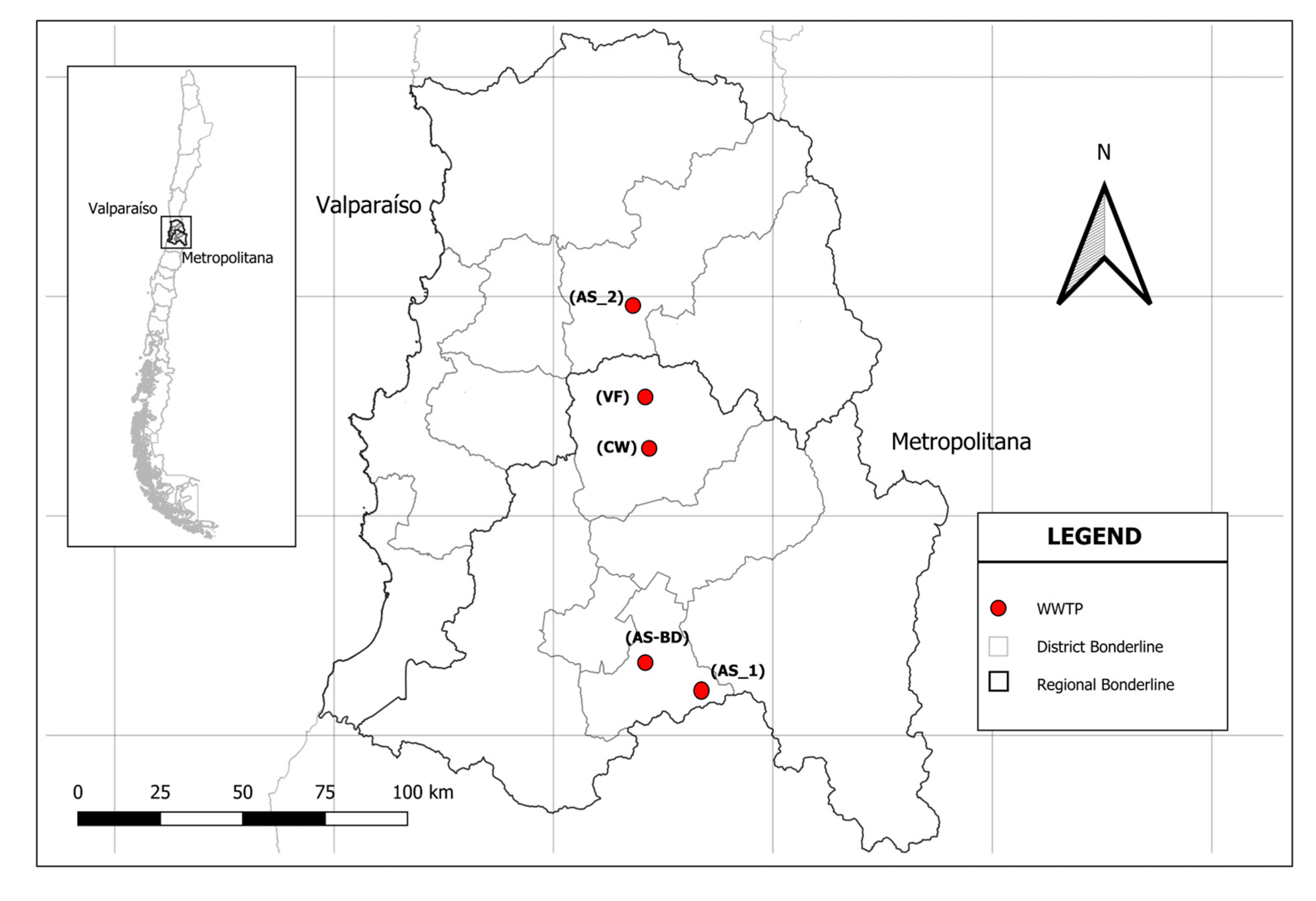
| WWTPs | Name | Location | Population WWTPs | Treatment | Date Autum–Winter | Date Spring–Summer | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q (m3/day) * | Kg BOD/day | Secondary | Tertiary | First Sample | Second Sample | Third Sample | Fourth Sample | ||||
| CW | Polpaico | 33°10′05.8″ S 70°53′12.3″ W | 267 | 40.05 | 10.21 | Constructed wetland | Ca (OCl)2 | 08/06/22 | nd | 30/11/22 | nd |
| VF | Rungue | 33°00′29.7″ S 70°53′22.7″ W | 372 | 55.80 | 22.38 | Verm filters | NaClO | 15/06/22 | 20/07/22 | 16/11/22 | 21/12/22 |
| AS_1 | Huelquén | 33°49′59.6″ S 70°38′49.6″ W | 279 | 41.85 | 13.32 | Activated sludge | NaClO | 06/07/22 | 03/08/22 | 02/11/22 | 07/12/22 |
| AS_2 | Los Loros | 32°50′44.4″ S 70°56′23.5″ W | 4850 | 727.5 | 124.49 | Activated sludge | Ca (OCl)2 | 13/07/22 | 24/08/22 | 23/11/22 | 28/12/22 |
| AS_BD | Bicentenario | 33°44′45.0″ S 70°52′18.0″ W | 4340 | 651.00 | 118.04 | Activated sludge | NaClO | 22/06/22 | 10/08/22 | 09/11/22 | 14/12/22 |
| WWTPs | Visit | Sample | Total Coliforms | Fecal Coliforms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFU/100 mL | Removal Efficiency (log10) | CFU/100 mL | Removal Efficiency (log10) | |||||||
| Autum Winter | Spring Summer | Autum Winter | Spring Summer | Autum Winter | Spring Summer | Autum Winter | Spring Summer | |||
| CW | 1 | Influent | 1.31 × 108 | 1.33 × 109 | 0.13 | 1.85 | 1.52 × 107 | 1.50 × 105 | 0.65 | 6.48 |
| Effluent | 9.7 × 107 | 1.88 × 107 | 3.39 × 106 | 0.00 × 100 | ||||||
| 2 | Influent | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | |
| Effluent | n.d | n.d | n.d | n.d | ||||||
| VF | 1 | Influent | 7.78 × 107 | 5.50 × 107 | 1.04 | nr | 4.77 × 107 | 3.67 × 105 | 1.91 | 0.31 |
| Effluent | 7.08 × 106 | 3.35 × 108 | 5.85 × 105 | 1.80 × 105 | ||||||
| 2 | Influent | 6.50 × 107 | 1.03 × 108 | 1.17 | 0.42 | 3.69 × 107 | 8.64 × 106 | 2.21 | 1.35 | |
| Effluent | 4.35 × 106 | 3.88 × 107 | 2.25 × 105 | 3.83 × 105 | ||||||
| AS_1 | 1 | Influent | 7.25 × 106 | 1.83 × 107 | 5.46 | 1.85 | 5.00 × 103 | 9.89 × 105 | 5.00 | 2.73 |
| Effluent | 0.00 × 100 | 2.60 × 105 | 0.00 × 100 | 1.83 × 103 | ||||||
| 2 | Influent | 3.25 × 106 | 6.45 × 107 | 5.11 | 1.96 | 8.33 × 103 | 6.67 × 103 | 1.48 | 1.23 | |
| Effluent | 0.00 × 100 | 7.00 × 105 | 2.78 × 102 | 3.89 × 102 | ||||||
| AS_2 | 1 | Influent | 1.98 × 107 | 4.78 × 107 | 3.42 | 0.78 | 7.22 × 103 | 3.67 × 105 | 1.64 | 6.87 |
| Effluent | 7.50 × 103 | 8.00 × 106 | 1.67 × 102 | 0.00 × 100 | ||||||
| 2 | Influent | 2.63 × 107 | 4.83 × 107 | 2.32 | 6.29 | 3.67 × 105 | 1.17 × 105 | 6.87 | 6.37 | |
| Effluent | 1.25 × 105 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | ||||||
| AS_BD | 1 | Influent | 7.63 × 109 | 5.25 × 109 | 0.40 | 0.94 | 3.20 × 107 | 2.07 × 107 | 1.43 | 1.17 |
| Effluent | 3.05 × 109 | 6.00 × 108 | 1.20 × 106 | 1.39 × 106 | ||||||
| 2 | Influent | 6.45 × 109 | 4.38 × 109 | 0.96 | 0.90 | 2.49 × 107 | 9.00 × 106 | 1.23 | 0.90 | |
| Effluent | 7.00 × 108 | 5.55 × 108 | 1.46 × 106 | 1.13 × 106 | ||||||
| WWTPs | Visit | Coliforms | Amoxicillin | Cefadroxil | Ciprofloxacin | Azithromycin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Autum–Winter | Spring–Summer | Autum–Winter | Spring–Summer | Autum–Winter | Spring–Summer | Autum–Winter | Spring–Summer | |||
| CW | 1 | Total | 1.87 | 1.12 | 1.00 | 0.82 | 0.11 | 0.18 | 0.49 | 0.37 |
| Fecal | 0.60 | 0.00 | 1.02 | 4.54 | 0.00 | 0.00 | 0.18 | 3.30 | ||
| 2 | Total | nd | nd | nd | nd | nd | nd | nd | nd | |
| Fecal | nd | nd | nd | nd | nd | nd | nd | nd | ||
| VF | 1 | Total | 0.58 | 0.65 | 0.79 | 0.81 | 0.12 | 0.30 | 0.22 | 0.26 |
| Fecal | 1.00 | 1.60 | 1.08 | 1.60 | 0.00 | 0.00 | 0.05 | 3.00 | ||
| 2 | Total | 0.48 | 0.60 | 0.76 | 0.76 | 0.15 | 0.22 | 0.40 | 0.15 | |
| Fecal | 0.90 | 3.85 | 1.38 | 1.41 | 0.00 | 0.00 | 0.30 | 0.00 | ||
| AS_1 | 1 | Total | 2.78 | 2.78 | 1.88 | 2.78 | 1.18 | 0.78 | 1.38 | 2.35 |
| Fecal | 4.65 | 4.54 | 5.20 | 5.29 | 3.70 | 4.00 | 0.00 | 0.00 | ||
| 2 | Total | 2.67 | 2.70 | 2.28 | 2.58 | 1.30 | 1.30 | 1.69 | 2.30 | |
| Fecal | 1.58 | 4.66 | 4.99 | 5.27 | 3.30 | 3.60 | 0.05 | 0.00 | ||
| AS_2 | 1 | Total | 2.30 | 2.78 | 2.48 | 2.78 | 1.38 | 1.00 | 2.23 | 2.04 |
| Fecal | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 3.30 | 3.00 | ||
| 2 | Total | 2.78 | 2.78 | 2.70 | 1.46 | 1.20 | 0.48 | 2.26 | 1.65 | |
| Fecal | 3.60 | 0.00 | 3.70 | 4.45 | 0.00 | 0.00 | 0.00 | 3.30 | ||
| AS_BD | 1 | Total | 1.00 | 0.91 | 1.20 | 0.93 | 0.70 | 0.78 | 0.33 | 0.30 |
| Fecal | 0.00 | 0.00 | 0.70 | 3.48 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 2 | Total | 1.01 | 1.03 | 1.22 | 0.90 | 0.30 | 0.27 | 0.37 | 0.20 | |
| Fecal | 0.00 | 0.00 | 0.58 | 0.48 | 0.48 | 3.00 | 0.00 | 0.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Garrido, A.; Villamar-Ayala, C.A.; Ampuero, M.; Gaggero, A. SARS-CoV-2, Noroviruses, Adenoviruses, and Antibiotic-Resistant Coliforms Within Chilean Rural Wastewater Treatment Plants. Water 2025, 17, 3197. https://doi.org/10.3390/w17223197
Plaza-Garrido A, Villamar-Ayala CA, Ampuero M, Gaggero A. SARS-CoV-2, Noroviruses, Adenoviruses, and Antibiotic-Resistant Coliforms Within Chilean Rural Wastewater Treatment Plants. Water. 2025; 17(22):3197. https://doi.org/10.3390/w17223197
Chicago/Turabian StylePlaza-Garrido, Angela, Cristina A. Villamar-Ayala, Manuel Ampuero, and Aldo Gaggero. 2025. "SARS-CoV-2, Noroviruses, Adenoviruses, and Antibiotic-Resistant Coliforms Within Chilean Rural Wastewater Treatment Plants" Water 17, no. 22: 3197. https://doi.org/10.3390/w17223197
APA StylePlaza-Garrido, A., Villamar-Ayala, C. A., Ampuero, M., & Gaggero, A. (2025). SARS-CoV-2, Noroviruses, Adenoviruses, and Antibiotic-Resistant Coliforms Within Chilean Rural Wastewater Treatment Plants. Water, 17(22), 3197. https://doi.org/10.3390/w17223197








