Abstract
Microbial fuel cells (MFCs) represent a sustainable alternative for wastewater treatment by simultaneously removing organic pollutants and generating energy. In this work, ceramic membranes were fabricated from low-cost locally available clays and tested as separators in MFCs. The systems achieved chemical oxygen demand (COD) removal efficiencies of up to 95%, comparable to those obtained with conventional Nafion membranes. In terms of energy performance, the ceramic membranes maintained open-circuit voltages of 0.80 ± 0.05 V during batch operation with voltage generation cycles ranging from 6 to 3 days, and delivered power densities between 140 and 180 mW/m2 under closed-circuit conditions. These values were very similar to those obtained with Nafion. The ceramic membranes maintained consistent COD removal performance during successive batch feeding cycles, confirming their stability under repeated operation. Overall, these results highlight the potential of ceramic materials as cost-effective and robust alternatives for large-scale wastewater treatment using MFC technology.
1. Introduction
The increasing production of wastewater and the rising global demand for energy represent two of the most critical and interconnected environmental challenges of our time [1,2]. Addressing these issues requires the development of sustainable, low-cost, and energy-efficient technologies, and in this context, microbial fuel cells (MFCs) have gained attention as an innovative bio electrochemical system capable of simultaneously degrading organic pollutants in wastewater and generating electrical energy through the metabolic activity of electroactive microorganisms [3]. MFCs offer several advantages, including ambient temperature operation, low external energy input, resource recovery (e.g., treated water and electricity), and scalability, making them particularly suitable for decentralized and off-grid applications [4].
A conventional MFC consists of an anode and a cathode chambers separated by a membrane. In the anodic compartment, microorganisms oxidize biodegradable organic matter, releasing electrons that cross the anode and then flow through an external circuit to the cathode where they reduce an electron acceptor—typically oxygen—forming water or hydrogen peroxide [5]. Concurrently, protons produced in the anode chamber migrate through the membrane to the cathode to complete the electrochemical reaction. MFCs have been successfully applied to a wide range of wastewaters, including domestic, industrial, and agricultural effluents, showing promising removal efficiencies and power output values [6,7,8].
Among the critical components of MFCs, the membrane plays a pivotal role in enabling selective proton transfer while maintaining electrochemical separation between the anodic and cathodic environments. Although polymeric membranes such as Nafion® are widely used due to their high proton conductivity and chemical stability, their high cost and limited durability under real-world conditions constrain their use in large-scale and/or long-term applications [9,10]. In this context, ceramic membranes have been proposed as a cost-effective and robust alternative, offering favorable ion exchange properties, high mechanical strength, and excellent stability under harsh conditions [11,12,13]. These attributes position ceramic membranes as a promising solution for the scale-up of MFCs, particularly in scenarios where affordability and durability are essential [14]. Therefore, these membranes constitute a suitable option for MFCs intended for real-world deployment, especially in rural or decentralized wastewater treatment scenarios [15,16].
Ceramic membranes can be fabricated from naturally occurring clays through simple processes such as shaping, drying, and sintering. Their performance depends on properties such as porosity, thickness, and mineralogical composition [17]. Various shaping techniques—including pressing, casting, and molding—have been employed to modulate membrane porosity and enhance electrochemical efficiency [18]. Several types of natural clays—such as bentonite, kaolinite, illite, and clay mixtures—have been investigated for membrane fabrication due to their varying structural, chemical, and thermal characteristics [19,20]. For instance, Cheraghipoor et al. demonstrated that red pottery clay with a sponge-like morphology enabled coulombic efficiencies of up to 27% [21]. Pasternak et al. on the other hand, reported enhanced biofilm formation and electrochemical performance using pyrophyllite, earthenware, mullite, and alumina clays. The improved performance of these materials was attributed to higher porosity and silica content [15]. Salar-García and Ieropoulos also found that sintering temperature significantly influenced the electrochemical behavior of terracotta membranes, with optimal performance observed between 860 and 1030 °C [22]. Later, the same group demonstrated that the incorporation of iron oxide in refractory clays improved porosity and enhanced power generation, with the best results achieved at 1100 °C and 5.75 vol% iron oxide [23]. In terms of power generation, recent research has demonstrated the promising performance of clay-based ceramic membranes in microbial fuel cells. For example, Gong et al. reported power densities of up to 182.8 mW m−2, while Khalili et al. achieved 321 mW m−2 using commercially available unglazed ceramics [24,25].
Despite these promising results, research on the systematic evaluation of commercially available clays for ceramic membrane fabrication is still incipient and, in this context, this study aims to assess the performance and applicability of ceramic membranes made from four different commercial clays in MFCs treating synthetic wastewater. The results contribute to the growing evidence supporting the development of cost-effective and scalable MFC technologies for sustainable wastewater treatment and energy recovery.
2. Materials and Methods
2.1. Clay Selection
Four commercially available clays—readily accessible on the market and used in various industrial applications—were selected for the fabrication of ceramic membranes in this study: Zacatecas clay (A-Z, USD 0.66/kg), Pottery clay (R-4, USD 0.5/kg), sodium bentonite (B-S, USD 0.91/kg), and calcium bentonite (B-C, USD 1.19/kg). Zacatecas clay (Materiales Cerámicos Salazar, Dolores Hidalgo, México) is primarily used in red tile production, while R-4 clay (Materiales Cerámicos Salazar, Dolores Hidalgo, México) is commonly employed in low-cost pottery. Sodium and calcium bentonites (Fosforita de México, Puebla de Zaragoza, Mexico), often referred to as “Fuller’s clay,” are widely used in industrial processes due to their high versatility. Prior research has shown promising results for these clays when used as ceramic membranes in MFCs [26].
2.2. Fabrication of Ceramic Membranes
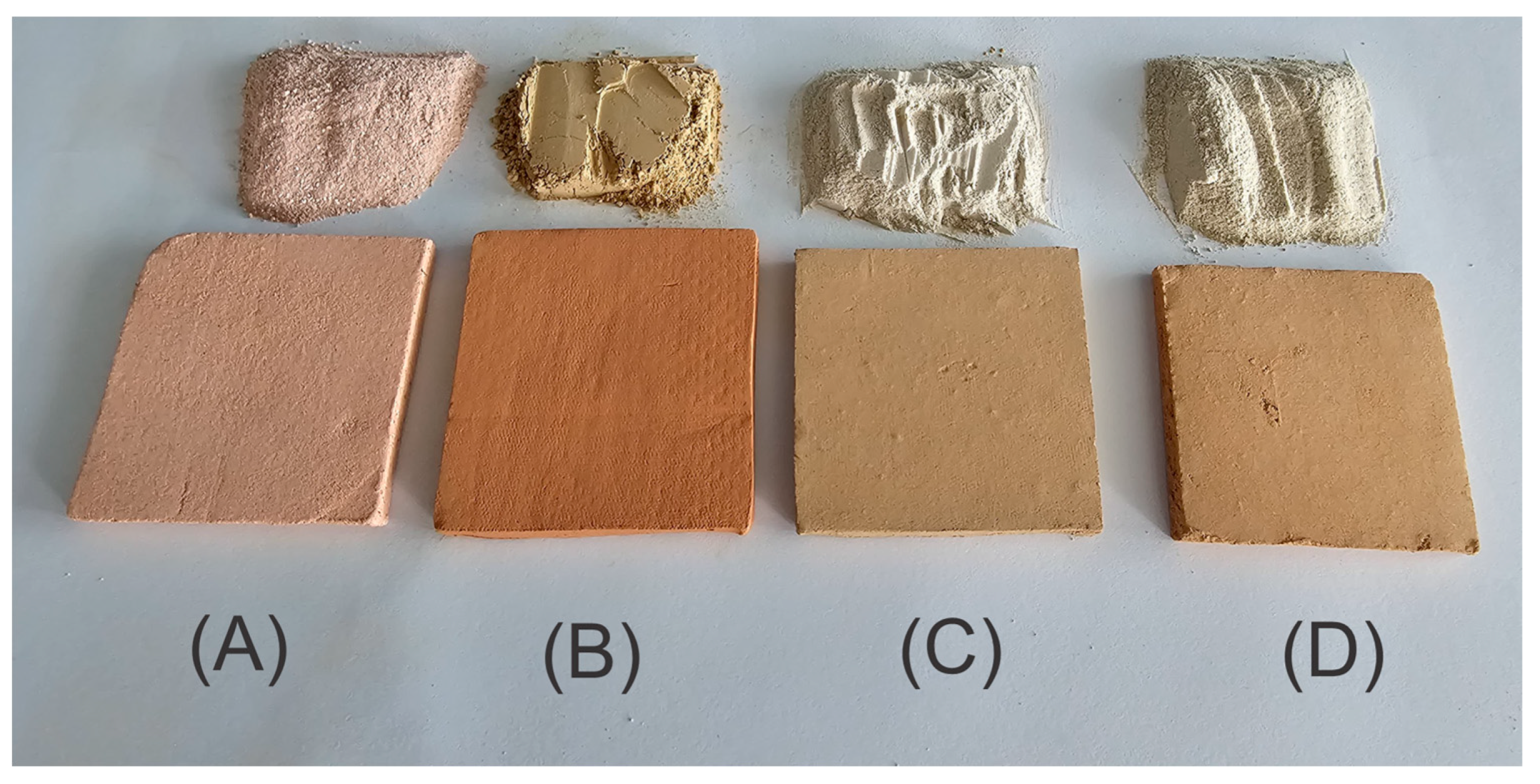
To prepare the membranes, 125 g of clay powder was mixed with 50 mL of water in a plastic container and manually kneaded until a homogeneous, plasticine-like consistency was achieved. The resulting paste was rolled and shaped into square plates (6.5 cm × 6.5 cm, 0.5 cm thick). The plates were placed between two acrylic sheets with an absorbent interlayer to ensure uniform drying and to prevent warping. Later, a glass container filled with water, with a total weight of 1 kg, was placed on the acrylic surface for 72 h in a shaded environment, followed by 24 h of sun exposure. Plates were then oven-dried at 105 °C for 2 h (Felisa FE-291AD, Guadalajara, México) and sintered in a muffle furnace (Whisterm Wisd J2) at 650 °C for 1 h with a controlled ramp rate of 10 °C/min, yielding the final ceramic membranes (Figure 1).
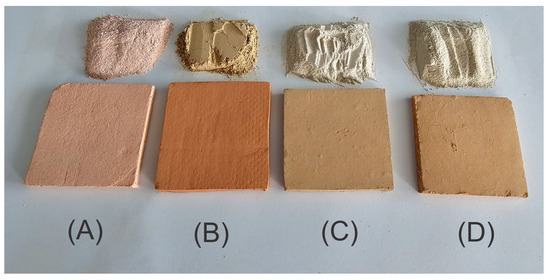
Figure 1.
Clay powders used and ceramic membranes fabricated: (A) A-Z; (B) R-4; (C) B-S; (D) B-C.
2.3. MFC Design and Construction
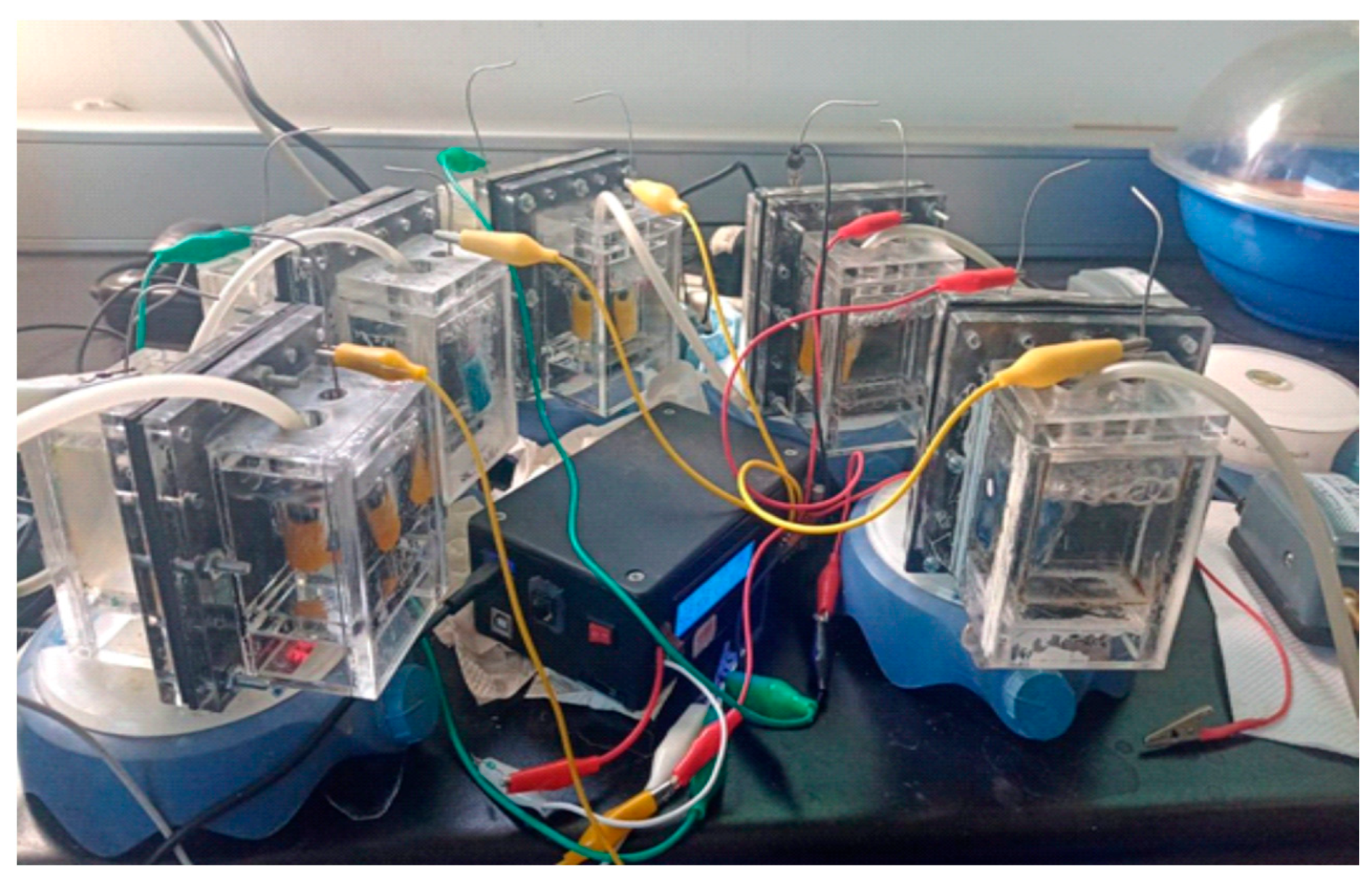
Five dual-chamber MFCs were constructed using 125 mL acrylic chambers and each MFC incorporated two carbon felt electrodes (5 cm × 5 cm × 0.5 cm, working as anode and cathode), placed on each side of a ceramic membrane. The electrode-membrane assembly was secured using stainless steel screws. Electrical connections were made using 24-gauge stainless steel wire and a control MFC using a Nafion 115 membrane was employed for comparison purposes (See Figure 2).
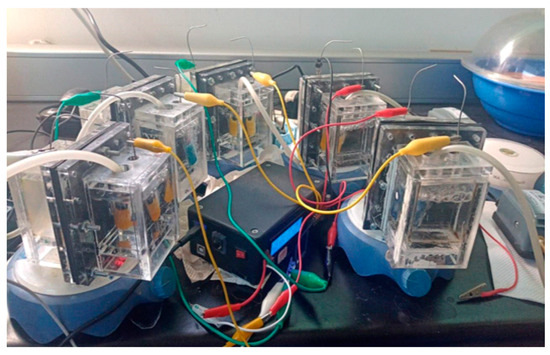
Figure 2.
Experimental system with the five MFCs used and the continuous monitoring system for voltage, current, and power.
2.4. MFC Inoculation and Operation
The anode chambers were inoculated by completely filling them with wastewater collected from the effluent generated at the Centro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ), thereby ensuring full electrode submersion and suitable conditions for microbial activity. Biofilm formation and microbial acclimation were conducted under batch-fed operation and open-circuit conditions. Upon voltage stabilization near zero, 125 mL of synthetic wastewater—previously validated by our group [27,28]—was introduced. The anode chamber was stirred at 300 RPM using a magnetic stirrer (IKA Color Squid Number One, Staufen im Breisgau, Germany), while the cathode chamber was aerated at 3 L/min using an air pump (Aquapura 1592). The MFCs voltage was continuously monitored via a custom-built Arduino-based data acquisition system developed in-house. After confirming stable operation and mature biofilm formation, an external resistor of 650 Ω was connected to each MFC, and electrical performance was continuously recorded using the data acquisition system.
2.5. COD (Chemical Oxygen Demand) Determination
COD was measured using a standardized spectrophotometric procedure [28]. Effluent samples from the experimental tests were centrifuged at 3500 rpm and subsequently mixed with the digestion solution. The samples were then heated at 150 °C for 2 h. After digestion, absorbance at 620 nm was measured, and COD concentration was calculated accordingly.
2.6. Coulombic Efficiency
The coulombic efficiency (CE) of the MFC was determined under Bach operating conditions, according next equation.
where M is the molecular weight of O2, ∫ Idt represents the total charge generated over the operation time, ne is the number of electrons involved in the oxygen reduction reaction, F is the Faraday constant, V is the volume of the anode chamber, and ∆COD is the variation in chemical oxygen demand during the experimental run [29].
2.7. Physicochemical Characterization
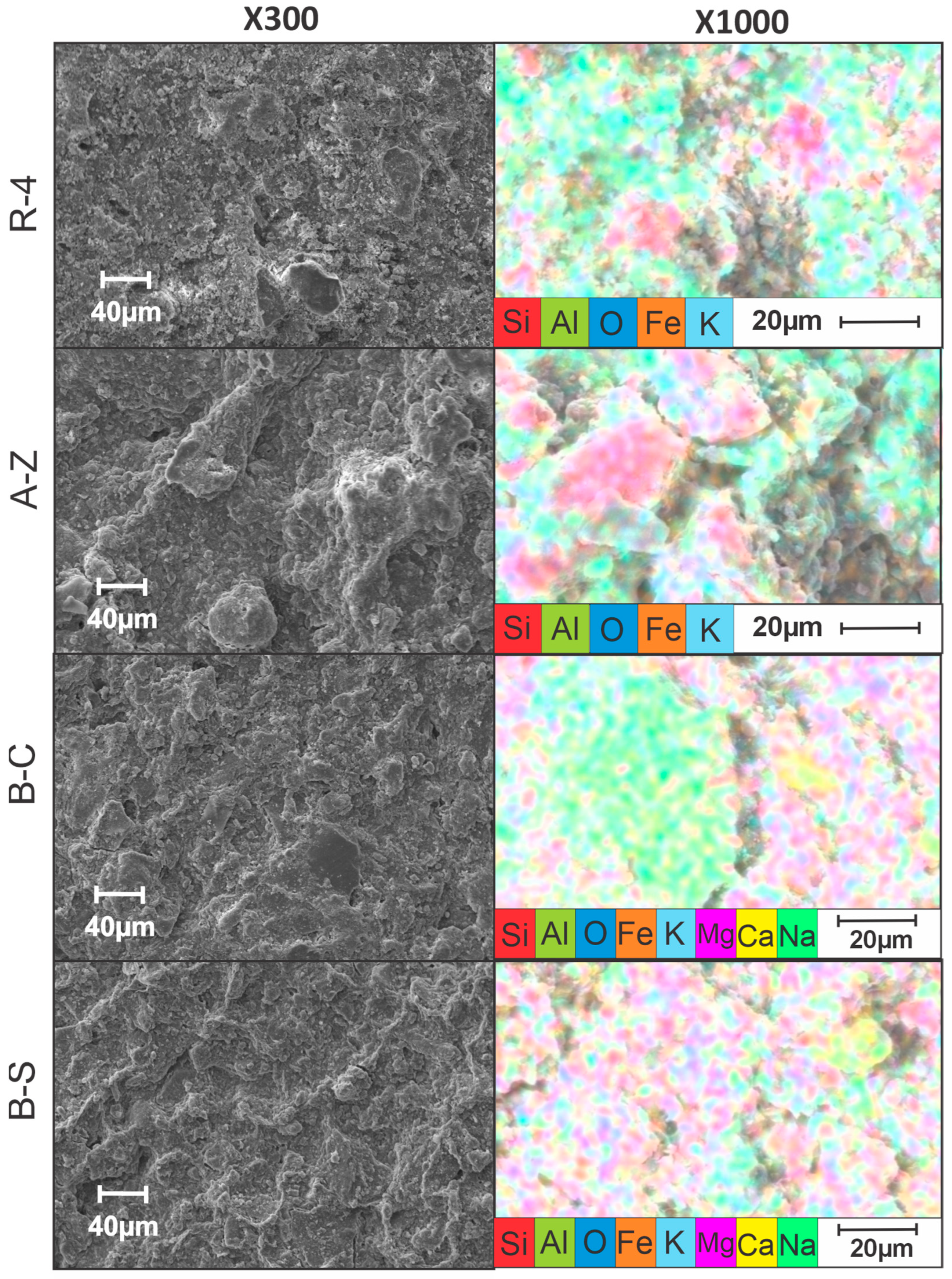
Morphological and physicochemical characterization of the ceramic membranes were performed using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM, Carl Zeiss EVO MA15, Cambridge, Reino Unido—EDS, Bruker XFlash, Berlín, Alemania). Measurements were taken at 300× and 1000× magnifications.
3. Results and Discussion
3.1. First Stage (Open-Circuit Conditions)
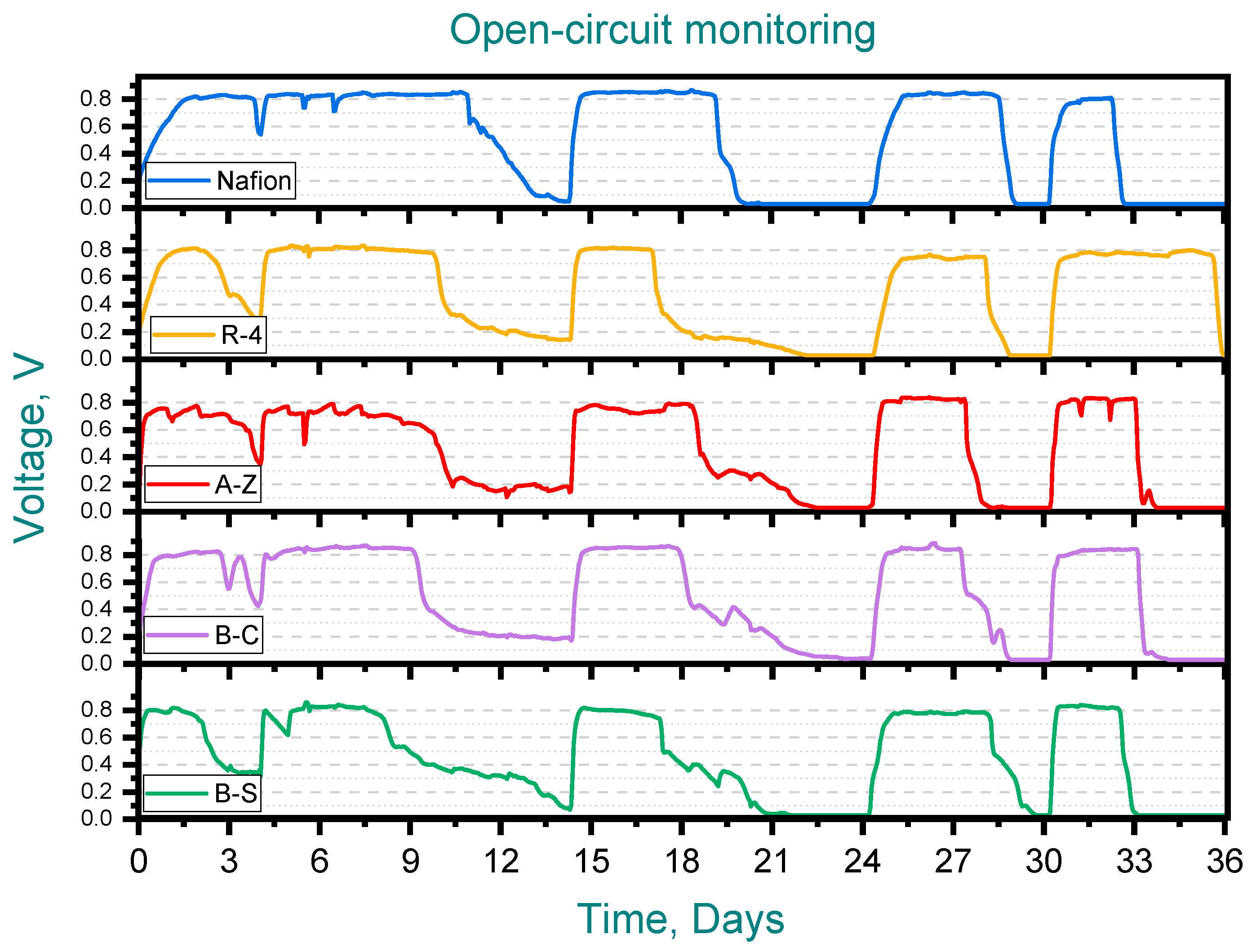
Figure 3 shows the performance of the ceramic membrane MFCs during the first (open-circuit) stage. In all cases maximum voltage outputs were consistently within the range of 0.75–0.85 V, reflecting values that are similar to those observed with Nafion. Also, a progressive reduction in the duration of the feeding cycles was observed, decreasing from 6 to 3 days for most membranes, including Nafion. However, the MFC with the R-4 membrane exhibited atypical behavior in the final cycle, requiring twice as long (6 days) to complete.
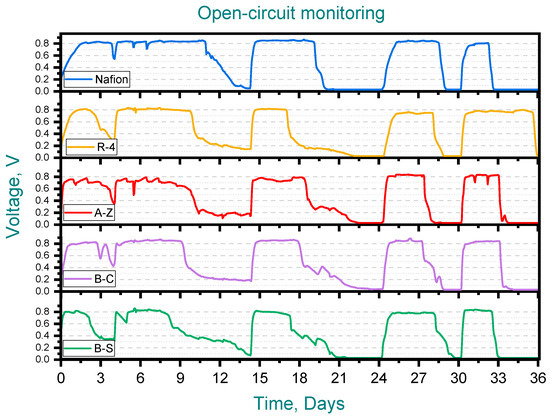
Figure 3.
Open-circuit monitoring, Pottery clay (R-4), Zacatecas clay (A-Z), calcium bentonite (B-C) and sodium bentonite (B-S).
3.2. Second Stage (Closed-Circuit Conditions)
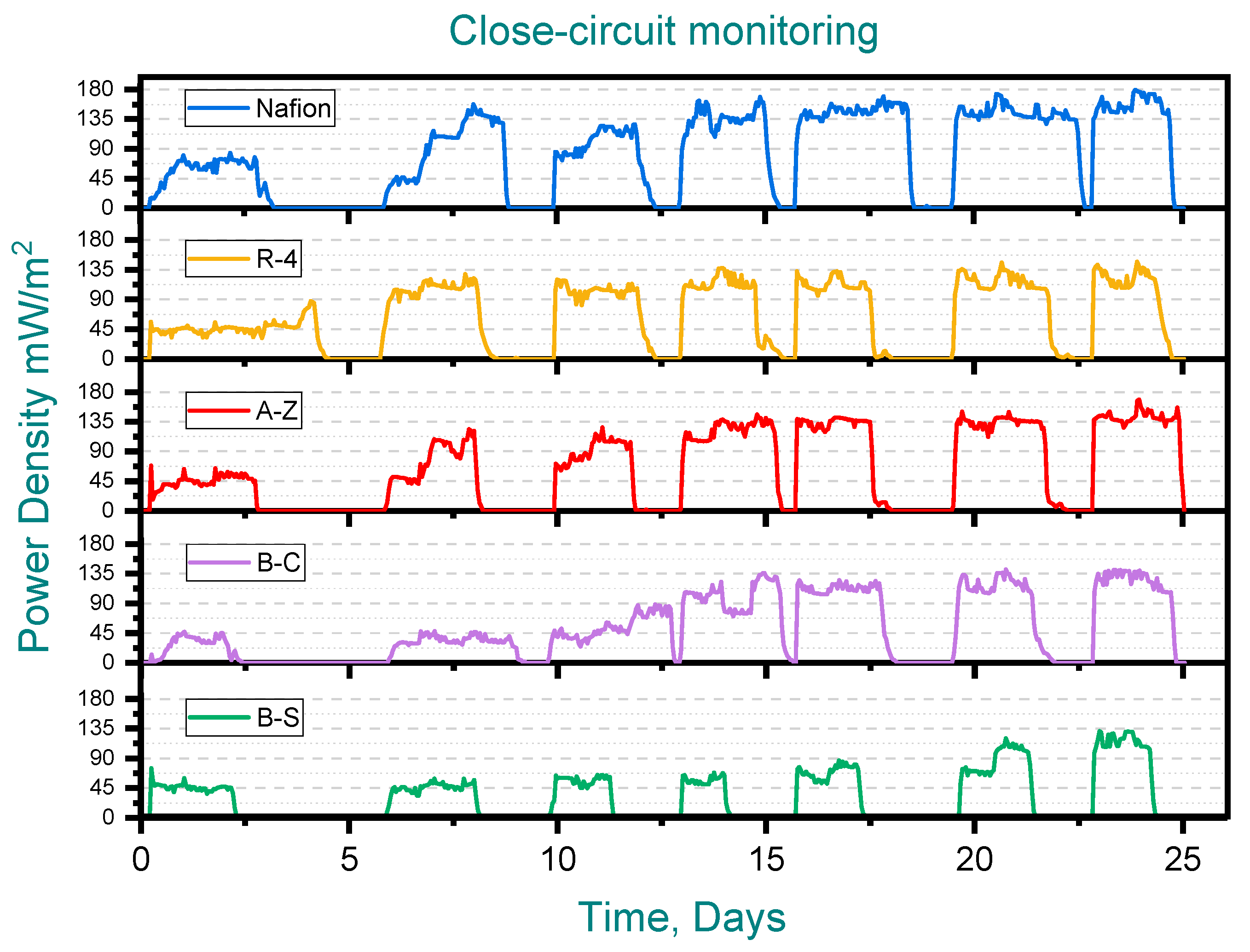
During the second (closed-circuit) stage, voltage, current and power densities were recorded over seven runs across 25 days (See Figure 4). Considering the power generated at the initial stage, ceramic membrane MFCs produced ~40 mW/m2, while Nafion delivered ~80 mW/m2. However, during successive feedings of synthetic wastewater into the MFCs, the maximum power density recorded was 180 mW/m2 for Nafion, 170 mW/m2 for A-Z, 145 for R-4 and 140 for B-C. The lowest performing membrane in this stage was B-S (120 mW/m2). It is important to note that the normalization of power output was carried out with respect to the cross-sectional area of the anode (5 × 5 cm).The results obtained are consistent with previous studies, where comparable performance values have been reported for both unmodified and modified clay-based membranes [24,25,28].
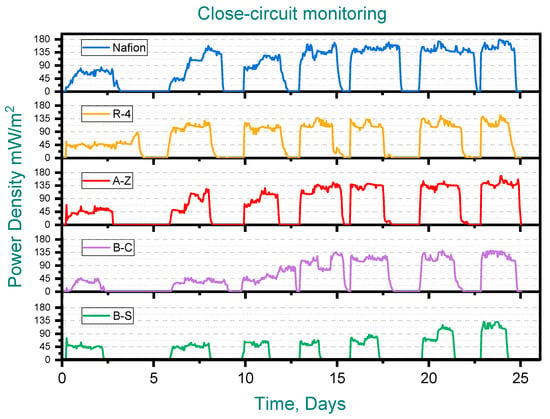
Figure 4.
Closed-circuit monitoring. Pottery clay (R-4), Zacatecas clay (A-Z), calcium bentonite (B-C) and sodium bentonite (B-S).
3.3. COD Removal
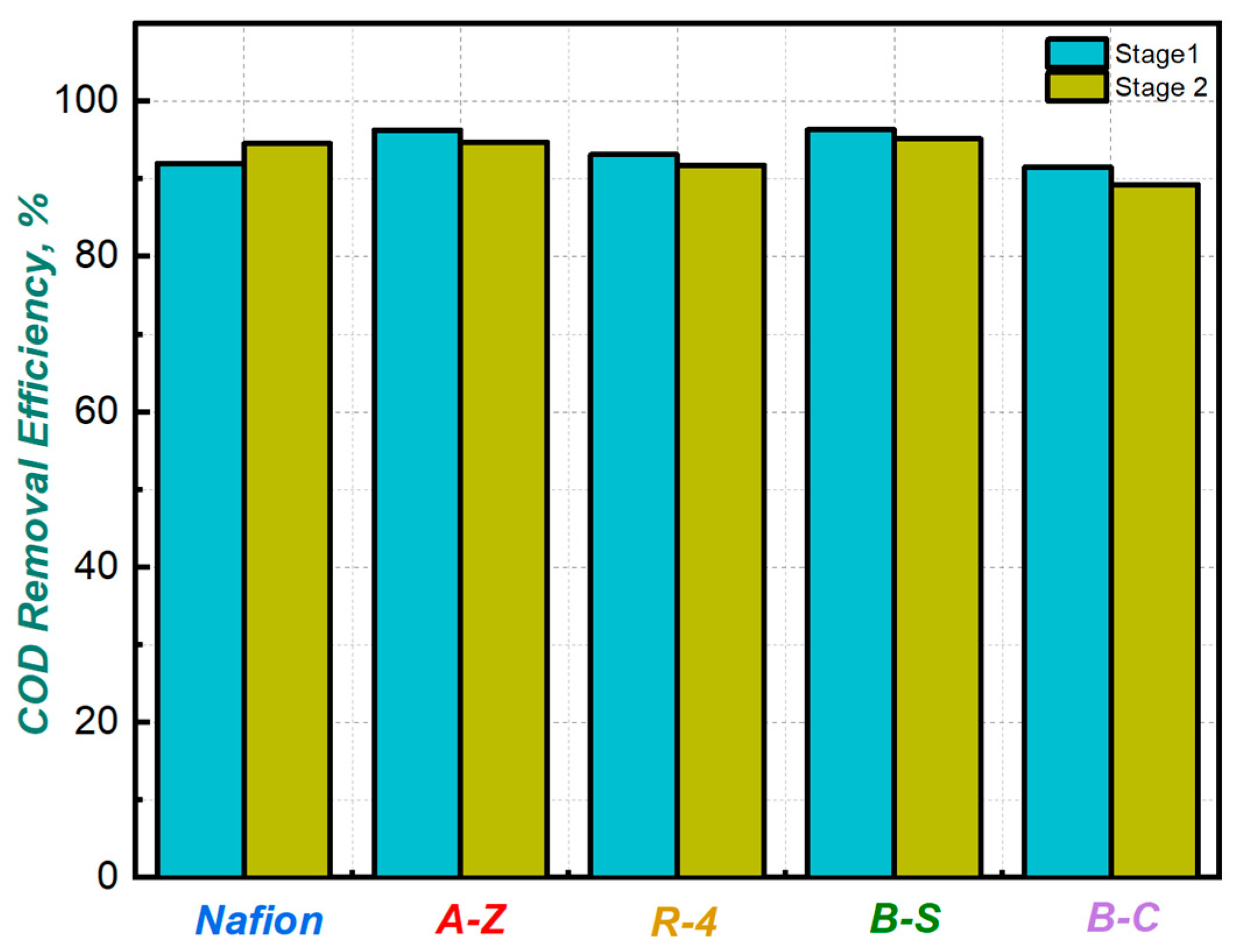
As shown in Figure 5 the average COD removal efficiency remained high in both stages, ranging between 89% and 95%. According to this, the evaluated cells not only generated power levels comparable to those reported in previous studies, but also demonstrated efficient removal of organic substrates.
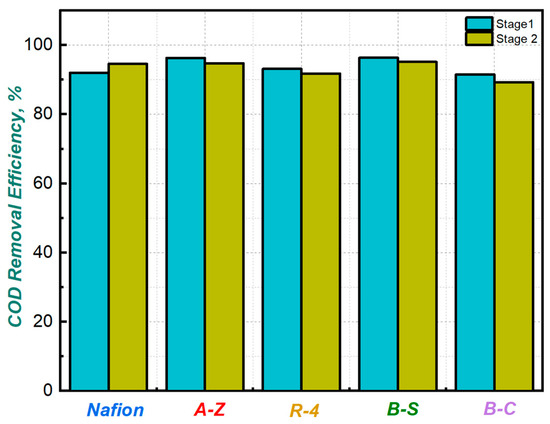
Figure 5.
COD removal efficiency for MFCs with different membranes.
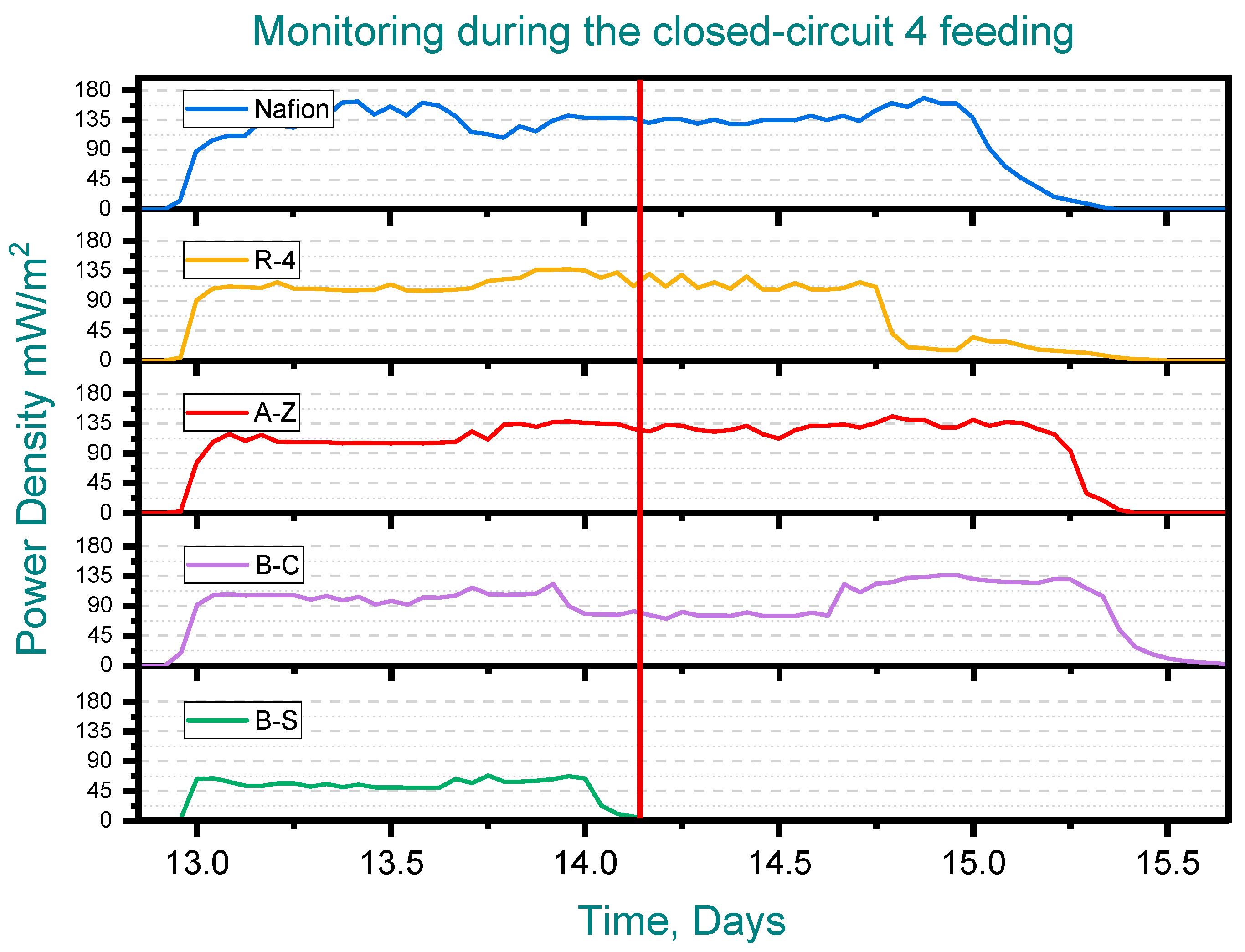
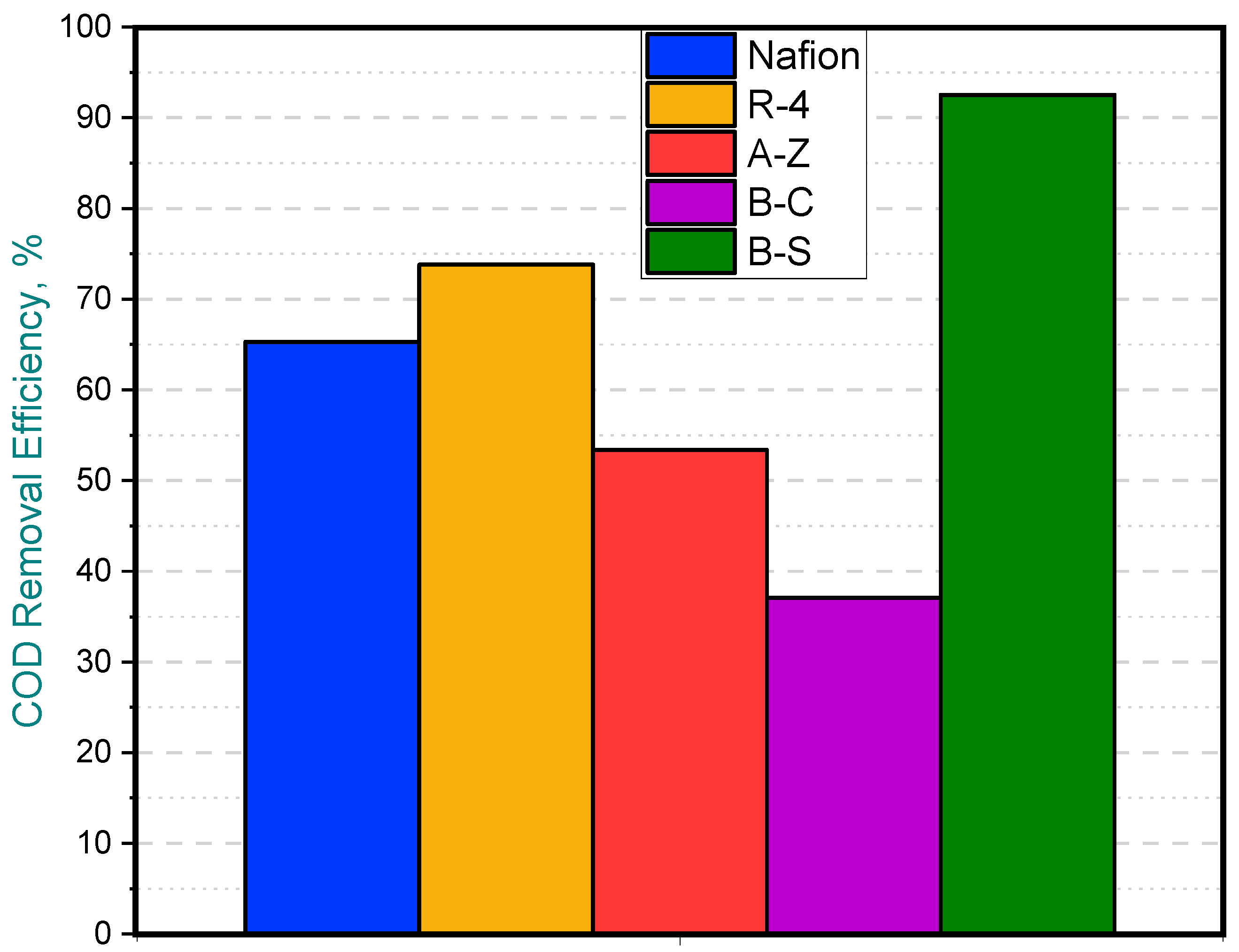
When water samples were taken from the anode chamber at the point where the potential of the first cell dropped to zero—while the remaining cells continued generating potential—the chemical oxygen demand (COD) of the solution was analyzed. Clear differences in COD removal were observed at this point, marked by red line (See Figure 6). In the B-C cell, where the voltage had already fallen to zero, COD removal reached 92%, indicating that the substrate available for voltage generation had been completely depleted. In contrast, the other cells exhibited COD removals ranging from 36% to 74%, which explains their continued ability to generate voltage. Notably, the lowest COD removal was observed in the cell equipped with the B-C membrane and the removal achieved with the cell containing the A-Z membrane is higher than that obtained with Nafion (Figure 7).
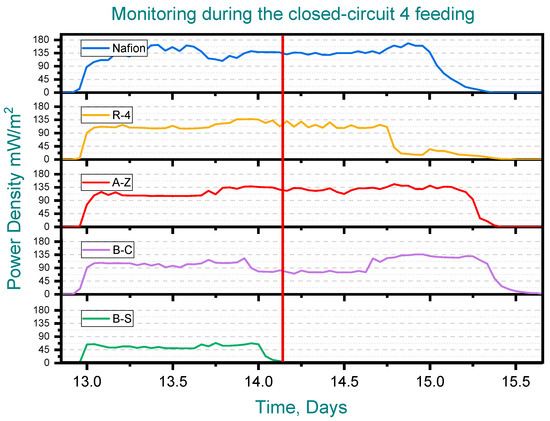
Figure 6.
Monitoring during the closed-circuit 4 feeding, Pottery clay (R-4), Zacatecas clay (A-Z), calcium bentonite (B-C) and sodium bentonite (B-S).
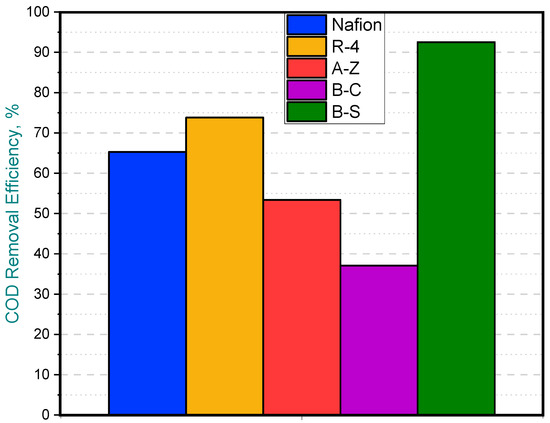
Figure 7.
Partial COD removal efficiency for MFCs with different membranes.
According to the above, it can be observed that some membranes, such as R-4, promote a slower COD removal, higher power generation, and longer voltage duration within the cycle, while others, such as B-S, favor a faster COD removal but with lower energy generation and shorter voltage cycle duration (See Figure 6).
3.4. Coulombic Efficiency
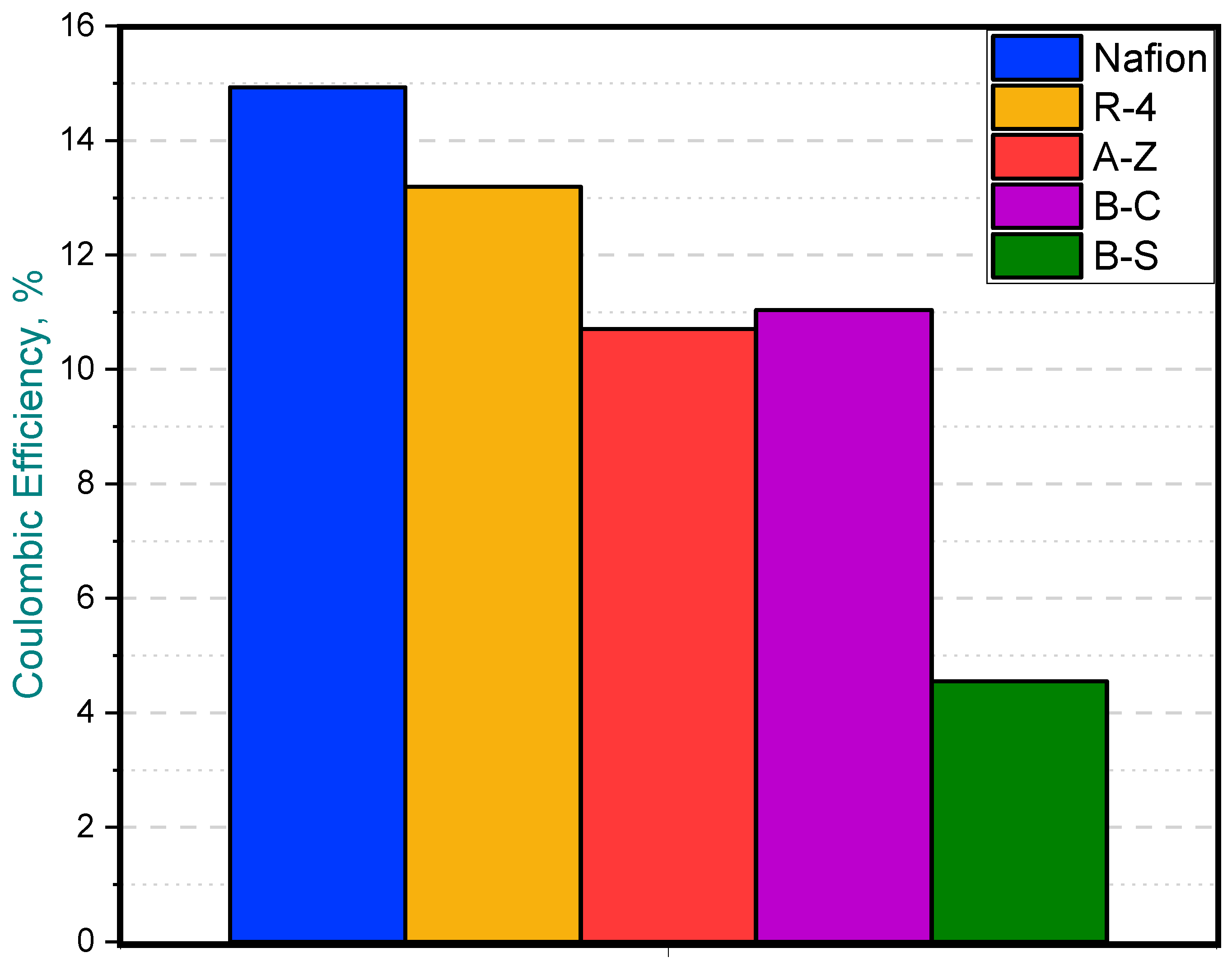
Regarding Coulombic efficiency, the MFC equipped with the Nafion membrane achieved the highest average value at 14.92%, closely followed by the R-4 ceramic membrane, which reached a comparable efficiency of 13.18%. On the other hand, the B-S membrane MFC exhibited the lowest CE (4.54%), likely due to its reduced power output (Figure 8).
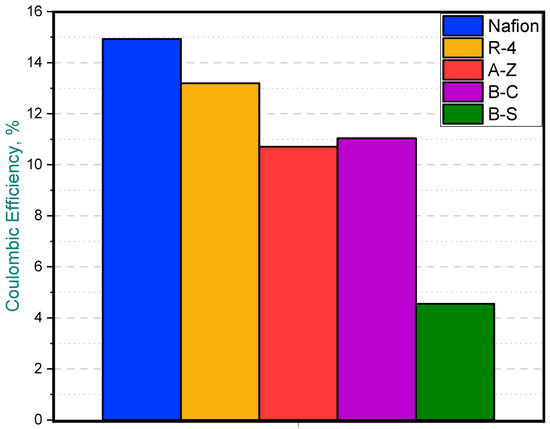
Figure 8.
Coulombic efficiency in MFCs with different membranes.
3.5. SEM/EDS Analysis
SEM micrographs obtained at 300 and 1000 magnification levels, reveal distinct morphological differences among the ceramic membranes. In this way, the A-Z and R-4 samples exhibit relatively compact and homogeneous surface textures, with uniform grain distribution, suggesting effective sintering and structural stability. In contrast, the B-C and B-S membranes display more heterogeneous and porous surfaces, which may indicate inconsistencies during the fabrication or sintering processes (Figure 9).
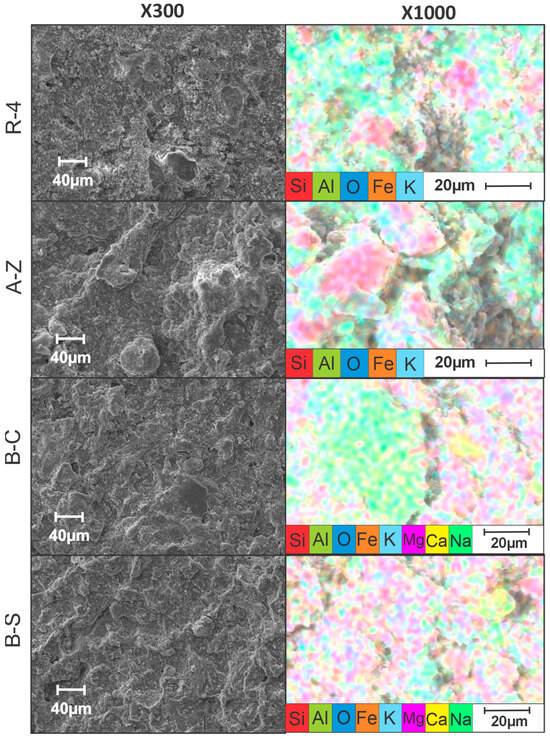
Figure 9.
SEM images of ceramic membranes.
Elemental mapping by EDS confirms the predominant presence of aluminum (Al) and silicon (Si) in all membranes, consistent with the expected composition of clay-based materials. In particular, the B-C and B-S membranes show significant concentrations of calcium (Ca) and sodium (Na), respectively, confirming their nature as calcium and sodium bentonites. Notably, the R-4 membrane demonstrates a more uniform elemental distribution across the surface, which is likely associated with its superior electrochemical performance.
Supplementary Figures S1 and S2 show photographs of the Nafion membrane and the R-4 ceramic membrane after use. The Nafion membrane presented some signs of degradation, whereas the ceramic membrane exhibited only minor physical alterations.
4. Conclusions
Although all the tested ceramic membranes worked in the MFC systems under study, their performance varied significantly, indicating that not all clays are suitable for the fabrication of ion-exchange membranes that support efficient energy generation and charge transport. The ceramic membrane made with R-4 clay is, however, characterized by a performance comparable to that of Nafion, both in terms of voltage generation during the initial open-circuit stage and of power output during the closed-circuit stage. It is noteworthy that the power density curves from the second stage revealed that the MFC equipped with the R-4 membrane reached a higher maximum power output (174 mW/m2) than that of the cell prepared with Nafion (153 mW/m2). Unlike Nafion, which showed clear signs of degradation after multiple operational cycles, the R-4 ceramic membrane showed no significant visible signs of deterioration. Overall, R-4 ceramic membranes represent a cost-effective and robust alternative to polymeric membranes in MFC applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17213064/s1, Figure S1: Ceramic membranes after being used; Figure S2: Nafion membranes after being used.
Author Contributions
Conceptualization, F.J.R.-V.; methodology, F.A.R.A.; formal analysis F.A.R.A. and V.A.R.C.; investigation, F.A.R.A. and F.J.R.-V.; resources, V.A.R.C., L.A.G. and F.J.R.-V.; writing—original draft preparation, F.A.R.A. and F.J.R.-V.; writing—review and editing, L.A.G. and V.A.R.C.; visualization, V.A.R.C.; supervision, F.J.R.-V.; project administration, F.J.R.-V.; funding acquisition, F.J.R.-V. All authors have read and agreed to the published version of the manuscript.
Funding
Projects number LN 2025-I-16 from the Mexican Secretariat of Science, Humanities, Technology and Innovation.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Acknowledgements are extended to the CONAHCYT National Laboratory for the Science and Technology of Water.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Sharma, A.; Đelević, L.; Herkendell, K. Next-Generation Proton-Exchange Membranes in Microbial Fuel Cells: Overcoming Nafion’s Limitations. Energy Technol. 2024, 12, 2301346. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M. Effect of various carbon-based cathode electrodes on the performance of microbial fuel cell. Biofuel Res. J. 2015, 2, 296–300. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Tan, W.H.; Chong, S.; Fang, H.W.; Pan, K.L.; Mohamad, M.; Lim, J.W.; Tiong, T.J.; Chan, Y.J.; Huang, C.-M.; Yang, T.C.-K. Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues. Processes 2021, 9, 985. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Chakma, R.; Hossain, M.K.; Paramasivam, P.; Bousbih, R.; Amami, M.; Toki, G.F.I.; Haldhar, R.; Karmaker, A.K. Recent Applications, Challenges, and Future Prospects of Microbial Fuel Cells: A Review. Glob. Chall. 2025, 9, 2500004. [Google Scholar] [CrossRef]
- Vidhyeswari, D.; Surendhar, A.; Bhuvaneshwari, S. General aspects and novel PEMss in microbial fuel cell technology: A review. Chemosphere 2022, 309, 136454. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 23354–23362. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Ceramic-based microbial fuel cells (MFCs): A review. Int. J. Hydrogen Energy 2017, 42, 1672–1690. [Google Scholar] [CrossRef]
- Daud, S.M.; Noor, Z.Z.; Mutamim, N.S.A.; Baharuddin, N.H.; Aris, A.; Faizal, A.N.M.; Ibrahim, R.S.; Suhaimin, N.S. A critical review of ceramic microbial fuel cell: Economics, long-term operation, scale-up, performances and challenges. Fuel 2024, 365, 131150. [Google Scholar] [CrossRef]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Comprehensive Study on Ceramic Membranes for Low-Cost Microbial Fuel Cells. ChemSusChem 2016, 9, 88–96. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Park, S.G.; Eisa, T.; Mungray, A.K.; Madenli, E.C.; Olabi, A.G.; Abdelkareem, M.A.; Chae, K.-J. Current outlook towards feasibility and sustainability of ceramic membranes for practical scalable applications of microbial fuel cells. Renew. Sustain. Energy Rev. 2022, 167, 112769. [Google Scholar] [CrossRef]
- Roslee Ab Jamal, N.A.S.; Othman, N.H.; Abdul Razak, N.A.; Alias, N.H.; Mat Shayuti, M.S.; Marpani, F.; Razlan, M.R.M.; Jumahat, A.; Othman, M.H.D.; Lau, W.J.; et al. Fabrication of low-cost granite dust ceramic hollow fibre membrane: Effects of sintering temperature. Mater. Today Proc. 2023; in press. [Google Scholar]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. C. R. Chim. 2018, 22, 175–187. [Google Scholar] [CrossRef]
- Tiwari, A.; Yadav, N.; Jadhav, D.A.; Saxena, D.; Anghan, K.; Sandhwar, V.K.; Saxena, S. A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells. Water 2024, 16, 1597. [Google Scholar] [CrossRef]
- Ahmad, S.T.; Ahmad, R.; Shaukat, H.; Rout, P.R.; Fazal, T.; Dumfort, A. Bioenergy production from wastewater using cost-effective ceramic membranes: A review. Environ. Chem. Lett. 2025, 23, 463–490. [Google Scholar] [CrossRef]
- Cheraghipoor, M.; Mohebbi-Kalhori, D.; Noroozifar, M.; Maghsoodlou, M.T. Comparative study of bioelectricity generation in a microbial fuel cell using ceramic membranes made of ceramic powder, Kalporgan’s soil, and acid leached Kalporgan’s soil. Energy 2019, 178, 368–377. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Ieropoulos, I. Optimisation of the internal structure of ceramic membranes for electricity production in urine-fed microbial fuel cells. J. Power Sources 2020, 451, 227741. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Walter, X.A.; Gurauskis, J.; de Ramón Fernández, A.; Ieropoulos, I. Effect of iron oxide content and microstructural porosity on the performance of ceramic membranes as microbial fuel cell separators. Electrochim. Acta 2021, 367, 137385. [Google Scholar] [CrossRef]
- Gong, K.; Zhang, X.; Tan, B.; Tang, J.; Wang, L. Development and characterization of clay based ceramic membrane with high proton conductivity and low cost for application in microbial fuel cell. Mater. Res. Bull. 2025, 184, 113278. [Google Scholar] [CrossRef]
- Khalili, H.-B.; Mohebbi-Kalhori, D.; Afarani, M.S. Microbial fuel cell (MFC) using commercially available unglA-Zed ceramic wares: Low-cost ceramic separators suitable for scale-up. Int. J. Hydrogen Energy 2017, 42, 8233–8241. [Google Scholar] [CrossRef]
- Ghadge, A.N.; Ghangrekar, M.M. Development of low cost ceramic separator using mineral cation exchanger to enhance performance of microbial fuel cells. Electrochim. Acta 2015, 166, 320–328. [Google Scholar] [CrossRef]
- González-Nava, C.; Godínez, L.A.; Chávez, A.U.; Cercado, B.; Arriaga, L.G.; Rodríguez-Valadez, F.J. Study of different carbon materials for their use as bioanodes in microbial fuel cells. Water Sci. Technol. 2016, 73, 2849–2857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rojas, F.A.; Hernández-Benitez, C.; Ramírez, V.; Ieropoulous, I.; Godínez, L.A.; Robles, I.; Meza, D.B.; Rodríguez-Valadez, F.J. Impact of electrode arrangement and electrical connections on the power generation of ceramic membrane microbial fuel cell. Fuel Cells 2024, 24, e202300241. [Google Scholar] [CrossRef]
- Pandit, S.; Das, D. Principles of Microbial Fuel Cell for the Power Generation. In Microbial Fuel Cell; Springer International Publishing: Cham, Switzerland, 2018; pp. 23–24. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).