Optimization of Irrigation Efficiency and Water Retention in Agroecological Systems Through Organic Matter Management
Abstract
1. Introduction
2. Materials and Methods
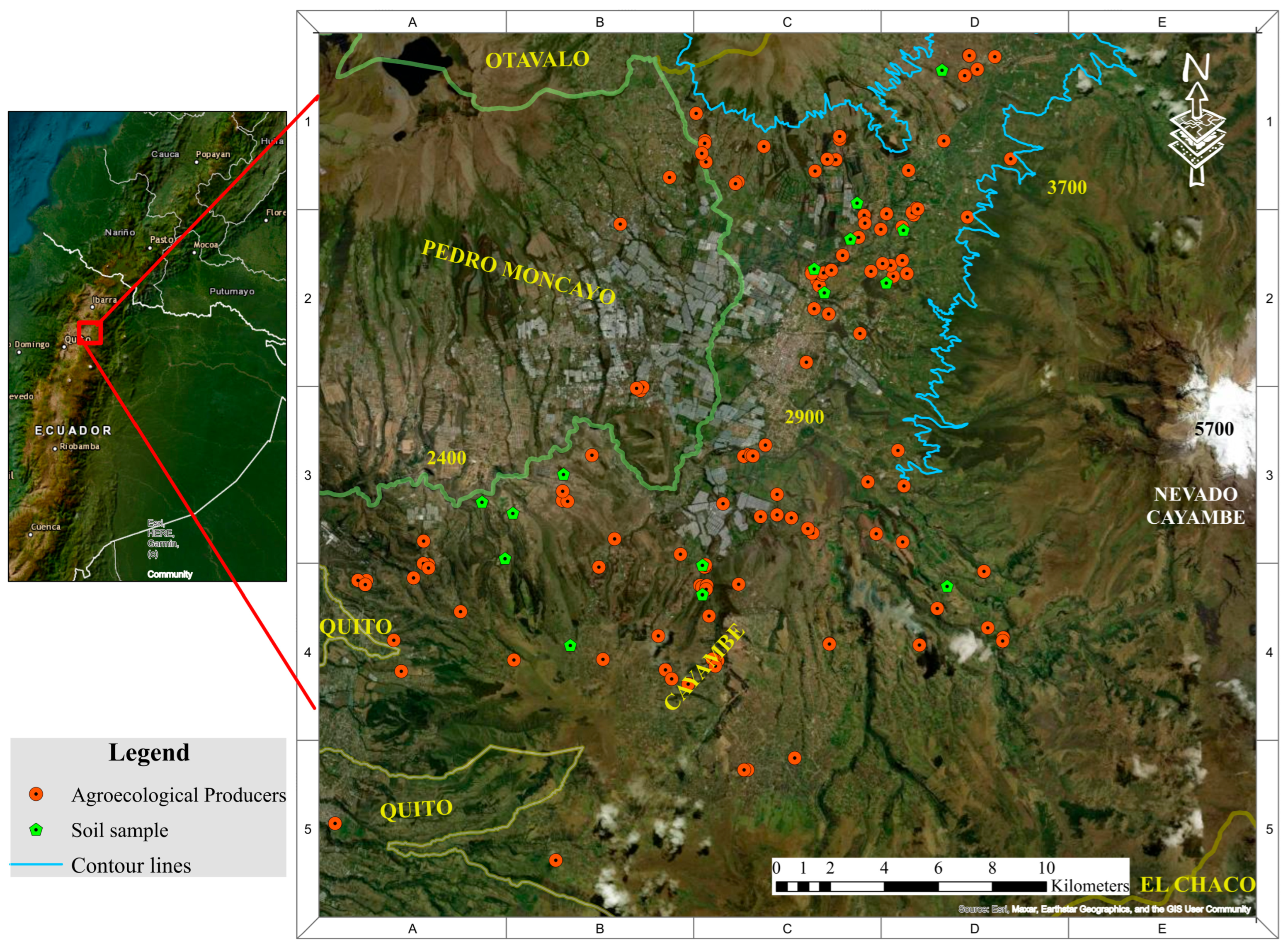
2.1. Study Area
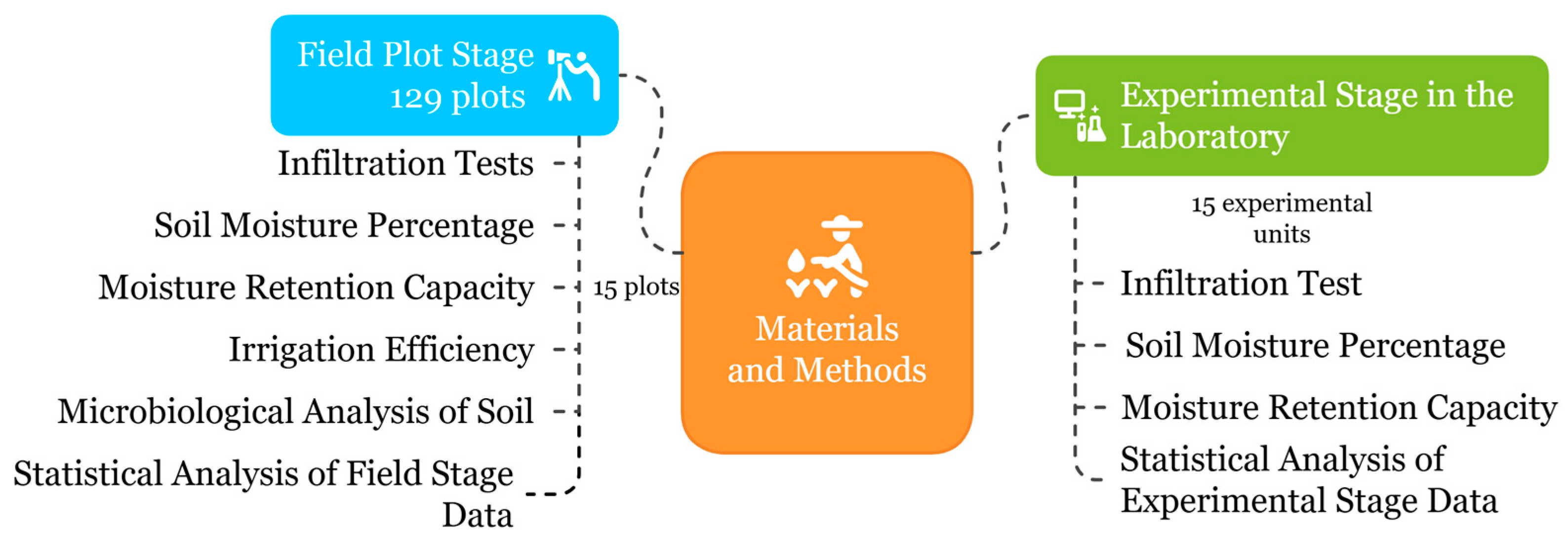
2.2. Methodological Approach
2.3. Field Stage
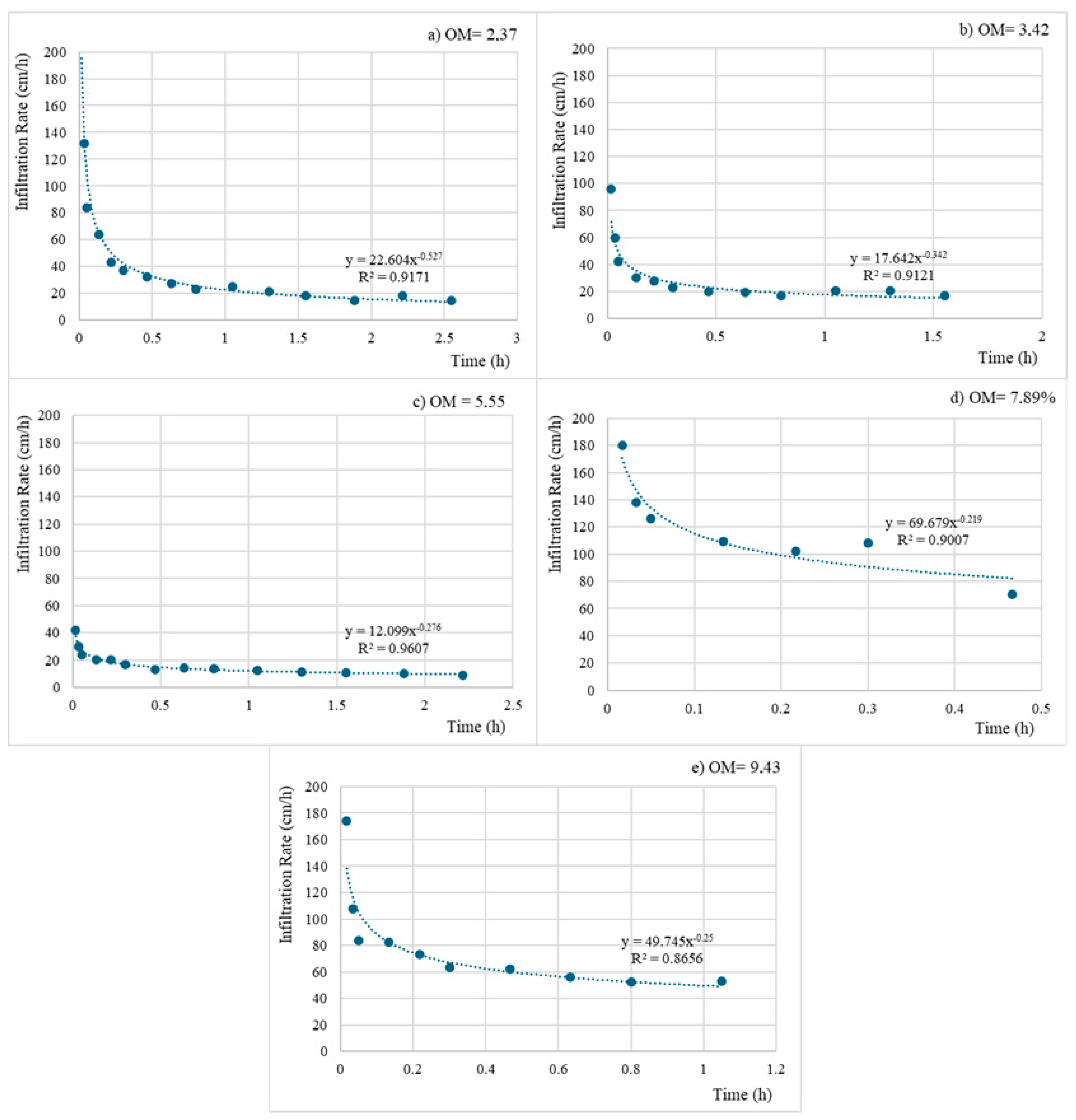
2.3.1. Infiltration Rate
2.3.2. Soil Moisture
2.3.3. Soil Moisture Retention Capacity
2.3.4. Evaluation of Irrigation Efficiency
2.3.5. Soil and Microbiological Analysis
2.3.6. Statistical Analysis of Field Data
2.4. Laboratory Experimental Stage
Infiltration Trial
3. Results
3.1. Organic Matter and Its Relationship with Infiltration and Soil Moisture
3.2. Soil Moisture Content and Water Retention Capacity
3.3. Relationship Between Organic Matter and Microorganisms
3.4. Irrigation Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OM | Organic Matter |
References
- Salazar, O.; Chinchilla-Soto, C.; de los Santos-Villalobos, S.; Ayala, M.; Benavides, L.; Berriel, V.; Cardoso, R.; Chavarrí, E.; Meigikos dos Anjos, R.; Liz González, A.; et al. Water Consumption by Agriculture in Latin America and the Caribbean: Impact of Climate Change and Applications of Nuclear and Isotopic Techniques. Int. J. Agric. Nat. Resour. 2022, 49, 1–21. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). El Estado Mundial de la Agricultura y la Alimentación 2020. Superar los Desafíos Relacionados Con el Agua en la Agricultura; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Zúniga, D.; Mendoza, R.; Wetson, G.; Instituto Interamericano de Cooperación para la Agricultura (IICA); Fondo Internacional de Desarrollo Agrícola (FIDA); Red de Comercialización Comunitaria Alternativa (Red COMAL); INNOVA Agricultura Familiar. Gestión y Manejo del Agua en la Agricultura; IICA: San José, Costa Rica, 2021; Available online: https://hdl.handle.net/11324/19866 (accessed on 20 May 2024).
- Betancourt-Aguilar, C.; Tartabull-Puñales, T.; Labaut-Betancourt, Y. El Manejo Integrado del Agua en la Agricultura: Necesidades de Implementación y Aspectos Vinculados. Rev. Científica Agroecosistemas 2017, 5, 40–54. Available online: https://aes.ucf.edu.cu/index.php/aes/article/view/119/156 (accessed on 3 January 2025).
- Omer, E.; Szlatenyi, D.; Csenki, S.; Alrwashdeh, J.; Czako, I.; Láng, V. Farming Practice Variability and Its Implicatios for Soil Health in Agriculture: A Review. Agriculture 2024, 14, 2114. [Google Scholar] [CrossRef]
- Cachipuendo, C. The Technification of Irrigation as a Strategy of Community Resilience. In Case Study: Pisque River Basin, Ecuador; Springer: Berlin/Heidelberg, Germany, 2022; pp. 207–216. [Google Scholar]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Gárgano, C. Agroecología Para Salir Del Agroextractivismo. Experiencias de Vida y Producción En La Provincia de Buenos Aires. Geograficando 2024, 20, e146. [Google Scholar] [CrossRef]
- Cachipuendo, C.; Sandoval, C.; Sandoval, J. Sentinel-2A Imaging in Mapping Greenhouse Rose Production and Rainwater Harvesting for Agricultural Irrigation Use. Environ. Res. Commun. 2024, 6, 111005. [Google Scholar] [CrossRef]
- López-Silva, M.; Carmenates-Hernández, D.; Mujica-Cervantes, A.; Paneque-Rondon, P. Criterios de Eficiencia Para La Evaluación Del Riego Por Aspersión. Cienc. Técnicas Agropecu. 2019, 28, 1–8. Available online: https://revistas.unah.edu.cu/index.php/rcta/article/view/1130 (accessed on 14 January 2025).
- Cisneros Zayas, E.; González, Á.; García, A.; Placeres, Z.; Jiménez, E. Evaluación y Propuesta de Medidas En Diferentes Técnicas de Riego Por Aspersión Para Un Uso Eficiente Del Agua. Rev. Ing. Agrícola 2014, 4, 22–28. [Google Scholar]
- Julca-Otiniano, A.; Meneses-Florián, L.; Blas-Sevillano, R.; Bello-Amez, S. La Materia Orgánica, Importancia y Experiencia de Su Uso En La Agricultura. Idesia 2006, 24, 49–61. [Google Scholar] [CrossRef]
- Assan, M.; Gorosito, S. Relación Suelo Agua Planta: Infiltración; Universidad Nacional de Catamarca: Catamarca, Argentina, 2018; Available online: https://editorial.unca.edu.ar/Publicacione%20on%20line/CUADERNOS%20DE%20CATEDRA/Guia%20didactica%20RASPA%20Assan.pdf (accessed on 23 October 2024).
- García, A. La Materia Orgánica (MOS) y su Papel en la Lucha contra la Degradación del Suelo. In Proceedings of the XI Congreso Ecuatoriano de la Ciencia del Suelo, Quito, Ecuador, 29–31 May 2008. [Google Scholar]
- Cisneros, F.; Pacheco, T.E.; Feyen, J. Evaluación Del Rendimiento de Sistemas de Riego Por Aspersión de Baja Pluviosidad Como Resultado de La Aplicación de La Extensión Como Soporte Técnico. Ing. Agua 2007, 14, 177. [Google Scholar] [CrossRef]
- Murillo Montoya, S.A.; Mendoza Mora, A.; Fadul Vásquez, C.J. La Importancia de Las Enmiendas Orgánicas En La Conservación Del Suelo y La Producción Agrícola. Rev. Colomb. Investig. Agroindustr. 2019, 7, 58–68. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Wang, W.; Bi, S.; Li, F.; Degen, A.A.; Liu, Y.; Zhang, C.; Ren, H.; Liu, N. Soil organic matter composition affects ecosystem multifunctionality by mediating the composition of microbial communities in long-term restored meadows. Environ. Microbiome 2025, 20, 22. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.P.; Álvarez-Herrera, J.G.; Jaime-Guerrero, M. Propiedades Físicas de Un Suelo Sometido a La Aplicación de Diferentes Cantidades de Materia Orgánica de Escarabajo. Rev. U.D.C.A Actual. Divulg. Cient. 2024, 27, 1–9. [Google Scholar] [CrossRef]
- Rosabal Ayan, L.; Macías Coutiño, P.; Maza González, M.; López Vázquez, R.; Guevara Hernández, F. Microorganismos Del Suelo y Sus Usos Potenciales En La Agricultura Frente Al Escenario Del Cambio Climático. Magna Sci. UCEVA 2021, 1, 104–119. [Google Scholar] [CrossRef]
- Osorio-Vega, N.W. Microorganismos del suelo y su efecto sobre la disponibilidad y absorción de nutrientes por las plantas. In Materia Orgánica, Biología del Suelo y Productividad Agrícola: Segundo Seminario Regional Comité Regional Eje Cafetero; Cenicafé: Manizales, Colombia, 2009; pp. 43–71. [Google Scholar]
- Murphy, D.V.; Stockdale, E.A.; Brookes, P.C.; Goulding, K.W.T. Impact of Fauna on Chemical Transformations in Soil. In Soil Biological Fertility; Abbott, L.K., Murphy, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 17–35. ISBN 978-1-4020-6618-4. [Google Scholar]
- Rytioja, J.; Hildén, K.; Yuzon, J.; Hatakka, A.; de Vries, R.P.; Mäkelä, M.R. Plant-Polysaccharide-Degrading Enzymes from Basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Ticona, A.R.P.; Hamann, P.R.V.; Quirino, B.F.; Noronha, E.F. Deconstruction of Lignin: From Enzymes to Microorganisms. Molecules 2021, 26, 2299. [Google Scholar] [CrossRef] [PubMed]
- Morell, F.; Hernández, A.; Borges, Y.; Marentes, F.L. La Actividad de Los Hongos Micorrízicos Arbuscurales En La Esructura Del Suelo. Cult. Trop. 2009, 30, 25–31. Available online: https://www.redalyc.org/pdf/1932/193221673005.pdf (accessed on 12 October 2024).
- Vallejo, V.; Afanador, L.; Hernández, M.; Parra, D. Efecto de la Implementación de Diferentes Sistemas Agrícolas Sobre la Calidad del Suelo en El Municipio de Cachipay, Cundinamarca, Colombia. Bioagro 2018, 30, 27–38. Available online: https://revistas.uclave.org/index.php/bioagro/article/view/2708 (accessed on 4 October 2024).
- Delgadillo, O.; Pérez, L. Medición de la Infiltración del Agua en el Suelo; Universidad Mayor de San Simón, Centro Agua: Cochabamba, Bolivia, 2016; Available online: http://www.centro-agua.umss.edu.bo/wp-content/uploads/2022/04/S_T_12016_Medicion_infiltracion_doble_anilla.pdf (accessed on 11 November 2024).
- Ruiz, E.; Martínez, M. Hidrología Aplicada. Tema 4: Infiltración y Humedad del Suelo; OpenCourseWare, Universidad del País Vasco (EHU/UPV): Bilbao, España, 2020; Available online: https://ocw.ehu.eus/pluginfile.php/47724/mod_resource/content/1/Material_Docente/Tema_4.pdf (accessed on 8 October 2024).
- Dane, J.H.; Topp, C.G. (Eds.) Methods of Soil Analysis; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 2002; 1692p, ISBN 978-0-89118-893-3. [Google Scholar]
- Klute, A. (Ed.) Methods of Soil Analysis; SSSA Book Series; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1986; ISBN 978-0-89118-864-3. pp. 493–544. [Google Scholar]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; ISBN 978-6-6110-1170-3. [Google Scholar]
- Richards, L.A. Porous Plate Apparatus for Measuring Moisture Retention and Transmission by Soil. Soil. Sci. 1948, 66, 105–110. [Google Scholar] [CrossRef]
- Moratelli, F.A.; Alves, M.A.B.; Borella, D.R.; Kraeski, A.; Almeida, F.T.D.; Zolin, C.A.; Souza, A.P.D. Effects of land use on soil physical-hydric attributes in two watersheds in the Southern Amazon, Brazil. Soil. Syst. 2023, 7, 103. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- AOAC International. AOAC International. AOAC Official Method 990.12: Aerobic Plate Count in Foods. In Official Methods of Analysis of AOAC International; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Ávila Dávila, L. Gestión del Riego Basado en la Velocidad de Infiltración del Agua en el Suelo Mediante Lisimetría de Pesada. Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, Universidad Autónoma de Zacatecas “Francisco García Salinas”, Zacatecas, Mexico, 2021. Available online: http://hdl.handle.net/10317/9347 (accessed on 17 February 2024).
- Úbeda Rivera, J.S.; Delgado Dallatorre, Y. La Infiltración Del Agua En Los Suelos y Componentes Artificiales y Materia Orgánica Que Se Utilizan En Ellos Para La Agricultura. Rev. Iberoam. Bioecon. Cambio Clim. 2018, 4, 889–896. [Google Scholar] [CrossRef]
- Tapia, R.; Carmona Crocco, J.; Martinelli, M. Evaluación de La Infiltración En Dos Complejos Suelo- Vegetación En El Monte de San Juan (Argentina). Bol. Soc. Argent. Bot. 2022, 57, 769–784. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2006, 131, 59–79. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil. Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Caycedo Lozano, L.; Corrales Ramírez, L.C.; Trujillo Suárez, D.M. Las bacterias, su nutrición y crecimiento: Una mirada desde la química. Nova 2021, 19, 49–94. [Google Scholar] [CrossRef]
- Cruz O’Byrne, R.K.; Piraneque Gambasica, N.V.; Aguirre Forero, S.E. Introducción a la Biología y Microbiología de Suelos; Universidad del Magdalena: Santa Marta, Colombia, 2022; ISBN 978-958-746-574-7. [Google Scholar]
- Khan, M.T.; Supronienė, S.; Žvirdauskienė, R.; Aleinikovienė, J. Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands. Agronomy 2025, 15, 1928. [Google Scholar] [CrossRef]
- Salcedo, E.; Hernández, T.; Galvis, A.; Rodríguez, R.; Zamora, F.; Bugarin, R.; Carrillo, R. La Humedad Aprovechable y Su Relación Con La Materia Orgánica y Superficie Específica Del Suelo. Terra Latinoam. 2017, 25, 419–425. [Google Scholar]
- Dec, D. El Concepto de la Relación Suelo–Agua–Planta. In Estructura del Suelo; Instituto de Investigaciones Agropecuarias (INIA): Santiago, Chile, 2012; pp. 1–18. Available online: https://biblioteca.inia.cl/server/api/core/bitstreams/29957e0c-7383-40ad-b274-02c51f93382e/content (accessed on 3 March 2024).
- Piraneque Gambasica, N.V.; Aguirre Forero, S.E.; Cruz O’Byrne, R. Introducción a la Génesis y Física de Suelos; Universidad del Magdalena: Santa Marta, Colombia, 2023; ISBN 978-958-746-595-2. [Google Scholar]
- Sisouvanh, P.; Trelo-ges, V.; Isarangkool Na Ayutthaya, S.; Pierret, A.; Nunan, N.; Silvera, N.; Xayyathip, K.; Hartmann, C. Can Organic Amendments Improve Soil Physical Characteristics and Increase Maize Performances in Contrasting Soil Water Regimes? Agriculture 2021, 11, 132. [Google Scholar] [CrossRef]
- Bondì, C.; Castellini, M.; Iovino, M. Compost Amendment Impact on Soil Physical Quality Estimated from Hysteretic Water Retention Curve. Water 2022, 14, 1002. [Google Scholar] [CrossRef]
- Matula, S.; Mojrová, M.; Špongrová, K. Estimation of the Soil Water Retention Curve (SWRC) Using Pedotransfer Functions (PTFs). Soil. Water Res. 2007, 2, 113–122. [Google Scholar] [CrossRef]
- Simón, M.; Peralta, N.; Costa, J.L. Relación entre la Conductividad Eléctrica Aparente con Propiedades del Suelo y Nutrientes. Cienc. Suelo 2013, 31, 45–55. Available online: https://www.scielo.org.ar/pdf/cds/v31n1/v31n1a05.pdf (accessed on 22 May 2024).
- Cornelis, W.M.; Khlosi, M.; Hartmann, R.; Van Meirvenne, M.; De Vos, B. Comparison of Unimodal Analytical Expressions for the Soil-Water Retention Curve. Soil. Sci. Soc. Am. J. 2005, 69, 1902–1911. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Gao, L.; Tian, Y. Irrigation Has More Influence than Fertilization on Leaching Water Quality and the Potential Environmental Risk in Excessively Fertilized Vegetable Soils. PLoS ONE 2018, 10, e0134172. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Aldaz, J.C.; Herrera-Sánchez, D.J. El Rol de La Agroecología En El Desarrollo Rural Sostenible En Ecuador. Rev. Científica Zambos 2022, 1, 16. [Google Scholar] [CrossRef]
- Sandoval Aparicio, J.C.; Gutierrez Castorena, M.d.C.; Cruz Flores, G.; Ortiz Solorio, C.A. Reservas de Carbono y Micromorfología de La Materia Orgánica En Suelos Ribereños En Tres Ecosistemas de Alta Montaña: Volcán Iztaccíhuatl. Madera Bosques 2022, 28, e2822469. [Google Scholar] [CrossRef]
- Zaiets, O.; Poch, R.M. Micromorphology of Organic Matter and Humus in Mediterranean Mountain Soils. Geoderma 2016, 272, 83–92. [Google Scholar] [CrossRef]
- Martínez, H.E.; Fuentes, E.J.P.; Acevedo, H.E. Carbono Orgánico y Propiedades Del Suelo. Cienc. Suelo Nutr. Veg. 2008, 8, 68–96. [Google Scholar] [CrossRef]
- Covarrubias, S.A.; Barrera-Galicia, G.C.; Martínez-Acuña, M.I.; Cardoso-Ortiz, J.; Flores de la Torre, J.A. Poblaciones Microbianas y Su Contribución En La Generación de Suelos Supresivos. ECOAgropecuaria. Rev. Cient. Ecol. Agropecu. 2024, 3, 1–12. [Google Scholar] [CrossRef]
| Field Plots | Laboratory Experimental Trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | Organic Matter (%) | Moisture Content (%) | Moisture Retention Capacity (%) | Treatment | Organic Matter (%) | Moisture Content (%) | Moisture Retention Capacity (%) | ||||
| I | 2.37 | 16.87 ± 0.42 | a | 10.08 ± 0.14 | a | T1 | 1.56 | 13.65 ± 0.66 | a | 8.95 ± 0.30 | a |
| II | 3.42 | 20.85 ± 2.71 | ab | 11.61 ± 1.03 | ab | T2 | 2.89 | 17.86 ± 0.64 | ab | 10.44 ± 0.50 | ab |
| III | 5.55 | 21.82 ± 4.44 | ab | 12.05 ± 1.86 | ab | T3 | 4.77 | 22.84 ± 0.93 | bc | 12.40 ± 0.70 | bc |
| IV | 7.89 | 23.28 ± 4.69 | b | 12.67 ± 1.93 | b | T4 | 7.69 | 25.55 ± 0.76 | cd | 13.58 ± 1.09 | cd |
| V | 9.43 | 27.39 ± 3.24 | b | 14.48 ± 1.55 | b | T5 | 9.2 | 28.11 ± 0.92 | d | 14.78 ± 0.89 | d |
| Field Plots | Laboratory Experimental Trial | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Le-vel | Organic Matter (%) | Log CFU/g Mesophilic | Log CFU/g Fungi | Log CFU/g Yeasts | Treatment | Organic Matter (%) (Post) | Log CFU/g Mesophilic (Post) | Log CFU/g Fungi (Post) | Log CFU/g Yeasts (Post) | EC Start | EC End | ||||||||
| I | 2.37 | 9.50 ± 0.05 | b | 5.30 ± 0.05 | b | 6.40 ± 0.05 | b | T1 | 1.6 | 7.80 ± 0.10 | c | 5.30 ± 0.10 | b | 6.00 ± 0.10 | d | 215.1 ± 5.00 | a | 171.1 ± 6.27 | a |
| II | 3.42 | 9.50 ± 0.05 | b | 5.50 ± 0.10 | c | 6.00 ± 0.05 | b | T2 | 2.9 | 7.60 ± 0.10 | b | 5.10 ± 0.10 | a | 5.70 ± 0.10 | b | 374.4 ± 4.40 | b | 339.1 ± 4.04 | b |
| III | 5.55 | 9.80 ± 0.05 | c | 5.80 ± 0.10 | d | 6.40 ± 0.10 | b | T3 | 4.8 | 6.80 ± 0.10 | a | 5.40 ± 0.10 | b | 5.60 ± 0.10 | a | 589.1 ± 9.00 | c | 337.4 ± 7.50 | b |
| IV | 7.89 | 9.30 ± 0.05 | a | 4.70 ± 0.10 | a | 4.30 ± 0.10 | a | T4 | 7.7 | 7.60 ± 0.10 | b | 5.50 ± 0.10 | c | 5.90 ± 0.10 | c | 758.1 ± 8.00 | d | 389.0 ± 9.00 | c |
| V | 9.43 | 9.30 ± 0.05 | a | 4.80 ± 0.10 | a | 5.00 ± 0.10 | a | T5 | 9.2 | 7.70 ± 0.10 | b | 5.60 ± 0.10 | d | 5.60 ± 0.10 | a | 917.6 ± 7.50 | c | 433.5 ± 8.50 | d |
| Organic Matter (%) | Application Rate (mm/h) | Uniformity Coefficient (%) | Required Water Depth (mm) | Irrigation Frequency (Days) | Irrigation Duration (Hours) | Applied Water Depth (mm) | Application Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 2.37 | 14.08 | 69.2 | 3.33 | 5 | 3.00 | 42.24 | 39% |
| 3.42 | 7.89 | 80.7 | 2.55 | 5 | 2.00 | 15.78 | 81% |
| 5.55 | 10.58 | 69.5 | 2.53 | 4 | 3.00 | 31.74 | 31% |
| 7.89 | 11.36 | 74.7 | 2.66 | 8 | 2.00 | 22.72 | 84% |
| 9.43 | 14.29 | 83 | 3.11 | 8 | 4.00 | 57.16 | 41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cachipuendo, C.; Pacheco, A.; Contero, R.; Sandoval, J. Optimization of Irrigation Efficiency and Water Retention in Agroecological Systems Through Organic Matter Management. Water 2025, 17, 3037. https://doi.org/10.3390/w17213037
Cachipuendo C, Pacheco A, Contero R, Sandoval J. Optimization of Irrigation Efficiency and Water Retention in Agroecological Systems Through Organic Matter Management. Water. 2025; 17(21):3037. https://doi.org/10.3390/w17213037
Chicago/Turabian StyleCachipuendo, Charles, Alison Pacheco, Rocío Contero, and Jorge Sandoval. 2025. "Optimization of Irrigation Efficiency and Water Retention in Agroecological Systems Through Organic Matter Management" Water 17, no. 21: 3037. https://doi.org/10.3390/w17213037
APA StyleCachipuendo, C., Pacheco, A., Contero, R., & Sandoval, J. (2025). Optimization of Irrigation Efficiency and Water Retention in Agroecological Systems Through Organic Matter Management. Water, 17(21), 3037. https://doi.org/10.3390/w17213037







