Novel Microwave-Synthesized Bimetallic Ce-Al-MOFs with Efficient Phosphate Removal from Aquaculture Effluent: Synthesis, Characterization and Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ce-Al-MOFs
2.3. Material Characterization
2.4. Batch Experiments of Adsorption
3. Results and Discussion
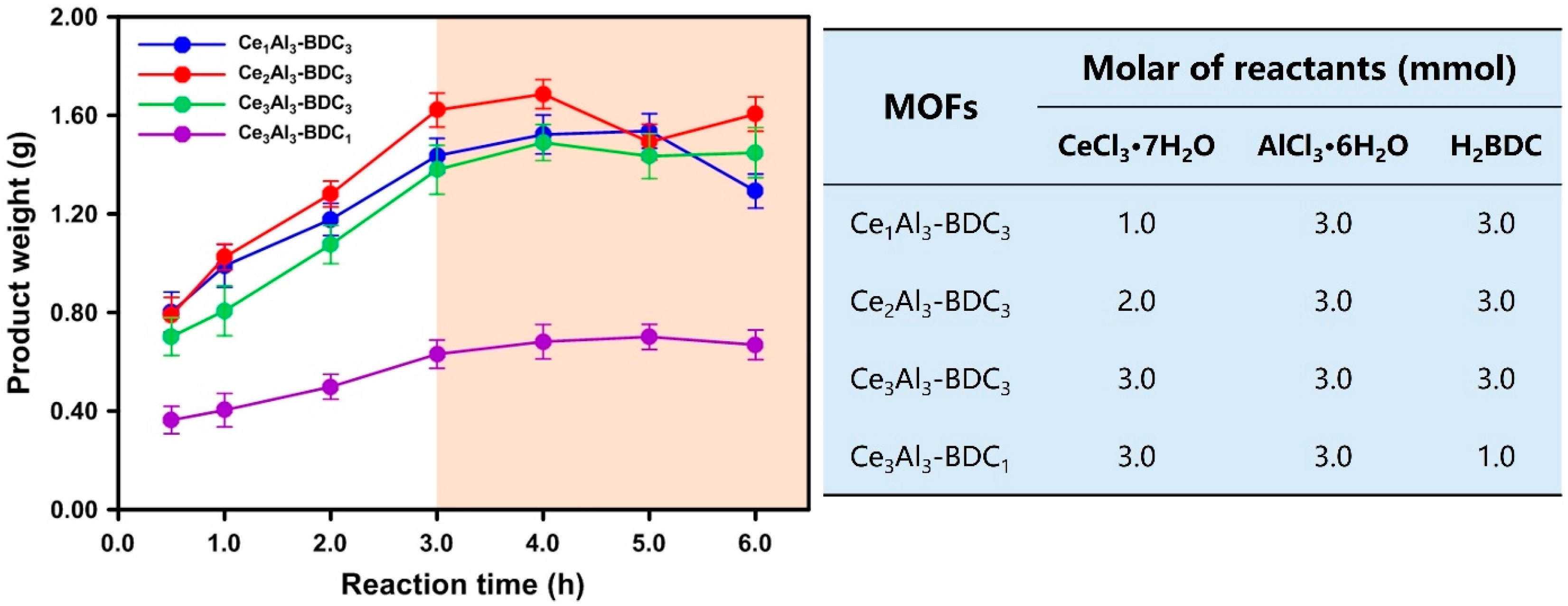
3.1. Effect of Microwave Reaction Time on Materials Yield
3.2. Adsorption Behavior of Phosphate
3.2.1. Effect of the Precursor-Ratios
3.2.2. Effect of Initial Phosphate Concentration
3.2.3. Effect of Interfered Co-Existing Anions
3.2.4. Influence of pH Conditions
3.3. Regeneration and Phosphorus Recovery of Adsorbents
3.4. Characterization
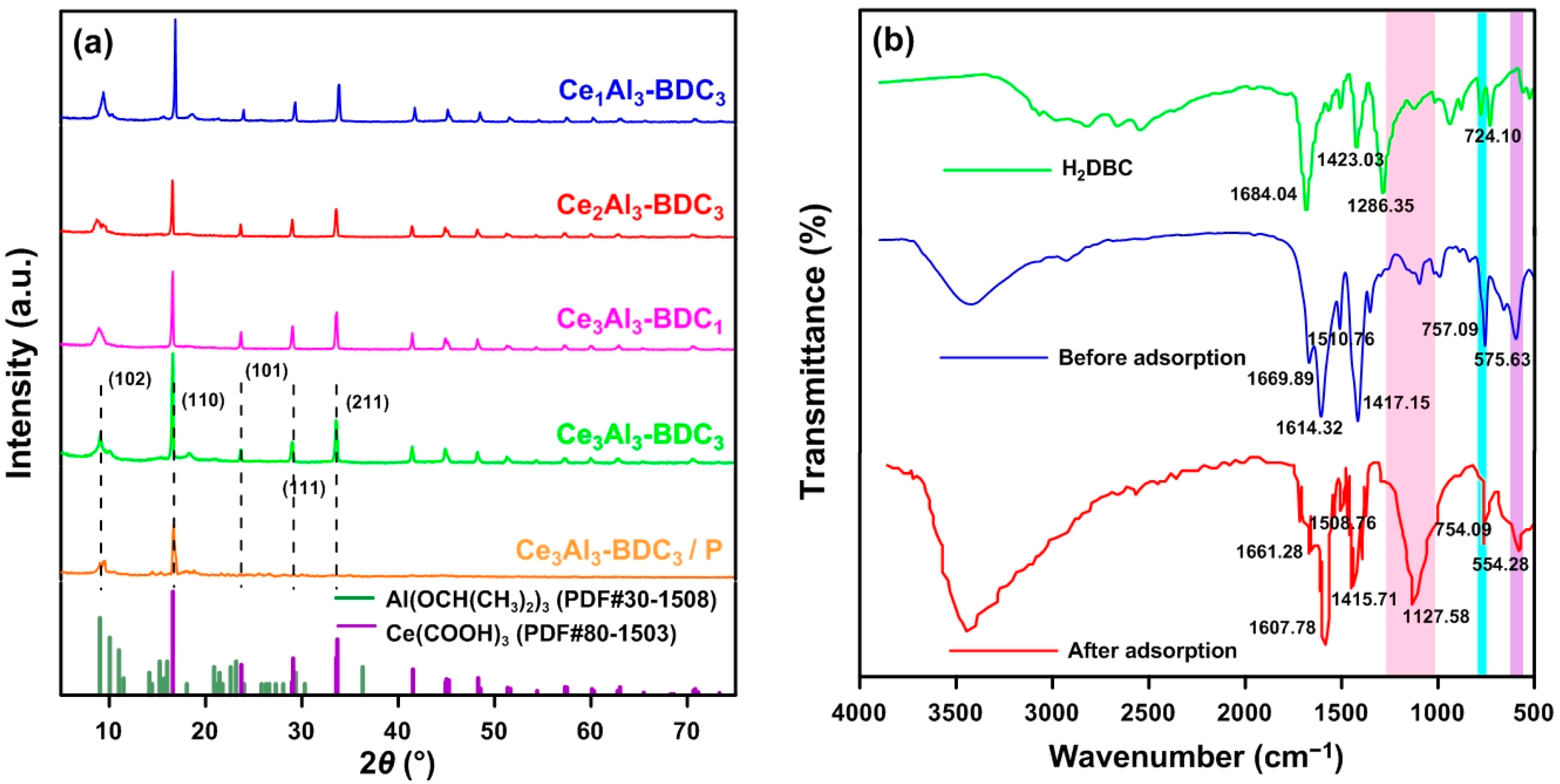
3.4.1. XRD Analysis
3.4.2. FT-IR Analysis
3.4.3. SEM and Energy-Dispersive Spectroscopy (EDS) Analyses
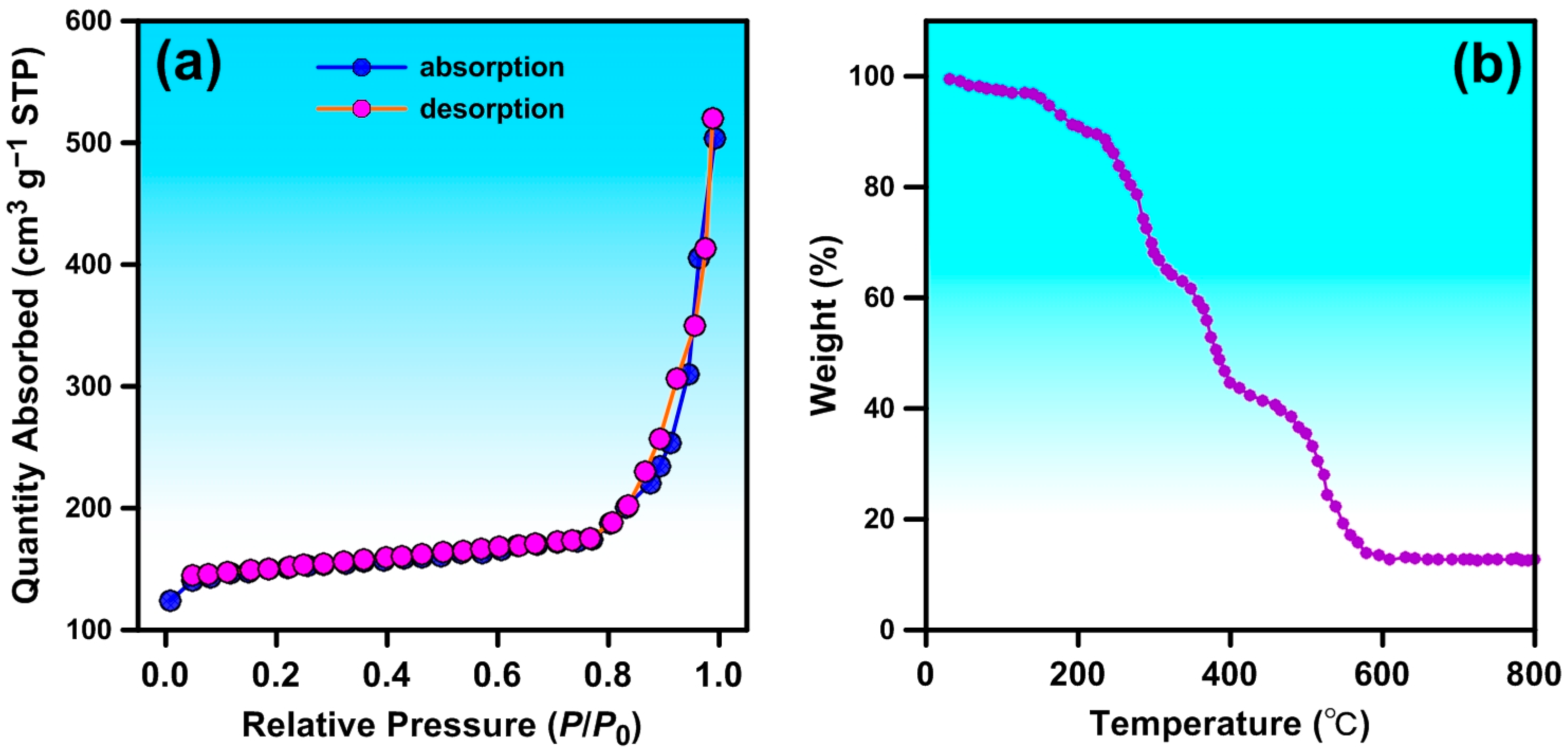
3.4.4. N2 Adsorption–Desorption Tests
3.4.5. TGA
3.5. Adsorption Kinetics
| Models | C0 (mg L−1) | K1 | K2 | qe (mg g−1) | R2 |
|---|---|---|---|---|---|
| Pseudo-first order | 100 | (5.4 ± 0.3) × 10−3 | - | 29.61 ± 1.50 | 0.9656 |
| 200 | (4.5 ± 0.2) × 10−3 | - | 57.81 ± 2.21 | 0.9708 | |
| Pseudo-second order | 100 | - | (4.5 ± 15) × 10−4 | 48.54 ± 1.18 | 0.9920 |
| 200 | - | (1.6 ± 5.3) × 10−4 | 77.52 ± 2.41 | 0.9831 |
| C0 (mg L−1) | Equation | R2 |
|---|---|---|
| 100 | y = 10.9077 + 2.0622x | 0.9952 |
| y = 19.1953 + 1.3234x | 0.9189 | |
| 200 | y = 7.7802 + 3.1840x | 0.9939 |
| y = 23.1588 + 2.1424x | 0.8092 |
3.6. Thermodynamic and Isothermal Explorations
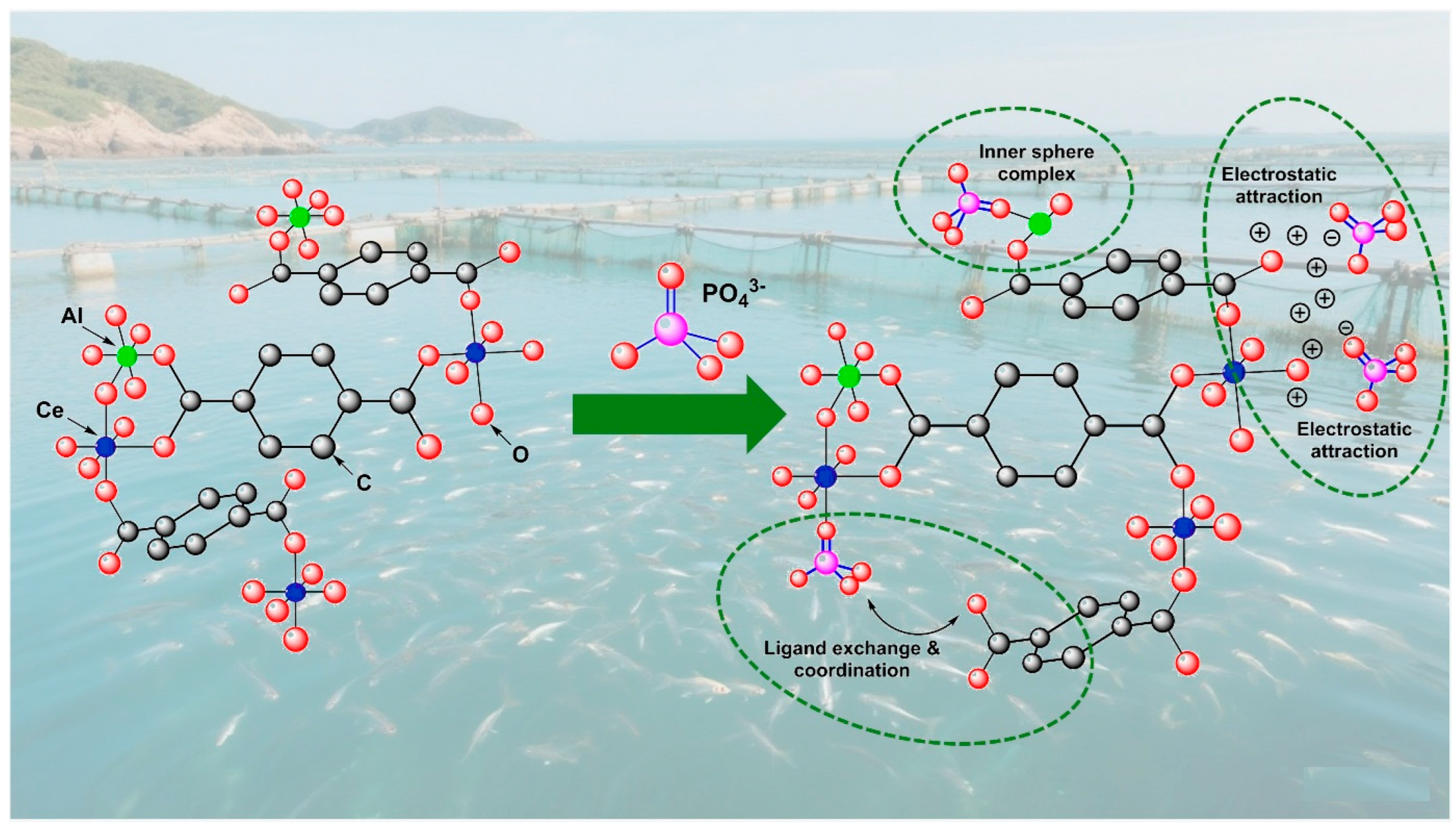
3.7. A Brief Summary of the Adsorption Mechanism
3.8. Performance Comparison of MOFs Adsorbents for Phosphate Removal
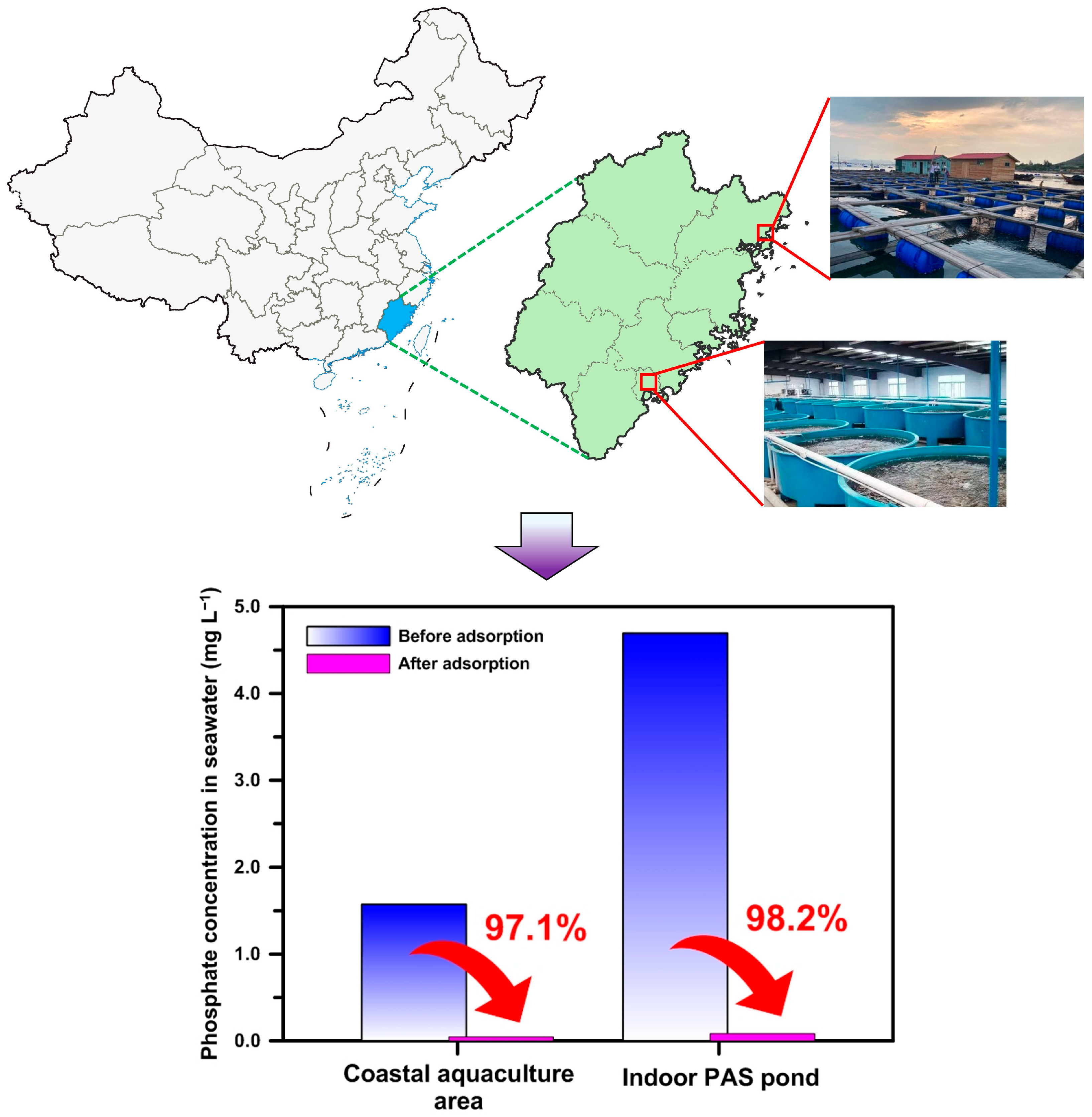
3.9. Field Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: https://coilink.org/20.500.12592/x69pbvz (accessed on 16 October 2025).
- Deng, X.; Song, M.; Li, Z.; Zhang, F.; Liu, Y. Renewable and Common Resources: Marine Fishery Resources. In Environmental and Natural Resources Economics; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.; Wang, Y.; Xie, H.; Zhu, M.; Chen, J. Presence and environmental risk assessment of selected antibiotics in coastal water adjacent to mariculture areas in the Bohai Sea. Ecotoxicol. Environ. Saf. 2019, 177, 117–123. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Li, Q.; Liu, Y.; Song, J.; Zhang, Y. Environmental response to long-term mariculture activities in the Weihai coastal area, China. Sci. Total Environ. 2017, 601–602, 22–31. [Google Scholar] [CrossRef]
- Lao, Q.; Chen, F.; Liu, G.; Chen, C.; Jin, G.; Zhu, Q.; Wei, C.; Zhang, C. Isotopic evidence for the shift of nitrate sources and active biological transformation on the western coast of Guangdong provinces, south China. Mar. Pollut. Bull. 2019, 142, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, W.; Jiang, J. Analysis of spatial-temporal variation and nutritional status of environmental quality in the mariculture zone at the Yueqing Bay. Mar. Environ. Sci. 2021, 40, 724–731. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Noga, E.J.; Hobbs, C.H.; Glasgow, H.B., Jr. New phantom dinoflagellate is the causative agent of major estuarine fish kills. Nature 1992, 360, 768–769. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Justic, D.; Dortch, Q.; Wiseman, W.J.; Sen Gupta, B.K. Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf. Estuaries 1996, 19, 386–407. [Google Scholar] [CrossRef]
- Indeewari, K.M.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis and characterization of 2D magnesium oxide nanoflakes: A potential nanomaterial for effective phosphate removal from wastewater. ChemistrySelect 2022, 7, e202103973. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Cheng, P.; Arbid, Y.; Liu, H.; Zou, X.; Chen, T. Utilization of Waste Adsorbent Generated after Ca/Al-LDH Adsorption of High-Concentration Phosphate: Fluorine Removal. J. Environ. Eng. 2023, 149, 04022090. [Google Scholar] [CrossRef]
- An, W.; Wang, Q.; Chen, H.; Liu, Y.; Hu, X.; Di, J. Efficient recovery of phosphate from water media by iron-magnesium functionalized lignite: Adsorption evaluation, mechanism revelation and potential application exploration. Molecules 2024, 29, 1252. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Wan, J.; Wu, B.; Lo, I.M.C. Development of FeO/Fe3O4 composites with tunable properties facilitated by Fe2+ for phosphate removal from river water. Chem. Eng. J. 2020, 388, 124242. [Google Scholar] [CrossRef]
- Ma, P.; Zhu, J.; Du, X.; Yang, Y.; Hao, X.; An, X.; Hao, X.; Prestigiacomo, C. Specific separation and recovery of phosphate anions by a novel NiFe-LDH/rGO hybrid film based on electroactivity-variable valence. J. Colloid Interface Sci. 2022, 626, 47–58. [Google Scholar] [CrossRef]
- Di, J.; Ruan, Z.; Zhang, S.; Dong, Y.; Fu, S.; Li, H.; Jiang, G. Adsorption behaviors and mechanisms of Cu2+, Zn2+ and Pb2+ by magnetically modified lignite. Sci. Rep. 2022, 12, 1394. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal-organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 12, 4912–4947. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, D.; Chang, Z.; Li, R.; Meng, S. Phosphorus removal from water by the metal-organic frameworks (MOFs)-based adsorbents: A review for structure, mechanism, and current progress. Environ. Res. 2024, 243, 117816. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, Y.; Lv, Z.; Zhou, H.; Yang, X.; Chen, J.; Guo, H. Effective adsorption and removal of phosphate from aqueous solutions and eutrophic water by Fe-based MOFs of MIL-101. Sci. Rep. 2017, 7, 3316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, S.; Khan, S.; Zhao, Y.; Guan, Q.; Geng, Z.; Guo, Y.; Chen, L.; Yang, X. Rapid removal and mechanism investigation of low concentration phosphate from wastewater by CuFe2O4/MIL-101(Fe) composite. J. Nanostructure Chem. 2022, 12, 117–127. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Yin, C.; Chen, J.; Yang, X.; Wang, S. Tuning microscopic structure of Al-based metal-organic frameworks by changing organic linkers for efficient phosphorus removal. J. Clean. Prod. 2021, 292, 125998. [Google Scholar] [CrossRef]
- Zhu, Z.; Qin, L.; Liu, Y.; Zhang, Q.; Cheng, P.; Liang, W. Fabrication and mechanism of La/Al bimetallic organic frameworks for phosphate removal. Chem. Eng. J. 2023, 479, 147081. [Google Scholar] [CrossRef]
- Song, J.; Yang, W.; Han, X.; Jiang, S.; Zhang, C.; Pan, W.; Jian, S.; Hu, J. Performance of Rod-Shaped Ce Metal-Organic Frameworks for Defluoridation. Molecules 2023, 28, 3492. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Han, R. Adsorption of phosphate on UiO-66-NH2 prepared by a green synthesis method. J. Environ. Chem. Eng. 2021, 9, 106672. [Google Scholar] [CrossRef]
- Shams, M.; Dehghani, M.H.; Nabizadeh, R.; Mesdaghinia, A.; Alimohammadi, M.; Najafpoor, A.A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: Modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016, 224, 151–157. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Moumen, E.; Bazzi, L.; El Hankari, S. Metal-organic frameworks and their composites for the adsorption and sensing of phosphate. Coord. Chem. Rev. 2022, 455, 214376. [Google Scholar] [CrossRef]
- Lien, H.-L.; Yu, C.-H.; Kamali, S.; Sahu, R.S. Bimetallic Fe/Al system: An all-in-one solid-phase Fenton reagent for generation of hydroxyl radicals under oxic conditions. Sci. Total Environ. 2019, 673, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseelan, A.; Viswanathan, N. Design of Amino-Functionalized Benzene-1,4-Dicarboxylic Acid-Fabricated Lanthanum-Based Metal–Organic Frameworks for Defluoridation of Water. J. Environ. Chem. Eng. 2020, 65, 5328–5340. [Google Scholar] [CrossRef]
- Haghighat, G.A.; Saghi, M.H.; Anastopoulos, I.; Javid, A.; Roudbari, A.; Talebi, S.S.; Ghadiri, S.K.; Giannakoudakis, D.A.; Shams, M. Aminated graphitic carbon derived from corn stover biomass as adsorbent against antibiotic tetracycline: Optimizing the physicochemical parameters. J. Mol. Liq. 2020, 313, 113523. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Xiong, Z.; Lai, B.; Sun, Y.; Yang, Y.; Yang, L. The enhanced removal of phosphate by structural defects and competitive fluoride adsorption on cerium-based adsorbent. Chemosphere 2020, 256, 127056. [Google Scholar] [CrossRef]
- Song, Q.-X.; Xu, Y.-X.; Fang, N.; Liu, J.; Zhu, H.-L. Novel aluminum/lanthanum-based metal organic frameworks for phosphate removal from water. J. Environ. Chem. Eng. 2023, 11, 111418. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, T.; Xiong, M.; Sun, A.; Xu, Y.; Wu, Y.; Shu, W.; Xu, Z. Tuning adsorption capacity of metal-organic frameworks with Al3+ for phosphorus removal: Kinetics, isotherm and regeneration. Inorg. Chem. Commun. 2021, 132, 108804. [Google Scholar] [CrossRef]
- Liu, R.; Chi, L.; Wang, X.; Wang, Y.; Sui, Y.; Xie, T.; Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168. [Google Scholar] [CrossRef]
- Gao, Z.; Wei, Y.; Tian, X.; Liu, Y.; Lan, X.; Zhang, D.; Han, S.; Huo, P. A novel Ce/Fe bimetallic metal-organic framework with ortho-dodecahedral multilevel structure for enhanced phosphate adsorption. Chem. Eng. J. 2024, 486, 150284. [Google Scholar] [CrossRef]
- Pandi, K.; Viswanathan, N. A facile synthesis of metal ion-imprinted graphene oxide/alginate hybrid biopolymeric beads for enhanced fluoride sorption. RSC Adv. 2016, 6, 75905–75915. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Hu, Y.; Han, R. Preparation of Novel Magnetic Microspheres with the La and Ce-Bimetal Oxide Shell for Excellent Adsorption of Fluoride and Phosphate from Solution. J. Environ. Chem. Eng. 2019, 64, 3641–3651. [Google Scholar] [CrossRef]
- Huo, Y.; Ding, W.; Huang, X.; Xu, J.; Zhao, M. Fluoride Removal by Lanthanum Alginate Bead: Adsorbent Characterization and Adsorption Mechanism. Chin. J. Environ. Chem. Eng. 2011, 19, 365–370. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Kumar, I.A.; Naushad, M.; Viswanathan, N. Fabrication of hydroxyapatite embedded cerium-organic frameworks for fluoride capture from water. J. Mol. Liq. 2022, 354, 118830. [Google Scholar] [CrossRef]
- Han, X.; Liu, Z.; Bakhtari, M.F.; Luo, J.; Deng, L. Preparation of novel Ce (IV)-based MOF/GO composite and its highly effective phosphate removal from aqueous solution. Environ. Eng. Res. 2023, 28, 220647. [Google Scholar] [CrossRef]
- Zhu, M.; Fu, W.; Zou, G. Urothermal synthesis of an unprecedented pillar-layered metal–organic framework. J. Coord. Chem. 2012, 65, 4108–4114. [Google Scholar] [CrossRef]
- Wei, F.; Ren, Q.; Liang, Z.; Chen, D. Synthesis of Graphene Oxide/Metal-Organic Frameworks Composite Materials for Removal of Congo Red from Wastewater. ChemistrySelect 2019, 19, 5755–5762. [Google Scholar] [CrossRef]
- Wei, F.; Chen, D.; Liang, Z.; Zhao, S.; Luo, Y. Synthesis and characterization of metal-organic frameworks fabricated by microwave-assisted ball milling for adsorptive removal of Congo red from aqueous solutions. RSC Adv. 2017, 7, 46520–46528. [Google Scholar] [CrossRef]
- Wei, F.; Ren, Q.; Zhang, H.; Yang, L.; Chen, H.; Liang, Z.; Chen, D. Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites. RSC Adv. 2021, 11, 9977–9984. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wu, M.; Hu, Q.; Wang, L.; Lv, C.; Zhang, L. Iron-based metal-organic frameworks as novel platforms for catalytic ozonation of organic pollutant: Efficiency and mechanism. J. Hazard. Mater. 2019, 367, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. A new MOFs/polymer hybrid membrane: MIL-68(Al)/PVDF, fabrication and application in high-efficient removal of p-nitrophenol and methylene blue. Sep. Purif. Technol. 2019, 215, 217–226. [Google Scholar] [CrossRef]
- Farrag, M. In situ preparation of palladium nanoclusters in cerium metal-organic frameworks Ce-MOF-808, Ce-UiO-66 and Ce-BTC as nanoreactors for room temperature Suzuki cross-coupling reaction. Microporous Mesoporous Mater. 2021, 312, 110783. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, D.; Qu, Y.; Chen, X.; Zhang, J.; Huang, M.; Wang, J. Facile Synthesis of Ce-MOF for the Removal of Phosphate. Fluoride Arsen. Nanomater. 2023, 13, 3048. [Google Scholar] [CrossRef]
- Yao, Z.-Q.; Li, G.-Y.; Xu, J.; Hu, T.-L.; Bu, X.-H. A Water-Stable Luminescent Zn-II Metal-Organic Framework as Chemosensor for High-Efficiency Detection of Cr-VI-Anions (Cr2O72- and CrO42-) in Aqueous Solution. Chem. A Eur. J. 2018, 24, 3192–3198. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, L.-X.; Xing, Y.-H.; Bai, F.-Y. A Double-Walled Thorium-Based Metal-Organic Framework as a Promising Dual-Function Adsorbent for Efficiently Capturing Iodine and Dyes. Cryst. Growth Des. 2019, 19, 5686–5695. [Google Scholar] [CrossRef]
- Zhuang, H.; Yan, G.; Wang, J.; Hu, Z.; Yan, S.; Sun, Y.; Guo, T.; Hu, J.; Reheman, A. Efficient Adsorption of Hg(II) Ions Benefits from the Synergistic Effect between Sulfur-Containing MOF-808 and C3N4. J. Environ. Eng. 2024, 150, 04024050. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y.; Jochems, A.P. Zirconium-based metal organic frameworks: Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Liu, H.; Guo, W.; Liu, Z.; Li, X.; Wang, R. Effective adsorption of phosphate from aqueous solution by La-based metal-organic frameworks. RSC Adv. 2016, 6, 105282–105287. [Google Scholar] [CrossRef]
- Luo, F.; Feng, X.; Li, Y.; Zheng, G.; Zhou, A.; Xie, P.; Wang, Z.; Tao, T.; Long, X.; Wan, J. Magnetic amino-functionalized lanthanum metal-organic framework for selective phosphate removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125906. [Google Scholar] [CrossRef]
- SC/T 9103-2007; Water Drainage Standard for Sea Water Mariculture. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2007. Available online: https://www.cssn.net.cn/cssn/list?sub_type=ST_N_CSIC&orderby=-a101 (accessed on 16 October 2025).






| T (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J (mol·K)−1) |
|---|---|---|---|
| 298 | −8.46 ± 0.24 | 23.196 ± 1.929 | 106.087 ± 6.186 |
| 308 | −9.41 ± 0.24 | ||
| 318 | −10.72 ± 0.50 | ||
| 328 | −11.55 ± 0.41 |
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | Kf (L g−1) | n | R2 | |
| 298 | 103.09 ± 2.12 | 0.0304 ± 0.0030 | 0.9967 | 8.32 ± 2.08 | 2.204 ± 0.298 | 0.8451 |
| 308 | 120.48 ± 1.45 | 0.0394 ± 0.0037 | 0.9980 | 11.40 ± 2.65 | 2.297 ± 0.313 | 0.8435 |
| 318 | 123.46 ± 1.56 | 0.0577 ± 0.0110 | 0.9978 | 14.64 ± 3.13 | 2.578 ± 0.367 | 0.8317 |
| 328 | 136.99 ± 1.88 | 0.0692 ± 0.0103 | 0.9978 | 20.04 ± 4.51 | 2.757 ± 0.465 | 0.7790 |
| Adsorbents | pH | Adsorption Capacity (mg P g−1) | References |
|---|---|---|---|
| CuFe2O4/MIL-101(Fe) | 2~10 | 3~30 | [19] |
| Al-BDC | 7.0 | 97.5 | [20] |
| Al-PMA | 7.0 | 42.25 | |
| La/Al-BTC | 7.0~8.0 | 85.2~210.3 | [21] |
| F-Al/La MOFs | 7.0 | 94.77~46.67 | [31] |
| UiO-66-NH2 | 6.0~7.0 | 50.5 | [23] |
| ZIF-8 | 2.8 | 38.22 | [24] |
| Fe-Al-MOF | 7.0 | 38.33 | [32] |
| NH2-MIL-101(Fe) | 5.0~7.0 | 94.34 | [33] |
| NH2-MIL-101(Al) | 5.0~7.0 | 87.85 | |
| UiO-66 | N.A. | 85.0 | [55] |
| UiO-66-NH2 | N.A. | 92.0 | |
| La-MOFs | 6.3 | 142.04 | [56] |
| Fe3O4/NH2-La-MOF | 8.0 | 111.22 | [57] |
| Ce3Al3-BDC3 | 6.0 | 136.99 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Zhao, J.; Cai, Z.; Hu, J.; Zhuo, Z.; Miao, X. Novel Microwave-Synthesized Bimetallic Ce-Al-MOFs with Efficient Phosphate Removal from Aquaculture Effluent: Synthesis, Characterization and Applications. Water 2025, 17, 3019. https://doi.org/10.3390/w17203019
Zeng J, Zhao J, Cai Z, Hu J, Zhuo Z, Miao X. Novel Microwave-Synthesized Bimetallic Ce-Al-MOFs with Efficient Phosphate Removal from Aquaculture Effluent: Synthesis, Characterization and Applications. Water. 2025; 17(20):3019. https://doi.org/10.3390/w17203019
Chicago/Turabian StyleZeng, Jian, Jiangnan Zhao, Zhenzhen Cai, Jianshe Hu, Zesheng Zhuo, and Xiongping Miao. 2025. "Novel Microwave-Synthesized Bimetallic Ce-Al-MOFs with Efficient Phosphate Removal from Aquaculture Effluent: Synthesis, Characterization and Applications" Water 17, no. 20: 3019. https://doi.org/10.3390/w17203019
APA StyleZeng, J., Zhao, J., Cai, Z., Hu, J., Zhuo, Z., & Miao, X. (2025). Novel Microwave-Synthesized Bimetallic Ce-Al-MOFs with Efficient Phosphate Removal from Aquaculture Effluent: Synthesis, Characterization and Applications. Water, 17(20), 3019. https://doi.org/10.3390/w17203019









