Abstract
This study reports the development of polyvinylidene fluoride (PVDF) membranes decorated with a copper(II) complex (CuL) for the removal of organic pollutants from wastewater. Using Drimaren Red X-6BN (DRX-6BN) as a probe, the PVDF membrane with the lowest CuL loading (PVDF/PDA/CuL-4) reached an adsorption capacity of 19.78 mg/g at 300 min, with removal of up to 50% DRX-6BN. Kinetic analysis favored Elovich (R2 > 0.9928; RMSE < 0.4489) and the pseudo-second-order model (R2 > 0.9540; RMSE < 1.1388), consistent with chemisorption. Intraparticle diffusion occurred in two steps. In the presence of 20 mg/L of hydrogen peroxide (H2O2), the removal was >80% within 180 min at higher CuL loadings (PVDF/PDA/CuL-40). In oily wastewater, PVDF/PDA/CuL-4 achieved ~100% COD removal in 120 min with H2O2, whereas pristine PVDF achieved 38.5%. Storage stability tests demonstrated the preservation of catalytic and separation performance for at least three months. All tests were conducted at pH ≈ 6.0 and a temperature of 25 °C. In contrast to many catalytic membranes, these membranes operate at near-neutral pH and ambient temperature in the absence of radiation. The results highlight PVDF membranes decorated with CuL as a robust and sustainable approach for the treatment of oily effluents, particularly by combining Fenton-like processes under mild conditions.
1. Introduction
The expansion of industrial activities has led to significant environmental challenges [1]. Among these, the generation of increasingly complex effluents poses a widespread global threat [2,3]. In line with the Sustainable Development Goals (SDGs), ensuring safe drinking water and sanitation (SDG 6), as well as good health and well-being (SDG 3), life below water (SDG 14), and life on land (SDG 15) have become a major focus of the United Nations (UN) and its partners in recent years [4,5,6].
Therefore, the development of technologies for the removal of organic pollutants has received considerable attention. Processes such as coagulation/flocculation, chemical precipitation, biological treatments, adsorption, ion exchange, electrochemical techniques, advanced oxidation processes (AOPs), and membrane separation processes (MSPs) have been employed [7,8,9,10,11]. However, in the case of MSPs, the deposition and accumulation of rejected materials—including organic and inorganic compounds and microorganisms—on the membrane surface and within its pores severely reduce treatment efficiency and may ultimately lead to membrane failure [12,13,14]. This phenomenon, known as fouling, depends on several factors, such as feedwater characteristics, membrane properties (material, porosity, hydrophobicity, etc.), operating conditions, and concentration polarization [12].
Emerging strategies, such as surface modification, aim to enhance the antifouling properties of membranes [12,15]. Among these, surface modification with catalysts is of particular interest because it enables chemical reactions to occur on the membrane surface. The incorporation of metal catalysts capable of promoting Fenton-type reactions into membranes represents an innovative approach that adds a chemical degradation function to the system.
The classical Fenton reaction is based on the generation of hydroxyl radicals (•OH, E0 = 2.80 V) through the catalytic decomposition of hydrogen peroxide (H2O2) by ferrous ions (Fe2+) under acidic conditions (pH 2.0–4.0) [16,17,18,19]. These radicals non-selectively attack organic matter, often leading to mineralization [16,17,18,19]. Other iron species and metals, such as copper and cobalt, have also been reported as catalysts in Fenton-like reactions [20,21].
In this context, catalyst development for Fenton reactions has been widely explored for water and wastewater treatment. Coordination compounds (CCs), especially those based on copper(II), are particularly attractive because they can operate over a wider pH range, including near-neutral conditions [20,22], where the competition between contaminant degradation and membrane degradation is less critical than when using simple metal salts. Furthermore, iron catalysts are more susceptible to deactivation by hydroxide/oxide precipitation than copper catalysts [20]. Owing to the difficulty of recovering such catalysts for reuse, their immobilization onto matrices such as activated carbon and membranes has emerged as a relevant strategy for addressing this issue [23].
Textile industry effluents are typically characterized by dyes, high chemical oxygen demand (COD), and total dissolved solids [24], whereas petroleum industry wastewaters contain hydrocarbons, oils and greases (O&G), COD, aromatic compounds, and other pollutants [14,25,26]. Combining the physical separation capacity of porous membranes with simultaneous oxidative activity to create a dual-function system holds promise for minimizing fouling while improving overall treatment efficiency compared with conventional processes.
Although catalytic membranes may represent an effective technology for the treatment of aquatic pollutants [27,28,29], to the best of our knowledge, they remain a neglected strategy for more complex effluents, such as those from the oil industry. In this study, poly(vinylidene fluoride) (PVDF) was selected as the membrane substrate owing to its chemical and mechanical stability, which is particularly suitable for oily and dye-rich wastewater. To introduce catalytic functionality, a copper(II) complex (CuL), previously reported to be effective for organic pollutant degradation under mild conditions [21], was immobilized onto the membrane surface using a polydopamine (PDA) interlayer, ensuring strong adhesion and preserving catalytic activity. This strategy led to the development of a dual-function Cu–Fenton-like membrane system capable of combining adsorption, physical separation, and chemical degradation without requiring radiation. Unlike conventional photocatalytic or iron-based systems, this approach addresses a notable gap in the literature regarding the use of Cu(II) coordination compounds on membranes for treating complex wastewater matrices, including reactive dyes and petroleum oil-in-water emulsions. The results demonstrated high removal efficiency and storage stability, highlighting the potential of the system for practical application in wastewater treatment.
2. Materials and Methods
2.1. Reagents and Supplies
Salicylaldehyde (C7H6O2, 98.00%), ethanolamine (C2H7NO, ≥99.00%), tris (hydroxymethyl) aminomethane hydrochloride (Tris-HCl, C4H11NO3·HCl, ≥99.00%), and dopamine hydrochloride (DA, C8H11NO2·HCl) were purchased from Sigma-Aldrich (Rio de Janeiro, Brazil). Methanol (MeOH, CH3OH, ≥99.80%) and copper(II) acetate monohydrate (Cu(OAc)2·H2O, ≥98.00%) were obtained from Vetec (Rio de Janeiro, Brazil). Commercial hydrophilic poly(vinylidene fluoride) (PVDF) membranes (Ø47 mm, pore size 0.45 μm) were acquired from Chromastore Comércio e Importação Ltda. (São Paulo, Brazil). Hydrogen peroxide (H2O2, 50% v/v) used in catalytic experiments was supplied by Sumatex Produtos Químicos Ltda. (Rio de Janeiro, Brazil). Drimaren Red X-6BN (DRX-6BN, CI Reactive Red 243), provided as powder, was obtained from Clariant (Rio de Janeiro, Brazil). Oil-in-water (o/w) emulsions were prepared using crude oil (28° API) collected from an offshore Brazilian production unit, in order to reproduce the characteristics of real produced water (PW), following a similar procedure reported by Ferreira et al. (2021) [14]. Emulsification was carried out with the aid of a Turrax homogenizer (Ultra-Turrax T-25, IKA® Works, Staufen, Germany) under continuous stirring (15,000 rpm) and heating (60 °C). All reagents were used without further purification, and solutions were prepared with deionized water.
2.2. Obtaining and Characterizing PVDF Membranes Decorated with CuL
The synthesis of [Bis-N-(2-hydroxyethyl)salicylaldiminate]copper(II) (CuL) was performed in situ following the methodology described by Silva et al. (2024) [21]. The ligand (L) was prepared by an equimolar reaction of salicylaldehyde with ethanolamine in MeOH under magnetic stirring for 120 min. A methanolic solution of Cu(OAc)2·H2O was then added, yielding a dark-green solution that was further stirred for 120 min. After filtration and standing, olive-green crystals were obtained with a 90% yield. The stoichiometric ratio between Cu(OAc)2·H2O and L was 1:2. Both L and CuL were synthesized at room temperature (~25 °C). The characterization of CuL has been previously reported [21,30].
The PVDF membranes were modified according to the methodology developed by Sun et al. (2022), with some adaptations [31]. The arrows correspond to the passage from one stage of the procedure to the next. Initially, the membranes were immersed in ethanol for 60 min to remove surface impurities and plasticizers, followed by rinsing with distilled water for further use. A Tris-HCl buffer solution (40 mmol/L, pH 8.5) was prepared, and the deposition solution was obtained by dissolving 0.2 g of DA in 40 mL of buffer in 250 mL Erlenmeyer flasks while stirring at 160 rpm for 60 min. After 60 min, PDA was formed by the crosslinking of DA, and each membrane was immersed in the polymer-containing medium for an additional 60 min to obtain PVDF/PDA membranes. Different amounts of CuL (mCuL) were added to the PVDF/PDA membranes and stirred for 30 min. The CuL fractions were then filtered onto the membrane surfaces, followed by the filtration of 5 mL of glutaraldehyde. The resulting membranes were designated as PVDF/PDA/CuL-4 (2.89 g/m2), PVDF/PDA/CuL-20 (14.46 g/m2), and PVDF/PDA/CuL-40 (28.92 g/m2), based on the ratio between mCuL and membrane area. Figure 1 shows the steps involved in obtaining PVDF membranes decorated with CuL.
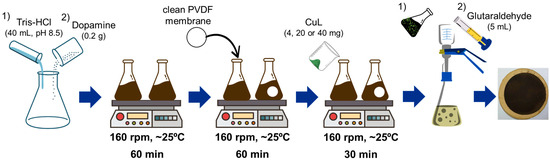
Figure 1.
Steps involved in obtaining PVDF membranes decorated with CuL.
The membranes were characterized by Fourier-transform infrared spectroscopy (FT-IR) using a Shimadzu IRSpirit FT-IR spectrophotometer (Kyoto, Japan) in the spectral range of 4000–400 cm−1. The samples were coated with a thin layer of gold, and their morphologies and surface compositions were analyzed using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS) using a FEI TESCAN VEGA 3 (Brno, Czech Republic). The water contact angle (WCA) was measured using a Phoenix-i contact-angle analyzer. Hydraulic permeance was evaluated in a recirculating filtration system under a transmembrane pressure ranging from 0.5 to 2.5 bar with a compaction time of 120 min.
FT-IR spectroscopy was used to verify the chemical modifications on the membrane surfaces after functionalization with polydopamine (PDA) and subsequent incorporation of the copper(II) complex (CuL), based on the identification of functional groups. The sample with the highest CuL content was selected because it represented the most favorable condition for demonstrating chemical interactions and avoiding spectral signal overlap.
The surface morphology was analyzed using SEM, and the elemental composition was analyzed using coupled EDS. Samples with the lowest and highest CuL loadings were chosen to allow a representative comparison of the distribution and copper content on the surfaces. The functional properties of the membranes were evaluated by measuring the water contact angle (WCA) and hydraulic permeance, which are parameters directly related to wettability, compatibility with the aqueous phase, and performance in liquid–liquid separations.
2.3. Evaluation of Membrane Performance in Agitated Systems
The experiments were carried out on a shaking table using 250 mL Erlenmeyer flasks containing 200 mL of DRX-6BN solution at an initial concentration (CDRX0) of 20 mg/L. The tests were performed at the natural pH of the solution (~6.0), temperature of 25 °C, and stirring speed of 200 rpm. In brief, 200 mL of DRX-6BN solution was transferred into a 250 mL Erlenmeyer flask, followed by the addition of the membranes. The flasks were sealed with plastic film and placed on a shaking table.
To evaluate the catalytic activity, a similar procedure was followed; however, 20 mg/L of H2O2 was added to the solution after the membrane was placed in the Erlenmeyer flask. In both cases, the concentration of DRX-6BN was monitored as a function of time.
2.4. Evaluation of Membrane Performance in Filtration Systems
The performance of the prepared membranes was evaluated in a filtration system operating mainly in the dead-end mode under a pressure of 1 bar. The setup consisted of a 2 L feed tank, permeation cell, diaphragm pump, and regulating valves. The feed volume of wastewater was 1.5 L.
To assess the catalytic activity, a similar procedure was employed; however, 20 mg/L of H2O2 was added to the solution or to the o/w emulsion in the feed tank. In both cases, the concentration of DRX-6BN was quantified at predetermined time intervals. For the oily wastewater, the concentrations of O&G and COD were determined, with an initial O&G concentration (CO&G0) of 100 ± 5 mg/L.
2.5. Analytical Determinations
The concentration of DRX-6BN was determined using a Shimadzu UV-1800 UV–Vis spectrophotometer (Kyoto, Japan) at 516 nm wavelength. COD was measured using a Hach DR-2800 spectrophotometer (Loveland, CO, USA) following the colorimetric method (5220 D) [32]. The concentration of O&G was determined using the standard extraction method (5520 D), with absorbance readings at 257 and 300 nm in a Shimadzu UV-1800 UV–Vis spectrophotometer [32,33,34]. Residual H2O2 was quantified according to the methodology described by Nogueira et al. (2005) [35] using a Hach DR-2800 spectrophotometer (Loveland, CO, USA) and a colorimetric method.
2.6. Data Analysis
Hydraulic permeance was determined from the permeate flux (J) values, given by Equation (1), which relates the permeate volume (V), effective membrane area (m2), and filtration time (t). The data were plotted as a function of transmembrane pressure, and the slope of the linear fit corresponded to the permeance, expressed in L/(h·m2·bar).
The removal efficiency in the tests in agitated systems was calculated according to Equation (2), where C0 represents the initial CDRX0, CO&G0 or COD (COD0), and C represents the concentration over time (mg/L) of DRX-6BN, or the COD or O&G of the oily effluent.
In membrane filtration systems, rejection (Rej) is calculated according to Equation (3), where CP refers to the solute concentration in the permeate and CA refers to the solute concentration in the feed. The solutes were DRX-6BN, COD, or O&G.
To evaluate the adsorption kinetics, the adsorptive capacity (q) was calculated using Equation (4), based on C0 and C of the adsorbate, mass of the adsorbent (m), and volume of the solution (V). The kinetic behavior was interpreted using the linearized forms of the pseudo-first-order (PFO) and pseudo-second-order (PSO) models, as shown in Equations (5) and (6), respectively. The Elovich model (Equation (7)) was also considered to account for chemisorption on heterogeneous surfaces. In addition, the intraparticle diffusion model proposed by Weber and Morris was applied (Equation (8)) to investigate the contribution of internal mass transfer resistance to adsorption. In this model, qt is the amount of adsorbate at time t, kd is the intraparticle diffusion rate constant (mg/g−1·min−0.5), and BL is the intercept, which is related to the thickness of the boundary layer. The parameters used in the models included the adsorption rate constants (k1 for PFO and k2 for PSO), equilibrium adsorption capacity (qe), initial adsorption rate (α), and constants β and kd related to surface heterogeneity and diffusion, respectively. The half-life (t1/2) and boundary layer thickness were also derived, where applicable. Fitting to nonlinear and intraparticle diffusion models (Figures S5–S9) was performed in Python 3.10 with NumPy 1.26, SciPy 1.11, and Matplotlib 3.8.
To evaluate the kinetics in the presence of an oxidant, the equations used were pseudo-first order (PFOr) (Equation (9)) and pseudo-second order (PSOr) (Equation (10)). The equations relate C, C0, the rate constant for PFOr (k1r), and PSOr. In the context of removal/degradation, k1r and k2r can be considered apparent rate constants (kap).
Hermia models were used to evaluate the fouling. They relate J, fouling constant (K), and t. Equations (11)–(14) present the linearized models for complete pore blockage (CPB), pore obstruction (PO), partial pore blockage (PPB), and filtration cake (FC), respectively.
3. Results and Discussion
3.1. Characterization
The FT-IR spectrum of the pristine PVDF membrane (Figure S1) exhibited characteristic peaks in the range of 1400–1000 cm−1 and at ~874 cm−1, attributed to –CF2– stretching and –C–H stretching, respectively [36,37]. Surface modification with PDA led to a slight shift in these bands. The incorporation of CuL was evidenced by the appearance of a new band at ~1613 cm−1, corresponding to the –C=N (imine) stretching vibration, which is characteristic of this complex [21,30]. The PDA layer provides active sites (catechol and amine groups) that can chemically coordinate with the CuL, facilitating its stable immobilization on the membrane surface [38]. Given the conditions for obtaining the membranes and the data obtained by FT-IR, the interaction between CuL and the membrane is probably of a chemical nature.
As shown in Figure S2, the pristine PVDF membrane exhibited a relatively smooth and porous surface (Figure S2A,B). A possible increase in surface roughness was observed after modification, suggesting successful PDA coating and CuL deposition (Figure S2C). This process generated a denser and more granular morphology, with CuL likely being distributed finely and continuously across the pores (Figure S2D). At higher CuL loadings (Figure S2E,F), quadrangular prism-like structures, typical of CuL [23], became evident, imparting greater heterogeneity to the surface of the film.
Elemental mapping (Figure S3A,C) revealed a weak Cu signal for PVDF/PDA/CuL-4 (lower copper loading) and a strong signal for PVDF/PDA/CuL-40 (higher copper loading) EDS spectra (Figure S3B,D) confirmed the presence of copper in both membranes, with a 9.08-fold increase in the atomic percentage for PVDF/PDA/CuL-40 compared to PVDF/PDA/CuL-4. Furthermore, since the signals of C, O, and N remained constant, it is reasonable to assume that both the polymeric matrix and PDA layer were present at comparable levels in both samples.
The initial water contact angle (WCA) of the pristine PVDF membrane was 53.33 ± 2.98°. After PDA modification, a decrease in the WCA was observed for all membranes compared to the pristine membrane (Figure S4A). This indicates that PDA rendered the membranes more hydrophilic, thereby facilitating water interaction [38], a phenomenon that has been widely documented in the literature [39,40]. However, increasing CuL loading led to an increase in the WCA from 22.00 ± 0.27° to 30.41 ± 0.75°, suggesting a slight reduction in the surface affinity toward water.
The effect of PVDF modification was also evident in the permeance results (Figure S4B). The pristine membrane exhibited a hydraulic permeance of 952.07 L/(h·m2·bar), which decreased to 753.07 L/(h·m2·bar) after PDA modification. As PDA formed a coating layer on the PVDF, coverage likely occurred, resulting in reduced permeance. In contrast, increasing the CuL loading led to a 2–3-fold enhancement in the permeance compared to PVDF/PDA (PVDF/PDA/CuL-4: 1479.59 L/(h·m2·bar); PVDF/PDA/CuL-20: 2272.64 L/(h·m2·bar); PVDF/PDA/CuL: 2229.91 L/(h·m2·bar)). Thus, even with the slight reduction in hydrophilicity for PVDF/PDA/CuL-40, the results suggest that the interaction of CuL with PDA may have generated additional pores or improved internal wettability, thereby making the surface less susceptible to fouling [41,42].
3.2. Evaluation of DRX-6BN Adsorption on Membranes in Stirred Systems
The membranes were first evaluated for the removal of DRX-6BN in the absence of H2O2 to assess their intrinsic adsorption capacity under controlled batch conditions. This configuration ensured uniform mixing and minimized the concentration gradients typically encountered in flow-through systems, thereby isolating the adsorption contribution from the catalytic effects. The corresponding results are shown in Figure 2.
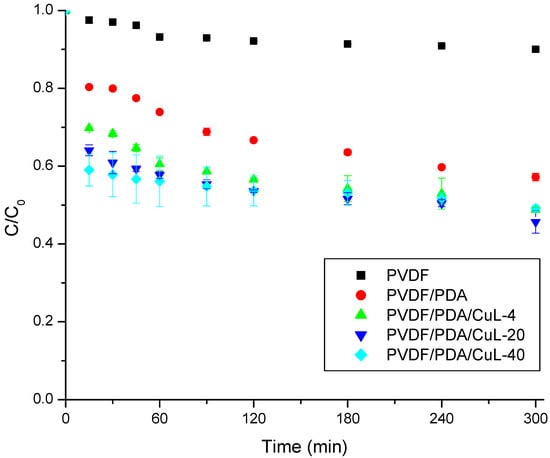
Figure 2.
Removal of DRX-6BN in agitated systems using pristine and modified PVDF membranes. Conditions: pH = ~6.0, CDRX-6BN0 = 20 mg/L, Vsolution = 200 mL, T = 25 °C, stirring speed = 200 rpm.
As illustrated in Figure 2, the adsorption of DRX-6BN by the pristine PVDF membrane was only 9.97% after 300 min. In contrast, the PVDF/PDA membrane achieved 19.67% removal within the first 15 min and reached 42.79% at 300 min, indicating that the presence of PDA significantly enhanced dye removal under stirred conditions. This improvement can be attributed to the increased hydrophilicity conferred by PDA, as previously evidenced in Figure S4, which facilitates the diffusion of soluble species towards the membrane surface. Moreover, PDA provides abundant active functional groups that promote stronger interactions between the pollutant and membrane surface [43,44]. The incorporation of the CuL complex further improved the removal efficiency, increasing the adsorption capacity to slightly above 50%.
The highest q was obtained for the PVDF/PDA/CuL-4 membrane, followed by PVDF/PDA/CuL-20 and PVDF/PDA/CuL-40, with values of 19.78 ± 0.20, 17.94 ± 0.99, and 14.51 ± 0.18 mg/g at 300 min, respectively. The decrease in q was expected because the PVDF/PDA/CuL-40 and PVDF/PDA/CuL-20 membranes had higher CuL loadings. Although the increase in CuL content resulted in a lower adsorption capacity (mg/g), no impact was observed on the DRX-6BN removal efficiency. This behavior may indicate reduced accessibility of the active sites due to possible agglomeration or excessive coverage of the adsorbent surface, reducing the effective contact area. It should be noted that part of the adsorption was due to CuL, as observed by Silva et al. (2024) [21].
Kinetic models were applied to elucidate the adsorption dynamics. Figure 3 presents the linearized fittings of the data from Figure 2 according to the pseudo-first-order (PFO), pseudo-second-order (PSO), and Elovich models. The fits to the nonlinear and intraparticle diffusion models are shown in Figures S5–S9. The corresponding correlation coefficients (R2) and root mean square errors (RMSE) for the models are summarized in Table 1. Table 2 presents the parameters and statistical metrics for the intraparticle diffusion model.
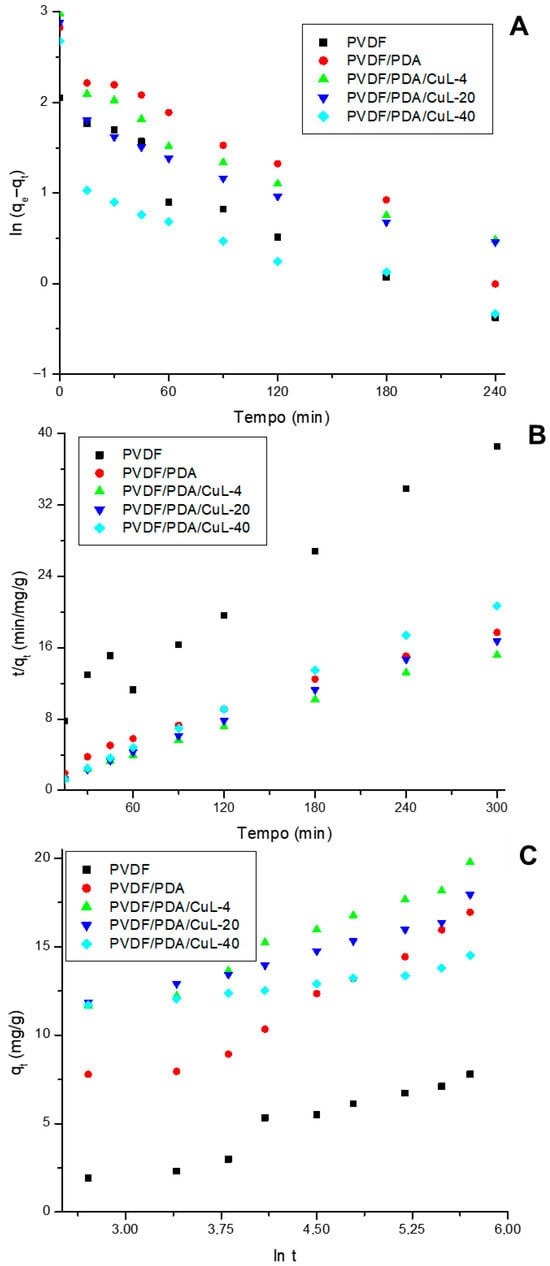
Figure 3.
Kinetic fitting of DRX-6BN removal in stirred systems using pristine PVDF, PVDF/PDA, and PVDF/PDA/CuL membranes. The experimental data were treated according to the PFO (A), PSO (B), and Elovich (C) models.

Table 1.
Correlation coefficient (R2) for the data fits to the adsorption kinetics models for the adsorption of DRX-6BN on pristine and CuL decorated membranes.

Table 2.
Parameters and statistical metrics for the intraparticle diffusion model.
As shown in Figure 3 and Figures S5–S9 and Table 1, with the exception of the pristine PVDF membrane, the adsorption kinetics were best described by the Elovich model (R2 > 0.9928; RMSE < 0.4489) and PSO (R2 > 0.9540; RMSE < 1.1388). The good fit of the data to the PSO and Elovich models indicates the predominance of chemisorption as the mechanism [45,46]. The best fit by the Elovich model suggests that the surface of the decorated membranes is heterogeneous, with different types of active sites and adsorption energies, reflecting the complexity of the CuL-containing structure of the membranes [29]. The visible coloration of the membrane after adsorption reinforces the occurrence of strong chemical interactions between DRX-6BN and the active sites on the membrane surface, which is evidence of chemisorption suggested by kinetic models, especially the Elovich model, which also indicates a heterogeneous surface with different adsorption affinities.
The Elovich model parameters showed a significant increase in the initial adsorption rate (α) with membrane modification. The pristine PVDF membrane exhibited the lowest α (0.8285) and β (0.6795) values, indicating low adsorptive activity (Figure 2). With the introduction of PDA, α increased to 2.263, and β decreased to 0.3545, indicating greater active site availability. α increased as the CuL loading in the membranes increased, with values of 14.08, 76.3, and 2410 for PVDF/PDA/CuL-4 and PVDF/PDA/CuL-20 and a value of 2410 for PVDF/PDA/CuL-40, respectively. Despite this, the β value was higher for the membrane with intermediate loading (0.5588), which suggests that, although the initial adsorption was very fast, the coverage of active sites did not increase proportionally, indicating surface saturation in the membrane with higher loading.
The intraparticle diffusion model (Figures S5B–S9B and Table 2) revealed a two-stage adsorption process for all the membranes. The intercept values, which are higher than zero, indicate that the initial stage is governed by film diffusion through the boundary layer. This effect was less pronounced for pristine PVDF, with an intercept of 0.4643 mg/g. The second stage corresponded to the gradual adsorption of DRX-6BN onto the membrane surface, which was attributed to intraparticle diffusion. In most cases, the intraparticle diffusion constant (kd, slope of the linear region) was lower than that in the first stage, indicating a reduced diffusion rate at later times. These results suggest that adsorption occurs via a combined mechanism: chemisorption as the dominant pathway, with film diffusion in the initial stage, followed by intraparticle diffusion in the later stage across all evaluated materials.
In summary, the adsorption experiments revealed that PDA incorporation enhanced the hydrophilicity and functionalization of PVDF membranes, resulting in higher dye removal efficiency, while CuL loading further improved the adsorption capacity. Kinetic analysis confirmed that the process was predominantly governed by chemisorption, with film diffusion as the initial step, followed by intraparticle diffusion. These findings provide a mechanistic basis for understanding the improved performance of the functionalized membranes and establish a reference for their subsequent evaluation under catalytic conditions in the presence of H2O2.
3.3. Evaluation of the Catalytic Activity of CuL Decorated Membranes
The catalytic activity of the CuL decorated membranes was first evaluated under batch conditions in the presence of H2O2, with the specific aim of assessing the role of CuL in peroxide decomposition. Figure 4 depicts the removal of DRX-6BN, H2O2 consumption, and kinetic model fittings (PFO and PSO) for the PVDF-based membranes. As previously reported, the use of H2O2 alone results in negligible DRX-6BN removal [21].
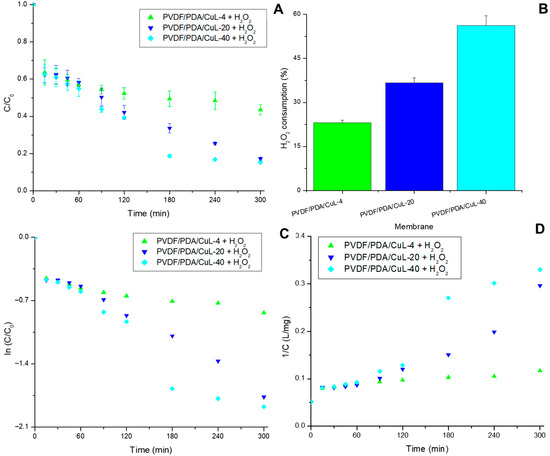
Figure 4.
Removal of DRX-6BN (A) H2O2 consumption in stirred systems over 300 min (B), fitting of kinetic data by PFOr (C) and PSOr (D) using neat and CuL decorated PVDF membranes. Conditions: pH = ~6.0, CDRX-6BN0 = CH2O20 = 20 mg/L, Vsolution = 200 mL, T = 25 °C, stirring speed = 200 rpm.
Membranes containing CuL exhibited continuous removal of DRX-6BN over time (Figure 4A), with higher CuL loadings leading to superior performance. Removals greater than 80% were achieved after 180 min, reaching 84.94 ± 0.44% for PVDF/PDA/CuL-40 after 300 min of exposure. Transition-metal-based catalysts, including iron, copper, cobalt, and nickel, are known to promote H2O2 decomposition into highly reactive •OH radicals, driving the mineralization of organic pollutants through Fenton and Fenton-like pathways [20,21,47,48]. The catalytic role of CuL in Fenton-like reactions was previously confirmed by Silva et al. (2024) [21]. The Fenton-like mechanism using copper has been reported in the literature and is shown by Silva et al. (2025) [23].
The gradual increase in DRX-6BN removal and reaction rate with increasing CuL loading (Figure 4A) can be attributed to the larger number of active sites, which enhances •OH generation via H2O2 decomposition under appropriate conditions [49,50,51,52].
The analysis of H2O2 consumption (Figure 4B) revealed incomplete utilization of peroxide. In membranes with higher CuL loading, approximately 56% of H2O2 was consumed, whereas in those with lower loading, consumption was below 25%. The H2O2 concentration is a critical parameter in Fenton-based processes: premature depletion compromises treatment efficiency, whereas excessive levels may result in radical scavenging, lowering overall performance [53,54,55,56]. Therefore, the results suggest that optimization requires adjusting the oxidant concentration to balance efficiency and radical availability.
Kinetic analysis (Table 3) showed satisfactory fittings to both the PFO and PSO models, with the apparent rate constants (kap) increasing proportionally to CuL loading. For comparison, CuL in a homogeneous solution exhibited a kap of 0.0152 min−1 under identical dye (20 mg/L) and H2O2 (0.0117 mol/L, ~400 mg/L) concentrations, with 0.000533 mol/L of CuL (~42 mg), reaching 69.14 ± 1.68% removal in 60 min [21]. This value is approximately three times higher than that obtained with membranes containing the same CuL mass; however, the peroxide concentration used in the solution was 20 times greater. Although adsorption contributed partially to dye removal, the supported CuL system demonstrated remarkable catalytic potential under milder oxidative conditions.

Table 3.
Kinetic parameters and R2 for data fits to DRX-6BN removal kinetic models on pristine and CuL decorated PVDF membranes.
Considering that free copper ions may be toxic to aquatic and terrestrial organisms [57,58], the immobilization of CuL onto membranes is an environmentally relevant strategy [23]. The PVDF/PDA/CuL membranes not only enhanced the catalytic efficiency in the presence of H2O2 but also increased the sustainability and safety of the process. Nevertheless, careful control of copper leaching and monitoring of potential oxidation byproducts remains essential to ensure the environmental applicability of this system in the future.
3.4. Enhanced Removal of Organic Compounds from Oily Effluents Aided by CuL Decorated Membranes in Filtration Systems
The previously conducted experiments served as a comparative basis for selecting the membranes to be employed in the treatment of oily wastewater. As shown in Figure 3, the adsorptive activities of the membranes were similar. Therefore, only the PVDF membrane with the lowest CuL loading was selected for assays with the oily effluent. Figure 5 presents the results obtained for the rejection of O&G and COD over time.
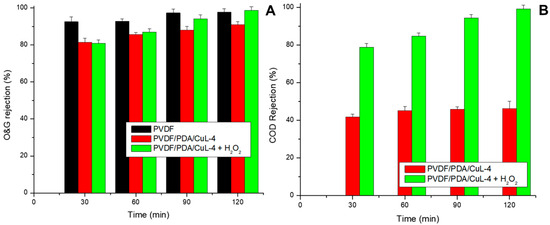
Figure 5.
Rejection of O&G (A) and COD (B) from oily effluent using pristine and CuL decorated PVDF membranes in a filtration system. Conditions: pH = ~6.0, CO&G0 = 100 ± 5 mg/L, CH2O20 = 20 mg/L (in oxidation tests), Vemulsion = 1.5 L, T = 25 °C, P = 1 bar.
As depicted in Figure 5, O&G rejection continuously increased from 0 to 120 min, particularly for the PVDF/PDA/CuL membrane, both in the presence and absence of H2O2, reaching 97.66 ± 1.92%, 91.02 ± 1.58%, and 98.67 ± 1.96% for the pristine membrane and those containing CuL without and with H2O2, respectively. However, COD rejection was limited to 38.52 ± 2.07% for the pristine membrane after 120 min, while nearly complete removal (~100%) was achieved with the CuL decorated membrane in the presence of H2O2 within the same period, surpassing 80% after 60 min. These results clearly demonstrate the ability of CuL to catalyze H2O2 decomposition and enhance the oxidative removal of organic contaminants [21].
Previous studies have reported alternative strategies for modifying PVDF membranes to improve their performance in treating oily wastewater. For instance, Xu et al. (2018) [59] described a PVDF membrane modified with a zinc-based metal–organic framework (MOF) using imidazolate ligands (ZIF-8) and achieved a separation efficiency of 92.93%. Similarly, Sun et al. (2020) [60] reported the modification of PVDF membranes by N-sulfonated, N-diethylenediamine co-deposited with tannic acid, resulting in separation efficiencies above 96%.
Nevertheless, a direct comparison with the system employed in the present work is not straightforward, because the performance of catalytic membranes based on Fenton or Fenton-like oxidation is more frequently reported for the degradation of dyes and pharmaceuticals in batch systems, often under visible or ultraviolet (UV) irradiation [61].
Considering the enhanced removal efficiency promoted by the incorporation of a small CuL loading into the PVDF membrane, the decorated membrane proved to be a promising material, particularly in the presence of H2O2.
3.5. Fouling Mechanisms
The permeate flux was expected to decline continuously over time owing to oil deposition on the membrane surface and within the pores, approaching an asymptotic value depending on the feed oil concentration, flow regime, and transmembrane pressure. Both the permeate flux and normalized flux (J/J0) were monitored (Figure 6).
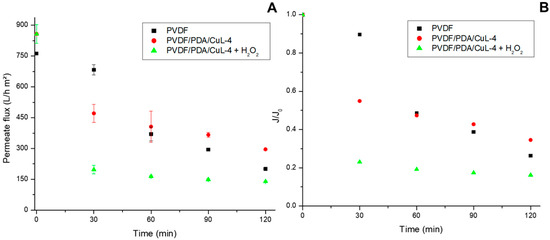
Figure 6.
Permeate flux (A) and J/J0 for the pristine and CuL decorated PVDF membranes (B) during oily wastewater treatment. Conditions: pH = ~6.0, CO&G0 = 100 ± 5 mg/L, CH2O20 = 20 mg/L (in the oxidation tests), Vemulsion = 1.5 L, T = 25 °C, P = 1 bar.
The pristine PVDF membrane exhibited lower flux than the CuL decorated membranes, likely due to the adsorption of partially oxidized by-products that exacerbate fouling [62,63]. For comparison, PVDF/ZIF-8 and PVDF/Fe3O4 membranes have reported fluxes of 1.11 L/(m2·h) and 175.8 L/(m2·h) at 1 bar, respectively, comparable to the PVDF/PDA/CuL-4 membrane in the presence of H2O2 [59,64].
To better understand the mechanism of O&G fouling during oily wastewater treatment, the experimental permeate flux data over time (Figure 6) were fitted using Hermia’s fouling models, including complete pore blocking (CPB), pore obstruction (PO), partial pore blocking (PPB), and cake filtration (CF) models. Figure 7 shows the corresponding fit, and Table 4 presents the fouling parameters (K) and R2 values obtained for each fouling mechanism.
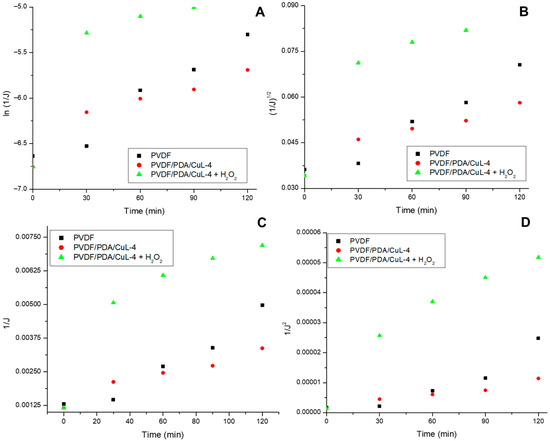
Figure 7.
Graphs associated with the Hermia models applied to the treatment data with pristine and CuL decorated PVDF membranes: BTP (A), OP (B), BPP (C), and e TF (D).

Table 4.
Fouling parameters and R2 for the Hermia fouling models of the pristine and CuL decorated PVDF membranes.
The most appropriate fouling models for PVDF-based membranes were analyzed (Figure 7 and Table 4). Considering the R2 values, fouling on the pristine PVDF membrane can be explained by a combination of the CPB, PO, and PPB effects, indicating the complexity of the phenomenon. In contrast, the CF model provided the best fit for the CuL decorated membrane in the absence and presence of H2O2. The CF model represents the successive formation of particle layers on the membrane surface, generating a dense particulate layer that acts as an additional barrier, increasing the resistance to permeate flow [65,66,67]. According to Zhang et al. (2020) [68], cake formation on the membrane surface may result from oil stains, dispersed oil, and emulsified oil, that is, a broad range of oil particle sizes.
The fouling constant (K) suggests cake formation was more pronounced in the presence of H2O2, likely due to the formation of less-soluble by-products. Additionally, BPP described fouling for the CuL decorated membrane in the absence of H2O2, indicating a combined mechanism of CF and BPP, where only a fraction of the particles contacts the membrane directly, while others deposit onto previously adhered particles.
3.6. Storage Stability of CuL Decorated Membranes
The use of copper-based catalytic membranes with Fenton-like activity demonstrates high potential for the oxidation of organic compounds, exhibiting reduced leaching and in situ regeneration of active sites through efficient redox mechanisms [69,70]. However, assessing the long-term stability of these membranes after storage, a common scenario in industrial operations, is essential to ensure their practical adoption. Figure 8 presents the storage stability results for both modified membranes tested in the presence and absence of H2O2.
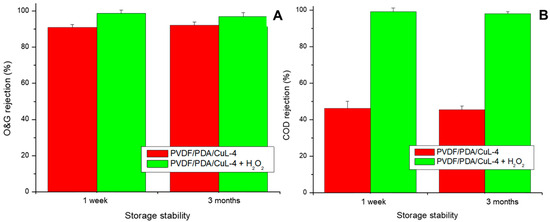
Figure 8.
O&G rejection (%) (A) and COD removal (%) (B) by CuL decorated PVDF membranes as a function of storage time. Conditions: pH = ~6.0, CO&G0 = 100 ± 5 mg/L, CH2O20 = 20 mg/L (in oxidation tests), Vemulsion = 1.5 L, T = 25 °C, P = 1 bar.
As shown in Figure 8, the rejection of both O&G and COD remained virtually unchanged over a period of three months, regardless of whether the membranes were operated under permeation without H2O2 or under coupled oxidative conditions. These results indicate that the membranes retained their catalytic properties for at least three months.
3.7. Comparison Between the Obtained PVDF Membranes and Other Membranes in o/w Separation and Removal of Organic Compounds
A comparative evaluation with the literature aims to contextualize the results obtained in this study relative to the existing research. Table 5 presents a direct comparison of studies involving modified membranes applied to oily wastewater treatment and, when available, dye degradation, including performance analyses in the presence and absence of H2O2.

Table 5.
Comparison of membranes in o/w separation and catalytic activity [71,72,73,74].
Table 5.
Comparison of membranes in o/w separation and catalytic activity [71,72,73,74].
| Membrane | o/w Separation | Permeate Flux (L/m2 h) | Target Compound (Removal) | Catalytic Conditions | Ref. |
|---|---|---|---|---|---|
| Celulose/PVDF-HFP | Petroleum in water (90.1%) | 124.62 (0.65 bar) | - | - | [71] |
| PVDF/PEMA/β-FeOOH | Soybean oil in water stabilized with surfactant (98.8%) | 694.56 | Methylene blue (99.8%) | 50 mL of target compound at 10 mg/L, 1 mL of H2O2 (30%), removal 7 h after equilibration, UV light, stirred system. | [72] |
| Fe@PVDF | Rapeseed oil, toluene, liquid paraffin, petroleum ether, carbon tetrachloride stabilized with Tween 80 (>99.0%) | ~2300 | Tetracycline and other drugs (>99.0%) | 100 mL of target compound at 50 mg/L, 0.1 mL of H2O2, removal after 50 min, visible light, thermostatic stirrer. | [73] |
| PVDF/PAMPS/β-FeOOH | Hexadecane in water stabilized by sodium dodecyl sulfate (>99.0%) | - | Methylene blue (~100%) | 50 mL of target compound at 10 mg/L, 10 mmol H2O2, removal after 40 min after equilibration (30 min), visible light. | [74] |
| PVDF/PDA/CuL | Petroleum in water (91.02% and 98.67%) | 857.34 | DRX-6BN (up to 84.94 ± 0.44%) | 200 mL of the target compound at 20 mg/L, 20 mg/L of H2O2, removal after 120 min and 300 min, absence of light, stirred system. | This study |
Notes: HFP—fluoride-co-hexafluoropropylene, PEMA—poly(ethylene-alt-maleic anhydride), PAMPS—poly(2-acrylamido-2-methyl-1-propanesulfonic acid.
As shown in Table 5, there is a clear interest in developing catalytic membranes for the removal of organic compounds, particularly dyes and pharmaceuticals, under light irradiation conditions. Studies based on copper-containing membranes are relatively scarce. Notably, most studies do not report the stability of membranes, limiting broader comparisons.
The PVDF/PDA/CuL membrane was distinguished by its combination of high performance and operational simplicity, achieving a flux of 857.34 L/m2 h and removal of up to 84.94% of the DRX-6BN dye, as well as an efficiency exceeding 98% in the separation of oil-in-water emulsions, all in the absence of radiation and without the need for heating or aggressive pH conditions. In comparison, the PVDF/PEMA/β-FeOOH membrane required UV light and long reaction times to achieve the removal of nearly 99.8% of methylene blue under more specific conditions [74]. This demonstrates that even without achieving complete removal, the PVDF/PDA/CuL membrane offers significant operational advantages, making it a promising alternative for practical applications in the treatment of complex wastewaters. The catalytic activity for Fenton-like degradation was confirmed and maintained for at least three months, which is crucial for industrial applications.
4. Conclusions
PVDF/PDA/CuL membranes were prepared and characterized using FT-IR, SEM/EDS, contact angle measurements, and hydraulic permeability. Modification with PDA significantly increased the adsorption of the DRX-6BN dye due to increased surface diffusion and the presence of active functional groups. After 300 min, removal reached 42.79% for PVDF/PDA, compared to 9.97% for pristine PVDF, and increased to ~50% with the incorporation of the CuL.
The adsorption kinetics were best described by the Elovich model, with increasing α values and CuL loading, indicating chemisorption and a heterogeneous process. The PSO model also showed a good fit, whereas the intraparticle diffusion model suggested a two-stage mechanism involving film diffusion followed by pore diffusion.
In the presence of H2O2, the membranes with the highest CuL loading removed over 80% of the DRX-6BN within 180 min. PVDF/PDA/CuL with the lowest CuL content achieved nearly 100% COD removal in 120 min compared to only 38.52 ± 2.07% for pure PVDF. Stability tests demonstrated the maintenance of catalytic activity after three months of storage at room temperature.
Overall, the CuL-functionalized PVDF membranes exhibited robust and versatile performance in the removal of reactive dyes and pollutants from petroleum oil-in-water emulsions. The combination of adsorption, catalytic oxidation at near-neutral pH, and storage stability highlights their potential for practical applications, such as the treatment of produced water onshore. These results indicate a promising technological solution aligned with the demand for more sustainable and efficient wastewater treatment processes.
Despite the promising results, this study has some limitations that point to important directions for future research. First, characterizing the materials after use is essential to deepen our understanding of adsorption, degradation, and filtration mechanisms. Assessing potential copper leaching, especially in the presence of H2O2, is also necessary, along with optimizing the H2O2 concentration to avoid excess residuals in the treated water.
Additionally, it is important to characterize the surface charge of membranes and their roles in the separation mechanism. Structural stability tests on the membranes after prolonged and cyclic testing should be performed using techniques such as FT-IR, SEM and X-ray diffraction (XRD). Studies using real matrices are also recommended to evaluate the background effects caused by variables such as competing ions, turbidity, natural organic matter, and pH variation. Finally, long-term storage stability tests should be conducted to confirm the practical applicability of the developed materials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w17202988/s1. Figure S1. FT-IR spectra of CuL and PVDF membranes. Figure S2. Micrographs of PVDF 500× (A), PVDF 5000 × (B), PVDF/PDA/CuL-4 500× (C), PVDF/PDA/CuL-4 5000× (D), PVDF/PDA/CuL-40 500× (E), e PVDF/PDA/CuL-40 5000× (F) membranes. Figure S3. Elemental mapping of PVDF/PDA/CuL-4 membrane (A) EDS spectrum of PVDF/PDA/CuL-4 membrane (B), Elemental mapping of PVDF/PDA/CuL-40 membrane (C), EDS spectrum of PVDF/PDA/CuL-40 membrane (D). Figure S4. Water contact angle (A) and permeance (B) of the PVDF membranes. Figure S5. Fitting of DRX-6BN adsorption data on PVDF membrane to the nonlinear PPO, PSO and Elovich models (A) and to the intraparticle diffusion model (B). Figure S6. Fitting of DRX-6BN adsorption data on PVDF/PDA membrane to the nonlinear PPO, PSO and Elovich models (A) and to the intraparticle diffusion model (B). Figure S7. Fitting of DRX-6BN adsorption data on PVDF/PDA/CuL-4 membrane to the nonlinear PPO, PSO and Elovich models (A) and to the intraparticle diffusion model (B). Figure S8. Fitting of DRX-6BN adsorption data on PVDF/PDA/CuL-20 membrane to the nonlinear PPO, PSO and Elovich models (A) and to the intraparticle diffusion model (B). Figure S9. Fitting of DRX-6BN adsorption data on PVDF/PDA/CuL-40 membrane to the nonlinear PPO, PSO and Elovich models (A) and to the intraparticle diffusion model (B).
Author Contributions
F.P.d.S.: conceptualization, data curation, methodology, formal analysis, investigation, visualization, writing—original draft; A.C.F.P.: investigation, writing—review and editing; J.C.P.: investigation, writing—review and editing; A.C.: conceptualization, resources, project administration, supervision, validation, visualization, writing—review and editing; C.P.B.: conceptualization, project administration, supervision, validation, visualization, writing—review and editing; F.V.d.F.: conceptualization, resources, funding acquisition, project administration, supervision, validation, visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Agência Nacional de Petróleo, Gás Natural e Biocombustíveis (ANP) (Grant number 041319), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Funding number: E-26-204.190/2024), for the financial support and scholarship granted to carry out this work.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Electron Microscopy Center of COPPE at the Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil, for their support in using their equipment. The authors would also like to thank the financial support of the ANP and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), through the ANP Human Resources Program for the Petroleum, Natural Gas and Biofuels Sector (PRH-ANP), in particular PRH 3.1, of the School of Chemistry–Training in Processes and Systems of the Petroleum and Biofuels Industry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bani-Atta, S.A.; Darwish, A.A.A.; Shwashreh, L.; Alotaibi, F.A.; Al-Tweher, J.N.; Al-Aoh, A.H.; El-Zaidia, E.F.M. Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation. Processes 2024, 12, 2769. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198–126227. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.Q.; Sabbah, A.; Chen, L.; Chen, K.; Thi, C.M.; Viet, P.V. High-efficient Photocatalytic Degradation of Commercial Drugs for Pharmaceutical Wastewater Treatment Prospects: A Case Study of Ag/g-C3N4/ZnO Nanocomposite Materials. Chemosphere 2021, 282, 130971. [Google Scholar] [CrossRef]
- The Sustainable Development Goals in Brazil, United Nations Brazil. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 17 July 2025). (In Portuguese).
- Varatharajan, G.R.; Ndayishimiye, J.C.; Nyirabuhoro, P. Emerging Contaminants: A Rising Threat to Urban Water and a Barrier to Achieving SDG-Aligned Planetary Protection. Water 2025, 17, 2367. [Google Scholar] [CrossRef]
- Grigg, N.S. Integrated Water Resources Management After 2030: An Agenda for Educators. Water 2025, 17, 189. [Google Scholar] [CrossRef]
- Sen, T.K. Adsorptive Removal of Dye (Methylene Blue) Organic Pollutant from Water by Pine Tree Leaf Biomass Adsorbent. Processes 2023, 11, 1877. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, J.; Zhu, S.; Liu, Q. Co-modification of Lignocellulosic Biomass by Maleic Anhydride and Ferric Hydroxide for the Highly Efficient Biosorption of Methylene Blue. New J. Chem. 2021, 45, 19678–19690. [Google Scholar] [CrossRef]
- Dai, J.; Gao, Y.; Shah, K.J. Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater. Water 2025, 17, 2494. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Leones, A.R.; De Farias, B.S.; Da Silva, M.D.; Jaeschke, D.P.; Fernandes, S.S.; Ribeiro, A.C.; Junior, T.R.S.C.; Pinto, L.A.A. A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal. Water 2025, 17, 2141. [Google Scholar] [CrossRef]
- Al-Ajmi, F.; Al-Marri, M.; Almomani, F. Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water 2025, 17, 23. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Ullah, A.; Tanudjaja, H.J.; Ouda, M.; Hasan, S.W.; Chew, J.W. Membrane Fouling Mitigation Techniques for Oily Wastewater: A Short Review. J. Water Process Eng. 2021, 43, 102293. [Google Scholar] [CrossRef]
- Ferreira, A.D.; Coelho, D.R.B.; dos Santos, R.V.G.; Nascimento, K.S.; Presciliano, F.A.; Silva, F.P.; Campos, J.C.; Fonseca, F.V.; Borges, C.P.; Weschenfelder, S.E. Fouling Mitigation in Produced Water Treatment by Conjugation of Advanced Oxidation Process and Microfiltration. Environ. Sci. Pollut. Res. 2021, 28, 12803–12816. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, full-scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Rodrigues, R.A.; de Campos, M.B.M.; Tonello, P.S. Degradation of Phenolic Compounds and Organic Matter from Real Winery Wastewater by Fenton and Photo-Fenton Processes Combined with Ultrasound. Water 2025, 17, 763. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Krzemińska, D.; Ratman-Kłosińska, I. Hybrid System of Fenton Process and Sequencing Batch Reactor for Coking Wastewater Treatment. Water 2025, 17, 751. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, C.; Su, P.; Lu, W.; Zhang, Z.; Wang, X.; Hu, W. Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism. Water 2022, 14, 2913. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced Oxidation Process for the Treatment of Industrial Wastewater: A Review on Strategies, Mechanisms, Bottlenecks and Prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.S.F.; Rodrigues, A.C.C.; Lima, J.F. Photocatalytic Degradation of Dyes by Mononuclear Copper(II) Complexes from Bis-(2-pyridylmethyl)amine NNN-Derivative Ligands. Inorganica Chim. Acta 2020, 512, 119924. [Google Scholar] [CrossRef]
- da Silva, F.P.; Casellato, A.; da Fonseca, F.V. Organic Compounds Removal Aided by a Copper(II) Complex: Kinetic Investigation, Mechanism Evaluation, and Catalyst Reuse and Stability. Int. J. Environ. Sci. Technol. 2024, 21, 1605–1618. [Google Scholar] [CrossRef]
- Li, J.; Pham, A.N.; Dai, R.; Wang, Z.; Waite, T.D. Recent Advances in Cu-Fenton Systems for the Treatment of Industrial Wastewaters: Role of Cu Complexes and Cu Composites. J. Hazard. Mater. 2020, 392, 122261–122279. [Google Scholar] [CrossRef]
- da Silva, F.P.; Felippe, L.C.; Borges, C.P.; Casellato, A.; da Fonseca, F.V. Enhanced Removal of Organic Compounds Assisted by Activated Carbon/Copper (II) Complex Composite. Processes 2025, 13, 447. [Google Scholar] [CrossRef]
- Araújo, F.V.F.; Yokoyama, L.; Teixeira, L.A.C.; Campos, J.C. Heterogeneous Fenton Process Using the Mineral Hematite for the Discolouration of a Reactive dye Solution. Braz. J. Chem. Eng. 2011, 28, 605–6016. [Google Scholar] [CrossRef]
- Danforth, C.; Chiu, W.A.; Rusyn, I.; Schultz, K.; Bolden, A.; Kwiatkowski, C.; Craft, E. An Integrative Method for Identification and Prioritization of Constituents of Concern in Produced Water from Onshore Oil and Gas Extraction. Environ. Int. 2020, 134, 105280. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced Water Characteristics, Treatment, and Reuse: A Review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Yu, R.; Chen, W.; Zhang, J.; Liu, J.; Li, X.-Y.; Lin, L. Catalytic Membranes for Water Treatment: Perspectives and Challenges. J. Hazard. Mater. Adv. 2024, 13, 100414. [Google Scholar] [CrossRef]
- Ma, X.; Deng, X.; Yuan, F.; Wang, Q.; Low, Z.-X.; Zhong, Z.; Wang, H.; Xing, W. Challenges and Opportunities in Catalytic Membrane-Based Oxidation-Filtration Systems for Water Remediation. Chem. Eng. J. 2025, 519, 165546. [Google Scholar] [CrossRef]
- da Silva, F.P.; Borges, C.P.; da Fonseca, F.V. Trends in Fouling Resistant Membranes Containing Metals or Metallic Nanoparticles for the Separation of Oil-in-Water Emulsions. ACS Omega 2025, 10, 7510–7529. [Google Scholar] [CrossRef]
- Dionízio, T.P.; dos Santos, A.C.; da Silva, F.P.; Moura, F.S.; D’Elia, E.; Garrido, F.M.S.; Medeiros, M.E.; Casellato, A. Copper(II) Schiff Base Complex with Electrocatalytic Activity Towards the Oxygen Reduction Reaction and Its Catalase Activity. Electrocatalysis 2021, 12, 137–145. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, H.; Jiang, S.; Zhu, M.; Zhou, Y.; Wang, D.; Fan, Y.; Zhang, D.; Zhang, L. Fabrication of FeOCl/MoS2 Catalytic Membranes for Pollutant Degradation and Alleviating Membrane Fouling with Peroxymonosulfate Activation. J. Environ. Chem. Eng. 2022, 10, 107717. [Google Scholar] [CrossRef]
- American Public Health Association—APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2012. [Google Scholar]
- Zhang, S.; Li, Y.; Yuan, Y.; Jiang, L.; Wu, H.; Dong, Y. Biomimetic Hydrophilic Modification of Poly (vinylidene fluoride) Membrane for Efficient Oil-in-Water Emulsions Separation. Sep. Purif. Technol. 2024, 329, 125227. [Google Scholar] [CrossRef]
- Ni, P.; Zeng, J.; Chen, H.; Yang, F.; Yi, X. Effect of Different Factors on Treatment of Oily Wastewater by TiO2/Al2O3-PVDF Ultrafiltration Membrane. Environ. Technol. 2022, 43, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Anari, Z.; Sengupta, A.; Sardari, K.; Wickramasinghe, S.R. Surface modification of PVDF membranes for treating produced waters by direct contact membrane distillation. Sep. Purif. Technol. 2019, 224, 388–396. [Google Scholar] [CrossRef]
- Kamaz, M.; Sengupta, A.; Gutierrez, A.; Chiao, Y.-H.; Wickramasinghe, R. Surface Modification of PVDF Membranes for Treating Produced Waters by Direct Contact Membrane Distillation. Environ. Res. Public Health 2019, 16, 685. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Zhu, L.-P.; Zhang, H.-T.; Zhu, B.-K.; Xu, Y.-Y. Improved hydrodynamic permeability and antifouling properties of poly (vinylidene fluoride) membranes using polydopamine nanoparticles as additives. J. Membr. Sci. 2014, 457, 73–81. [Google Scholar] [CrossRef]
- Li, G.; Liu, B.; Bai, L.; Shi, Z.; Tang, X.; Wang, J.; Liang, H.; Zhang, Y.; Van der Bruggen, B. Improving the performance of loose nanofiltration membranes by poly-dopamine/zwitterionic polymer coating with hydroxyl radical activation. Sep. Purif. Technol. 2020, 238, 116412. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Q.; Wang, T.; Zhang, L. Effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; He, W.; Chen, M.; Tu, W.; Zhu, M.; Gan, D.; Liu, S. Multifunctional stable PDA/RGO/MOFs&SiO2-COOH membrane with excellent flux and anti-fouling performance for the separation of organic dye and oil/water. Surf. Interfaces 2022, 33, 102183. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine Nanostructures as Biomaterials for Medical Applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-Inspired Surface Engineering for Water-Remediation Materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef]
- Badu Latip, N.M.; Gopal, K.; Suwaibatu, M.; Hashim, N.M.; Rahim, N.Y.; Raoov, M.; Yahaya, N.; Mohamad Zain, N.N. Removal of 2,4-dichlorophenol from wastewater by an efficient adsorbent of magnetic activated carbon. Sep. Sci. Technol. 2020, 56, 252–265. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, X.; Ye, S.; Yin, G.; Zeng, Q. Catalyzed oxidative degradation of methylene blue by in situ generated cobalt (II)-Bicarbonate complexes with hydrogen peroxide. Appl. Catalysis B Environ. 2011, 102, 37–43. [Google Scholar] [CrossRef]
- El-Baradie, K.; El-Sharkawy, R.; El-Ghamry, H.; Sakai, K. Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes of a number of sulfadrug azodyes and their application for wastewater treatment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 180–187. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Ngo, Q.N.; Van, H.T.; Thai, V.N.; Nguyen, T.P.; Phan Thi, K.O. Reutilization of Fe-containing tailings ore enriched by iron(III) chloride as a heterogeneous Fenton catalyst for decolorization of organic dyes. RSC Adv. 2021, 11, 15871–15884. [Google Scholar] [CrossRef]
- Zhou, P.; Dai, Z.; Lu, T.; Ru, X.; Ofori, M.A.; Yang, W.; Hou, J.; Jin, H. Degradation of Rhodamine B in Wastewater by Iron-Loaded Attapulgite Particle Heterogeneous Fenton Catalyst. Catalysts 2022, 12, 669. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, F.; Shen, C.; Zhu, C.; Li, A. A green and energy-saving microwave-based method to prepare magnetic carbon beads for catalytic wet peroxide oxidation. J. Clean. Prod. 2019, 215, 232–244. [Google Scholar] [CrossRef]
- Acisli, O.; Khataee, A.; Soltani, R.D.C.; Karaca, S. Ultrasound-assisted Fenton process using siderite nanoparticles prepared via planetary ball milling for removal of reactive yellow 81 in aqueous phase. Ultrason. Sonochem. 2017, 35, 210–218. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Env. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Aanchal; Basu, S. Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 32–42. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Garcia, J.; Cirés, S.; de Pedro, Z.M.; Quesada, A.; Casas, J.A. Photo-Fenton oxidation of cylindrospermopsin at neutral pH with LEDs. Environ. Sci. Pollut. Res. 2023, 30, 21598–21607. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Cheng, J.; Xia, Y.; Yang, Y. Copper exposure causes alteration in the intestinal microbiota and metabolites in Takifugu rubripes. Ecotoxicol. Environ. Saf. 2024, 272, 116064. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Chen, L.; Xu, B.; Feng, C.; Bai, Y.; Liao, H.; Sun, S.; Giesy, J.P.; Guo, W. Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environ. Pollut. 2016, 219, 1069–1076. [Google Scholar] [CrossRef]
- Xu, S.; Ren, L.-F.; Zhou, Q.; Bai, H.; Li, J.; Shao, J. Facile ZIF-8 functionalized hierarchical micronanofiber membrane for high-efficiency separation of water-in-oil emulsions. J. Appl. Polym. Sci. 2018, 135, 46462. [Google Scholar] [CrossRef]
- Sun, Y.; Zong, Y.; Yang, N.; Zhang, N.; Jiang, B.; Zhang, L.; Xiao, X. Surface hydrophilic modification of PVDF membranes based on tannin and zwitterionic substance towards effective oil-in-water emulsion separation. Sep. Purif. Technol. 2020, 234, 116015. [Google Scholar] [CrossRef]
- Liang, L.; Ji, L.; Ma, Z.; Ren, Y.; Zhou, S.; Long, X.; Cao, C. Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review. Membranes 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Che, A.-F.; Huang, X.-J.; Xu, Z.K. Polyacrylonitrile-based nanofibrous membrane with glycosylated surface for lectin affinity adsorption. J. Membr. Sci. 2011, 366, 272. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zhang, X.; Wang, Y.-X.; Sun, J.-Y.; Zhang, H.; Liu, W.-L.; Li, M.-P.; Ma, X.-H.; Xu, Z.-L. Fe3O4/PVDF catalytic membrane treatment organic wastewater with simultaneously improved permeability, catalytic property and anti-fouling. Environ. Res. 2020, 187, 109617. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 16919. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, F.-X.; Li, X.-C.; Liu, Q.; Chen, J.-F.; Guo, C.-M. Comparison of the performance of prepared pristine and TiO2 coated UF/NF membranes for two types of oil-in-water emulsion separation. Chemosphere 2020, 244, 125386. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sun, M.; Huang, D.; Chu, C.; Hedtke, T.; Wang, X.; Zhao, Y.; Kim, J.-H.; Elimelech, M. Catalytic Membrane with Copper Single-Atom Catalysts for Effective Hydrogen Peroxide Activation and Pollutant Destruction. Environ. Sci. Technol. 2022, 56, 8733–8745. [Google Scholar] [CrossRef] [PubMed]
- del Castillo-Velilla, I.; Romero-Muñiz, I.; Marini, C.; Montoro, C.; Platero-Prats, A.E. Copper single-site engineering in MOF-808 membranes for improved water treatment. Nanoscale 2024, 16, 6627–6635. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF-HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Li, C.; Shi, M.; Xu, D.; Liao, Q.; Liu, G.; Guo, Y.; Zhang, H.; Zhu, H. Fabrication of photo-Fenton self-cleaning PVDF composite membrane for highly efficient oil-in-water emulsion separation. RSC Adv. 2022, 12, 35543–35555. [Google Scholar] [CrossRef]
- Wang, M.; Mu, L.; Zhang, H.; Mao, X.; Zhang, M.; Dong, C.; Lei, H.; Shen, R.; Ju, A.; Hu, J.; et al. A superwettable PVDF membrane with durably chelated Fe(III) for excellent photo-Fenton self-cleaning and effective oil-in-water emulsion separation. J. Membr. Sci. 2025, 713, 123245. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, T.; Liu, H.; Tang, Z.; Kuang, X.; Qiao, Y.; Zhang, H.; Zhu, C. Hydrogel/β-FeOOH-Coated Poly(vinylidene fluoride) Membranes with Superhydrophilicity/Underwater Superoleophobicity Facilely Fabricated via an Aqueous Approach for Multifunctional Applications. Polymers 2023, 15, 839. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).