1. Introduction
Legionella is an environmental bacterium widely distributed in aquatic ecosystems, capable of infecting both protozoa and humans. It is considered a significant public health concern worldwide, affecting both developed and developing countries. In developed regions, challenges often stem from complex water systems and aging infrastructure, whereas in developing regions, high population density and inadequate water supply and sanitation infrastructure contribute to the increasing risk of waterborne pathogens such as
Legionella [
1,
2,
3]. This bacterium is commonly found in freshwater environments such as lakes and rivers, as well as artificial systems, including water distribution networks, air conditioning systems, cooling towers, and spas.
Legionella survives only in humid conditions and does not persist in dry environments [
4].
Legionella is a facultative intracellular bacterium that relies on protozoa (e.g.,
Acanthamoeba,
Naegleria,
Hartmannella, and
Tetrahymena) as natural hosts for replication [
5]. This symbiotic relationship provides essential nutrients and protects the bacterium from adverse conditions [
6]. Additionally, biofilm formation on aquatic surfaces is a key mechanism for its persistence. These microbial communities, embedded in an extracellular matrix, enhance resistance to disinfectants and facilitate colonization of artificial systems [
7,
8]. The
Legionellaceae family consists of a single genus,
Legionella. The number of recognized species and serogroups has steadily increased since the 1976 outbreak in Philadelphia. Currently, over 60 species and 80 serogroups have been identified. Among them,
Legionella pneumophila is the primary causative agent of both community-acquired and nosocomial infections [
5,
9], with
L. pneumophila serogroup 1 responsible for 84% of global Legionnaires’ disease cases [
10]. These bacteria are Gram-negative bacilli (0.3–0.9 μm wide; 2–20 μm long), motile (with polar flagella), catalase-positive, and urease-negative. Although they do not form spores, they adapt to oligotrophic environments through interactions with protozoa [
11].
Human infection occurs through inhalation of contaminated aerosols generated from various water sources, such as showers, cooling towers, decorative fountains, spa pools, humidifiers, and certain medical equipment [
3]. In the lungs,
Legionella bacteria are phagocytosed by alveolar macrophages, where they evade phagosome–lysosome fusion and replicate within vacuoles [
12]. Once the host cell’s resources are depleted, the bacteria induce cell lysis, releasing new bacteria into the extracellular environment and perpetuating the infection cycle. This process triggers pulmonary inflammation, leading to the symptoms of Legionnaires’ disease [
13,
14]. Legionellosis manifests in two main forms: Legionnaires’ disease, a severe pneumonia characterized by high fever, dry cough, dyspnea, and potential systemic complications (e.g., delirium), with a mortality rate exceeding 10% without treatment [
6]; and Pontiac fever, a self-limiting flu-like illness causing fever and myalgia, but without pulmonary involvement [
6]. The most vulnerable individuals are men over 50, smokers, immunocompromised individuals, and those with chronic lung diseases. Transmission does not occur through water ingestion, and person-to-person spread has not been documented [
15].
Effective strategies to prevent
Legionella proliferation include regular maintenance of water systems by avoiding stagnation and controlling temperatures between 20 and 50 °C, the use of corrosion-resistant materials, and periodic disinfection through methods such as chlorination, UV treatment, or copper-silver ionization [
16]. In Portugal, legislation mandates the implementation of prevention plans with laboratory monitoring conducted by accredited entities [
17]. Detection methods vary in sensitivity and applicability. The cultural method (ISO 11731:2017) is considered the “gold standard”, but obtaining conclusive results may take up to 15 days post-sampling and does not detect viable but non-culturable bacteria [
18]. RT-qPCR, i.e., reverse transcription quantitative polymerase chain reaction (ISO/TS 12869:2019), detects bacterial DNA in less than 24 h, but the lack of a direct correlation between genomic units (GU) and colony-forming units (CFU) limits its legal applicability [
19]. Alternative methods, such as immunoassays like Legiolert™, quantify
Legionella through the most probable number (MPN) approach [
20].
European surveillance of Legionnaires’ disease was initially coordinated by the European working group for Legionella infections (EWGLINet), with Portugal participating since 1986. In April 2010, coordination shifted to the European center for disease prevention and control (ECDC) under the European Legionnaires’ disease surveillance network (ELDSNet). Portugal is represented by two collaborating centers. The directorate-general of health (DGS) for epidemiology and the national health institute Dr. Ricardo Jorge (INSA) for microbiology.
Legionella was gradually recognized as a significant cause of pneumonia, with its importance as a pathogen becoming evident after an outbreak during the 58th annual convention of the American Legion in the summer of 1976. Of the 221 members who developed acute respiratory infection, 34 died. After months of epidemiological and microbiological studies,
L. pneumophila was isolated from lung tissue samples of four fatal cases [
6]. The clinical and epidemiological description of this outbreak was later linked to previous pneumonia epidemics, including those at a meatpacking plant in Minnesota in 1957 and St. Elizabeth Hospital in 1965. The 2021 Annual Epidemiological Report for Legionnaires’ disease, published by the European Center for Disease Prevention and Control in June 2023, is the latest official report currently available. It indicates a disease rate of 2.4 cases per 100,000 inhabitants in the European economic area, with variations between countries. Italy, France, Spain, and Germany account for 75% of cases, with men over 65 being the most affected group. Most cases are community-acquired, but only 11% are culture-confirmed, potentially underestimating cases caused by other
Legionella species. In 2021, 19 outbreaks were reported across 8 countries. The travel-associated Legionnaires’ disease surveillance scheme recorded a 38% increase in cases compared to 2020, primarily affecting individuals aged 45 or older [
21].
In recent years, some cases of
Legionella infection have also been reported in Portugal. In November 2014, the municipality of Vila Franca de Xira experienced the largest outbreak of Legionnaires’ Disease in the country, considered the second-largest documented in the literature. A total of 403 pneumonia cases were identified, 377 of which were confirmed to be caused by
L. pneumophila, resulting in 14 deaths. A match was confirmed between the bacterial strain isolated from a cooling tower at Adubos de Portugal and the strain found in patients’ bronchial secretions [
22]. Similarly, in March 2017, five cases of Legionnaires’ disease were identified in the municipality of Maia. All affected individuals were employees of Sakthi Portugal SA. Health authorities considered occupational exposure, as the workers may have contracted the infection before the completion of decontamination procedures in the company’s cooling towers [
23,
24]. In November 2017, another outbreak of Legionnaires’ disease occurred at Hospital S. Francisco Xavier in Lisbon, resulting in 56 confirmed cases and 5 suspected cases. Unfortunately, 6 deaths occurred among the confirmed cases, representing a mortality rate of 11% [
25]. Then, in January 2018, Hospital CUF Descobertas, also in Lisbon, was linked to 15 confirmed cases of Legionnaires’ disease. Of these, one patient did not require hospitalization, 12 were discharged, and 2 required intensive care [
26].
Public awareness is essential for prevention, and the manner in which information is conveyed to the population is a decisive factor in shaping understanding and mitigating exposure risks [
27,
28,
29]. The World Health Organization (WHO) defines health literacy as the ability to understand and apply health-related information [
30]. Informed communities adopt practices such as adjusting hot water temperatures to above 60 °C and regularly cleaning showers to reduce risks. Health institutions should publish clear guidelines to empower the population. Despite challenges and the need for more resources, Portugal has demonstrated leadership in implementing health literacy strategies aligned with behavioral sciences. The new health literacy and behavioral sciences plan 2023–2030 aims to improve preventive and health-promoting behaviors [
31]. Controlling
Legionella requires a multidisciplinary approach, ranging from environmental surveillance to public education. Investing in health literacy and rapid diagnostic technologies is an essential step to mitigate outbreaks and protect vulnerable populations. To effectively address
Legionella infection risks and promote preventive behaviors, it is essential to understand the population’s knowledge and attitudes towards the disease. In this context, providing insights into the public’s awareness of
Legionella transmission, their perceptions of risk, and their engagement with prevention measures is crucial. The aim of this study was to evaluate the Portuguese population’s understanding of
Legionella infections and their readiness to adopt preventive actions.
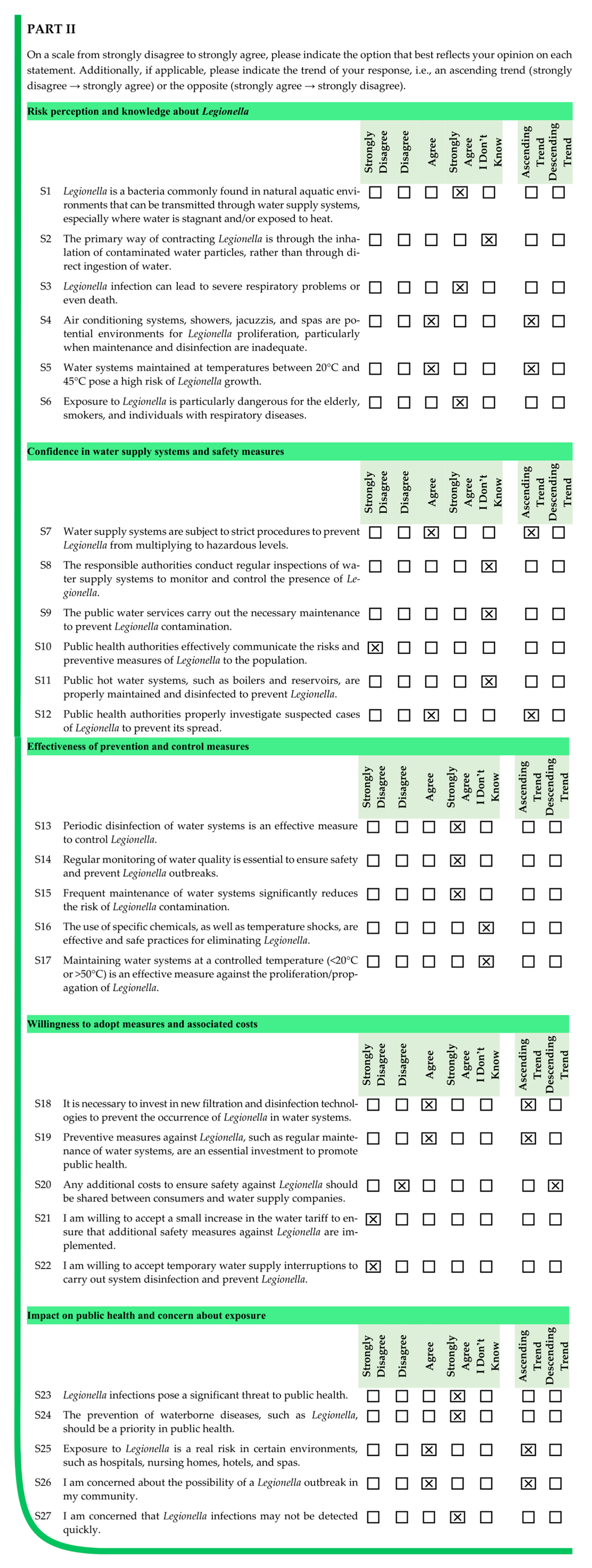
The primary contribution of this research is a thorough evaluation of public awareness concerning various aspects of Legionella prevention through a large-scale questionnaire. This evaluation highlights areas where knowledge is insufficient, particularly regarding risk factors and the importance of effective control measures. Additionally, the study introduces methods for assessing overall awareness levels and identifying areas for improvement, which are essential for grouping participants based on similar characteristics and for designing targeted interventions that can enhance public health behaviors and reduce the spread of Legionella.
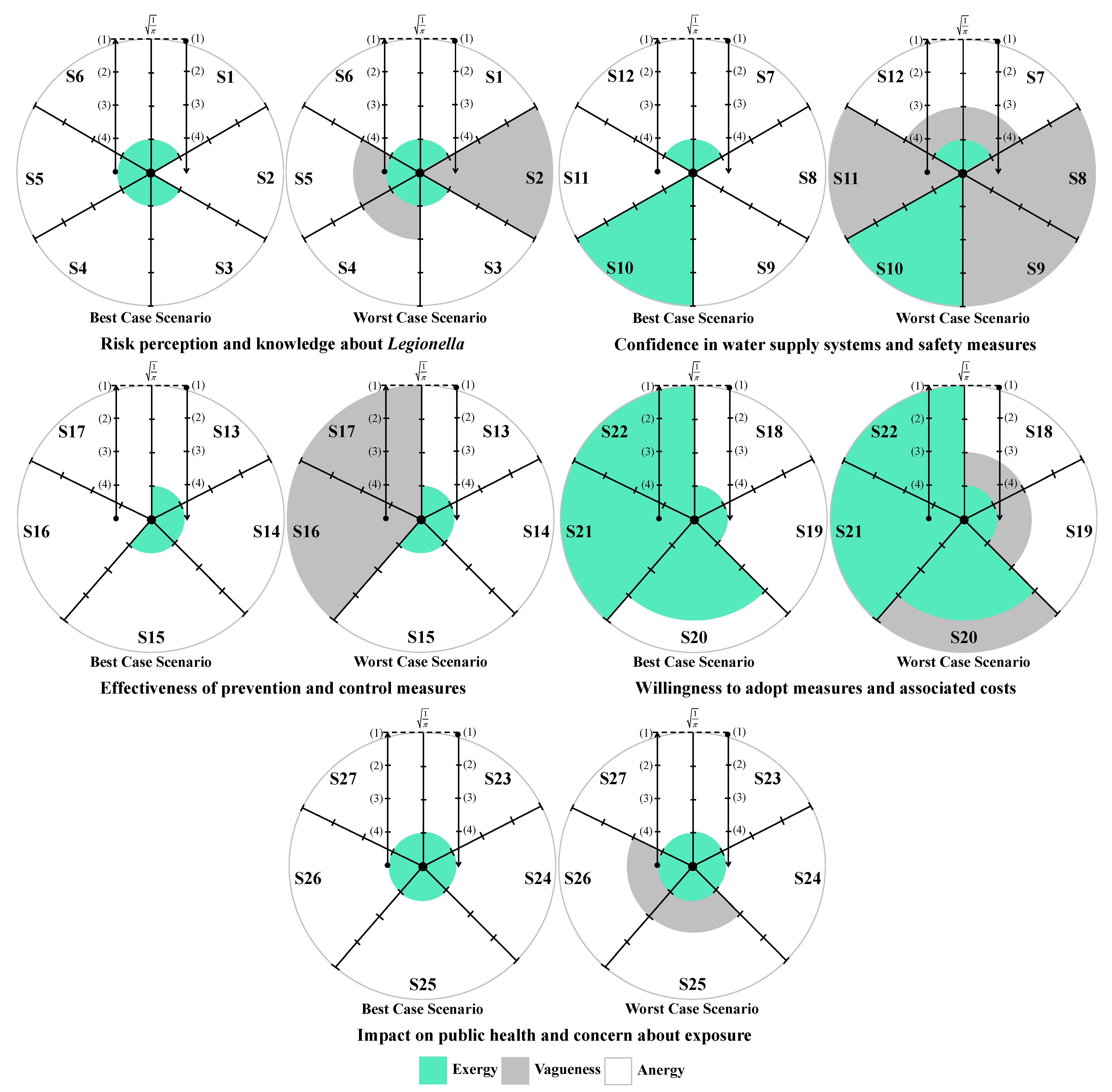
4. Discussion
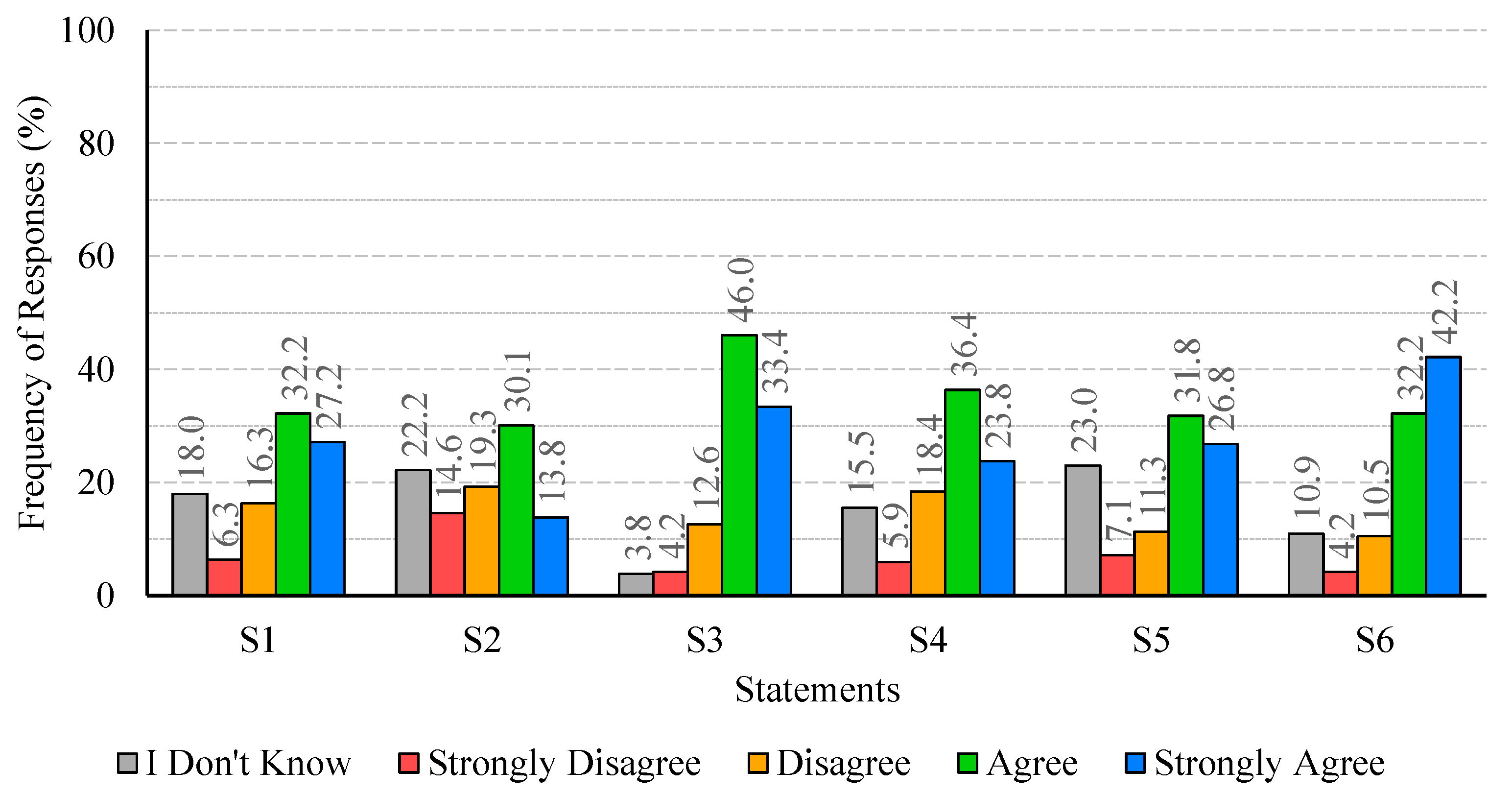
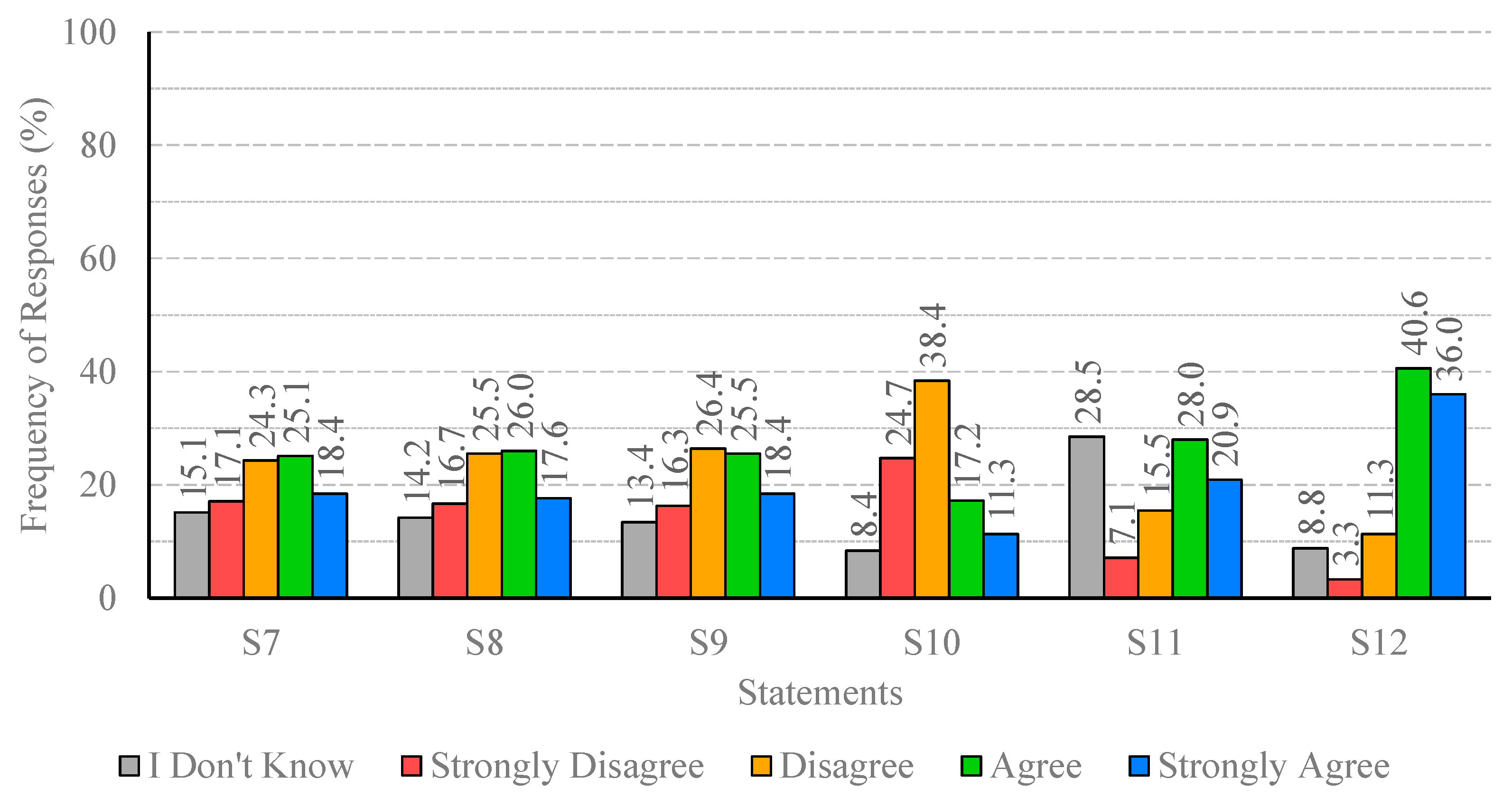
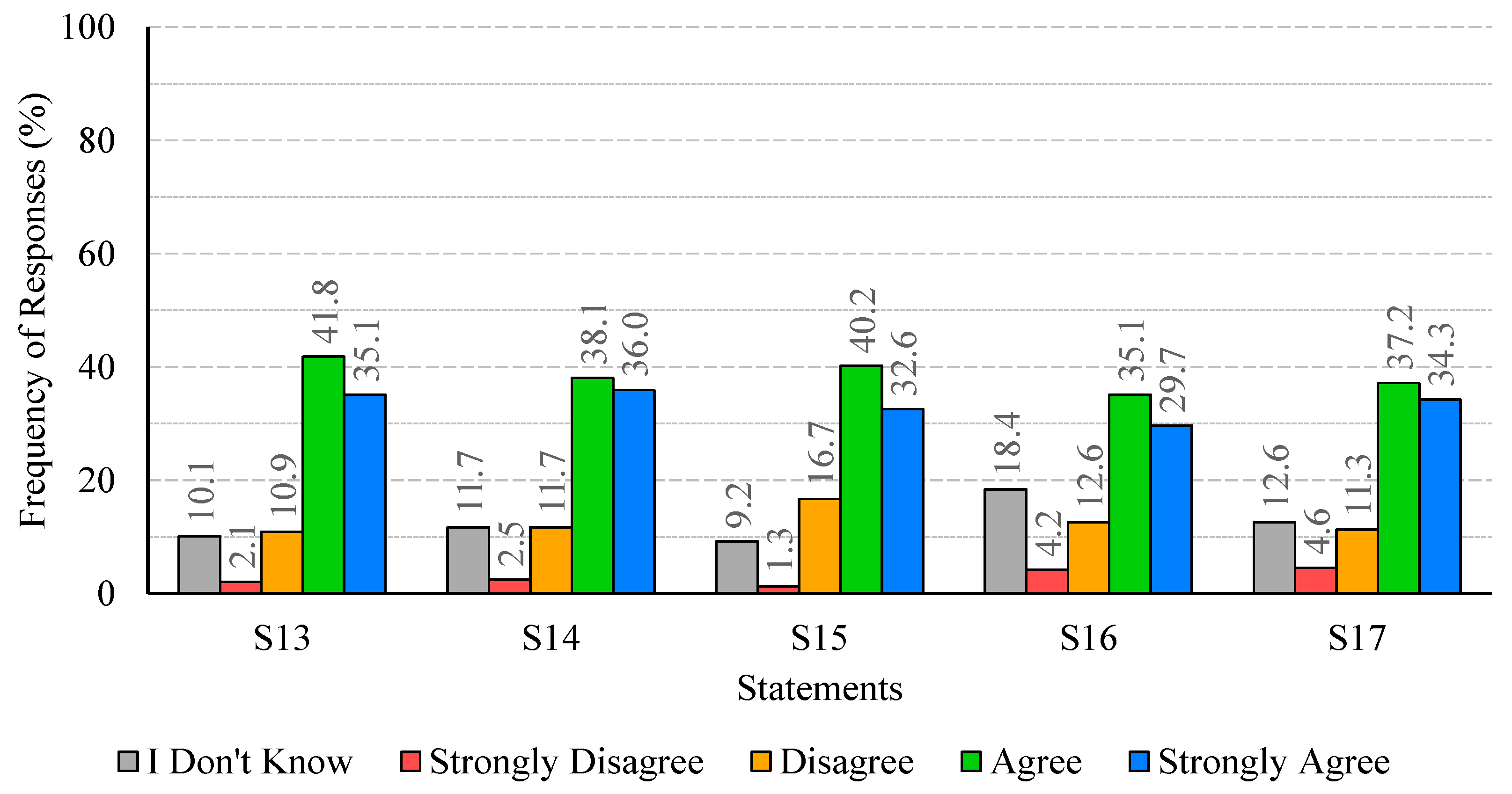
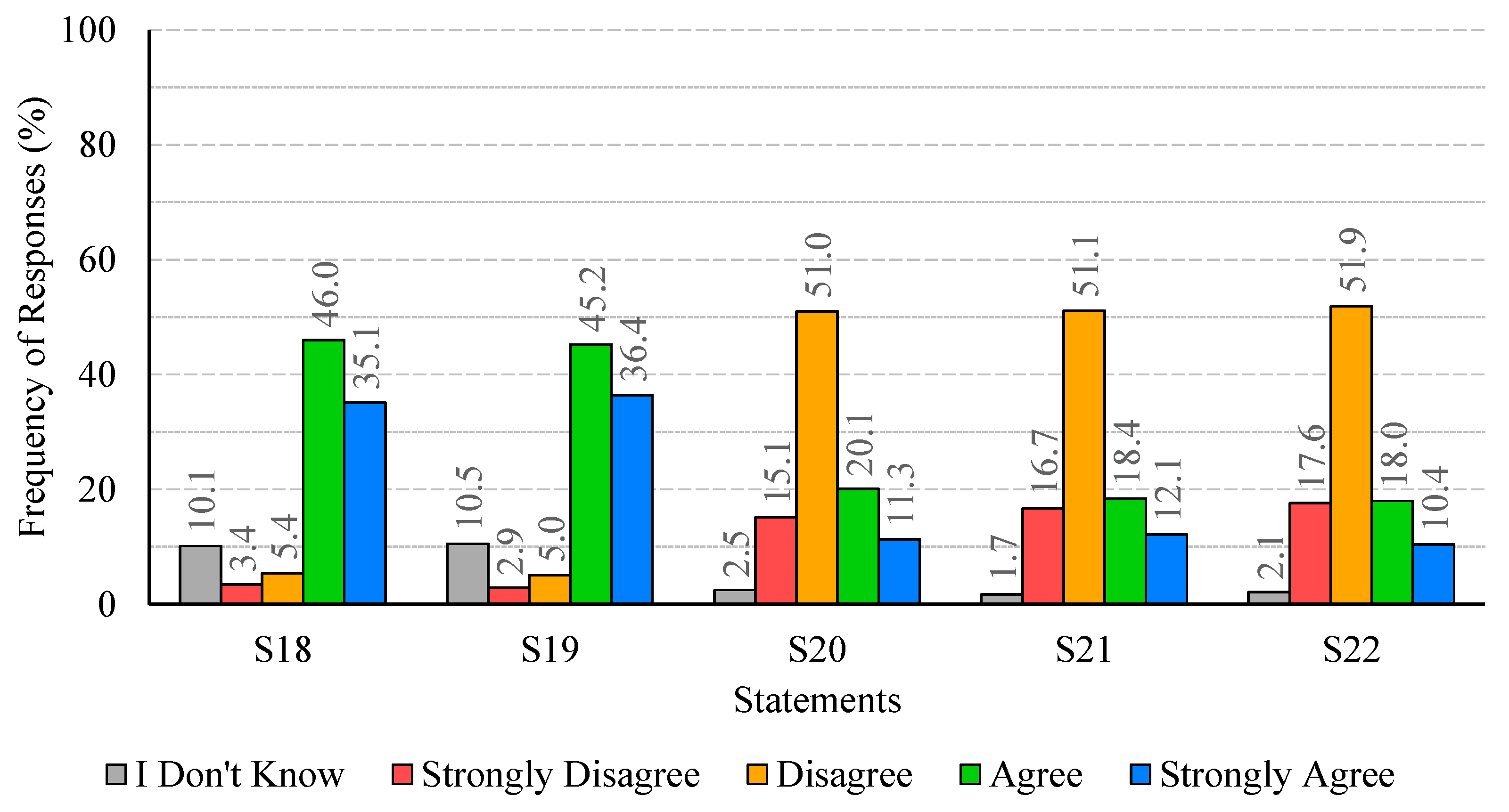
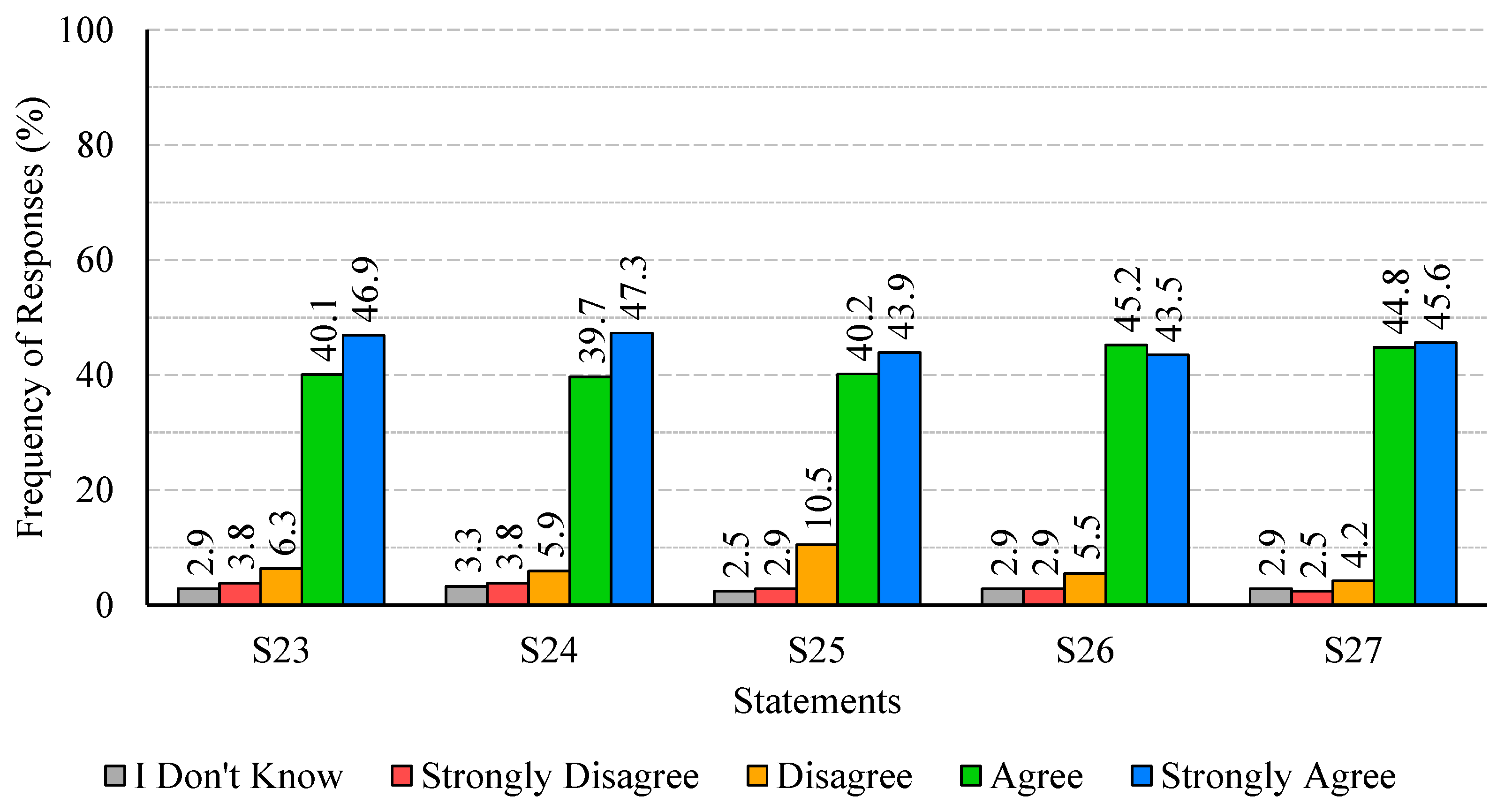
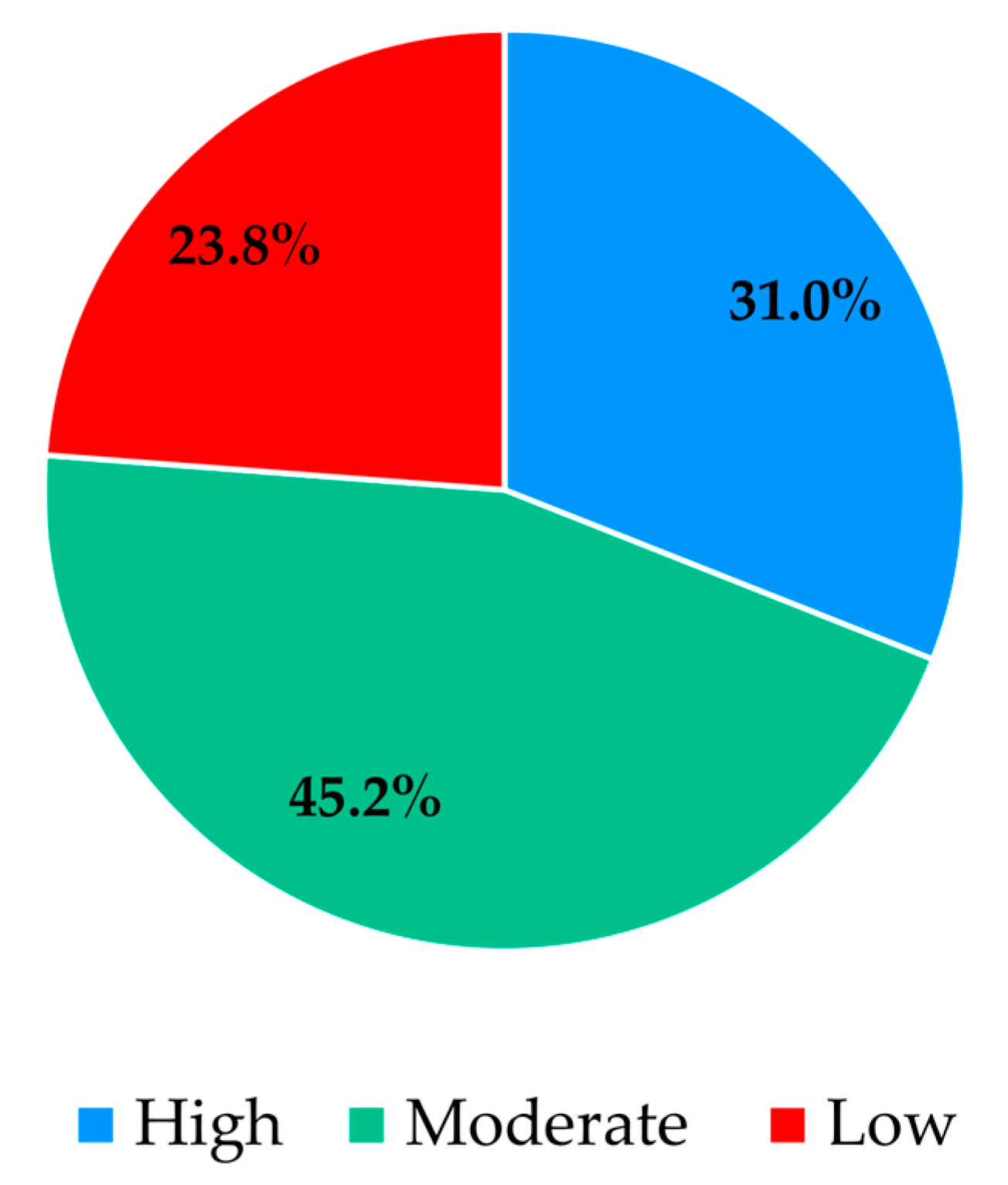
The analysis of participants’ responses reveals a generally high level of risk perception and knowledge regarding Legionella infections, indicating awareness of the severity and potential health impacts of the disease. Despite this, critical gaps persist, particularly in understanding the main transmission pathways and the influence of temperature on bacterial growth. These gaps suggest that targeted educational interventions are needed to reinforce knowledge on key aspects of infection dynamics and environmental factors that facilitate bacterial proliferation. With regard to confidence in water supply systems and associated safety measures, responses were heterogeneous. While certain procedures, such as the investigation of suspected cases by authorities, were positively recognized, substantial disagreement and uncertainty were observed regarding risk communication and routine maintenance practices. This highlights the importance of not only maintaining safe infrastructure but also providing transparent and clear information to foster public trust and understanding. Regarding the perceived effectiveness of prevention and control measures, participants generally acknowledged disinfection, maintenance, and technological interventions, though some information gaps were observed. Clear explanations regarding the rationale and expected outcomes of these measures may support greater understanding and engagement. Participants’ willingness to adopt preventive measures involving financial costs or service disruptions was limited, despite recognition of the importance of controlling Legionella. Indeed, they show limited acceptance of financial burdens or service disruptions, which are often perceived as unfair or disproportionate. Moreover, the results indicate that participants place particular importance on investment in new technologies and on the regular maintenance of water supply systems, which are perceived as essential responsibilities of system operators. Technological improvements and maintenance may be associated with efficiency gains and enhanced safety, without causing immediate personal inconvenience. The results also show that participants feel more confident in rejecting cost- and disruption-related measures, while expressing greater uncertainty regarding the technical aspects of new technologies and maintenance practices. This pattern reflects a degree of lack of knowledge or unfamiliarity with preventive strategies, underscoring the need for clearer communication from responsible authorities to strengthen public understanding and acceptance of Legionella control measures. Finally, participants exhibited strong awareness of the broader public health impact of Legionella and concern regarding exposure risks. The predominance of positive responses reflects recognition of both the severity of the disease and the importance of timely detection in preventing outbreaks. Nevertheless, a minority of participants underestimated the public health implications or questioned the effectiveness of preventive measures, indicating areas where additional education or clarification could further strengthen understanding, particularly in relation to preventing waterborne diseases.
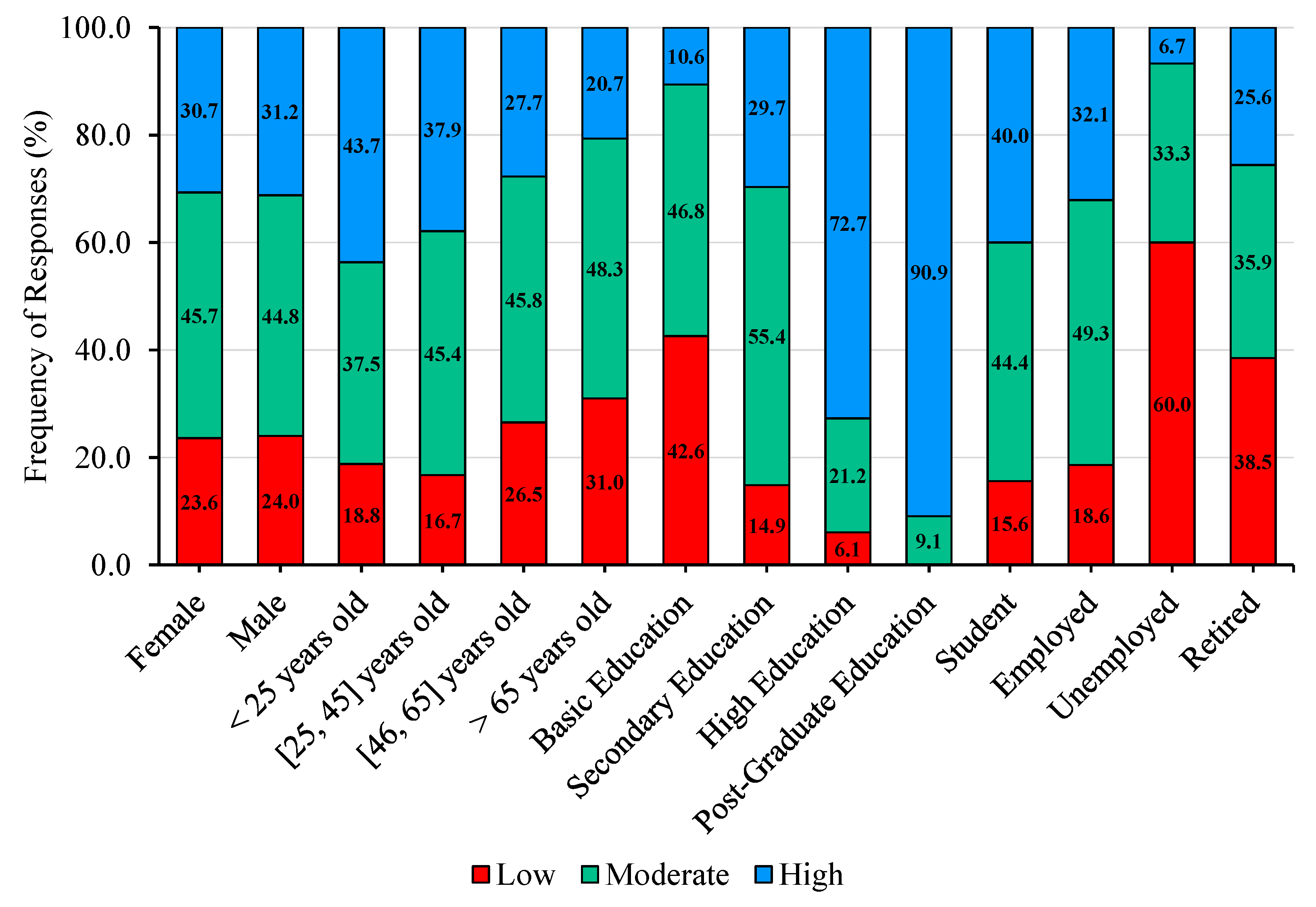
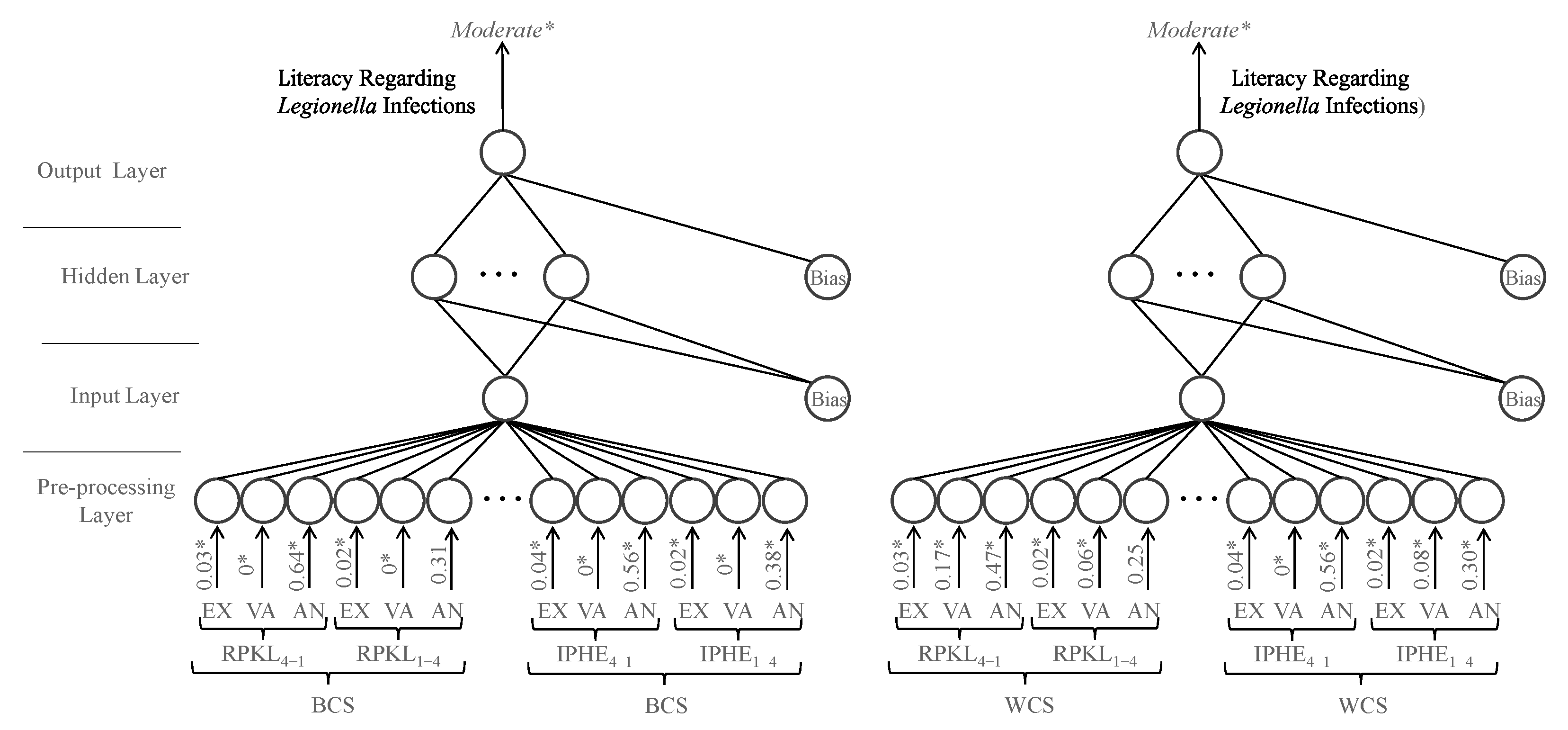
The study of the influence of socio-demographic factors on participants’ self-assessed literacy reveals a clear pattern, with the percentage of participants reporting high literacy decreasing with increasing age, while the percentage reporting low literacy generally increases across age categories. These results are consistent with the findings of Abu-Shakra [
51], who reported in a study conducted in an urban community in North Carolina that age was a strong predictor of awareness, with significant differences across age groups in knowledge regarding the causes, prevention, treatment, and affected body systems of
Legionella infections. Regarding educational attainment, the results suggest that participants with lower levels of education tend to report low or moderate literacy, whereas those with higher qualifications tend to report high literacy. This pattern contrasts with Abu-Shakra [
51], who found that education had a limited effect on literacy, possibly reflecting contextual or methodological differences between the studies. Finaly, a pattern emerges in which the percentage of participants reporting high literacy decreases among the unemployed and retired, while the proportion reporting low literacy increases. Conversely, students and employed participants exhibit the opposite trend. Overall, the findings highlight variations in self-assessed literacy according to socio-demographic factors, including age, educational attainment, and work status, suggesting that life experience, educational background, and professional engagement play a role in shaping participants’ knowledge about
Legionella infections.